Abstract
In order to save natural resources and protect the natural environment, the relevant performance of waste glass powder as a building material must be enhanced. In general, volcanic ash activity is enhanced through grinding the glass powder particles to a specific degree of fineness, thereby improving the overall strength of cementitious sand. Our current need is to study the effects of replacing standard sand with glass powder of varying particle size and dosage range on its resistance to sulfate erosion as well as the corresponding mechanisms. To further examine the durability properties of glass powder cementitious sand, this study uses glass powder of 100–200 mesh and 200–500 mesh to create cementitious sand samples, replacing 10% and 15% of standard sand with equal volume. After curing for 28 days in a standard curing room, the samples are submerged in tap water and a 5% concentration of sodium sulfate solution for 30, 60, 90, 120, and 150 days. Subsequently, the mass loss rate, flexural strength, and compressive strength are measured to reflect the sulfate erosion resistance of the cementitious sand samples containing glass powder. The findings indicate that the flexural and compressive strengths of cementitious sand with waste glass powder experience a swift decline in strength during the pre-erosion stage and a slow decline or even an increase in strength during the post-erosion stage as erosion age progresses. As the glass powder dosage increases, there is a noticeable decrease in the flexural and compressive strengths, in which the doping of 200–500 mesh doped with 15% of glass powder has the worst effect on the resistance to sulfate erosion. As the particle size increases, both flexural and compressive strengths significantly improve, suggesting that sulfate erosion properties are gradually enhanced. The primary reason for this phenomenon is that when glass powder substitutes fine aggregate, the activity develops more slowly in the initial stage, primarily filling the pores and cracks. However, the activity increases rapidly later, more fully integrating with the cement mortar in sodium hydroxide’s hydration product to create dense hydrated calcium silicate crystals. This enhances the overall strength of the sample, filling the pore structure within the system, and is more conducive to resisting the erosion effect of sulfate ions on the sample.
1. Introduction
The utilization of glass products inevitably results in a significant quantity of waste glass, leading to both land resource occupation and substantial environmental pollution [1]. Nevertheless, recycling glass conserves raw materials, saves energy, and reduces greenhouse gas emissions, thus significantly alleviating environmental pollution and promoting the sustainable use of resources [2]. However, waste glass contains large amounts of harmful heavy metals such as iron, zinc, and copper and cannot be directly incinerated, landfilled, or treated and decomposed through physical and chemical methods [3]. Conversely, using glass powder as an admixture in the construction industry provides an effective solution to the issues of waste glass recycling and environmental protection.
In recent decades, scholars, both domestically and abroad, have primarily studied the application of glass as an aggregate and additive gel material in concrete. When waste glass powder is used as a fine aggregate, the quantity mixed in significantly affects the performance of concrete. If the glass powder substitution rate is below 30%, it aids in filling gaps and improving the compactness of concrete, thereby enhancing its mechanical properties and durability. Conversely, a substitution rate exceeding 30% tends to decrease the overall performance of the concrete [4]. Glass powder can inhibit alkali-silicate reactions when replacing fine aggregate or serving as an auxiliary cementitious material, with the inhibition effect becoming more apparent as the particle size of the glass powder decreases [5,6,7,8,9,10]. When waste glass powder is used as an additive gel material, the concrete’s mechanical properties tend to increase and then decrease with the increase in the glass powder substitution rate, reaching optimal compressive and flexural strengths at a 20% substitution of cement [11,12,13]. Previous studies have shown that glass powder exhibits pozzolanic activity. Initially, this activity is not strong, and the glass powder primarily serves to fill system pores. However, as time progresses, the activity of the glass powder gradually enhances. The glass powder then reacts with calcium hydroxide, a hydration product within the cementitious sand, to generate dense hydrated calcium silicate. This process takes full advantage of the pozzolanic activity of smaller-particle-size glass powder, improving the weak zone of the interfacial transition zone and further enhancing the overall strength of the cementitious sand [14,15,16,17,18]. Despite these findings, most studies focusing on the sulfate erosion resistance performance of waste glass powder cement mortar have predominantly focused on equal mass admixture and large particle sizes of glass powder. Research regarding the effects of equal volume admixture and small-particle-size glass powder on concrete durability remains limited, especially in environments subject to salinization, such as coastal areas and Northwestern China. In these settings, research into the resistance of cementitious sand to sulfate erosion becomes increasingly critical.
Substituting a portion of cement with glass powder can enhance resistance to sulfate erosion. Matos et al. [19] found that the strength-activity indices of glass-powdered cement mortar specimens subjected to alkali-silicate reaction, sulfate attack, chloride penetration, and carbonation in the presence of concentrated sodium sulfate and alkali solutions significantly increased from 28 to 90 days when some of the cement was replaced with glass powder, implying the occurrence of pozzolanic activity. When the cement was mixed with glass powder or silica fume, the non-sulfate-resistant silicate cement used became sulfate-resistant, and the cementitious sand containing glass powder demonstrated increased resistance to chloride penetration. Siad et al. [20] indicated that the glass powder replacement of cement enhances the sulfate erosion resistance of cementitious materials, primarily due to the low chloride permeability of glass powder concrete and the potential of silica/aluminum-rich residue produced by the pozzolanic reaction of glass powder to inhibit the acidic ionic barrier. Tang et al. [21] studied the sulfate erosion resistance of sustainable concrete mixed with various solid wastes such as glass powder and fly ash. The results showed that regardless of the dosage, replacing cement with glass powder positively affects sulfate erosion, with the optimum dosage at 20%. Jain et al. [22] investigated the long-term sulfate erosion resistance effect of glass-powdered concrete with different admixtures. The results showed that when the admixture of glass powder is less than 20%, it positively affects sulfate resistance durability. An X-ray diffraction test was used to evaluate the pozzolanic effect of glass powders. When the dosage exceeds 20%, it negatively impacts the pozzolanic effect, primarily evidenced by the reduced calcium hydroxide crystals. Xu et al. [23] studied the synergistic effect of using glass powder and a sodium sulfate solution to evaluate the effect of waste glass powder in an alkaline solution. They found that the compressive strength of their 28-day specimens increased by 67% when the sodium sulfate content was 2.5% and the glass powder dosing was 10%. Rashidian-Dezfouli et al. [24] investigated the effect of three different matrixes consisting of fly ash, glass fibers, and glass powder in a sodium sulfate solution and analyzed the microstructural changes of geopolymers using SEM-EDX and XRD. Their results showed that glass powder-based polymers performed significantly lower than glass fiber and fly ash-based polymers in sodium sulfate solution, which may be related to less stable geopolymerization products that increase porosity and the presence of a large amount of available alkali in the raw glass powder.
In addition to substituting supplementary cementitious materials with glass powder, glass powder further positively impacts sulfate resistance when utilized as an aggregate. Luet et al. [25] demonstrated that in relation to drying shrinkage, the introduction of glass powder significantly reduces the drying shrinkage of glass-powdered concrete, regardless of the fineness of the glass powder particles. Simultaneously replacing aggregates and additive cementitious materials with glass powder can effectively enhance the resistance of glass powder concrete to sulfate attack. This effect becomes more pronounced with the increased fineness of the glass powder. Shalan et al. [26] investigated the long-term sulfate resistance of different glass powder mixtures as substitutes for fine aggregates in concrete. The selected fine aggregate was mixed with 50%, 60%, and 100% waste glass powders, and the volcanic ash activity of the glass powder was evaluated using an X-ray diffraction test, which revealed the degree of reduction in calcium hydroxide. The findings indicated that the sulfate resistance of the specimens gradually decreased with the increase in the glass powder admixture. However, concrete mixtures containing 50% glass powder aggregate displayed enhanced sulfate resistance, which was comparable to that of natural aggregates.
Accordingly, previous studies have primarily utilized a specific range of glass powder particle sizes and doses to replace portions of the cement or aggregate in the preparation of cementitious sand and concrete. Nevertheless, determining the optimal dosage of glass powder to replace standard sand, such that the resulting glass powder cementitious sand fulfills basic mechanical properties and durability requirements, has not been widely agreed upon. Likewise, experimental research and theoretical analysis regarding the use of glass powder as a fine aggregate in sulfate solutions are insufficient. This study investigates the effect of glass powder dosage, particle size, and erosion age on the durability properties of glass-powdered cementitious sand when glass powder is substituted for fine aggregate. Moreover, it performs mass loss rate, flexural strength, and compressive strength tests on glass powder cementitious sand specimens to comprehensively analyze glass powder cementitious sand.
