Abstract
To achieve the efficient utilization of magnesium slag, this study investigates the use of magnesium slag, fly ash, and metakaolin as partial substitutes for cement in cementitious materials. The reactivity of these materials is assessed based on the compressive strength of mortar. The response surface methodology is employed to explore the influence of material proportions on the strength performance of cement mortar. The mechanisms underlying strength development in the composite system are examined through XRD, SEM, TG-DTG, and BET analyses. Additionally, the effect of magnesium slag on the drying shrinkage properties of cement mortar is studied. The experimental results indicate that magnesium slag exhibits low reactivity and cannot be used alone as an active admixture. The optimal proportion of magnesium slag, fly ash, metakaolin, and cement is 10:10:10:70, achieving over 80% of the strength of pure cement mortar and approximately 1.5 times the strength of cement mortar containing 30% magnesium slag. Furthermore, magnesium slag helps mitigate the volume shrinkage caused by drying in cement mortar. Therefore, this study can facilitate the comprehensive utilization of magnesium slag in the construction sector, reducing its negative impact on the ecological environment.
1. Introduction
With the continuous advancement of the global economy, the surge in construction activities has led to an unprecedented increase in cement demand. [1,2,3]. However, the cement industry’s heavy reliance on energy and its substantial carbon footprint poses significant environmental challenges [4,5]. Furthermore, cement usage generates considerable waste, exacerbating environmental pollution and degradation. To address these issues and foster sustainable development within the cement sector, there is a pressing need to innovate and develop alternative materials that can partially or fully replace traditional cement [6,7].
In the realm of magnesium resource extraction and production, a byproduct known as magnesium slag is generated in vast quantities—up to 5 to 7 tons for every ton of magnesium metal produced [8,9,10]. The majority of magnesium slag, with a utilization rate below 30%, ends up in landfills or abandoned lands, consuming valuable land resources and polluting the environment [11,12]. Research indicates that industrial alkaline resources can be utilized through carbonation, resulting in carbonate products that serve as potential cementitious materials applicable to buildings or mining backfill materials [13,14]. However, the low hydration activity characteristic of magnesium slag presents challenges for its use as a standalone alternative to cementitious materials. In practical applications, it is often necessary to incorporate other reactive components to enhance the performance of the cementitious system. Magnesium slag exhibits unique reaction stability, which does not interfere with the hydration process of other highly reactive supplementary materials. This characteristic makes the integration of various highly reactive mineral admixtures with magnesium slag into the cementitious system a feasible approach to significantly enhance the hydration performance of the system.
Additionally, fly ash, a primary solid waste produced by thermal power plants, is highly versatile and is widely used in research on composite cementitious materials [15,16]. Another promising material is metakaolin, a highly reactive mineral admixture obtained from calcined ultrafine kaolin. The amorphous aluminosilicates in metakaolin exhibit pozzolanic activity, which interacts with calcium hydroxide during cement hydration to form gels, thereby enhancing the strength of concrete [17,18,19]. Currently, although there is extensive research on using magnesium slag, fly ash, and other cement substitutes, studies focusing on the synergistic mechanisms among various replacement components in cementitious systems remain limited [20,21,22]. This underscores the necessity for research focused on the application of metallurgical solid waste with potential hydration characteristics within multifaceted cementitious systems.
This study investigates the strength performance and volume stability of mortar containing magnesium slag, fly ash, metakaolin, and cement. The response surface methodology is used to analyze the mechanical properties of the mortar. Techniques such as X-ray diffraction (XRD), thermogravimetric analysis and derivative thermogravimetric analysis (TG-DTG), Brunauer–Emmett–Teller (BET) analysis, and scanning electron microscopy (SEM) are employed to explore the mechanisms by which various components affect mortar strength. This research aims to promote waste utilization and reduce environmental pollution.
2. Materials and Methods
2.1. Materials
The magnesium slag used in the experiment is from Jinchuan Magnesium Company in Yulin, China. Fly ash utilized in this study is sourced from a coal gangue power plant in China and underwent desulfurization in a furnace, with a fineness of 11% (45 μm sieve residue). Metakaolin is purchased from Inner Mongolia Chaopai Metakaolin Co., Ltd. in Inner Mongolia, China, with a median particle size of 4.02 μm. The cement utilized in this experiment is ordinary Portland cement (P.O.), specifically the Hai Luo brand, with a grade of 42.5. The chemical compositions of the magnesium slag, fly ash, metakaolin, and cement are detailed in Table 1. Key performance metrics of cement are listed in Table 2.

Table 1.
Main chemical composition of magnesium slag, fly ash, metakaolin, and cement.

Table 2.
Key performance metrics of cement.
2.2. Mix Proportions
2.2.1. Admixture Activity Test
The mix proportion of the admixture activity test of magnesium slag, fly ash, and metakaolin are provided in Table 3.

Table 3.
Mix proportions of the admixture activity test.
2.2.2. Mix Design of Strength Test
After consulting previous studies in the literature and conducting preliminary experiments, the dosage range for magnesium slag, fly ash, and metakaolin was set to be 0~20%, utilizing a water-to-cement ratio of 0.42. Thirteen experimental schemes were generated, as detailed in Table 4.

Table 4.
Mix proportions of the strength test.
2.2.3. Mix Design of Dry Shrinkage Test
Cement specimens decrease in volume due to internal hydration, which causes the specimen to shrink. The expansion of magnesium slag occurs post-hydration owing to the presence of unbound calcium oxide and magnesium oxide. Therefore, mixing magnesium slag into cement specimens can compensate for the self-shrinkage of cement specimens. In this paper, a separate experimental program is designed. A range of 0~30% of magnesium slag is mixed in cement mortar so as to explore the effect of magnesium slag on the dry shrinkage properties of cement mortar. The controlled drying environment is set at 20 °C with 50% relative humidity, and the mix proportions of the dry shrinkage test are provided in Table 5.

Table 5.
Mix proportions of the dry shrinkage test.
