Health and Safety Protocol for the Management of Building Demolition Waste with High Mercury Contamination
Abstract
1. Introduction
1.1. Brief State of the Art
1.2. SUBproducts4LIFE Project
1.3. Empirical Model: Description, Planning, Risk Assessment
1.4. Objectives of the Study
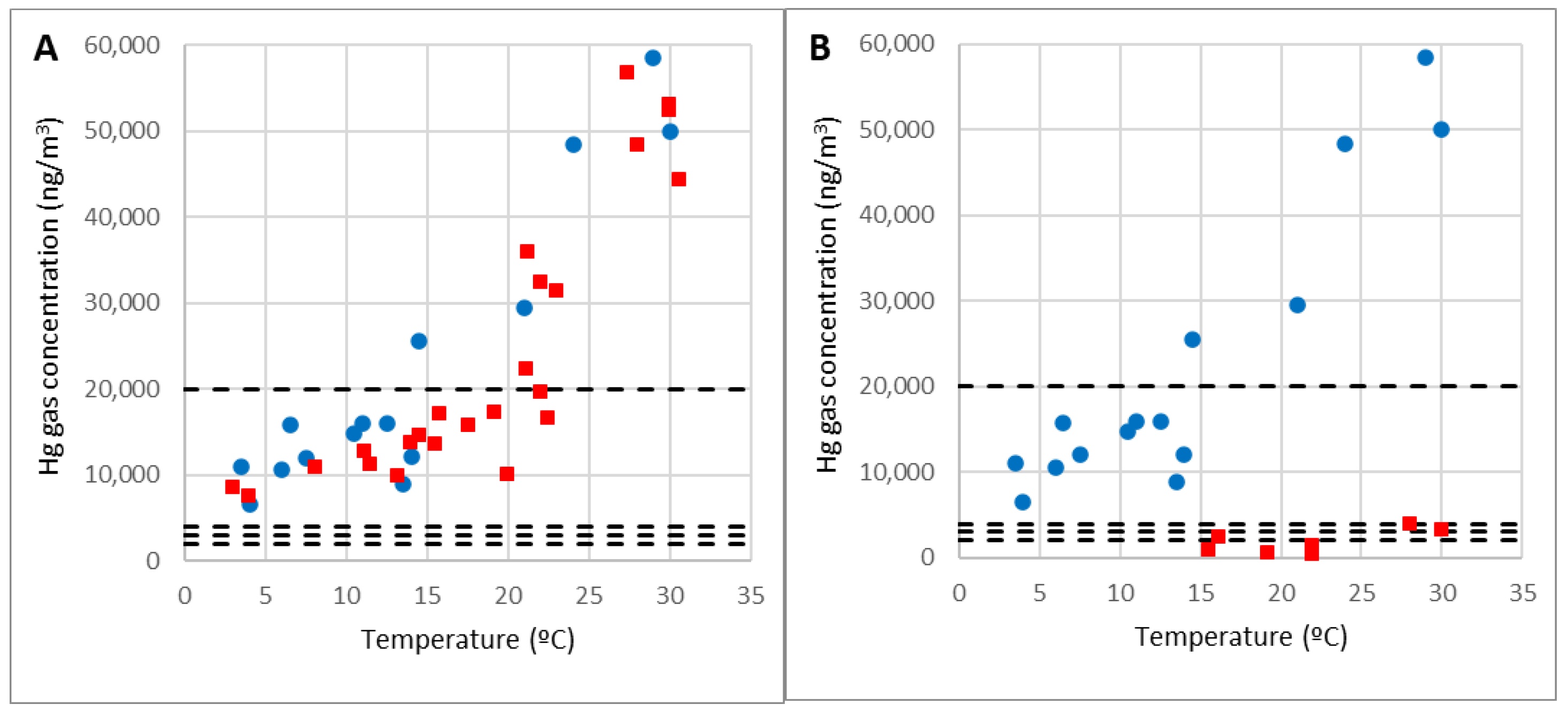
- A gaseous Hg measurement campaign was carried out in the area, with a sampling period of 2 hours following the EN-689:2018 standard [43]. The workplace was differentiated based on the Hg gas concentration in the environment. The accuracy of the empirical model proposed in previous work was verified, and it was shown that temperature is the crucial variable.
- A risk prevention criterion for exposure to gaseous Hg was defined following EN-689:2018, which limits working hours in the most contaminated areas based on ambient temperature; based on it, a protocol was developed to work in health and safety conditions for workers. On the other hand, it is stated that covering the contaminated material is an effective safety measure for worker protection.