2. Experimental
2.1. Experimental Raw Materials
2.1.1. Cement
The cement employed in this experiment is a P.O 42.5 ordinary Portland cement produced by Henan Xinxiang Yukui Tianrui Cement Co., Ltd. in Henan province of China. Its physical and chemical properties are provided in Table 1 and Table 2.

Table 1.
Physical performance indicators of Portland cement.

Table 2.
Chemical composition of cement as a percentage (%).
2.1.2. Fine Aggregate
The fine aggregate used in this study conforms to the Chinese ISO standard sand produced by Xiamen ISO Standard Sand Co., Ltd. (Xiamen, China), with an apparent density of 1350 kg/m3.
2.1.3. Glass Powder
In order to obtain clean glass powder, all the glass powder used in the experiment was made from white waste glass bottles. The waste glass powder was obtained through crushing waste glass bottles with a crusher, grinding them with an SM-500 cement test double-layer sound-proof small cement steel slag ternary catalytic ball mill, and separating them using a standard vibrating pendulum instrument. The glass is ground to the desired particle size using 100 mesh, 200 mesh, and 500 mesh sieves, resulting in two different particle size ranges: 100 mesh–200 mesh and 200 mesh–500 mesh. The flow chart for making waste glass powder is shown in Figure 1, and the chemical composition of the glass powder is shown in Table 3.

Figure 1.
Flow chart for making waste glass powder.

Table 3.
Chemical composition of waste glass powder as a percentage (%).
2.2. Pilot Program
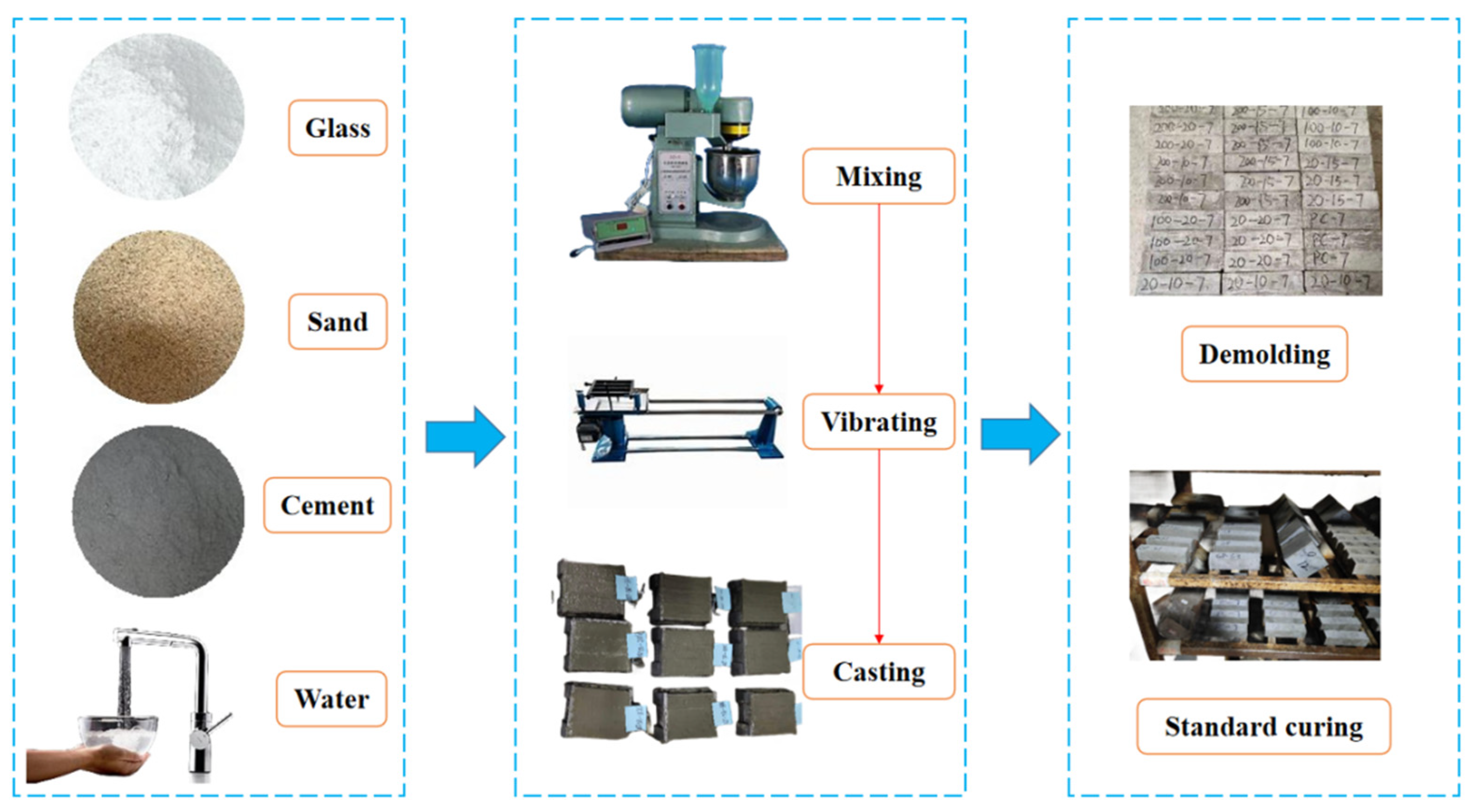
Initially, cement mortar test blocks without glass powder were prepared as a reference group (G0 group), using a standard mixing ratio of cement: standard sand: water of 1:3:0.5. The glass powder was incorporated by means of replacing standard sand on a volume-equal basis. The glass powder particle sizes were 100–200 mesh and 200–500 mesh. The sample of glass powder cement mortar with 100–200 mesh was labeled as GA-15 (15% mixing amount), and the two samples of glass powder cement mortar with 200–500 mesh were designated as GB-10 (10% mixing amount) and GB-15 (15% mixing amount), respectively. The fabrication and curing diagram of the waste glass powder cementitious sand test block is shown in Figure 2.
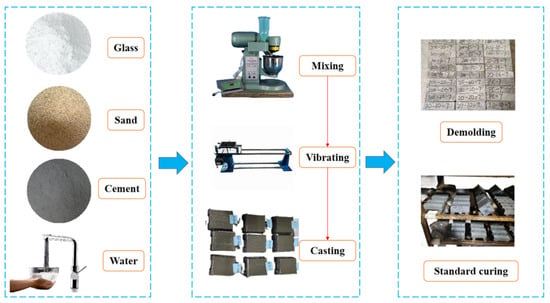
Figure 2.
Waste glass powder cement sand test block production, curing figure.
The sulfate corrosion resistance of cement mortar test blocks was tested following the Chinese standard GB/T 749-2008 “Cement Sulfate Corrosion Resistance Method” [27]. The total immersion method was adopted in this experiment, and the specimen size was taken as 40 × 40 × 160 mm. Initially, the specimen was naturally cured for 1 day, followed by mold removal, and then the specimen was cured in a standard curing room for 28 days. The specimen was placed in a 5% sodium sulfate solution and tap water, ensuring that the solution was at least 5 cm above the specimen to guarantee complete immersion. In addition, the 5% sodium sulfate solution was replaced every month to maintain the concentration of the sodium sulfate solution. The mass loss rate, compressive strength, and bending strength measurements were taken at 30, 60, 90, 120, and 150 days. The flexural strength of each set of ratios was determined through averaging the flexural strength results of three specimens, and when any of the three strength values exceeded ±10% of the average value, this value was removed and averaged. The compressive strength of each set of mixes is the average of the six compressive strength results at the end of the flexural strength as the test result, and when any of the six strength values exceeds ±10% of the average value, this value should be removed and then averaged.
The mass loss rate test is the ratio of the mass in the same batch of test pieces soaked in tap water to the specified age and the mass soaked in a sodium sulfate solution at the specified age. The mass loss rate calculation is given in Equation (1).
where is the mass loss rate; is the mass of the specimen immersed in tap water to a specified age in g; is the mass of the specimen immersed in sodium sulfate solution to a specified age in g.