2.3. Test Methods
2.3.1. Optimization Method Based on Response Surface Methodology
Response surface methodology is primarily used for optimizing experimental processes [23]. It optimizes experimental content while considering random errors as much as possible and aims to minimize the number of experiments while obtaining reliable results. By fitting the experimental results obtained from the response surface method, a surface model can be obtained, and reasonable ranges of various influencing factors can be determined [24,25]. In this experiment, response surface methodology will be used to determine the optimal proportions of raw materials based on the flexural and compressive strengths at 7 days and 28 days. The primary aim of this experiment is to optimize the proportion of magnesium slag while meeting certain strength conditions.
2.3.2. Strength and Activity Test
Mortar specimens are prepared in accordance with Chinese national standards, and the flexural and compressive strengths of the mortar are tested at 7 days and 28 days [26]. The activity indices of magnesium slag, fly ash, and metakaolin are also determined following national standards [27].
2.3.3. Dry Shrinkage Test
Cement mortar samples are prepared according to Chinese industry standards, and the 28-day drying shrinkage is measured [28].
2.3.4. Microscopic Test
Samples cured to the specified age are crushed, and fragments approximately 2 mm in diameter are selected from the core and immersed in anhydrous ethanol to halt the hydration reaction. The hydration-stopped samples are then transferred to an oven at around 40 °C to dry for 48 h. Afterward, the dried samples are ground into powder. X-ray diffraction (XRD) analysis of the powder samples was carried out using a model D8 ADVANCE X-ray diffractometer from Bruker, Karlsruhe, Germany, with a 2θ angle range set from 10° to 65° at a scan rate of 10°/min. Thermal analysis of the analyzed powder samples was carried out using a Simultaneous Thermal Analyzer Model STA 449 F3 from Netsch, Selb, Germany. The pore structure characteristics of the samples were analyzed using a surface area and porosity analyzer model 3Flex from Micromeritics, Norcross, GA, USA. To halt hydration, samples approximately 5 mm in size from the core are immersed in anhydrous ethanol and then dried in an oven. The surface of the dried sample was gold sprayed and examined in detail using a scanning electron microscope model Gemini Sigma 300 from Zeiss, Oberkochen, Germany. Note that the SEM and XRD tests in this paper are for qualitative analysis only.
3. Results and Discussion
3.1. Activity Analysis of Materials Used in Cementitious Systems
The 28-day activity index of magnesium slag, fly ash, and metakaolin is shown in Table 6, and their strength properties at various ages are shown in Figure 1. It can be observed from Table 6 that the 28-day activity index of magnesium slag stands at 55.15%, while that of fly ash and metakaolin is 92.49% and 94.64%. When supplementary materials are used as active admixtures, an activity index greater than 70% is required [27]. Magnesium slag does not meet this requirement, while the activity index of fly ash and metakaolin meets the requirements for active admixtures.

Table 6.
The 28-day activity index.
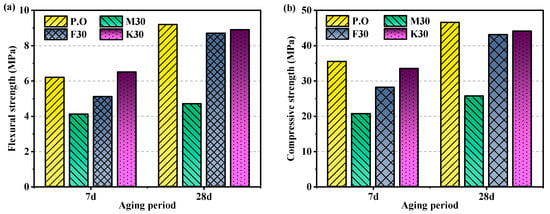
Figure 1.
Flexural and compressive strength at 7 days and 28 days: (a) flexural strength and (b) compressive strength.
The 7-day compressive and flexural strengths with 30% magnesium slag notably fall below those of the control group. As time progresses, both flexural and compressive strengths decrease significantly, with the 28-day compressive strength being only 55.15% of that of the control group. Analysis suggests that the low early strength of specimens is due to the low content of early hydration mineral C3S and the slow reaction rate of the main cementitious mineral C2S in magnesium slag compared to C3S. Although magnesium slag with longer placement time contains more C2S internally, its crystal form is γ-type, and γ-C2S has almost no hydration activity, leading to volume expansion and structural pulverization [29,30]. Additionally, the weaker early hydration capability of minerals in magnesium slag does not create a favorable environment for the hydration of β-C2S, resulting in low 28-day compressive strength [10]. This primarily accounts for why the compressive strength of magnesia cement mortar significantly lags behind that of cement mortar. Furthermore, the high replacement ratio (30%) significantly reduces the cement content in the system, slowing down the hydration rate and reducing the amount of hydration products. Therefore, this leads to a gradual decrease in strength [10]. Fly ash and metakaolin contain active substances that can react with cement hydration products and contribute to strength formation.
3.2. Strength Curve Fitting and Response Surface Analysis of Composite Cementitious Materials
3.2.1. Establishment of Regression Equations
The linear, two-factor, quadratic model for 28-day compressive and flexural strengths is regressed and analyzed. Table 7 and Table 8 present the goodness-of-fit analysis for the three models of compressive and flexural strengths. The coefficient of determination, R2, is used to evaluate the degree of fit and to search for the optimal model equations.

Table 7.
Analysis of compressive strength fitting.

Table 8.
Analysis of flexural strength fitting.
According to the data in Table 7, the R2 of the quadratic model is 0.9576, which indicates that the model has an explanatory rate of 0.9576 for the experimental results. Relative to the other two models, the quadratic model exhibits a higher degree of fit. Therefore, it is recommended to use the quadratic model for compressive strength. According to the data in Table 8, the R2 of the quadratic model is 0.9330, which indicates that the model explains the experimental results with a rate of 0.9330, and this model has a higher degree of fit relative to the other two models. Therefore, it is recommended to use the quadratic model for flexural strength. According to the quadratic model, the compressive and flexural strengths form a regression equation, refer to Equations (1) and (2):
In the equations, A represents magnesium slag, B represents fly ash, and C represents metakaolin.
To better illustrate the credibility of the fitted models for compressive and flexural strength, confirmation is provided through a comparison between measured values and predicted values in Figure 2. In the graph, the scattered points represent the measured values, while the diagonal line represents the predicted values. The greater the distance between points and the diagonal line, the lower the accuracy of prediction. The smaller deviations above and below the diagonal line indicate lower errors. From Figure 2, it can be observed that the points are evenly distributed and close to the diagonal line, with no outliers present. This indicates that the model’s predicted results closely align with the actual values, demonstrating a good fit for both compressive and flexural strength models and exhibiting high applicability.