- Analysis of the actual work completed was conducted, detailing the actual activities in the riskiest areas (something that is not typically published in the specialised bibliography); based on the empirical model and the risk prevention criterion, an appropriate way to respond was defined, first during the planning of the tasks, and then during their execution, so that the quality standards in terms of health and safety are met at all times.
2. Materials and Methods
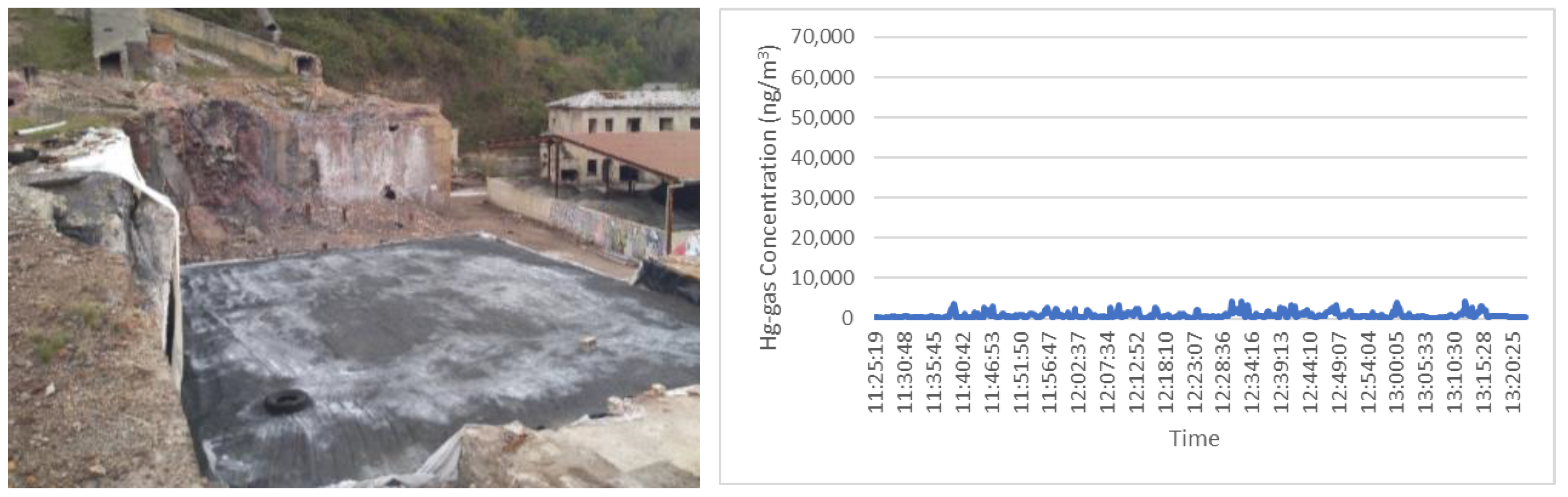
2.1. Measuring Device and Sampling Procedure
- Point 10: area with demolition debris in its original location;
- Point 23: area with demolition rubble covered with slag and ash (next to the previous one).
- Point 3 is an area where the store and welfare facilities are located; it is a rest area for workers.
- Point 4 is where ancillary tasks such as repair and machinery maintenance are carried out.
- Point 7 is a covered work area where the filter channels are located.
- Point 21 is an earthwork work area in the furnace slag heap.
2.2. EN-689:2018 Standard Application
- If j < 0.25, then T = 36 months.
- If 0.25 < j < 0.5, then T = 30 months.
- If j > 0.5, then T = 24 months.
2.3. Biological Markers
3. Results and Discussion
3.1. Results in Areas with Demolition Debris from the Metallurgical Plant Building
- Point 10: area with demolition debris in its original location;
- Point 23: area with demolition rubble covered with slag and ash (next to the previous one).
3.2. Application of the EN-689:2018 Standard and Development of a Risk Prevention Criterion
3.3. Analysis of the Actual Work with the Demolition Rubble of the Metallurgical Plant



- The dates on which the work will be carried out are defined.
- The temperature θ (°C) is defined with the average monthly value of the last 10 years according to the Spanish Agency for Meteorology (Agencia Española de Meteorología, AEMET).
- The allowed work period nmax = 6 h/day is established as a first approximation.
- The concentration at point 10 and the concentration at other alternative points are estimated (for simplicity, it is assumed that they are at least 50 m from point 10):
- The weighted average exposure to gaseous Hg in an 8 h workday is estimated as
- The average concentration of inorganic Hg in urine and blood is estimated:
- It is checked if the Ceq weighted average exposure is in accordance with the OELV = 20,000 ng/m3.
- The concentrations in urine CHg-U and blood CHg-B are compared with the OELV 30 µg/L and 10 µg/L.