3. Experimental Results and Analysis
3.1. The Influence of Sulfate Attack on the Mass Loss of Test Blocks
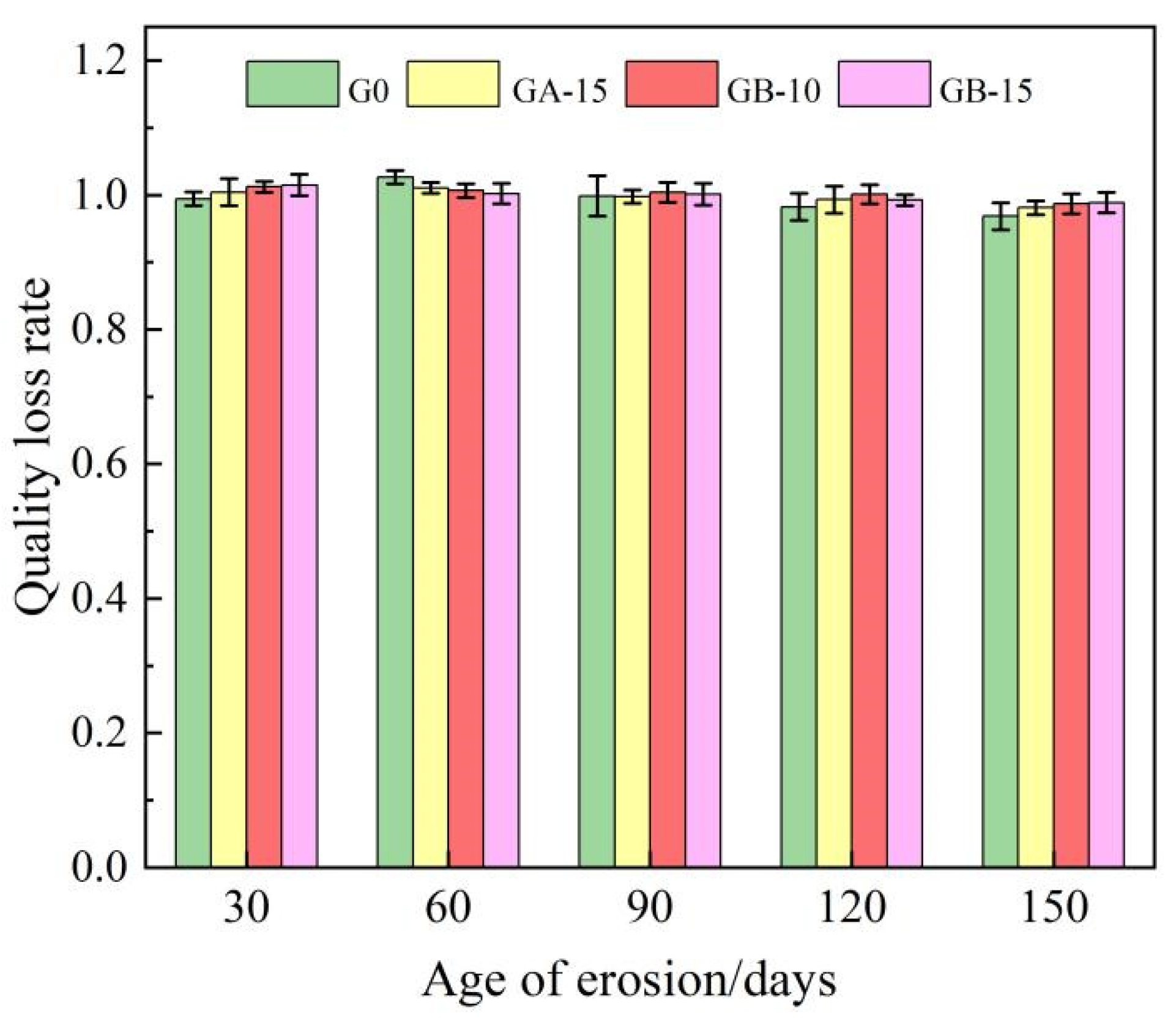
The mass loss rates of the G0 group, GA-15 group, GB-10 group, and GB-15 group at various ages after erosion by tap water and a 5% sodium sulfate solution are presented in Table 4. Figure 3 illustrates the impact of erosion age on the mass loss rate of the glass powder cement mortar test blocks. The data from Table 4 and Figure 3 reveal that the variance in sulfate resistance between the ordinary cement mortar test block and the waste glass powder cement mortar test block is consistent, with numerical coefficients ranging from 0.96 to 1.03. The variation patterns of the G0 group and the GA-15 group align as the mass loss rate increases and then decreases with the progression of the erosion age. Conversely, the GB-10 group and GB-15 group exhibit a consistent pattern, demonstrating a continual decrease in the mass loss rate as erosion age advances. When the GA-15 group is compared to the G0 group, adding 15% glass powder with 100–200 meshes stabilizes and improves the test block’s erosion rate after 60 days. Beyond the 60-day erosion mark, the test block’s erosion rate decreases slowly as the erosion age gradually increases. The mass loss rate at 150 days was markedly higher than that of the G0 group. This is largely due to the glass powder’s pozzolanic activity when its particle size is less than 100 meshes, which further reacts with the sodium hydroxide in the cement mortar to generate dense calcium silicate hydrate crystals, enhancing the specimen’s overall strength and resistance to the corrosion of sulfate ions. In contrast to the G0 group, the GB-10 group and the GB-15 group indicate that the addition of 200–500 mesh glass powder at 10% and 15% contents progressively reduces the mass loss rate at each age, a downward trend that is markedly more stable than those observed in the G0 and GA-15 groups. This suggests that while the early activity of glass powder replacing fine aggregate develops slowly, it primarily serves to fill holes and joints. However, the activity rapidly escalates in later stages, which is more conducive to the specimen’s sulfate attack resistance. The effect becomes increasingly noticeable as the glass powder’s particle size decreases. The smaller the particle size, the greater the compaction in the microstructure’s internal pores, which enhances the weak area between the fine aggregate and the cement slurry, thereby improving the specimen’s strength. For the reference group G0, the mass loss rate drops slowly in the initial stages of erosion and then decreases rapidly in the later stages. This phenomenon aligns with the findings of Patel et al. [13], attributed to excessive sodium sulfate gradually causing micro-cracks on the cement mortar specimens’ surface, with a small number of specimens breaking off in the early erosion stage prior to experiencing high tensile strain. During the later erosion stages, the sulfate ions penetrated the cement paste through these micro-cracks, inevitably forming expansive products such as gypsum and ettringite. As the volume of these hydration products is one to three times that of the original hydration products, rapid deterioration occurred due to the internal expansion cracking in the specimen, leading to the ultimate failure of the specimen.

Table 4.
Quality loss index of waste glass powder cement mortar specimen.
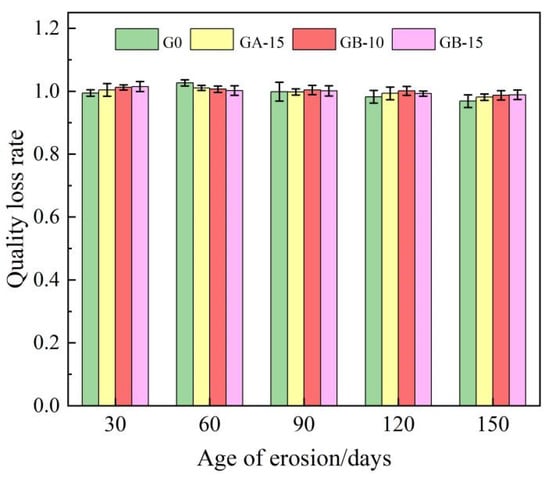
Figure 3.
Effect of erosion age on mass loss rate of glass powder cement mortar test block.