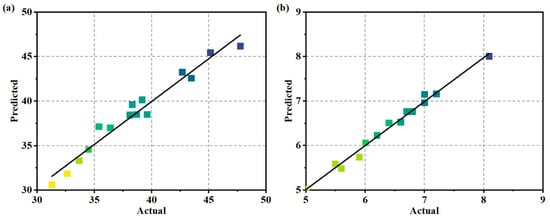
Figure 2.
Distribution of actual and predicted values for strength: (a) compressive strength and (b) flexural strength.
It is important to note that Equations (1) and (2) are onlyapplicable to materials with similar chemical compositions. The chemical composition of fly ash and other hydraulic binders has a significant impact on strength, as will be confirmed in subsequent studies. Therefore, when necessary, the quadratic model should be reconstructed for further research.
3.2.2. Analysis of Variance
The models and parameters used in this experiment are within a 95% confidence interval, implying that when the p-value drops below 0.05, the selected models and parameters are considered significant. Table 9 and Table 10 list the variance analyses for the influencing factors of 28-day compressive and flexural strengths, respectively. From the data in the tables, the p-values for both strengths fitted models are less than 0.05, indicating that the models established using the response surface method are all significant, with a confidence level exceeding 95%. The significance ranking of the influencing factors for compressive and flexural strength is C > A > B.

Table 9.
Analysis of variance for 28-day compressive strength.

Table 10.
Analysis of variance for 28-day flexural strength.
3.2.3. Response Surface Model Analysis
Contour plots and surface response plots of the changes in the compressive strength response index under the action of the two factors are plotted in Figure 3 and Figure 4, where the third factor is kept at the median value. The greater the degree of deformation of the response surface plot, the greater the degree of influence between the factors on the response index.
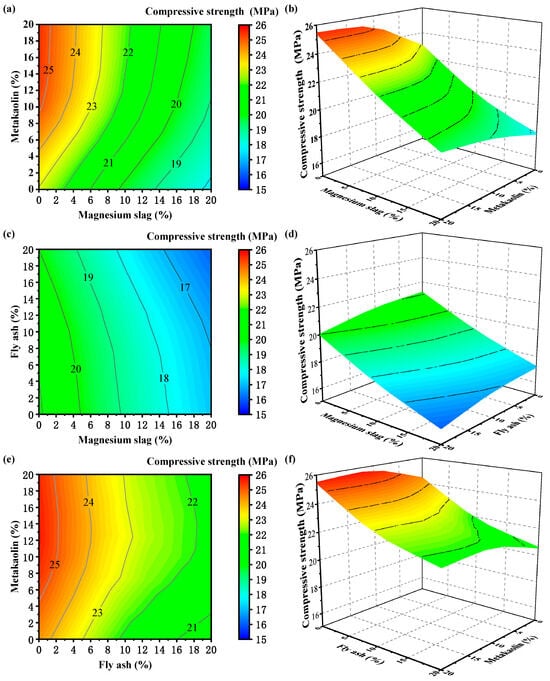
Figure 3.
Contour and 3D surface plot of compressive strength at 3 days: (a) contour plot—magnesium slag and metakaolin; (b) 3D surface plot—magnesium slag and metakaolin; (c) contour plot—magnesium slag and fly ash; (d) 3D surface plot—magnesium slag and fly ash; (e) contour plot—fly ash and metakaolin; and (f) 3D surface plot—fly ash and metakaolin.
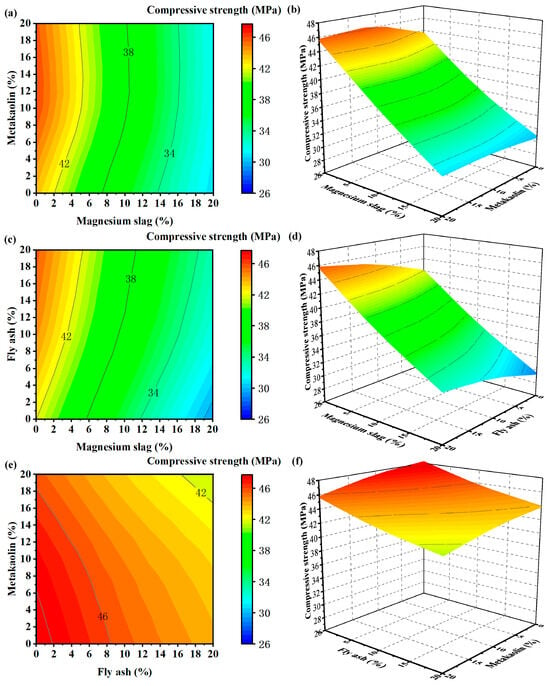
Figure 4.
Contour and 3D surface plot of compressive strength at 28 days: (a) contour plot—magnesium slag and metakaolin; (b) 3D surface plot—magnesium slag and metakaolin; (c) contour plot—magnesium slag and fly ash; (d) 3D surface plot—magnesium slag and fly ash; (e) contour plot—fly ash and metakaolin; and (f) 3D surface plot—fly ash and metakaolin.