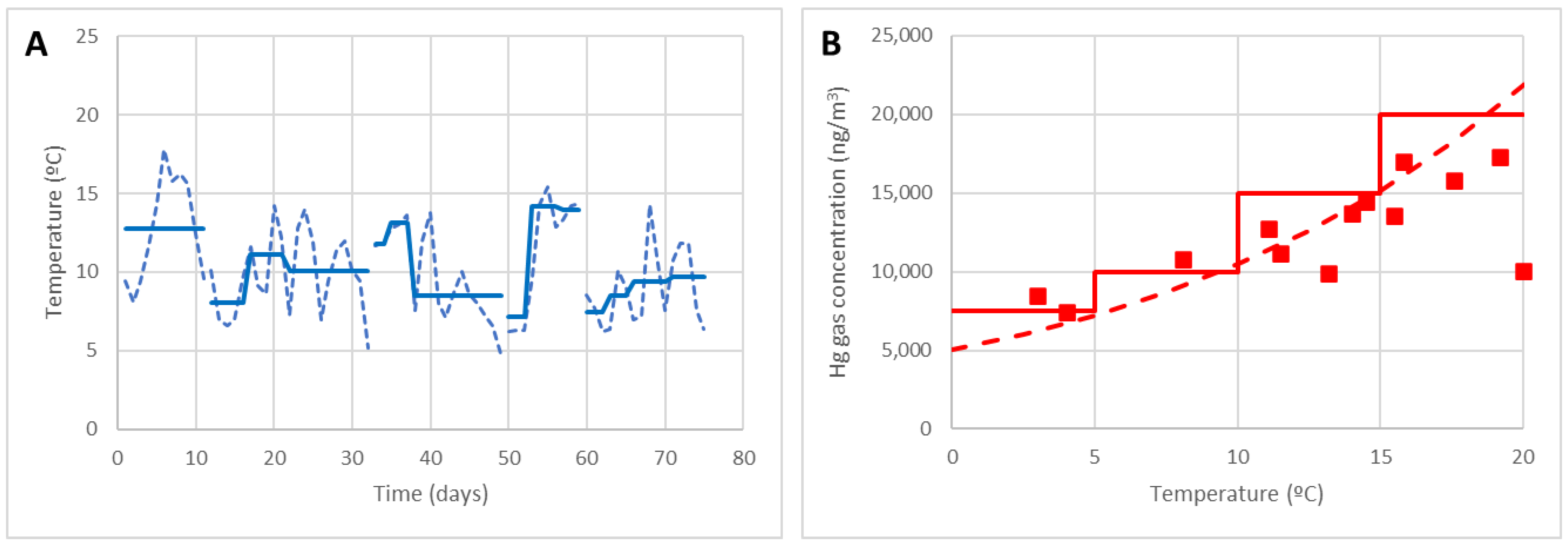
- The best months to work were chosen: those in which the average temperature is below 15 °C according to the prevention criteria (in Asturias, all except those in summer, June, July, August and September).
- The daily temperature was recorded, or the average daily temperature in the area provided by the meteorological services (AEMET) was used. The graph of Figure 13A shows the daily variation of the real temperature, as well as the average temperature of the period.
- The allowed work time was established according to the prevention criteria: nmax = 6 h/day as the temperature is always θ < 15 °C.
- The concentration in point 10 was estimated based on the prevention criteria. In Figure 13B, it is seen that also the curve of the empirical model (dotted) could be used to define the concentration at point 10 at a given temperature; however, as said, it is easier to use the concentration specified by the risk prevention criteria (solid line) according to EN-689:2018.
- The equivalent exposure Ceq was calculated assuming that, with the regulatory breaks, 6 h/day was spent at point 10, 1 h/day at point 3 and 1 h/day at point 7 (the most unfavourable work point). Assuming C3 ≤ 1000 ng/m3 and C7 ≤ 4000 ng/m3, the weighted average exposure to gaseous Hg in an 8 h shift was estimated.
- (a)
- During planning (prediction);
- (b)
- After executing the jobs (back-analysis).
3.4. Brief Analysis under ESG Criteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hylander, L.D.; Meili, M. 500 Years of Mercury Production: Global Annual Inventory by Region until 2000 and Associated Emissions. Sci. Total Environ. 2003, 304, 13–27. [Google Scholar] [CrossRef]
- UN Environment Programme. Guideline for Decommissioning of Mercury Chlor-Alkali Plants. Available online: http://www.unep.org/resources/report/guideline-decommissioning-mercury-chlor-alkali-plants (accessed on 18 April 2023).