3.2. Effect of Age on Compressive and Flexural Strengths of the Specimens
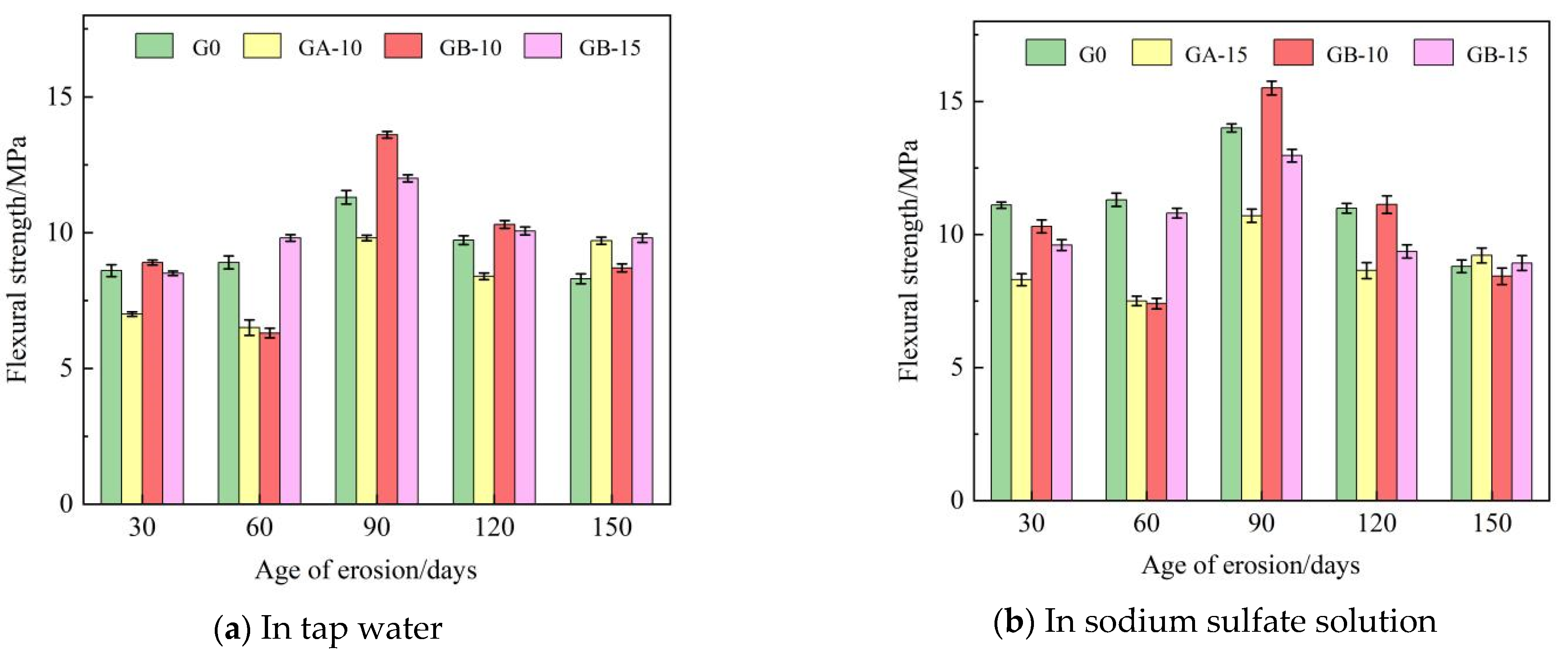
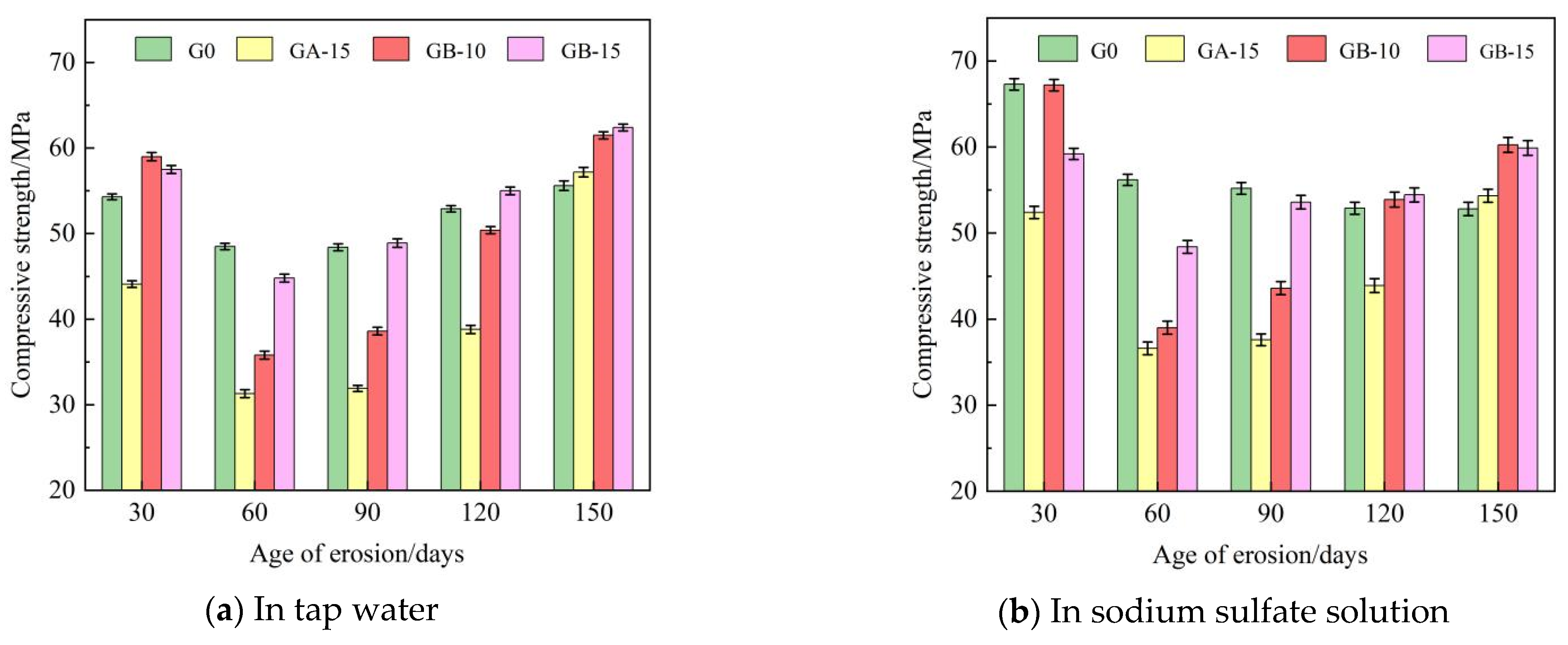
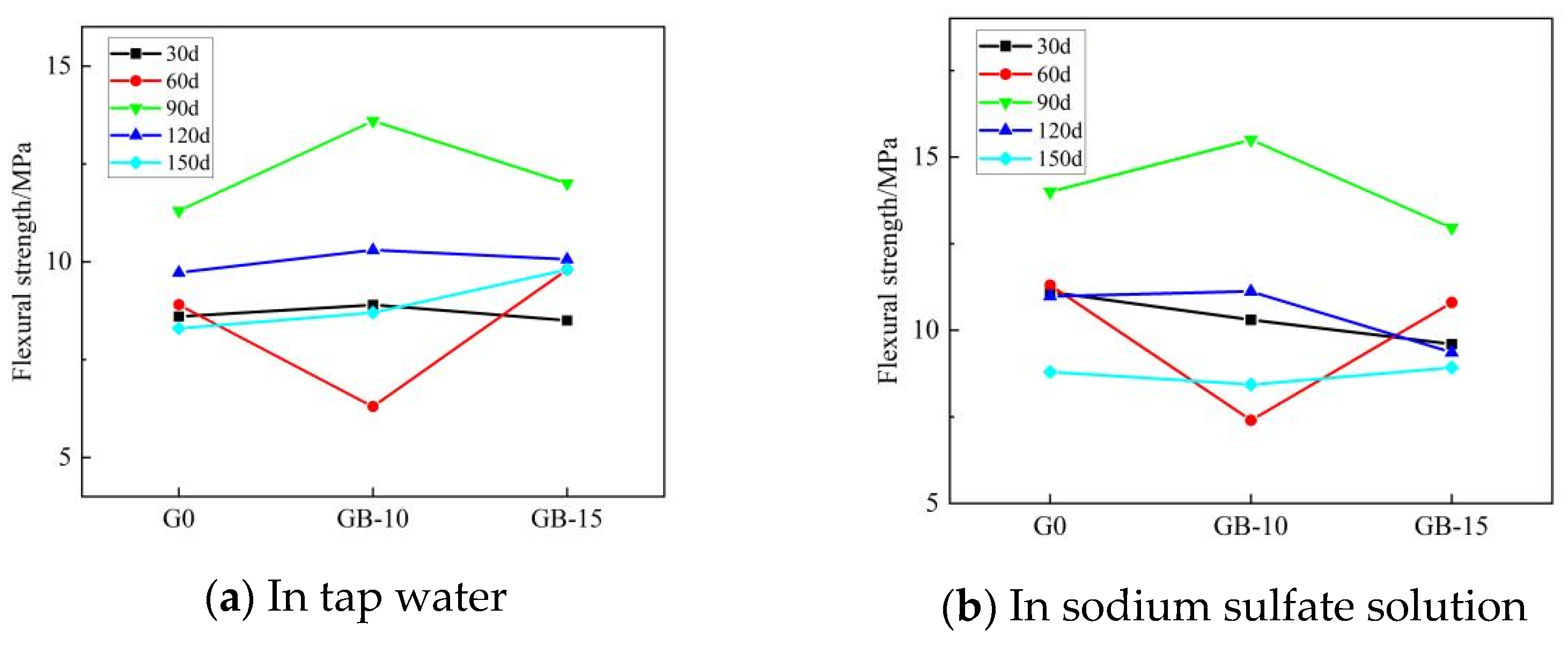
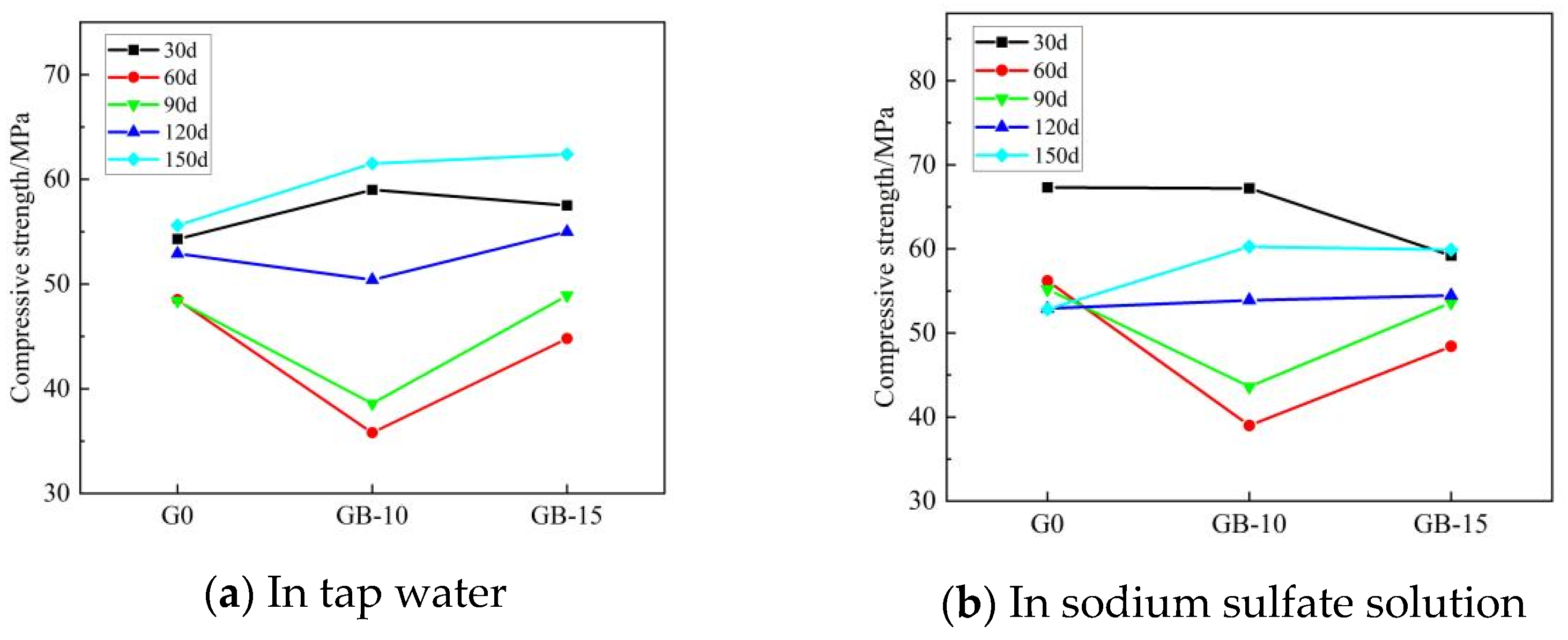
Table 5 presents the flexural and compressive strengths of Group G0, Group GA-15, Group GB-10, and Group GB-15 glass powder cementitious sand specimens under tap water erosion. Similarly, Table 6 details these same groups’ flexural and compressive strengths under the erosion of a sodium sulfate solution. Both Table 5 and Table 6 reveal that with the increase in age, the flexural and compressive strengths initially decline, then rise, and subsequently follow this pattern repeatedly. Flexural strength is in the range of 6.3–15.5 MPa, and compressive strength is in the range of 31.3–67.3 MPa. Figure 4 illustrates the effect of specimen age on cementitious sand’s flexural strength under tap water erosion and sodium sulfate solution erosion, respectively. Similarly, Figure 5 demonstrates the impact of specimen age on the compressive strengths of cementitious sand under tap water erosion and sodium sulfate solution erosion. Analysis of Figure 4 reveals that the flexural strength of the specimens of Group G0 and Group GB-15 demonstrate a growth-then-decline pattern with age, whereas those of Group GA-15 and Group GB-10 display a pattern of decline-to-growth and then back-to-decline with age. The specimens achieve their maximum flexural strength at 90 days and perform better in a sodium