Risk of Microbial Growth in Ventilation Ductwork Located in the Humid and Cold Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Ductwork and Measurement Equipment Setup
2.2. Microbial Media Preparation, Initial Duct Surface Contamination
2.3. Biological Sampling, Schematic and Schedule
2.4. Analysis of Biological Samples
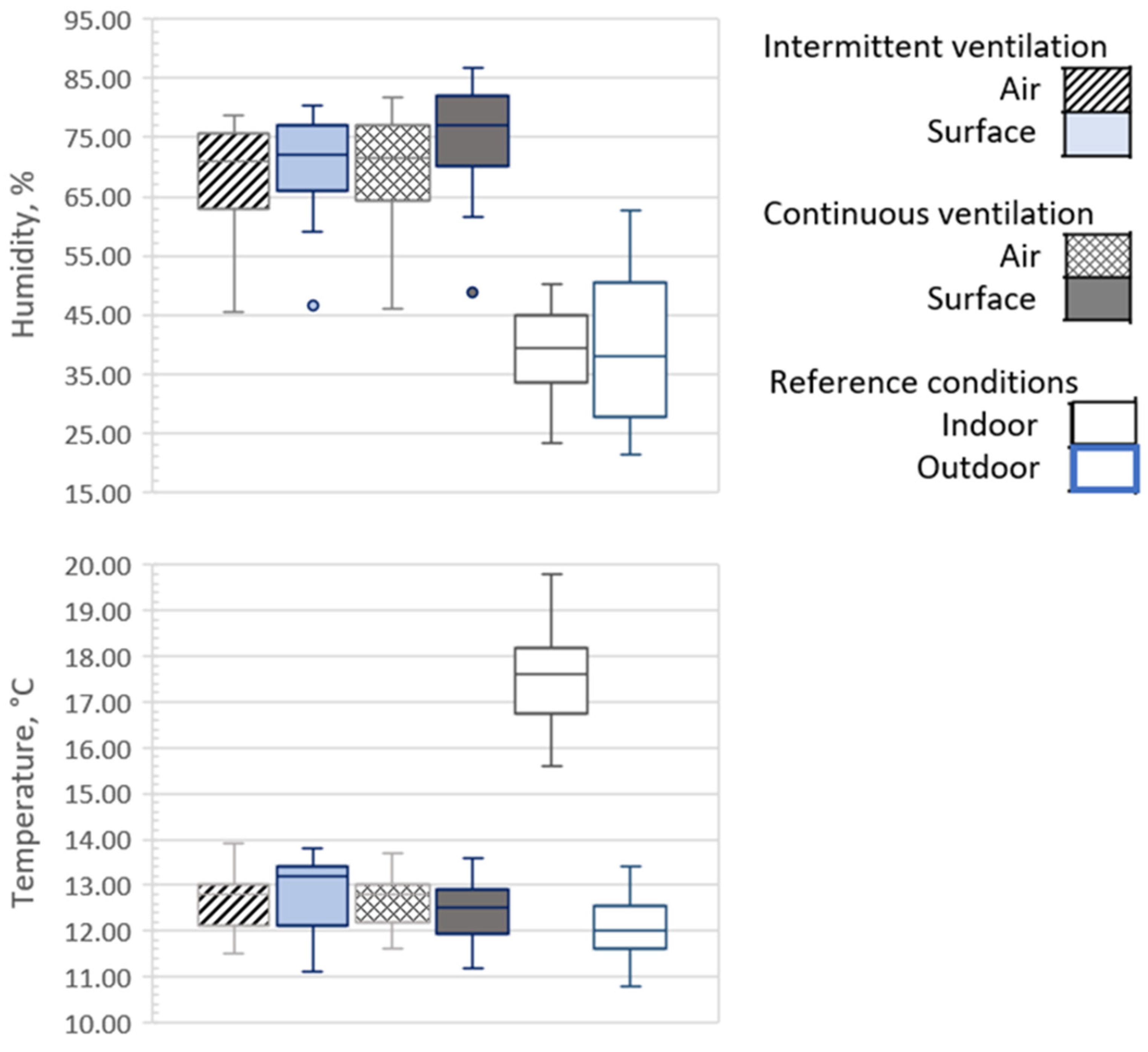
2.5. Ductowrk Conditions: Airflow, Temperature and Humidity during the Experiment
3. Results
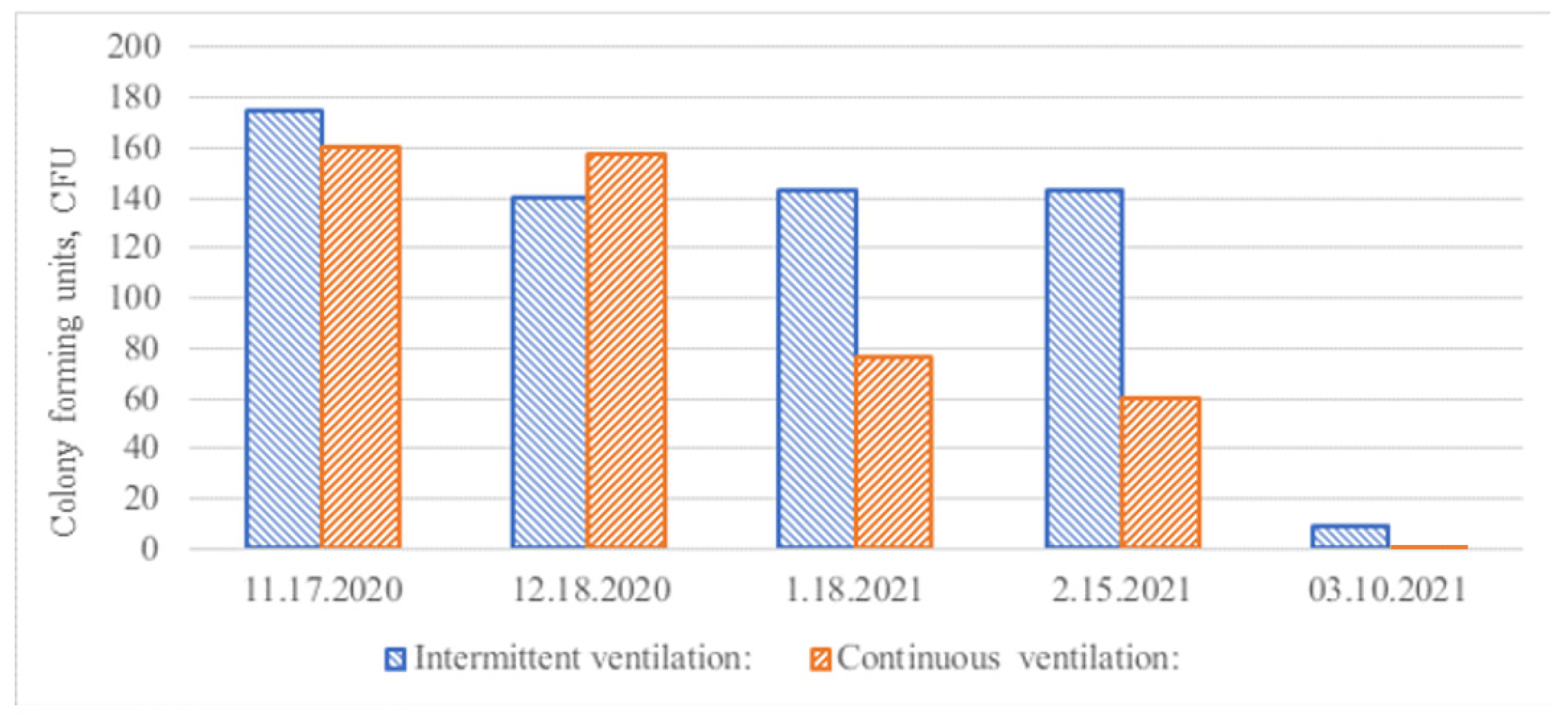
3.1. Duct Surface Contact Samples for Fungal Growth
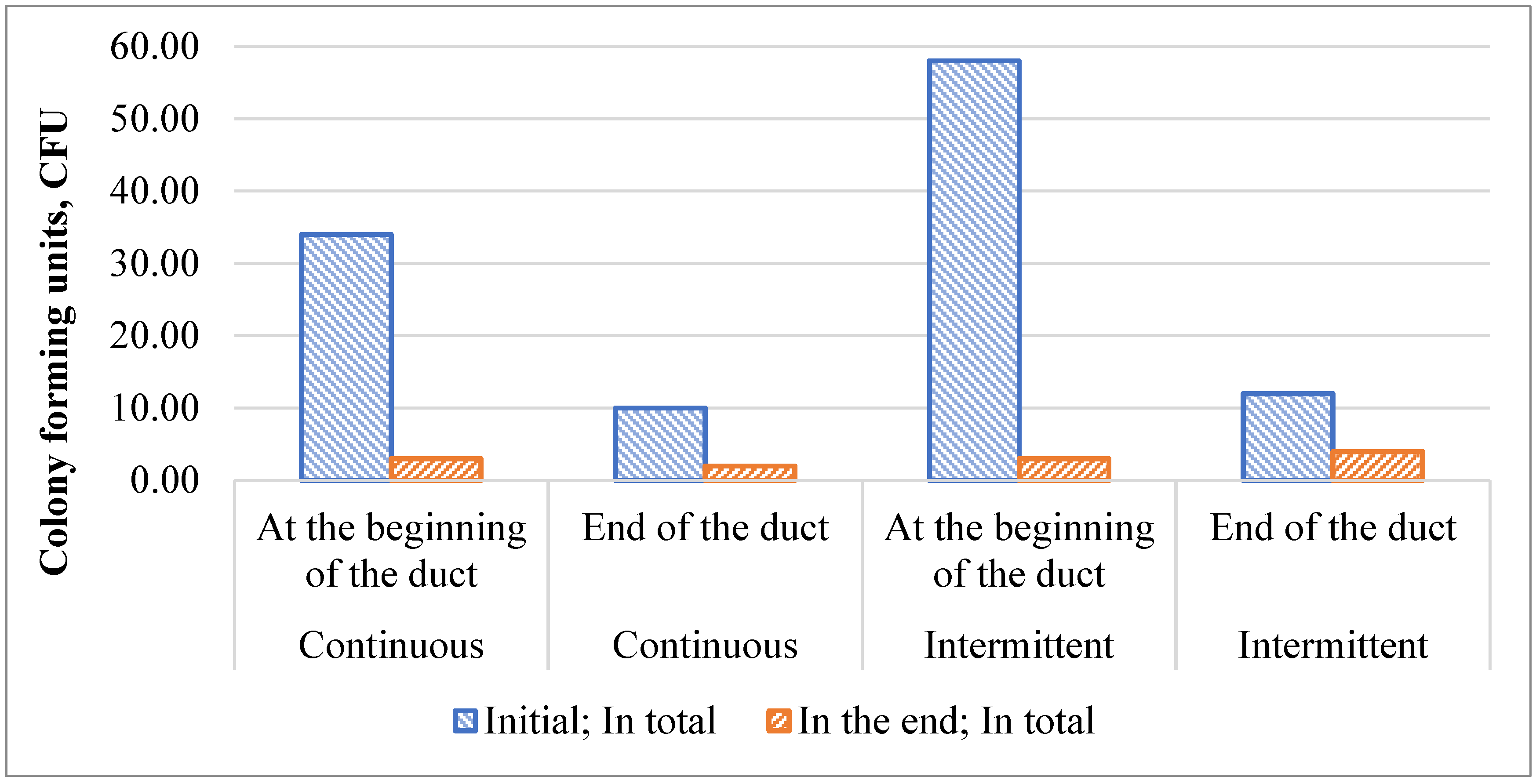
3.2. Duct Surface Swab Fungal and Bacterial Growth
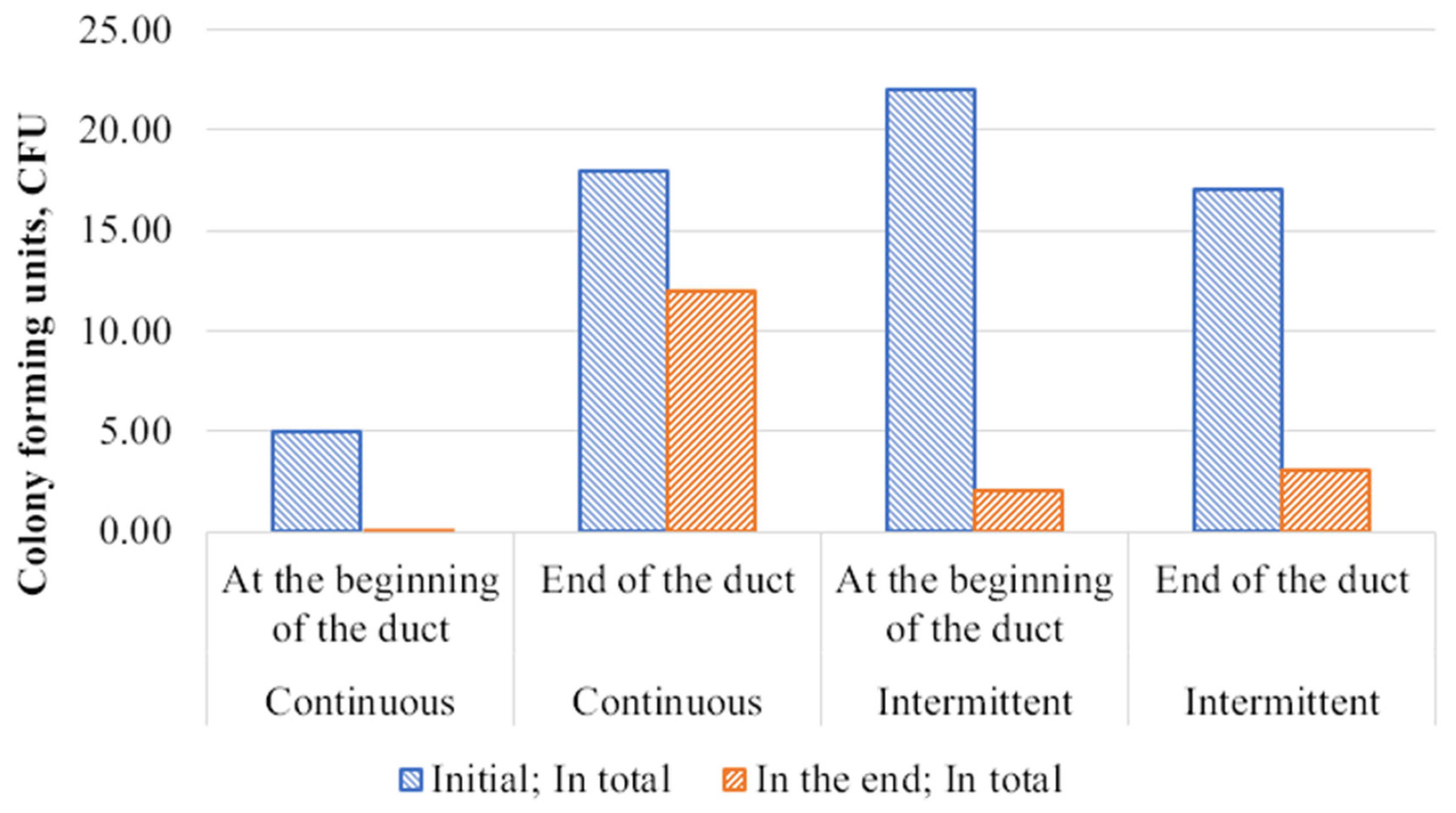
3.3. Ductwork Air Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. The World Health Report 2000: Health Systems: Improving Performance; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-156198-3. [Google Scholar]
- Wargocki, P.; Sundell, J.; Bischof, W.; Brundrett, G.; Fanger, P.O.; Gyntelberg, F.; Hanssen, S.O.; Harrison, P.; Pickering, A.; Seppänen, O.; et al. Ventilation and Health in Non-Industrial Indoor Environments: Report from a European Multidisciplinary Scientific Consensus Meeting (EUROVEN). Indoor Air 2002, 12, 113–128. [Google Scholar] [CrossRef]