- Finster, M.E.; Raymond, M.R.; Scofield, M.A.; Smith, K.P. Mercury-Impacted Scrap Metal: Source and Nature of the Mercury. J. Environ. Manag. 2015, 161, 303–308. [Google Scholar] [CrossRef]
- Randall, P.; Chattopadhyay, S. Advances in Encapsulation Technologies for the Management of Mercury-Contaminated Hazardous Wastes. J. Hazard. Mater. 2004, 114, 211–223. [Google Scholar] [CrossRef]
- Wang, S.; Feng, X.; Qiu, G.; Shang, L.; Li, P.; Wei, Z. Mercury Concentrations and Air/Soil Fluxes in Wuchuan Mercury Mining District, Guizhou Province, China. Atmos. Environ. 2007, 41, 5984–5993. [Google Scholar] [CrossRef]
- Gustin, S.M.; Coolbaugh, M.; Engle, M.; Fitzgerald, B.; Keislar, R.; Lindberg, S.; Nacht, D.; Quashnick, J.; Rytuba, J.; Sladek, C.; et al. Atmospheric Mercury Emissions from Mine Wastes and Surrounding Geologically Enriched Terrains. Environ. Geol. 2003, 43, 339–351. [Google Scholar] [CrossRef]
- Feng, X. Mercury Pollution in China—An Overview. In Dynamics of Mercury Pollution on Regional and Global Scales: Atmospheric Processes and Human Exposures around the World; Springer: Boston, MA, USA, 2005; pp. 657–678. ISBN 978-0-387-24493-8. [Google Scholar]
- Loredo, J.; Ordóñez, A.; Gallego, J.R.; Baldo, C.; García-Iglesias, J. Geochemical Characterisation of Mercury Mining Spoil Heaps in the Area of Mieres (Asturias, Northern Spain). J. Geochem. Explor. 1999, 67, 377–390. [Google Scholar] [CrossRef]
- Lin, Y.; Larssen, T.; Vogt, R.D.; Feng, X. Identification of Fractions of Mercury in Water, Soil and Sediment from a Typical Hg Mining Area in Wanshan, Guizhou Province, China. Appl. Geochem. 2010, 25, 60–68. [Google Scholar] [CrossRef]
- Ordóñez, A.; Álvarez, R.; Charlesworth, S.; Miguel, E.D.; Loredo, J. Risk Assessment of Soils Contaminated by Mercury Mining, Northern Spain. J. Environ. Monit. 2011, 13, 128–136. [Google Scholar] [CrossRef]
- Scholtz, M.T.; Van Heyst, B.J.; Schroeder, W.H. Modelling of Mercury Emissions from Background Soils. Sci. Total Environ. 2003, 304, 185–207. [Google Scholar] [CrossRef]
- Kotnik, J.; Horvat, M.; Dizdarevič, T. Current and Past Mercury Distribution in Air over the Idrija Hg Mine Region, Slovenia. Atmos. Environ. 2005, 39, 7570–7579. [Google Scholar] [CrossRef]
- Loredo, J.; Soto, J.; Alvarez, R.; Ordóñez, A. Atmospheric Monitoring at Abandoned Mercury Mine Sites in Asturias (NW Spain). Environ. Monit. Assess. 2007, 130, 201–214. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, D.; Liu, X.; Zhang, Y. Mercury Fluxes from Air/Surface Interfaces in Paddy Field and Dry Land. Appl. Geochem. 2011, 26, 249–255. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Meng, B.; Sommar, J.; Gu, C. Environmental Geochemistry of an Active Hg Mine in Xunyang, Shaanxi Province, China. Appl. Geochem. 2012, 27, 2280–2288. [Google Scholar] [CrossRef]
- Cabassi, J.; Tassi, F.; Venturi, S.; Calabrese, S.; Capecchiacci, F.; D’Alessandro, W.; Vaselli, O. A New Approach for the Measurement of Gaseous Elemental Mercury (GEM) and H2S in Air from Anthropogenic and Natural Sources: Examples from Mt. Amiata (Siena, Central Italy) and Solfatara Crater (Campi Flegrei, Southern Italy). J. Geochem. Explor. 2017, 175, 48–58. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Kim, K.H.; Meyers, T.P.; Owens, J.G. Micrometeorological Gradient Approach for Quantifying Air/Surface Exchange of Mercury Vapor: Tests over Contaminated Soils. Environ. Sci. Technol. 1995, 29, 126–135. [Google Scholar] [CrossRef]
- Llanos, W.; Kocman, D.; Higueras, P.; Horvat, M. Mercury Emission and Dispersion Models from Soils Contaminated by Cinnabar Mining and Metallurgy. J. Environ. Monit. 2011, 13, 3460–3468. [Google Scholar] [CrossRef]
- Matanzas, N.; Sierra, M.J.; Afif, E.; Díaz, T.E.; Gallego, J.R.; Millán, R. Geochemical Study of a Mining-Metallurgy Site Polluted with As and Hg and the Transfer of These Contaminants to Equisetum Sp. J. Geochem. Explor. 2017, 182, 1–9. [Google Scholar] [CrossRef]