sulfate solution than in tap water. Figure 5 shows that, except for the G0 group, the compressive strength of the other groups, in both tap water and sodium sulfate solution, follows a trend of initial decrease followed by an increase with the growth of the erosion age. Furthermore, the specimens perform better in sodium sulfate solution than in tap water, contrary to the development pattern of flexural strength. On the other hand, Group G0 shows a gradual decrease in compressive strength with age in the sodium sulfate solution. Compared to Group G0, the flexural strength decreases rapidly from 30 to 60 days in the initial erosion period, by 7.1% in tap water and 9.6% in sodium sulfate solution when 100–200 mesh glass powders with 15% dosing are incorporated. However, the decline in flexural strength from 90 to 150 days is slower, being only 1% in tap water and 13.9% in sodium sulfate solution. The flexural strength at 90 days is optimal, with 9.8 MPa in tap water and 10.7 MPa in sodium sulfate solution.

Table 5.
Flexural and compressive strength of waste glass powder cementitious sand specimens under tap water erosion.

Table 6.
Flexural and compressive strength of waste glass powder cementitious sand specimens under the erosion of a sodium sulfate solution.
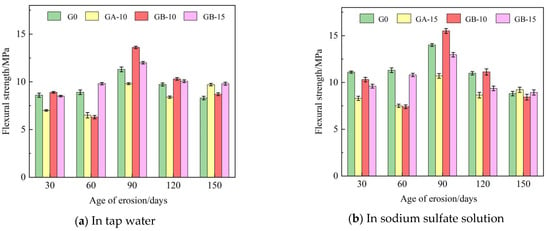
Figure 4.
Effect of different erosion ages on the flexural strength of cementitious sand.
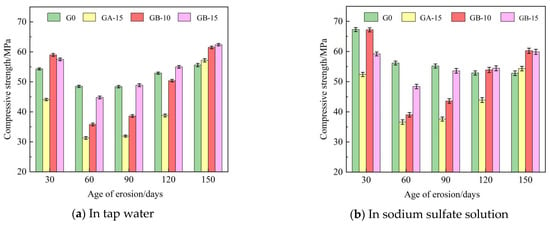
Figure 5.
Effect of different age of erosion on compressive strength of cementitious sand.