Figure 3 illustrates the influence patterns of fly ash, metakaolin, and magnesium slag on the 3-day compressive strength of glued sand specimens. Figure 3a,e show that the compressive strength initially rises and subsequently declines with the dosage escalation of metakaolin and attains a peak compressive strength within the dosage range of 10% to 15% metakaolin. Keeping the metakaolin dosage constant at 10%, the compressive strength gradually diminishes as the dosage of fly ash and magnesium slag increases. According to the contour sparsity, the unfavorable effect of magnesium slag on compressive strength is more obvious, and the adverse effect of fly ash also exists, but it is obviously smaller. And about 10% of metakaolin can reduce this adverse effect. Figure 3c demonstrates that fly ash is not able to combine with magnesium dross alone to enhance the strength of cement mortar. The 3D graph highlights the important role of metakaolin in the early enhancement of compressive properties with an optimum dosage of around 10%. It is evident from Figure 3b,d,f that the curvature of the surface plots varies significantly, and the specimens’ compressive strength experiences a notable decrease as the dosage of fly ash and magnesium slag increases. The reason may be twofold. On one side, metakaolin is a volcanic ash material with high activity and can react quickly with alkali to generate cementitious materials in the early stage to produce high early strength [17]. But the magnesium slag and fly ash did not undergo a significant hydration reaction, and the content of the gel material generated is small, which cannot play the role of gelling and bonding of the cement particles [31,32]. It mainly plays the role of filler in the early stage of hydration, which reduces the early strength. On the other side, in the case of large dosages, fly ash particles and magnesium slag particles are easy to wrap around the particles of kaolin and cement, hindering the early hydration of cement and kaolin, resulting in a decrease in the early compressive strength. Comprehensively, metakaolin has the most positive influence on the early strength of the system. The combination of metakaolin and fly ash had the best effect, followed by metakaolin and magnesium slag, and finally, fly ash and magnesium slag.
From Figure 4, once the hydration period extends to 28 days, the influence of metakaolin on the system’s strength gradually diminishes. Meanwhile, the impact of magnesium slag dosage on the compressive strength remains significant. The reason is that the magnesium slag is less active, and the active ingredients in the early-stage hydrate generate Ca(OH)2, which provides an exciter for the hydration of fly ash and metakaolin [33]. But the remaining components have basically no hydration activity, which does not help the growth of compressive strength in the later stage. However, after magnesium slag is compounded with fly ash and metakaolin, the mechanical characteristics of mortar specimens witness enhancement. This can be attributed to the heightened volcanic ash activity of metakaolin, facilitating a secondary hydration reaction with cement hydration products. According to Figure 4c, the late hydration of fly ash is obvious, which has a great role in compensating for the missing late strength of magnesium slag.
3.2.4. Multi-Objective Optimization Based on Response Surfaces
In order to obtain the optimum dosing of magnesium slag, fly ash, and metakaolin, the regression model is optimized simultaneously with multiple objectives so that strengths reach 80% and above of the strength value of cement mortar. Based on the 28-day test data, the metakaolin is controlled at the optimum dosage of 10%. Magnesium slag is the main factor considered in this test, and as much magnesium slag as possible needs to be doped in the experiment, so the dosing range was set to 0~20%. Fly ash, as the main complex of magnesium slag, is mainly for the later stage to improve the strength, and its dosage is set at 0~20%. The final objective optimized design is shown in Table 11.

Table 11.
Multi-objective optimization design table.
As shown in Table 12, based on the maximum expected value of 0.913, 10% magnesium slag, 10% fly ash, and 10% metakaolin are the optimal dosages with compressive and flexural strengths of 38.5 MPa and 6.7 MPa, which are enhanced by 44.2% and 42.6% relative to 30% magnesium slag dosage alone. Strength can be guaranteed to reach more than 80% of pure cement mortar.

Table 12.
Response surface optimization design results.
3.3. XRD Analysis
Figure 5 displays the XRD patterns of four sets of specimens with different ratios for 3 days and 28 days. The primary hydration products of pure cement are dominated by Ca(OH)2 and C-S-H, in which C-S-H exists in the form of gel. And it cannot be reflected as a sharp signal in the XRD pattern. While Ca(OH)2 exists in the form of crystals, its main diffraction peak is located near 26.87°, and other minor diffraction peaks are located at 28.80°, 54.92°, 59.85°. For the magnesium slag-cement cementitious material, the intensity of the C2S and C3S diffraction peaks decreased significantly at 3 days of hydration when 10% of magnesium slag is doped, indicating that the hydration reaction of the low doped magnesium slag occurs in the highly alkaline environment produced by cement hydration. When hydrated for 28 days, the diffraction peak intensities of C2S and C3S are still lower than that of pure cement, and the primary and secondary diffraction peak intensities of Ca(OH)2 are higher than that of pure cement samples in the 10% doped magnesium slag net slurry samples. The above two items indicate that magnesium slag promotes cement hydration and generates more Ca(OH)2 at low dosages. When magnesium slag, cement, and fly ash are mixed together, the intensity of Ca(OH)2 diffraction peaks at 3 days of hydration is lower than that of both the pure cement sample and the magnesium slag sample alone, which indicates that Ca(OH)2 is consumed in large amount in the process of hydration. And the content of the slurry with Ca(OH)2 is less. When the age reached 28 days, the Ca(OH)2 diffraction peak intensity was still lower than that of both pure cement samples and single-magnesium slag samples. The diffraction peak intensities of C2S and C3S are higher than that of pure cement but lower than that of the specimen with single-magnesium slag, indicating that the reaction of the fly ash up to 28 days is still consuming Ca(OH)2, which facilitated the hydration reaction of the system. When magnesium slag, fly ash, and metakaolin are compounded, the diffraction peak intensity at the 3-day hydration age is comparable to that when the first three are co-doped. It is because the metakaolin contained a large amount of SiO2 and Al2O3, which consumed a substantial portion of Ca(OH)2 during the initial stages of the hydration reaction to promote the hydration process of cement and magnesium slag. After 28 days of hydration, the intensity of the Ca(OH)2 diffraction peak is much higher than that of the pure cement sample, indicating that the fly ash in the cementitious system undergoes a secondary hydration reaction, which promotes the hydration process of cement and magnesium slag.
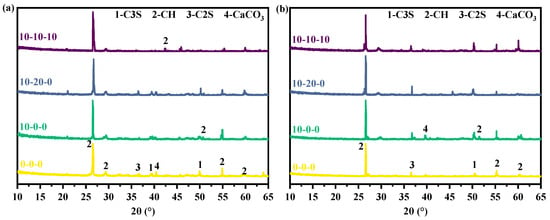
Figure 5.
XRD patterns of magnesium slag specimens: (a) 3 days and (b) 28 days.