- Daisey, J.M.; Angell, W.J.; Apte, M.G. Indoor Air Quality, Ventilation and Health Symptoms in Schools: An Analysis of Existing Information: Indoor Air Quality, Ventilation and Health Symptoms in Schools. Indoor Air 2003, 13, 53–64. [Google Scholar] [CrossRef]
- Mendell, M.J.; Heath, G.A. Do Indoor Pollutants and Thermal Conditions in Schools Influence Student Performance? A Critical Review of the Literature. Indoor Air 2005, 15, 27–52. [Google Scholar] [CrossRef]
- Han, O.; Li, A.; Kosonen, R. Hood Performance and Capture Efficiency of Kitchens: A Review. Build. Environ. 2019, 161, 106221. [Google Scholar] [CrossRef]
- Bocanegra-Yanez, M.C.; Rojas, G.; Zukowska, D.; Burman, E.; Cao, G.; Hamon, M.P.Y.; Kolarik, J. Design and Operation of Ventilation in Low Energy Residences—A Survey on Code Requirements and Building Reality from Six European Countries and China. In Proceedings of the 38th AIVC Conference “Ventilating Healthy Low-Energy buildings”, Nottingham, UK, 13–14 September 2017; Available online: http://www.aivc.org/resource/design-and-operation-ventilation-low-energy-residences-survey-code-requirements-and (accessed on 4 November 2020).
- Brelih, N.; Seppanen, O. Ventilation Rates and IAQ in European Standards and National Regulations. In Proceedings of the 32nd AIVC Conference and 1st TightVent Conference, Brussels, Belgium, 12–13 October 2011. [Google Scholar]