The compressive strength shows a pattern of decrease and then increase with age. Specifically, the compressive strength drops sharply from 30 to 90 days, by 27.7% in tap water and 28.2% in sodium sulfate solution. However, from 90 to 150 days, the compressive strength increases swiftly, 79% in tap water and 44.5% in sodium sulfate solution. This trend mainly occurs because when the particle size of the glass powder is less than 100 mesh, the early activity of the glass powder is low, thus leading to a decline in strength. As the age increases, the volcanic ash activity of the glass powder gradually rises, leading to dense hydrated calcium silicate crystals forming, which enhance the overall strength of the specimen and effectively fill the pore structure inside the system, increasing its resistance to the erosion effect of sulfate ions. When mixed with 200–500 mesh glass powder at a dosage of 10%, the flexural strength declines slowly from 30 to 60 days during the early erosion period, while the compressive strength decreases rapidly. However, in the late erosion period, from 90 to 150 days, the decline in flexural strength accelerates while the compressive strength increases. When 200–500 mesh glass powder with a dosage of 15% is mixed in, the development trend aligns with the specimens in the GB-10 group, although the overall effect is slightly less pronounced. When the particle size of the glass powder is less than 200 mesh, the finer the particle size of the glass powder cement sand, the larger the dosage, but the specimen’s resistance to sulfate erosion decreases. This is most effective when the dosage is 10%. Due to its larger contact area, the finer glass powder is more likely to react with the calcium hydroxide in cement hydration products, forming densely hydrated calcium silicate. As the age of erosion increases, this reaction becomes more efficient at compensating for the internal pores and micro-cracks caused by sulfate erosion. Therefore, an enhancement in compressive strength becomes apparent in the later stages of erosion.
In examining the effects of the erosion of waste glass powder cementitious sand specimens in sodium sulfate solution [19,20,21,22,23,24], it can be inferred that the early stages of erosion represent a typical expansive erosion. The erosion products are primarily dominated by calcovanadite and a small number of gypsum crystals. The later stages are largely influenced by the reaction of sodium sulfate with calcium hydroxide crystals from cement hydration, which forms gypsum and highly alkaline sodium hydroxide. This chemical reaction can be described using Equation (2). However, under high alkaline concentrations, the stabilized structure of the glass is prone to disruption. This is because the glass solution is weakly acidic (with silica being an acidic oxide), and the chemical bonds between silicon/aluminum and oxygen atoms are fragile in high alkali concentrations, leading to easy breakage. In addition to the highly alkaline conditions, the swelling agent, gypsum (CaSO4), can form via reacting with aluminates (C3A) to produce coarsely shaped needles and rod crystals of calcium alumina (AFt). An increase in AFt may cause expansion and cracking within the system. Moreover, the hydration reaction of C3S can be accelerated by sulfate ions, which generate a dense C–S–H gel. This augments the mortar’s internal density and decreases the internal porosity and micro-cracks in the mortar. This phenomenon aligns with the findings of Patel et al. [13]. These chemical reactions can be described using Equations (3)–(5).
3.3. Effect of Dosage on the Sulfate Erosion Resistance of Cementitious Sand
Figure 6 and Figure 7 show the trends for the flexural and compressive strengths of glass powder cementitious sands with the same particle size but different dosages in tap water and sodium sulfate solution across various erosion ages. When the particle size of the glass powder is 200–500 mesh, and its dosage varies, the strength of the cementitious sand specimen is significantly affected. Figure 6 illustrates that the flexural strength of glass powder cementitious sand specimens with the same particle size but different dosages in tap water and sodium sulfate solution increases then decreases with the advancement of erosion age. However, the strength in sodium sulfate solution is slightly superior to that in tap water. Compared to the benchmark group G0, the flexural strength of the glass powder cementitious sand specimens declines gradually at 30 days, with smaller dosages resulting in a slower decrease. The glass powder cementitious sand specimens with a dosage of 10% reach their lowest value at 60 days, while the specimens with a dosage of 15% are slightly higher than the benchmark group. When the erosion age reaches 90 days, specimen strength significantly improves and peaks, with a dosage of 10% of the glass powder enhancing the effect most significantly. From 120 to 150 days, compared to the benchmark group, the strength of the glass powder cement sand specimens experienced a slight increase. A 10% glass powder dosage is more effective than a 15% one. During early strength development, specimens mixed with 200–500 mesh glass powder cement sand develop strength slowly. However, due to the glass powder’s more complete volcanic ash activity reaction, the late strength development accelerates. Figure 7 reveals that the compressive strength of glass powder cement sand specimens with the same particle size and different dosages at each age in sodium sulfate solution is notably higher than in tap water. At an erosion age of 30 days, the strength of glass powder cementitious sand specimens eroded in tap water with 10% and 15% doping is higher than the benchmark group. The strength of specimens eroded in sodium sulfate solution with 10% doping surpasses that of the reference group, while those with 15% doping fall short. Here, 10% doping in sodium sulfate solution is more effective. During the 60-to-90-day erosion period, each glass powder cement sand specimen’s strength decreases, with the 10% glass powder specimen experiencing the most significant decline. In the later erosion period, from 90 to 150 days, all glass powder cementitious sand specimens’ strength increases when compared to the benchmark group. The optimum erosion age in tap water is 150 days, while in sodium sulfate solution, it is 30 days, followed by 150 days. Adding a 15% dosage of finer-particle-size glass powder is more conducive to filling the internal pores of cementitious sand, thereby preventing internal quality loss and promoting the reaction of calcium hydroxide, the internal cement hydration product of cementitious sand, to generate dense calcium-hydrated silicate (C–S–H) [13,22]. This process maximizes the volcanic ash activity of smaller particle size glass powder, improving the interfacial transition zone of the weak area and thereby enhancing the overall mechanical strength and resistance to sulfate erosion.
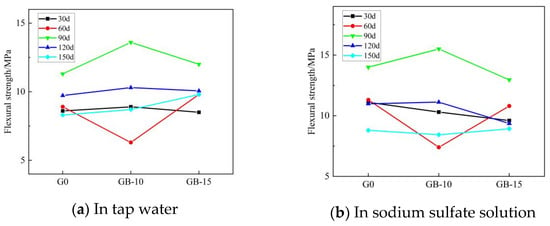
Figure 6.
Effect of different dosages of 200–500 mesh glass powder on flexural strength of cementitious sand.
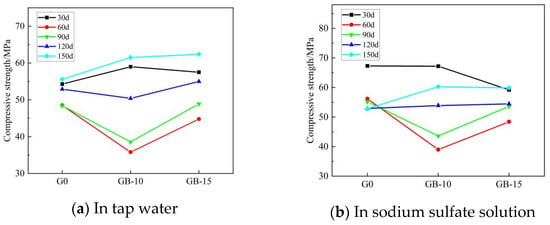
Figure 7.
Effect of different dosages of 200–500 mesh glass powder on the compressive strength of cementitious sand.