3.4. Microscopic Morphology Analysis
The microscopic morphology of hydration for 3 days is depicted in Figure 6. The hydration products of both pure cement and composite system cementitious materials are dominated by amorphous C-S-H gel, with a minimal portion of needle-and-rod shaped ettringite (AFt) interspersed and bridged in between. At 3 days of hydration, a large amount of C-S-H gel is generated, and a minimal portion of Ca(OH)2 and AFt are interspersed with it, forming a network-like structure. In the sample with 10% magnesium slag, a minimal portion of AFt, C-S-H gel, and Ca(OH)2 are generated, and some block structure is interspersed in the gel. That is because only a minimal portion of reactive CaO inside the magnesium slag particles could undergo the hydration reaction, and the unreacted magnesium slag particles split up the hydration reaction system. And the C-S-H could not be connected into a whole, which made the early strength lower. When magnesium slag, cement, and fly ash are co-mingled, the fly ash hardly undergoes a hydration reaction, and the quantity of C-S-H gel formed is reduced. This is due to the internal vitreous body of fly ash, which contains a dense vitreous surface layer, hindering the interaction of active substances with water [34]. When magnesium slag, fly ash, and metakaolin are compounded, the metakaolin contains a large amount of active SiO2 and Al2O3, which forms hydrated calcium silicate in the early stage. Al3+ also replaces Si4+ in the silica-oxygen tetrahedra to form aluminosilicate gels, which are uniformly distributed in the system to form a dense network-connected structure that provides high early strength [35].
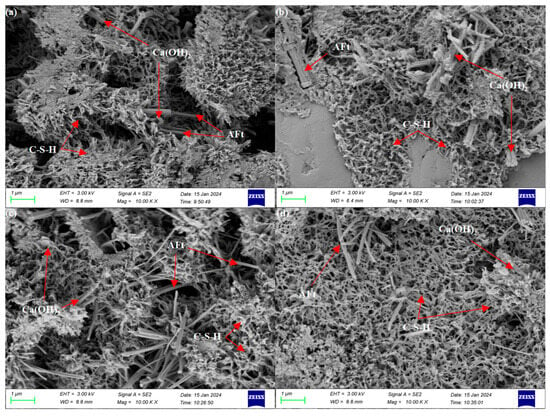
Figure 6.
SEM images of various samples at 3 days: (a) 0-0-0, (b) 10-0-0, (c) 10-20-0, and (d) 10-10-10.
The microscopic morphology of hydration for 28 days is shown in Figure 7. The C-S-H gel inside the pure cement sample formed an interconnected structure after continuous hydration and gradually formed a denser, hardened slurry. When magnesium slag is co-mingled with cement, a large amount of magnesium slag does not undergo hydration and still exists in the form of particles inside the hardened slurry. Since there is no network-like structure formed, it leads to a significant decrease in 28-day mechanical properties. Cement, magnesium slag, and fly ash composite can promote each other’s hydration process. The Ca(OH)2 generated through cement and magnesium slag hydration serves as the activator and material source for the fly ash hydration reaction. Ca(OH)2 increases the concentration of OH− and Ca2+ in the slurry, and fly ash particles are subjected to the action of OH− in the slurry. Fly ash surface ionizes SiO44− and AlO2− ions, which then react with the Ca2+ in the slurry to produce hydrated calcium aluminates and hydrated calcium silicate, thereby enhancing the strength in later stages. When cement, magnesium slag, fly ash, and metakaolin are compounded, a considerable number of C-S-H gels that had already coagulated and hardened existed inside, interconnecting to form a network-like dense structure. The early hydration reaction of metakaolin consumed more Ca(OH)2, resulting in slower erosion of fly ash vitreous and delayed hydration reaction. However, the continuous consumption of Ca(OH)2 by both metakaolin and fly ash can double promote the hydration of cement and magnesium slag. Therefore, after 28 days of hydration, there is still C-S-H gel being generated inside the hardened slurry, which promotes the continuous strength growth of the hardened slurry in the later stage.
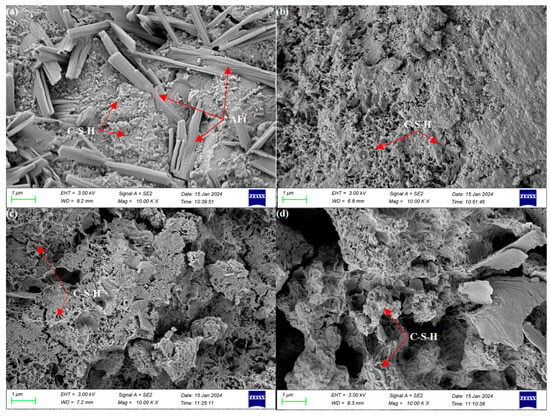
Figure 7.
SEM images of various samples at 28 days: (a) 0-0-0, (b) 10-0-0, (c) 10-20-0, and (d) 10-10-10.
3.5. Thermal Analysis Based on TG-DTG Curves
The TG-DTG curves of the composite cementitious system at 3-day and 28-day hydration are depicted in Figure 8. Dehydration of the main hydration products of cementitious materials occurs at 50 °C~600 °C. AFt dehydrates near 100 °C, and C-S-H gel dehydrates at 50 °C~450 °C. Ca(OH)2 dehydrates at 450 °C~550 °C to produce CaO. When the temperature increases to 700 °C~800 °C, CaCO3 decomposes to form CaO. It can be seen that the TG-DTG curves of several groups of blended pastes are basically the same, and three weight loss stages are evident, each corresponding to three peaks of heat absorption. C-S-H exists in a gel state without forming weight loss or heat absorption peaks. However, its dehydration occurs throughout the entire process. The DTG curves of both composite cementitious systems and pure cement have an obvious heat absorption peak near 100 °C, which is caused by the dehydration of ettringite and C-S-H gel [36]. The DTG curves of pure cement and specimens with magnesium slag alone have a distinct heat absorption peak near 470 °C. This is a dehydration reaction of Ca(OH)2, whereas the peak did not appear in the test group doped with fly ash, indicating that the cementitious material containing fly ash is completely consumed by the generated Ca(OH)2 at the late stage of the hydration reaction. At 3 days, the TG curves showed two significant weight loss changes in both pure cement and composite cementitious systems around 100 °C and 470 °C. With the overall weight loss of pure cement reaching, the weight loss of pure cement is 5.9%, while that of composite cementitious system is around 4.7%. At 28 days, the TG curves showed that there are two obvious weight loss changes in both pure cement and composite cementitious systems at around 150 °C and 470 °C, with an overall weight loss of 7%. Due to the high activity of metakaolin, Ca(OH)2 produced by early hydration is consumed in large quantities, and Ca(OH)2 is consumed by fly ash in large quantities at the hydration age of 28 days, so the content of Ca(OH)2 is low.