- Fernbas Energy Performance of Buildings Directive. Available online: https://ec.europa.eu/energy/topics/energy-efficiency/energy-efficient-buildings/energy-performance-buildings-directive_en (accessed on 5 November 2020).
- EN 15251:2007; Indoor Environmental Input Parameters for Design and Assessment of Energy Performance of Buildings Addressing Indoor Air Quality, Thermal Environment, Lighting and Acoustics. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2007. Available online: https://standards.iteh.ai/catalog/standards/cen/92485123-bf64-40e3-9387-9724a642eae8/en-15251-2007 (accessed on 2 September 2021).
- EN 16798-1:2019; Energy Performance of Buildings—Ventilation for Buildings—Part 1: Indoor Environmental Input Parameters for Design and Assessment of Energy Performance of Buildings Addressing Indoor Air Quality, Thermal Environment, Lighting and Acoustics—Module M1-6. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2019. Available online: https://standards.iteh.ai/catalog/standards/cen/b4f68755-2204-4796-854a-56643dfcfe89/en-16798-1-2019 (accessed on 2 September 2021).
- Dimitroulopoulou, C. Ventilation in European Dwellings: A Review. Build. Environ. 2012, 47, 109–125. [Google Scholar] [CrossRef]
- Norbäck, D.; Zock, J.-P.; Plana, E.; Heinrich, J.; Tischer, C.; Jacobsen Bertelsen, R.; Sunyer, J.; Künzli, N.; Villani, S.; Olivieri, M.; et al. Building Dampness and Mold in European Homes in Relation to Climate, Building Characteristics and Socio-Economic Status: The European Community Respiratory Health Survey ECRHS II. Indoor Air 2017, 27, 921–932. [Google Scholar] [CrossRef]
- Kokko, E.; Ojanen, T.; Salonvaara, M.; Hukka, A.; Viitanen, H. For Wooden Structures Technical Activities; VTT Technical Research Centre of Finland: Espoo, Finland, 1999; ISBN 978-951-38-5499-7. [Google Scholar]
- Viitanen, H.; Vinha, J.; Salminen, K.; Ojanen, T.; Peuhkuri, R.; Paajanen, L.; Lähdesmäki, K. Moisture and Bio-Deterioration Risk of Building Materials and Structures. J. Build. Phys. 2010, 33, 201–224. [Google Scholar] [CrossRef]
- Hamada, N.; Fujita, T. Effect of Air-Conditioner on Fungal Contamination. Atmos. Environ. 2002, 36, 5443–5448. [Google Scholar] [CrossRef]
- Morey, P.R.; Hodgson, M.J.; Sorenson, W.G.; Kullman, G.J.; Rhodes, W.W.; Visvesvara, G.S. Environmental Studies in Moldy Office Buildings: Biological Agents, Sources and Preventive Measures. Ann. Am. Conf. Gov. Ind. Hyg. 1984, 10, 21–35. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/22733 (accessed on 21 October 2021).