3.4. Effect of Grain Size on Sulfate Erosion Resistance of Cementitious Sand
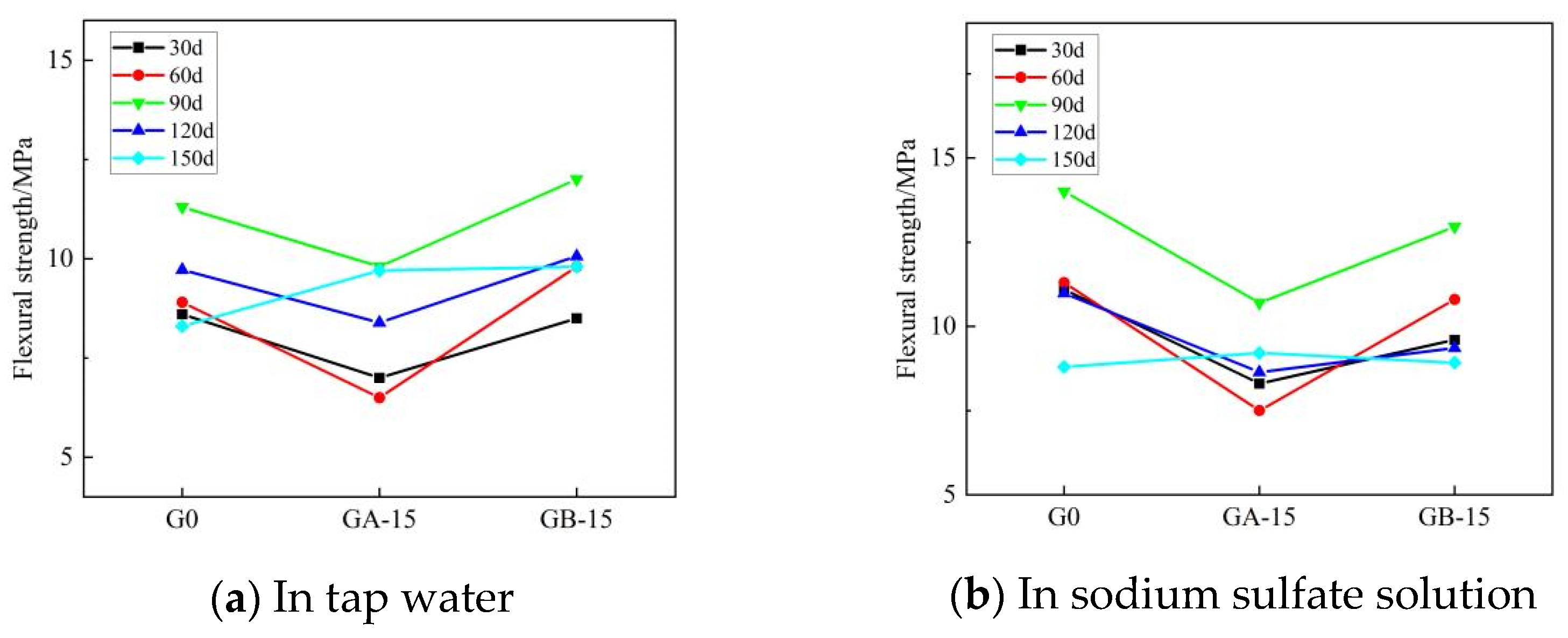
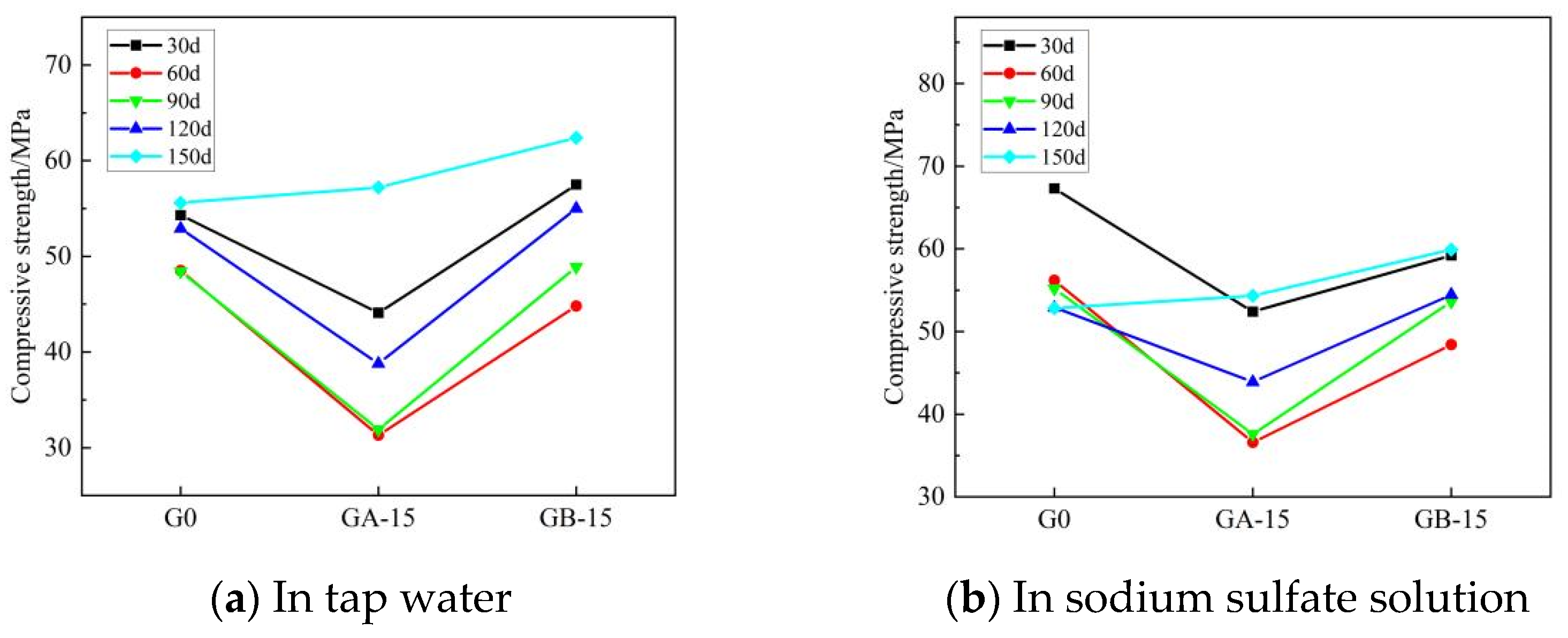
Figure 8 and Figure 9 illustrate the trends for flexural and compressive strengths of glass powder cementitious sands, with identical dosage and varying particle sizes, when immersed in tap water and sodium sulfate solution throughout erosion. Figure 8 indicates that during the early erosion stage, whether in tap water or sodium sulfate solution, the flexural strength of each glass powder cementitious sand sample at 30–60 days is lower than that of the benchmark group. The fastest decreasing trend occurs in the glass powder cementitious sand samples with a particle size of 100–200 mesh, while a slight increase is observed in the 200–500 mesh particle size. The finer the particle size, the higher the strength of the glass powder for the same dosage. The strength of the samples peaks at 90 days in the middle of the erosion period, where the samples with 200–500 mesh size yield better results than those with 100–200 mesh size. In the late stage of erosion, from 90 to 150 days, the flexural strength of the glass powder cementitious sand samples with a particle size of 200–500 mesh is, as a whole, higher than that of the samples with a particle size of 100–200 mesh, and even slightly higher than that of the benchmark group. Figure 9 reveals that, in tap water, the compressive strength of the glass powder cementitious sand samples with a particle size of 100–200 mesh is generally lower than that of the benchmark group, whereas the compressive strength of the samples with a particle size of 200–500 mesh is slightly higher. The effect becomes more significant as the age increases. However, in sodium sulfate solution, the compressive strength of glass powder cementitious sand samples is lower than the reference group until 120 days of erosion. The overall strength of the samples with 200–500 mesh size is higher than that of the samples with 100–200 mesh size, but it only surpasses that of the reference group when it reaches 150 days. This is primarily due to the ability of 200–500 mesh glass powder to better fill the pores between the aggregate, thereby increasing the internal density of the mortar and, consequently, the strength. Its inherent activity may also prompt a volcanic ash reaction in the later stage to generate a dense gel material, thus enhancing the system’s compactness. The sulfate ions infiltrate the specimen from the surface, slowly invading the interior. The densification makes it more challenging for the sulfate ions to penetrate the slurry, thereby limiting the production of large volumes of gypsum and calcium alumina crystals, which cause internal expansion and stress cracking, leading to specimen damage. This also improves the specimen’s resistance to sulfate erosion.
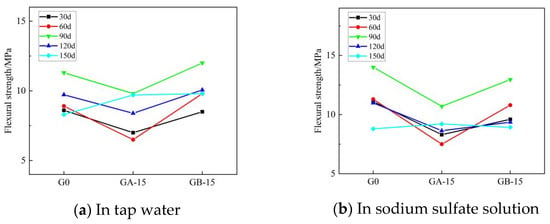
Figure 8.
Effect of the same dose of glass powder with different particle sizes on the flexural strength of cementitious sand.
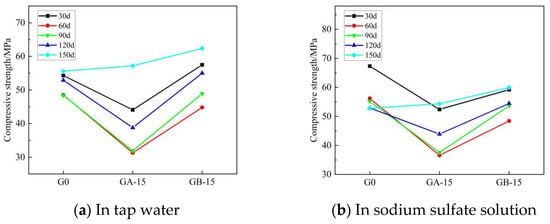
Figure 9.
Effect of the same dose of glass powder with different particle sizes on the compressive strength of cementitious sand.
4. Conclusions
The erosion of cementitious sand by sulfate is a multifaceted process, subject to numerous factors. This paper delves into the role of 100–200 mesh and 200–500 mesh glass powder in mitigating sulfate erosion when used to replace standard sand with cementitious sand. The primary conclusions are as follows:
(1) The erosion damage of cementitious sand initiates from the surface and progresses inward. Initially, sulfate ions act on the specimen’s surface, with pronounced erosion effects at the corners. As the surface erodes and begins to degrade, micro-cracks form on the specimen’s surface. This enables sulfate ions to reach the internal, cracked areas and react with Ca(OH)2 to generate expansive materials, such as gypsum and calcium alumina. This causes erosion products to deposit within the cracks and pore spaces. As more sulfate ions penetrate the specimen, the production of these expansive materials increases within the specimen, which subsequently reduces its original mechanical properties until complete disintegration occurs.