- McNutt, M. Mercury and Health. Science 2013, 341, 1430. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. A Review on the Distribution of Hg in the Environment and Its Human Health Impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, L.; Xia, T.; Jia, X.; Wang, S. Heavy Metal Pollution and Human Health Risk Assessment at Mercury Smelting Sites in Wanshan District of Guizhou Province, China. RSC Adv. 2020, 10, 23066–23079. [Google Scholar] [CrossRef]
- Phelps, R.W.; Clarkson, T.W.; Kershaw, T.G.; Wheatley, B. Interrelationships of Blood and Hair Mercury Concentrations in a North American Population Exposed to Methylmercury. Arch. Environ. Health Int. J. 1980, 35, 161–168. [Google Scholar] [CrossRef]
- Koenigsmark, F.; Weinhouse, C.; Berky, A.J.; Morales, A.M.; Ortiz, E.J.; Pierce, E.M.; Pan, W.K.; Hsu-Kim, H. Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2021, 18, 13350. [Google Scholar] [CrossRef]
- Garcia Gonzalez, H.; García-Ordiales, E.; Diez, R.R. Analysis of the Airborne Mercury and Particulate Arsenic Levels Close to an Abandoned Waste Dump and Buildings of a Mercury Mine and the Potential Risk of Atmospheric Pollution. SN Appl. Sci. 2022, 4, 76. [Google Scholar] [CrossRef]
- WHO. Mercury and Health. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 1 February 2021).
- Saturday, A. Mercury and Its Associated Impacts on Environment and Human Health: A Review. J. Environ. Health Sci. 2018, 4, 37–43. [Google Scholar] [CrossRef]
- Morton, J.; Sams, C.; Leese, E.; Garner, F.; Iqbal, S.; Jones, K. Biological Monitoring: Evidence for Reductions in Occupational Exposure and Risk. Front. Toxicol. 2022, 4, 836567. [Google Scholar] [CrossRef]
- Wcisło, E.; Bronder, J.; Bubak, A.; Rodríguez-Valdés, E.; Gallego, J.L.R. Human Health Risk Assessment in Restoring Safe and Productive Use of Abandoned Contaminated Sites. Environ. Int. 2016, 94, 436–448. [Google Scholar] [CrossRef]
- Lie, A.; Gundersen, N.; Korsgaard, K.J. Mercury in Urine—Sex, Age and Geographic Differences in a Reference Population. Scand. J. Work. Environ. Health 1982, 8, 129–133. [Google Scholar] [CrossRef]
- Yoshida, M. Relation of Mercury Exposure to Elemental Mercury Levels in the Urine and Blood. Scand. J. Work. Environ. Health 1985, 11, 33–37. [Google Scholar] [CrossRef]
- Rumiantseva, O.Y.; Ivanova, E.; Komov, V. High Variability of Mercury Content in the Hair of Russia Northwest Population: The Role of the Environment and Social Factors. Int. Arch. Occup. Environ. Health 2022, 95, 1027–1042. [Google Scholar] [CrossRef]
- Eckley, C.S.; Gilmour, C.C.; Janssen, S.; Luxton, T.P.; Randall, P.M.; Whalin, L.; Austin, C. The Assessment and Remediation of Mercury Contaminated Sites: A Review of Current Approaches. Sci. Total Environ. 2020, 707, 136031. [Google Scholar] [CrossRef]
- González-Valoys, A.C.; Jiménez Salgado, J.U.; Rodríguez, R.; Monteza-Destro, T.; Vargas-Lombardo, M.; García-Noguero, E.M.; Esbrí, J.M.; Jiménez-Ballesta, R.; García-Navarro, F.J.; Higueras, P. An Approach for Evaluating the Bioavailability and Risk Assessment of Potentially Toxic Elements Using Edible and Inedible Plants—The Remance (Panama) Mining Area as a Model. Environ. Geochem. Health 2021, 45, 151–170. [Google Scholar] [CrossRef]
- Wcisło, E.; Bronder, J.; Rodríguez-Valdés, E.; Gallego, J.L.R. Health Risk Assessment of Post-Mining Hg-As-Contaminated Soil: Implications for Land Remediation. Water Air Soil Pollut. 2022, 233, 306. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Tun, A.Z.; Chotpantarat, S.; Siriwong, W. Related Health Risk Assessment of Exposure to Arsenic and Some Heavy Metals in Gold Mines in Banmauk Township, Myanmar. Sci. Rep. 2021, 11, 22843. [Google Scholar] [CrossRef]
- Mina de La Soterraña (Lena—Asturias) a Vista de Dron; Ax1 Ahora: La Soterraña, Asturias, 2022.