- Jareemit, D.; Shu, S.; Heidarinejad, M.; Kim, Y.-S.; Liu, J.; Alhafi, Z.; Srebric, J. Evaluation of Indoor Mold Growth Relative to Indoor Humidity Using a Multi-Zone Modeling. In Proceedings of the CLIMA 2013. Available online: https://www.researchgate.net/publication/266375006_Evaluation_of_Indoor_Mold_Growth_Relative_to_Indoor_Humidity_Using_a_Multi-zone_Modeling (accessed on 27 June 2023).
- Al-Doory, Y.; Domson, J.F. Mould Allergy; Lea & Febiger: Philadelphia, PA, USA, 1984; ISBN 978-0-8121-0897-2. [Google Scholar]
- Tariq, S.M.; Matthews, S.M.; Stevens, M.; Hakim, E.A. Sensitization to Alternaria and Cladosporium by the Age of 4 Years. Clin. Exp. Allergy 1996, 26, 794–798. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2222.1996.tb00610.x (accessed on 25 October 2021). [CrossRef]
- Sakamoto, T.; Ito, K.; Miyake, M.; Doi, S.; Yamada, M.; Torii, S. Cross-Allergenicity between Aspergillus restrictus, Aspergillus fumigatus and Alternaria alternata Determined by Radioallergosorbent Test Inhibition. Allergol. Int. 1996, 45, 45–49. [Google Scholar] [CrossRef]
- Sekhar, S.C. Enhancement of Ventilation Performance of a Residential Split-System Air-Conditioning Unit. Energy Build. 2004, 36, 273–279. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, W.Z.; Zhang, J.L.; Sun, D.X. Microorganisms and Particles in AHU Systems: Measurement and Analysis. Build. Environ. 2009, 44, 694–698. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Mosso, S.; Sampò, S.; Carraro, E. Assessment of Microbiological Indoor Air Quality in an Italian Office Building Equipped with an HVAC System. Environ. Monit. Assess. 2010, 161, 473–483. [Google Scholar] [CrossRef]
- Piekarska, K.; Trusz, A.; Szczęśniak, S. Bacteria and Fungi in Two Air Handling Units with Air Recirculating Module. Energy Build. 2018, 178, 154–164. [Google Scholar] [CrossRef]
- Al-Abdalall, A.; Al-dakheel, S.; Al-Abkari, H. Impact of Air-Conditioning Filters on Microbial Growth and Indoor Air Pollution. In Low-Temperature Technologies; BoD—Books on Demand: Norderstedt, Germany, 2019; ISBN 978-1-83880-666-8. [Google Scholar]
- Pejtersen, J. Sensory Pollution and Microbial Contamination of Ventilation Filters. Indoor Air 1996, 6, 239–248. [Google Scholar] [CrossRef]
- Grbić, M.; Vukojevic, J.; Stupar, M. Fungal Colonization of Air-Conditioning Systems. Arch. Biol. Sci. 2008, 60, 201–206. [Google Scholar] [CrossRef]
- Abbasi, F.; Samaei, M.R.; Manoochehri, Z.; Jalili, M.; Yazdani, E. The Effect of Incubation Temperature and Growth Media on Index Microbial Fungi of Indoor Air in a Hospital Building in Shiraz, Iran. J. Build. Eng. 2020, 31, 101294. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, A.; Luhung, I.; Gall, E.T.; Cao, Q.; Chang, V.W.-C.; Nazaroff, W.W. Bioaerosol Deposition on an Air-Conditioning Cooling Coil. Atmos. Environ. 2016, 144, 257–265. [Google Scholar] [CrossRef]
- Siegel, J.; Walker, I. Deposition of Biological Aerosols on HVAC Heat Exchangers. In Proceedings of the ASHRAE IAQ 2001, San Francisco, CA, USA, 4–7 November 2001. [Google Scholar]
- Bakker, A.; Siegel, J.A.; Mendell, M.J.; Peccia, J. Building and Environmental Factors That Influence Bacterial and Fungal Loading on Air Conditioning Cooling Coils. Indoor Air 2018, 28, 689–696. [Google Scholar] [CrossRef]
- Luongo, J.C.; Miller, S.L. Ultraviolet Germicidal Coil Cleaning: Decreased Surface Microbial Loading and Resuspension of Cell Clusters. Build. Environ. 2016, 105, 50–55. [Google Scholar] [CrossRef]
- Pasanen, P.; Pasanen, A.-L.; Jantunen, M. Water Condensation Promotes Fungal Growth in Ventilation Ducts. Indoor Air 1993, 3, 106–112. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Kalliokoski, P.; Pasanen, P.; Jantunen, M.J.; Nevalainen, A. Laboratory Studies on the Relationship between Fungal Growth and Atmospheric Temperature and Humidity. Environ. Int. 1991, 17, 225–228. [Google Scholar] [CrossRef]
- Li, A.; Liu, Z.; Liu, Y.; Xu, X.; Pu, Y. Experimental Study on Microorganism Ecological Distribution and Contamination Mechanism in Supply Air Ducts. Energy Build. 2012, 47, 497–505. [Google Scholar] [CrossRef]
- Rämö, A. Statistics Finland—Building Stock 2019. Available online: http://www.stat.fi/til/rakke/2019/rakke_2019_2020-05-27_kat_002_en.html (accessed on 5 November 2020).