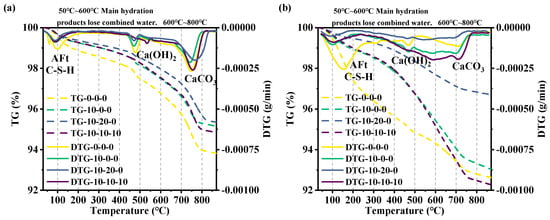
Figure 8.
Hydration TG-DTG curves: (a) 3 days and (b) 28 days.
3.6. Pore Analysis Based on BET Test
The adsorption of specimens with different ratios at different ages tested by BET is shown in Figure 9, and the pore distribution is shown in Figure 10 and Table 13. When P/Po is between 0 and 0.4, almost no N2 adsorption occurred. When P/Po is between 0.4 and 1, a hysteresis loop appears in the region. In accordance with the IUPAC classification criteria, the adsorption–desorption isotherms are found to belong to type IV, while the samples had H3 hysteresis loops in the region of high relative pressures, indicating that the pore structure is incomplete [37]. There is a significant difference in the pore size distribution among the four groups of samples. The average pore size increases in the hardened samples doped with magnesium slag alone and with both magnesium slag and fly ash at the hydration age of 3 days. This is because fly ash and magnesium slag have low hydration rates in the early stage and cannot form the gel material with a smaller pore size. In contrast, metakaolin can rapidly undergo a hydration reaction to yield C-S-H gel, resulting in significant production of gel material to occupy the large pores [38]. Therefore, the average pore size of the hardened specimen with magnesium slag, fly ash, and metakaolin is reduced. When the hydration age is 28 days, single doping of magnesium slag will still make the average pore size increase. There are two reasons for this. One is that the fineness of magnesium slag particles is larger than cement particles, which cannot fill some large pores by physical filling. The second is that the magnesium slag particles themselves are less active, and it is difficult to generate the gel material with smaller pore size by sufficient hydration reaction. It is noteworthy that the average pore size of the hardened specimens, post-compounding with magnesium slag and fly ash, is smaller than that of the reference specimens composed solely of pure cement. This is due to the involvement of fly ash in the later stages of the hydration reaction, which is consistent with the SEM observations. Whereas, when magnesium slag, fly ash, metakaolin, and cement are compounded, the highly reactive metakaolin and fly ash undergo a full hydration reaction, generating a large amount of gelatinous material and other products that fill the voids of the reactants, and hence the average pore size decreased.
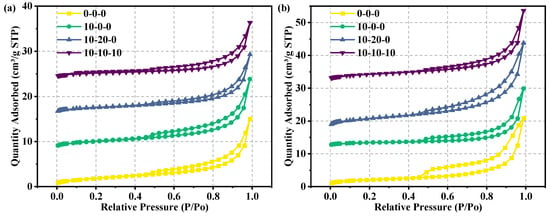
Figure 9.
BET test results: (a) 3 days and (b) 28 days.
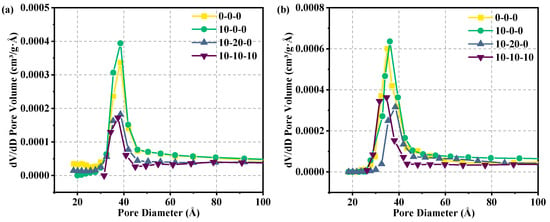
Figure 10.
Pore size distribution of various samples: (a) 3 days and (b) 28 days.

Table 13.
Corresponding structural pore structure parameters.
3.7. Analysis of Drying Shrinkage Test of Cement Mortar Specimens
Figure 11 illustrates the impact of magnesium slag on the dry shrinkage properties of cement mortar. The findings indicate that magnesium slag exerts a notable influence on the dry shrinkage of the specimens. With the increase in magnesium slag dosage, the specimen dry shrinkage decreased significantly. When the magnesium slag substitution amount is 10% and 20%, the dry shrinkage percentage of the specimen at the age of 28 days is 0.054% and 0.032%, which is obviously smaller than the dry shrinkage percentage of the pure cement specimen (0.089%). This indicates that magnesium slag can alleviate the dry shrinkage of cement mortar. The magnesium slag used in this test mainly contains CaO, Fe2O3, SiO2, and MgO. The content of CaO is about 56%, and the content of MgO is about 6.3%, which are the main reasons for the expansion observed in magnesium slag-cement composite mortar. During the prehydration phase, the CaO and β-C2S on the surface of magnesium slag particles undergo initial hydration, resulting in the formation of Ca(OH)2 crystals and hydrated calcium silicate gel. And the crystals and gel formed a structure between the fine sand and filled the pore space, slowing down the migration of water to the inside of the particles. With the prolongation of hydration time, water gradually penetrates into the interior of the specimen. The substantial presence of MgO and CaO within the magnesium slag facilitates a reaction with water, producing expansion products such as Mg(OH)2 and Ca(OH)2. This reaction induces volumetric expansion across the entire specimen [38]. Therefore, mixing magnesium slag in cement mortar can alleviate the dry shrinkage of cement mortar.