- Rodríguez, R.; Garcia-Gonzalez, H.; García-Ordiales, E. Empirical Model of Gaseous Mercury Emissions for the Analysis of Working Conditions in Outdoor Highly Contaminated Sites. Sustainability 2022, 14, 13951. [Google Scholar] [CrossRef]
- Rodríguez, R.; Fernández, B.; Malagón, B.; Garcia-Ordiales, E. Chemical-Physical Model of Gaseous Mercury Emissions from the Demolition Waste of an Abandoned Mercury Metallurgical Plant. Appl. Sci. 2023, 13, 3149. [Google Scholar] [CrossRef]
- Siegel, S.M.; Siegel, B.Z. Temperature Determinants of Plant-Soil-Air Mercury Relationships. Water Air Soil Pollut. 1988, 40, 443–448. [Google Scholar] [CrossRef]
- Zhang, H.; Lindberg, S.E.; Marsik, F.J.; Keeler, G.J. Mercury Air/Surface Exchange Kinetics of Background Soils of the Tahquamenon River Watershed in the Michigan Upper Peninsula. Water Air Soil Pollut. 2001, 126, 151–169. [Google Scholar] [CrossRef]
- Nagl, C.; Spangl, W.; Buxbaum, I. Sampling Points for Air Quality. In Representativeness and Comparability of Measurement in Accordance with Directive 2008/50/EC on Ambient Air Quality and Cleaner Air for Europe; European Parliament: Luxembourg, 2019. [Google Scholar]
- EN 689:2018+AC:2019; Workplace Exposure-Measurement of Exposure by Inhalation to Chemical Agents-Strategy for Testing Compliance with Occupational Exposure Limit Values. European Committee for Standardisation: Brussels, Belgium, 2018.
- Rodriguez, V. El Estado de Situación Del Mercurio Ambiental En Asturias. 2013. Available online: https://www.astursalud.es/documents/35439/38376/20130613+El+estado+de+situaci%C3%83%C2%B3n+del+mercurio+ambiental+en+Asturias.pdf/c7fecc72-43e4-7b00-1fe5-690e5f8152de?t=1564654288891 (accessed on 1 May 2023).
- INSST Límites de Exposición Profesional para Agentes Químicos 2022—Portal INSST. Available online: https://www.insst.es/documentacion/catalogo-de-Publicaciones/limites-de-exposicion-profesional-para-agentes-quimicos-2022 (accessed on 26 April 2022).
- Drake, P.; Rojas, M.; Reh, C.; Mueller, C.; Jenkins, F. Occupational Exposure to Airborne Mercury during Gold Mining Operations near El Callao, Venezuela. Int. Arch. Occup. Environ. Health 2001, 74, 206–212. [Google Scholar] [CrossRef]
- Español Cano, S. Toxicología Del Mercurio. In Actuaciones Preventivas En Sanidad Laboral y Ambiental; Gama Ed., Lima, Perú. 2001. Available online: http://www.gama-peru.org/jornada-hg/espanol.pdf (accessed on 1 May 2023).
- Iden, M.; Kira, S.; Miyaue, H.; Fukuda, M.; Yamaguchi, K.; Fujiki, Y. Biological Monitoring of Inorganic Mercury in Workers in a Fluorescent Lamp Plant. J. Occup. Health 1998, 40, 68–72. [Google Scholar] [CrossRef]
- Tejero Manzanares, J.; Español Cano, S.; Serrano García, J.J.; de Paula Montes Tubío, F. Niveles de mercurio en ambiente y en fluidos biológicos: Caso de la metalurgia en Almadén, España (1986–2001). Salud de los Trabajadores 2011, 19, 123–133. [Google Scholar]
- Kobal, A.; Dizdarevič, T. The Health Safety Programme for Workers Exposed to Elemental Mercury at the Mercury Mine in Idrija. Water Air Soil Pollut. 1997, 97, 169–184. [Google Scholar] [CrossRef]
- Ramírez, A.V. Mejora de los indicadores biológicos de exposición al mercurio en trabajadores de una refinería de oro. Anales de la Facultad de Medicina 2011, 72, 177–182. [Google Scholar] [CrossRef]
- Lovejoy, H.B.; Bell, Z.G. Mercury Exposure Evaluations and Their Correlation With Urine Mercury Excretions: 6. Recommendations for Medical Evaluation of Mercury Exposed Workers in the Chlor-Alkali Industry. J. Occup. Med. 1973, 15, 964–966. [Google Scholar]
- Euro Chlor Code of Practice: Control of Worker Exposure to Mercury in the Chlor-Alkali Industry | Global Mercury Partnership; Euro Chlor, Brussels. 2010. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/13103/Health_2_Edition_6.pdf?sequence=1&isAllowed=y (accessed on 1 May 2023).