- Wyon, D.P. The Effects of Indoor Air Quality on Performance and Productivity. Indoor Air 2004, 14 (Suppl. S7), 92–101. [Google Scholar] [CrossRef]
- Sundell, J.; Levin, H.; Nazaroff, W.W.; Cain, W.S.; Fisk, W.J.; Grimsrud, D.T.; Gyntelberg, F.; Li, Y.; Persily, A.K.; Pickering, A.C.; et al. Ventilation Rates and Health: Multidisciplinary Review of the Scientific Literature. Indoor Air 2011, 21, 191–204. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1600-0668.2010.00703.x?casa_token=q6_qKmmD3OcAAAAA%3AvnjNkNO2q9T-wZoJPeamiLw_riLgjcA7HYqeGm_s9sgqjLrEXSgY45q7Wt9QVFMf_UIYvf5DSCvzzj8 (accessed on 29 September 2021). [CrossRef]
- Tang, J.W.; Bahnfleth, W.P.; Bluyssen, P.M.; Buonanno, G.; Jimenez, J.L.; Kurnitski, J.; Li, Y.; Miller, S.; Sekhar, C.; Morawska, L.; et al. Dismantling Myths on the Airborne Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). J. Hosp. Infect. 2021, 110, 89–96. [Google Scholar] [CrossRef]
- Wargocki, P. What We Know and Should Know about Ventilation. REHVA J. 2021, 58, 5–13. [Google Scholar]
- Wierzbicka, A.; Pedersen, E.; Persson, R.; Nordquist, B.; Stålne, K.; Gao, C.; Harderup, L.-E.; Borell, J.; Caltenco, H.; Ness, B.; et al. Healthy Indoor Environments: The Need for a Holistic Approach. Int. J. Environ. Res. Public Health 2018, 15, 1874. [Google Scholar] [CrossRef]
- Bhagat, R.K.; Linden, P.F. Displacement Ventilation: A Viable Ventilation Strategy for Makeshift Hospitals and Public Buildings to Contain COVID-19 and Other Airborne Diseases. R. Soc. Open Sci. 2020, 7, 200680. [Google Scholar] [CrossRef]
- Chang, J.C.S.; Foarde, K.K.; VanOsdell, D.W. Assessment of Fungal (Penicillium chrysogenum) Growth on Three HVAC Duct Materials. Environ. Int. 1996, 22, 425–431. [Google Scholar] [CrossRef]
- Korpi, A.; Pasanen, A.-L.; Pasanen, P. Volatile Compounds Originating from Mixed Microbial Cultures on Building Materials under Various Humidity Conditions. Appl. Environ. Microbiol. 1998, 64, 2914–2919. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Cao, G.; Meng, C.; He, B.-J. Distribution Characteristics, Growth, Reproduction and Transmission Modes and Control Strategies for Microbial Contamination in HVAC Systems: A Literature Review. Energy Build. 2018, 177, 77–95. [Google Scholar] [CrossRef]
- Li, Y.; Wadsö, L. Simultaneous Measurements of Colony Size and Heat Production Rate of a Mould (Penicillium brevicompactum) Growing on Agar. J. Therm. Anal. Calorim. 2011, 104, 105–111. [Google Scholar] [CrossRef]
- Lab M—A NEOGEN Brand—Lab M. Available online: http://www.labm.com/ (accessed on 29 March 2022).
- Peitzsch, M.; Sulyok, M.; Täubel, M.; Vishwanath, V.; Krop, E.; Borràs-Santos, A.; Hyvärinen, A.; Nevalainen, A.; Krska, R.; Larsson, L. Microbial Secondary Metabolites in School Buildings Inspected for Moisture Damage in Finland, The Netherlands and Spain. J. Environ. Monit. 2012, 14, 2044–2053. [Google Scholar] [CrossRef]
- Li, C.-S. Sampling Performance of Impactors for Bacterial Bioaerosols. Aerosol Sci. Technol. 1999, 30, 280–287. [Google Scholar] [CrossRef]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef]
- Tryptic Soy Agar for Microbiology Tryptone Soya Agar. Available online: http://www.sigmaaldrich.com/ (accessed on 20 June 2023).
- Research Group of Indoor Environment and Occupational Health. Available online: https://uefconnect.uef.fi/en/group/research-group-of-indoor-environment-and-occupational-health/ (accessed on 20 June 2023).
- 3MTM PetrifilmTM Aerobic Count Plates (2 × 50). Labema—Life Science—Ja Bioteknologia-Alan Asiantuntija. Available online: https://www.labema.fi/tuote-C6400 (accessed on 20 June 2023).