(2) With the addition of 15% 100–200 mesh glass powder, flexural and compressive strengths decrease significantly between 30 to 60 days of the initial erosion. However, the decrease in flexural strength from 90 to 150 days slows down by only 1% in tap water and 13.9% in sodium sulfate solution. In contrast, compressive strength rapidly increases by 79% in tap water and 44.5% in sodium sulfate solution. With the addition of 10% and 15% 200–500 mesh glass powder, the flexural strength declination slows, and the compressive strength declination becomes quicker in the early erosion stage of 30 to 60 days, whereas in the later erosion stage of 90 to 150 days, the flexural strength declination accelerates while the compressive strength increases.
(3) When increasing the quantity of the same-particle-size glass powder, the flexural and compressive strengths of glass powder cement sand in tap water and sodium sulfate solution decrease. The optimal anti-sulfate erosion performance is observed in specimens of cement sand mixed with 10% 200–500 mesh glass powder. This is primarily because an excessive concentration of fine glass powder enlarges the specific surface area of the glass powder, causing an increase in moisture absorption and slurry consistency. Consequently, the water available for cement hydration is reduced, which affects the content of CH, the hydration product of cement, and ultimately inhibits the full activation of the glass powder, resulting in a slight decrease in strength.
(4) As particle size decreases, the mass loss rate’s decreasing trend noticeably slows while flexural and compressive strengths gradually enhance, indicating a progressive enhancement in sulfate erosion resistance. This is primarily because finer glass powder has a stronger pozzolanic effect, producing densely hydrated calcium silicate crystals. Additionally, finer glass powder has a stronger micro-aggregate filling effect, which reduces the internal pore structure of the mortar and compensates for the lower strength of the interfacial transition zone, thereby preventing sulfate ions from infiltrating the test specimen’s interior, leading to expansion, internal cracking, and eventual destruction.
Author Contributions
Conceptualization, S.Y. and Y.Z.; methodology, Y.Z.; validation, S.W. and H.Y.; formal analysis, S.W.; investigation, S.W.; resources, S.Y.; data curation, H.Y.; writing—original draft preparation, S.W.; writing—review and editing, Y.Z.; visualization, H.Y.; supervision, H.Y.; project administration, S.Y.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Foundation of China grant number [52104157].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This support is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majchrowska, S.; Mikołajczyk, A.; Ferlin, M.; Klawikowska, Z.; Plantykow, M.A.; Kwasigroch, A.; Majek, K. Deep learning-based waste detection in natural and urban environments. Waste Manag. 2022, 138, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Valuable Recycling of waste glass generated from the liquid crystal display panel industry. J. Clean. Prod. 2018, 174, 191–198. [Google Scholar] [CrossRef]
- Eun, H.T.; Kang, S.G.; Kim, Y.T.; Lee, G.K.; Kim, J.H. Heavy Metal Leaching Behavior of Glasses Containing EAF Dust. Mater. Sci. Forum 2005, 486–487, 382–386. [Google Scholar] [CrossRef]
- Limbachiya, M.C. Bulk engineering and durability properties of washed glass sand concrete. Constr. Build. Mater. 2009, 23, 1078–1083. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kassem, E.; Ibrahim, A. ASR mitigation using binary and ternary blends with waste glass powder. Constr. Build. Mater. 2021, 280, 122425. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Liao, S.; Huang, Y. Grinding kinetics of waste glass powder and its composite effect as pozzolanic admixture in cement concrete. Constr. Build. Mater. 2020, 239, 117876. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, M.; Shen, W.; Li, J.; Zhao, D.; Li, P.; Wu, J. Performance and microstructure of ultra-high-performance concrete (UHPC)with silica fume replaced by inert mineral powders. Constr. Build. Mater. 2022, 327, 126996. [Google Scholar] [CrossRef]
- Martín, C.M.; Scarponi, N.B.; Villagrán, Y.A.; Manzanal, D.G.; Piqué, T.M. Pozzolanic activity quantification of hollow glass microspheres. Cem. Concr. Compos. 2021, 118, 103981. [Google Scholar] [CrossRef]
- Amaral, M.; Macioski, G.; Medeiros, M.H.F.D. Atividade pozolânica da sílica ativa:análise em pastas cimentícias com diferentes teores de substituição. Matéria 2021, 26, e13023. [Google Scholar]
- Song, J.; Feng, S.; Xiong, R.; Ouyang, Y.; Zeng, Q.; Zhu, J.; Zhang, C. Mechanical properties, pozzolanic activity and volume stability of copper slag-filled cementitious materials. Mater. Sci. 2019, 26, 218–224. [Google Scholar] [CrossRef]
- Khan, Q.S.; Sheikh, M.N.; McCarthy, T.J.; Robati, M.; Allen, M. Experimental investigation on foam concrete without and with recycled glass powder:A sustainable solution for future construction. Constr. Build. Mater. 2019, 201, 369–379. [Google Scholar] [CrossRef]
- Khmiri, A.; Chaaboubi, M.; Samet, B. Chemical behaviour of ground waste glass when used as partial cement replacement in mortars. Constr. Build. Mater. 2013, 44, 74–80. [Google Scholar] [CrossRef]
- Patel, D.; Shrivastava, R.; Tiwari, R.P.; Yadav, R.K. Properties of cement mortar in substitution with waste fine glass powder and environmental impact study. J. Build. Eng. 2020, 27, 100940. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Wu, S.; Li, Q. Strength development and microcosmic mechanism of waste glass powder cement mortar. J. Exp. Nanosci. 2022, 17, 564–584. [Google Scholar] [CrossRef]
- Borges, A.L.; Soares, S.M.; Freitas, T.O.G.; Oliveira Júnior, A.D.; Ferreira, E.B.; Ferreira, F.G.D.S. Evaluation of the Pozzolanic Activity of Glass Powder in Three Maximum Grain Sizes. Mater. Res. 2021, 24, e20200496. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Kalakada, Z.; Doh, J.H.; Chowdhury, S. Glass powder as replacement of cement for concrete–an investigative study. Eur. J. Environ. Civ. Eng. 2022, 26, 1046–1063. [Google Scholar] [CrossRef]
- Kalakada, Z.; Doh, J.H.; Zi, G. Utilisation of coarse glass powder as pozzolanic cement—A mix design investigation. Constr. Build. Mater. 2020, 240, 117916. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Waste glass powder in cement:macro and micro scale study. Adv. Cem. Res. 2016, 28, 423–432. [Google Scholar] [CrossRef]
- Siad, H.; Lachemi, M.; Sahmaran, M.; Hossain, K.M.A. Effect of glass powder on sulfuric acid resistance of cementitious materials. Constr. Build. Mater. 2016, 113, 163–173. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Ke, G.; Zhou, J.L.; Tam, V.W. Sulfate attack resistance of sustainable concrete incorporating various industrial solid wastes. J. Clean. Prod. 2019, 218, 810–822. [Google Scholar] [CrossRef]
- Jain, K.L.; Sancheti, G.; Gupta, L.K. Durability performance of waste granite and glass powder added concrete. Constr. Build. Mater. 2020, 252, 119075. [Google Scholar] [CrossRef]
- Xu, Y.; He, T. Effect of Mitigating Strength Retrogradation of Alkali Accelerator by the Synergism of Sodium Sulfate and Waste Glass Powder. J. Renew. Mater. 2021, 9, 1991–1999. [Google Scholar] [CrossRef]
- Rashidian-Dezfouli, H.; Rangaraju, P.R. A comparative study on the durability of geopolymers produced with ground glass fiber, fly ash, and glass-powder in sodium sulfate solution. Constr. Build. Mater. 2017, 153, 996–1009. [Google Scholar] [CrossRef]
- Lu, J.X.; Zhan, B.J.; Duan, Z.H.; Poon, C.S. Using glass powder to improve the durability of architectural mortar prepared with glass aggregates. Mater. Des. 2017, 135, 102–111. [Google Scholar] [CrossRef]
- Shalan, A.H.; El-Gohary, M.M. Long-Term Sulfate Resistance of Blended Cement Concrete with Waste Glass Powder. Pract. Period. Struct. Des. Constr. 2022, 27, 04022047. [Google Scholar] [CrossRef]
- GB/T749-2008; Test Method For Determing Capability of Resisting Sulfate Corrode of Cement. China Standard Publishing House: Beijing, China, 2008. (In Chinese)
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).