- Mason, H. Biological Monitoring and Exposure to Mercury. Occup. Med. 2001, 51, 2–11. [Google Scholar] [CrossRef]
- Ellingsen, D.G.; Thomassen, Y.; Langård, S.; Kjuus, H. Urinary Mercury Excretion in Chloralkali Workers after the Cessation of Exposure. Scand. J. Work. Environ. Health 1993, 19, 334–341. [Google Scholar] [CrossRef]
- Díez, S.; Esbrí, J.M.; Tobias, A.; Higueras, P.; Martínez-Coronado, A. Determinants of Exposure to Mercury in Hair from Inhabitants of the Largest Mercury Mine in the World. Chemosphere 2011, 84, 571–577. [Google Scholar] [CrossRef]
- Packull-McCormick, S.; Ratelle, M.; Lam, C.; Napenas, J.; Bouchard, M.; Swanson, H.; Laird, B.D. Hair to Blood Mercury Concentration Ratios and a Retrospective Hair Segmental Mercury Analysis in the Northwest Territories, Canada. Environ. Res. 2022, 203, 111800. [Google Scholar] [CrossRef]
- Harada, M.; Nakachi, S.; Cheu, T.; Hamada, H.; Ono, Y.; Tsuda, T.; Yanagida, K.; Kizaki, T.; Ohno, H. Monitoring of Mercury Pollution in Tanzania: Relation between Head Hair Mercury and Health. Sci. Total Environ. 1999, 227, 249–256. [Google Scholar] [CrossRef]
- Ramos, M.; Carrasco, J.; Conde, A.; Delacasa, E.; Pujols, M.; Ballesteros, G.; Malet, A.; Caprino, A.; Chimenos, J.M. Guidelines on Best Environmental Practices for the Environmental Sound Management of Mercury Contaminated Sites in the Mediterranean United Nations Environment Programmes. Athens, Greece. 2015. Available online: https://wedocs.unep.org/handle/20.500.11822/9917 (accessed on 1 May 2023).
- BlackRock Financial Markets Advisory. Directorate-General for Financial Stability, F.S. and C.M.U. (European C. Development of Tools and Mechanisms for the Integration of ESG Factors into the EU Banking Prudential Framework and into Banks’ Business Strategies and Investment Policies: Final Study; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-17252-9. [Google Scholar]
- Li, T.-T.; Wang, K.; Sueyoshi, T.; Wang, D.D. ESG: Research Progress and Future Prospects. Sustainability 2021, 13, 11663. [Google Scholar] [CrossRef]
- Seth, R.; Gupta, S.; Gupta, H. ESG Investing: A Critical Overview. Hans Shodh Sudha 2021, 2, 69–80. [Google Scholar]
- Ehlers, T.; Elsenhuber, U.; Jegarasasingam, A.; Jondeau, E. Deconstructing ESG Scores: How to Invest with Your Own Criteria. BIS Work. Pap. Basel, Switzerland. 2022, pp. 1–48. Available online: https://www.bis.org/publ/work1008.pdf (accessed on 1 May 2023).