- Pasanen, A.-L.; Kujanpää, L.; Pasanen, P.; Kalliokoski, P.; Blomquist, G. Culturable and Total Fungi in Dust Accumulated in Air Ducts in Single-Family Houses. Indoor Air 1997, 7, 121–127. [Google Scholar] [CrossRef]
- Korpi, A.; Pasanen, A.-L.; Pasanen, P.; Kalliokoski, P. Microbial Growth and Metabolism in House Dust. Int. Biodeterior. Biodegrad. 1997, 40, 19–27. [Google Scholar] [CrossRef]
- Lestinen, S.; Kilpeläinen, S.; Kosonen, R.; Valkonen, M.; Jokisalo, J.; Pasanen, P. Effects of Night Ventilation on Indoor Air Quality in Educational Buildings—A Field Study. Appl. Sci. 2021, 11, 4056. [Google Scholar] [CrossRef]
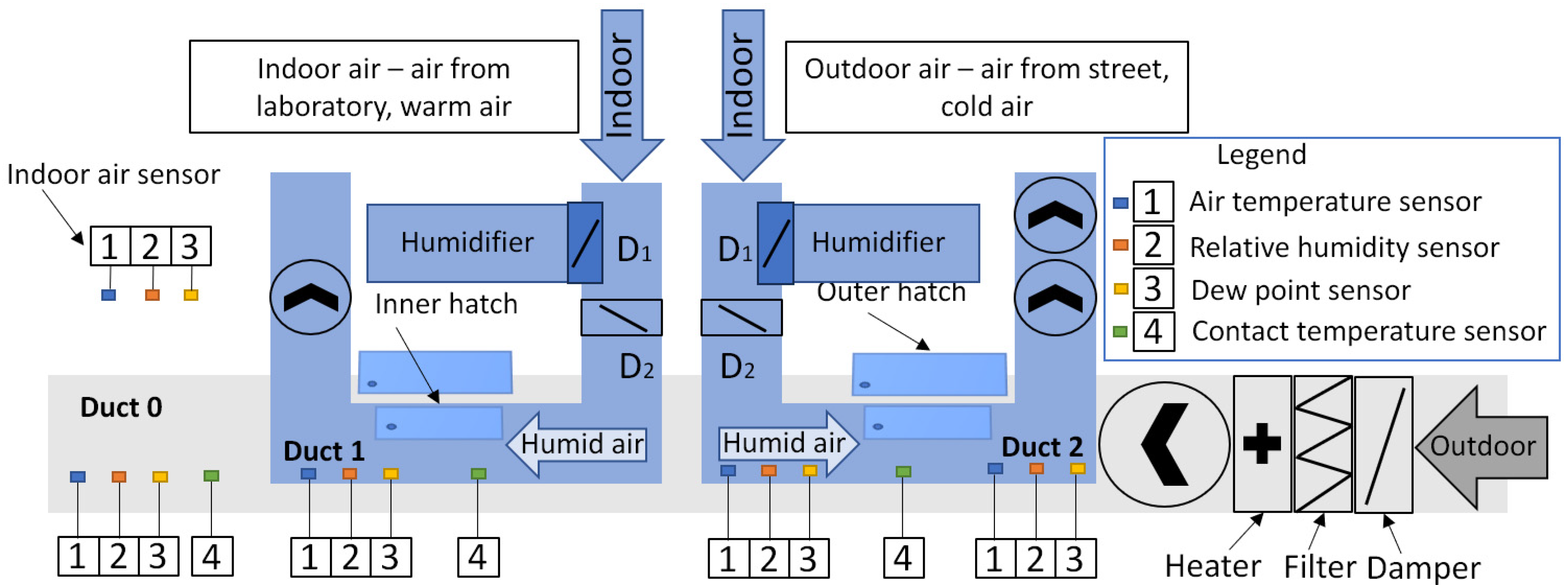
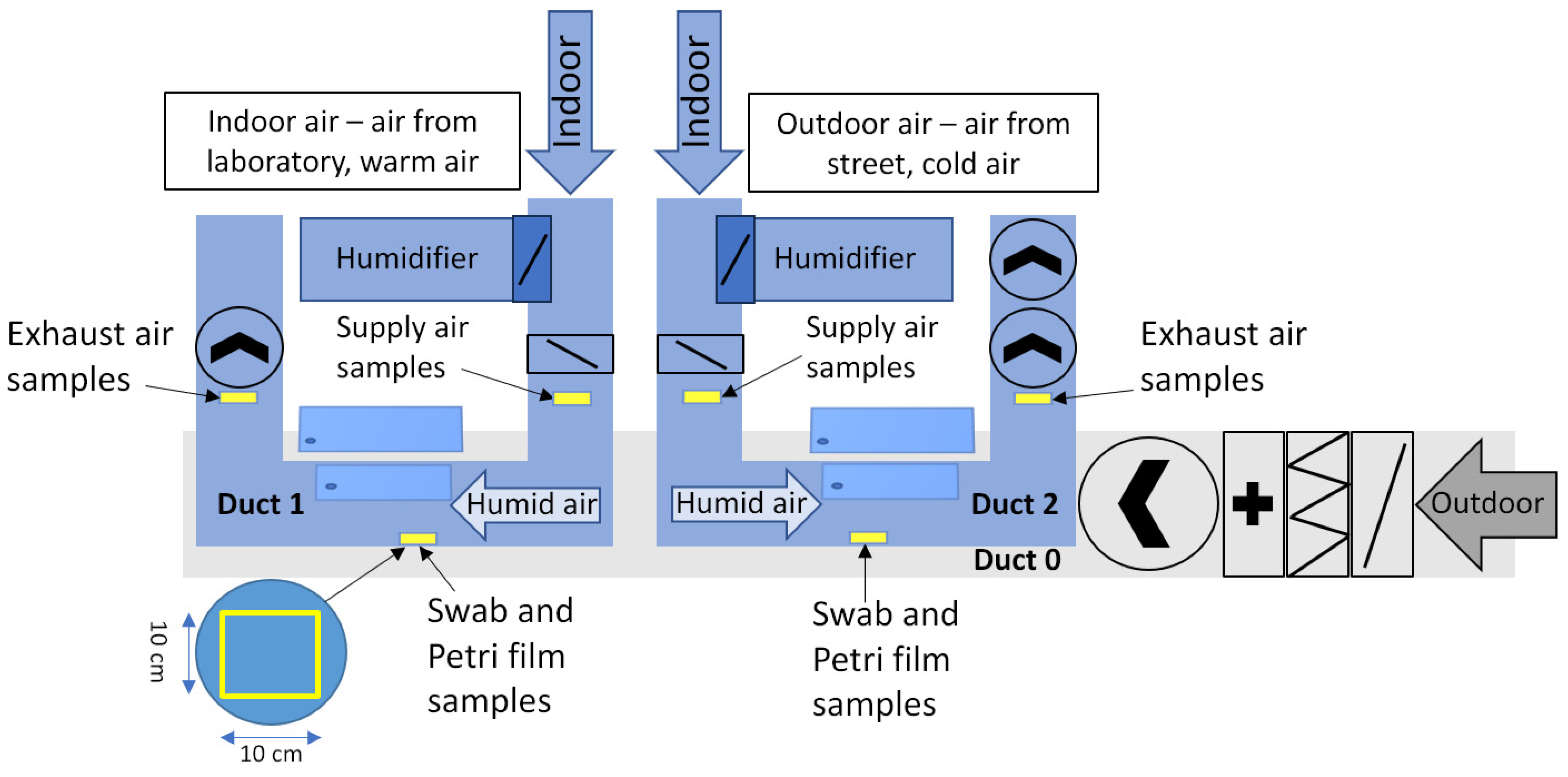

| Device | Parameter | Accuracy |
|---|---|---|
| Tinytag plus 2, TGP-4500 | Air temperature, Relative humidity | 0.5 °C (0… +45 °C); ±3% RH at 25 °C; 5 min interval |
| PT100 class A | Duct surface temperature | ±0.19 °C at 20 °C |
| Airflow LCA 6000 | Air velocity | 0.25 to 4.99 m/s: ±0.1 m/s 5 to 30 m/s: calibrated better than ±2% of reading |
| Sampling | Method | 11.17.2020 | 12.18.2020 | 01.18.2021 | 02.15.2021 | 03.10.2021 |
|---|---|---|---|---|---|---|
| Contact, Fungal | Petri film | ● | ● | ● | ● | ● |
| Swab, Fungal | 10 × 10 cm2 area swabing | ● | ● | |||
| Swab, Bacterial | 10 × 10 cm2 area swabing | ● | ● | |||
| Air, Fungal | Anderson collector | ● | ● | |||
| Air, Bacterial | Anderson collector | ● | ● |
| Swab Sample | Bacterial Colonies | Fungi Colonies |
|---|---|---|
| Change Ratio 1 | Change Ratio | |