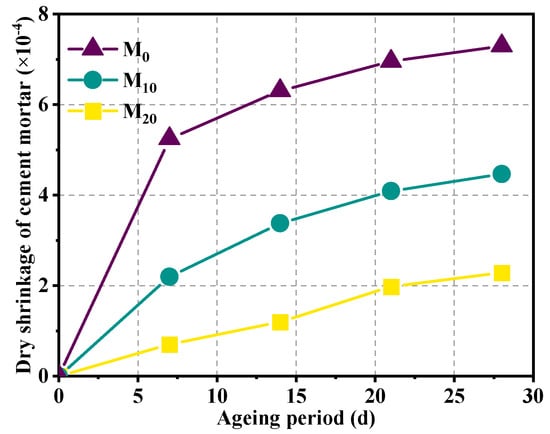
Figure 11.
Effect of magnesium slag on dry shrinkage of cement mortar.
4. Conclusions
This paper explores the feasibility of using fly ash, magnesium slag, and metakaolin as cementitious materials instead of cement. The optimal ratio of fly ash, magnesium slag, metakaolin and cement and the mechanism of strength formation are investigated from macro and micro perspectives. The effect of magnesium slag on the drying and shrinking properties of cement mortar is also investigated. The principal conclusions are outlined as follows:
- (1)
- The activity of magnesium slag is low, and the 28-day activity index is only 55.15%, which cannot be utilized as an admixture alone in cement mortar and concrete as a cementitious material. Fly ash and metakaolin have higher activity, with a 28-day activity index of 92.49% and 94.64%, and can be used alone as active mixing material.
- (2)
- The best ratio of magnesium slag, fly ash, metakaolin, and cement of the four elements of the composite system is 10:10:10:70, the flexural strength and compressive strength of the cement mortar is 142.6% and 144.2% of the cement mortar mixed with 30% of magnesium slag alone, and it can reach more than 80% of the strength of pure cement mortar.
- (3)
- Magnesium slag can alleviate the volume shrinkage caused by drying cement mortar. Magnesium slag contains CaO and MgO inside, which undergoes expansion after hydration, thus suppressing the volume change caused by dry shrinkage of cement mortar.
Author Contributions
Conceptualization, F.W.; software, H.X.; investigation, J.Z.; data curation, Z.H. writing—original draft preparation, F.W., Z.H., X.C. and B.G.; writing—review and editing, X.C. and B.G.; supervision, B.G.; funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
We received financial support from the China Construction Corporation Research Project (No. CSCEC-2022-Z-2).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Fulu Wei was employed by the company China Construction Eighth Engineering Division Co., Ltd. Author Hairong Xiao and Jia Zhang was employed by the company China Construction Civil Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ma, X.D.; He, T.S.; Da, Y.Q.; Xu, Y.D.; Zhao, F.W.; Sun, Y.H.; Yang, R.H. Improving the performance of incineration fly ash as cement admixture by high-temperature sintering and its toxic leaching characteristics. J. Clean. Prod. 2023, 416, 137875. [Google Scholar] [CrossRef]
- Lghalo, O.J.; Adeniyi, G.A. A perspective on environmental sustainability in the cement industry. Waste Dispos. Sustain. Energy 2020, 2, 161–164. [Google Scholar]
- Nwankwo, O.C.; Bamigboye, O.G.; Davies, E.E.L.; Michaels, A.T. High volume Portland cement replacement: A review. Constr. Build. Mater. 2020, 260, 120445. [Google Scholar] [CrossRef]
- Bentz, P.D.; Ferraris, F.C.; Jones, Z.S.; Lootens, D.; Zunino, F. Limestone and silica powder replacements for cement: Early-age performance. Cem. Concr. Compos 2017, 78, 43–56. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Luo, L.; Liu, T.T.; Hao, L.W.; Li, Y.M.; Liu, P.F.; Zhu, T.Y. A review of low-carbon technologies and projects for the global cement industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef]
- Tan, C.; Yu, X.; Guan, Y.R. A technology-driven pathway to net-zero carbon emissions for China’s cement industry. Appl. Energy 2022, 325, 119804. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.Y. Utilization of carbonated alkaline solid wastes in ordinary Portland cement-metakaolin-limestone ternary mixture: Underlying the role of low grade-sustainable calcium carbonate sources. J. Clean. Prod. 2024, 456, 142382. [Google Scholar] [CrossRef]
- Ruan, S.S.; Liu, L.; Zhu, M.B.; Shao, C.C.; Xie, L. Development and field application of a modified magnesium slag-based mine filling cementitious material. J. Clean. Prod. 2023, 419, 138269. [Google Scholar] [CrossRef]
- Ruan, S.S.; Liu, L.; Zhu, M.B.; Shao, C.C.; Xie, L.; Hou, D.Z. Application of desulfurization gypsum as activator for modified magnesium slag-fly ash cemented paste backfill material. Sci. Total Environ. 2023, 869, 161631. [Google Scholar] [CrossRef]
- Xie, G.; Liu, L.; Suo, Y.L.; Zhu, M.B.; Yang, P.; Sun, W.J. High-value utilization of modified magnesium slag solid waste and its application as a low-carbon cement admixture. J. Environ. Manag. 2023, 349, 119551. [Google Scholar] [CrossRef]
- Amini, O.; Ghasemi, M. Laboratory study of the effects of using magnesium slag on the geotechnical properties of cement stabilized soil. Constr. Build. Mater. 2019, 223, 409–420. [Google Scholar] [CrossRef]
- Liu, P.; Mo, L.; Zang, Z. Effects of carbonation degree on the hydration reactivity of steel slag in cement-based materials. Constr. Build. Mater. 2023, 370, 130653. [Google Scholar] [CrossRef]
- Song, Q.F.; Guo, M.Z.; Wang, L.; Ling, T.C. Use of steel slag as sustainable construction materials: A review of accelerated carbonation treatment. Resour. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- Li, Z.J.; Chen, J.; Lv, Z.Z.; Tong, Y.C.; Ran, J.Y.; Qin, C.L. Evaluation on direct aqueous carbonation of industrial/mining solid wastes for CO2 mineralization. J. Ind. Eng. Chem. 2023, 122, 359–365. [Google Scholar] [CrossRef]