| Intermittent ventilation: | 0.41 | 0.27 |
| Continuous ventilation: | 0.46 | 0.08 |
| Air Sample | At the Beginning of the Duct | End of the Duct |
|---|---|---|
| Change Ratio 1 | Change Ratio | |
| Intermittent ventilation: | 0.05 | 0.33 |
| Continuous ventilation: | 0.09 | 0.2 |
| Air Sample | At the Beginning of the Duct | End of the Duct |
|---|---|---|
| Change Ratio 1 | Change Ratio | |
| Intermittent ventilation: | 0.09 | 0.18 |
| Continuous ventilation: | 0.0 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravchenko, I.; Pasanen, P.; Lestinen, S.; Kilpeläinen, S.; Kosonen, R. Risk of Microbial Growth in Ventilation Ductwork Located in the Humid and Cold Conditions. Buildings 2023, 13, 1683. https://doi.org/10.3390/buildings13071683
Kravchenko I, Pasanen P, Lestinen S, Kilpeläinen S, Kosonen R. Risk of Microbial Growth in Ventilation Ductwork Located in the Humid and Cold Conditions. Buildings. 2023; 13(7):1683. https://doi.org/10.3390/buildings13071683
Chicago/Turabian StyleKravchenko, Ilia, Pertti Pasanen, Sami Lestinen, Simo Kilpeläinen, and Risto Kosonen. 2023. "Risk of Microbial Growth in Ventilation Ductwork Located in the Humid and Cold Conditions" Buildings 13, no. 7: 1683. https://doi.org/10.3390/buildings13071683
APA StyleKravchenko, I., Pasanen, P., Lestinen, S., Kilpeläinen, S., & Kosonen, R. (2023). Risk of Microbial Growth in Ventilation Ductwork Located in the Humid and Cold Conditions. Buildings, 13(7), 1683. https://doi.org/10.3390/buildings13071683