- Deng, C.Q.; Jiang, Y.J.; Tian, T.; Yi, Y. Laboratory mechanical properties and frost resistance of vibration-compacted cement-fly ash slurry and cement-fly ash-treated macadam mixtures. Constr. Build. Mater. 2024, 419, 135555. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.F.; Liu, J.P.; Chen, X.S.; Ren, F.Z.; Chen, W.B.; Cui, H.Z. Improvement of shrinkage resistance and mechanical property of cement-fly ash-slag ternary blends by shrinkage-reducing polycarboxylate superplasticizer. J. Clean. Prod. 2024, 447, 141493. [Google Scholar] [CrossRef]
- Li, M.Y.; Zheng, K.R.; Chen, L.; Prateek, G.; Zhou, X.F.; Yuan, Q. Using metakaolin to improve properties of aged Portland cement: Effectiveness and the mechanism. Constr. Build. Mater. 2024, 428, 136299. [Google Scholar] [CrossRef]
- Reza, H.; Akbar, A.R.; Mirdarsoltany, M. Influence of metakaolin on fresh properties, mechanical properties and corrosion resistance of concrete and its sustainability issues: A review. J. Build. Eng. 2021, 44, 103011. [Google Scholar]
- Zhan, P.M.; He, Z.H.; Ma, Z.M.; Liang, C.F.; Zhang, X.X.; Abreham, A.A.; Shi, J.Y. Utilization of nano-metakaolin in concrete: A review. J. Build. Eng. 2020, 30, 101259. [Google Scholar] [CrossRef]
- Jin, K.R.; Zhou, X.M.; Wang, D.Z.; Bi, W.L.; Lu, Y.; Wang, J.H. Performance of cementitious materials prepared with magnesium slag and concrete slurry waste. J. Build. Eng. 2024, 89, 109379. [Google Scholar] [CrossRef]
- Ji, G.X.; Peng, X.Q.; Wang, S.P.; Hu, C.; Ran, P.; Sun, K.K.; Zeng, L. Influence of magnesium slag as a mineral admixture on the performance of concrete. Constr. Build. Mater. 2021, 295, 123619. [Google Scholar] [CrossRef]
- Xie, G.; Suo, Y.L.; Liu, L.; Zhu, M.B.; Xie, L.; Qu, H.S.; Sun, W.J. Mechanical grinding activation of modified magnesium slag and its use as backfilling cementitious material. Case Stud. Constr. Mater. 2023, 18, e01778. [Google Scholar] [CrossRef]
- Abdellatief, M.; Elemam, W.E.; Alanazi, H.; Tahwia, A.M. Production and optimization of sustainable cement brick incorporating clay brick wastes using response surface method. Ceram. Int. 2023, 49, 9395–9411. [Google Scholar] [CrossRef]
- Hafez, H.; Kassim, D.; Kurda, R.; Silva, R.V.; Brito, J.D. Assessing the sustainability potential of alkali-activated concrete from electric arc furnace slag using the ECO2 framework. Constr. Build. Mater. 2021, 281, 122559. [Google Scholar] [CrossRef]
- Abdellatief, M.; Elrahman, M.A.; Elgendy, G.; Bassioni, G.; Tahwia, A.M. Response surface methodology-based modelling and optimization of sustainable UHPC containing ultrafine fly ash and metakaolin. Constr. Build. Mater. 2023, 388, 131696. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- GB/T 1596-2017; Fly Ash Used for Cement and Concrete. Standardization Administration of China: Beijing, China, 2017.
- JC/T 603-2004; Standard Test Method for Drying Shrinkage of Mortar. China National Building Material Industry Press: Beijing, China, 2004.
- Yang, X.B.; Dong, F.S.; Zhang, X.Z.; Li, C.Z.; Gao, Q. Review on Comprehensive Utilization of Magnesium Slag and Development Prospect of Preparing Backfilling Materials. Minerals 2022, 12, 1415. [Google Scholar] [CrossRef]
- Xie, D.M.; Zhang, Z.P.; Liu, Z.C.; Wang, F.Z.; Hu, S.G.; Fu, J. Utilization of magnesium slag to prepare CO2 solidified fiber cement board. Constr. Build. Mater. 2024, 411, 134345. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.X.; Song, W.D. Mechanical model and strength development evolution of high content fly ash–cement grouting material. Constr. Build. Mater. 2023, 398, 132492. [Google Scholar] [CrossRef]
- Gao, M.; Dai, J.; Jing, H.J.; Ye, W.J.; Sesay, T. Investigation of the performance of cement-stabilized magnesium slag as a road base material. Constr. Build. Mater. 2023, 403, 133065. [Google Scholar] [CrossRef]
- Overmann, S.; Weiler, L.; Haufe, J.; Vollpracht, A.; Matschei, T. Statistical assessment of the factors influencing fly ash reactivity. Constr. Build. Mater. 2024, 426, 136151. [Google Scholar] [CrossRef]
- Lee, J.; Lim, A.; Kim, J.; Moon, J. Durability study of Portland cement blended with metakaolin from thermodynamic modeling. J. Build. Eng. 2024, 89, 109369. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Ma, H.R.; Ba, M.F.; Fang, S.Y.; Wang, Y. The influence of secondary aluminum ash sintering and grinding fine powder on the mechanical properties and shrinkage characteristics of Portland cement matrix. J. Build. Eng. 2024, 89, 109244. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B.B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transf. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Nodehi, M.; Ren, J.; Shi, X.J.; Debbarma, S.; Ozbakkaloglu, T. Experimental evaluation of alkali-activated and Portland cement-based mortars prepared using waste glass powder in replacement of fly ash. Constr. Build. Mater. 2023, 394, 132124. [Google Scholar] [CrossRef]
- Ruan, S.S.; Liu, L.; Shao, C.C.; Xie, L.; Zhu, M.B.; Wang, R.F. Study on the source of activity and differences between modified and unmodified magnesium slag as a filling cementitious material. Constr. Build. Mater. 2023, 392, 132019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).