| n | UT | n | UT | n | UT | n | UT | n | UT |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 2.187 | 11 | 1.981 | 16 | 1.905 | 21 | 1.863 | 26 | 1.836 |
| 7 | 2.12 | 12 | 1.961 | 17 | 1.895 | 22 | 1.857 | 27 | 1.832 |
| 8 | 2.072 | 13 | 1.944 | 18 | 1.886 | 23 | 1.851 | 28 | 1.828 |
| 9 | 2.035 | 14 | 1.929 | 19 | 1.878 | 24 | 1.846 | 29 | 1.824 |
| 10 | 2.005 | 15 | 1.917 | 20 | 1.870 | 25 | 1.841 | 30 | 1.820 |
| Fluid | Biological Indicator | Moment of Sampling | Year of Update |
|---|---|---|---|
| Urine | Total inorganic mercury 30 μg/gcreatinine | Before working hours | 2013 |
| Blood | Total inorganic mercury 10 μg/L | End of the workweek | 2013 |
| Point 10 | Point 23 | ||
|---|---|---|---|
| Temp. (°C) | C10 (ng/m3) | Temp. (°C) | C23 (ng/m3) |
| 3 | 8505 | 16.2 | 2394 |
| 4 | 7420 | 15.5 | 814 |
| 13.2 | 9915 | 22 | 445 |
| 11.1 | 12,738 | 19.2 | 641 |
| 11.5 | 11,195 | 28 | 3912 |
| 8.1 | 10,800 | 30 | 3317 |
| 21.2 | 35,883 | 22 | 1383 |
| 14.5 | 14,481 | - | - |
| 23 | 31,404 | - | - |
| 14 | 13,680 | - | - |
| 15.5 | 13,570 | - | - |
| 15.8 | 17,000 | - | - |
| 17.6 | 15,805 | - | - |
| 22 | 19,606 | - | - |
| 22.5 | 16,600 | - | - |
| 19.2 | 17,315 | - | - |
| 21.1 | 22,323 | - | - |
| 30 | 52,313 | - | - |
| 30.6 | 44,308 | - | - |
| 28 | 48,334 | - | - |
| 30 | 53,095 | - | - |
| 22 | 32,327 | - | - |
| 27.4 | 56,746 | - | - |
| 20 | 10,082 | - | - |
| Hg Gas Concentration on the Rubble C10 (ng/m3) | Scenario | Hg Gas Concentration Ranges C10 (ng/m3) | Temperature Ranges θ (°C) | Permissible Working Time EN689 Standard (h/day) | Working Time Assumed in the Protocol (h/day) | Hg Gas Monitoring Interval | Additional Measures |
|---|---|---|---|---|---|---|---|
| ≤10,000 | Green | 5000–7500 | 0–5 °C | 8 | 6 * | 1 month | No |
| 7500–10,000 | 5–10 °C | 8 | 6 * | 1 month | No | ||
| 10,000–20,000 | Orange | 10,000–15,000 | 10–15 °C | 8 | 6 * | 1 month | No |
| 15,000–20,000 | 15–20 °C | 5 | 5 | 2 weeks | Recommended | ||
| ≥20,000 | Red | 20,000–40,000 | 20–25 °C | 3 | 3 | 2 weeks | Recommended |
| 40,000–60,000 | 25–30 °C | 1 ** | 1 ** | Continuous | Necessary |
| Task | Work to Be Carried Out | Plant Machinery | Number of Workers |
|---|---|---|---|
| 1 | Debris removal, demolition of benches and scaling the work area | Backhoe, excavator with a hydraulic hammer, diesel generator with irrigation equipment | 3 |
| 2 | Recessing and spreading of the concrete slab | Backhoe, concrete tank, diesel generator, compressor | 3 |
| 3 | Construction of perimeter retaining wall and placement of drainage pipe | Backhoe, diesel generator, compressor, concrete mixer | 4 |
| 4 | Waterproofing with HDPE sheet | Backhoe, diesel generator, compressor, HDPE welding equipment | 3 (+4) |
| 5 | Application slag surface layer on an HDPE sheet | Backhoe, track chain excavator, tipper truck | 3 |
| 6 | Filling of the treatment area with demolition rubble | Backhoe, track chain excavator | 3 |
| 7 | Filling of the treatment area with furnace slag | Backhoe, track chain excavator, tipper truck | 3 |
| 8 | Ash coating | Backhoe, track chain excavator, tipper truck | 3 |
| Task | Year | Month | Temp. (°C) | Working Hours (h/day) | C10 (ng/m3) | Ci (ng/m3) | Ceq (ng/m3) | CHg-U (µg/L) | CHg-B (µg/L) | Ceq/20,000 | CHg-U/30 | CHg-B/10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | October | 14.8 | 6 | 19,159 | 1446 | 14,731 | 14.7 | 3.1 | 0.74 | 0.49 | 0.31 |
| 2 | 2 | February | 8.1 | 6 | 11,955 | 902 | 9191 | 9.2 | 2.3 | 0.46 | 0.31 | 0.23 |
| 3 | 2 | February | 8.1 | 6 | 11,955 | 902 | 9191 | 9.2 | 2.3 | 0.46 | 0.31 | 0.23 |
| 4 | 2 | March | 9.7 | 6 | 13,380 | 1010 | 10,287 | 10.3 | 2.4 | 0.51 | 0.34 | 0.24 |
| 5 | 2 | October | 14.8 | 6 | 19,159 | 1446 | 14,731 | 14.7 | 3.1 | 0.74 | 0.49 | 0.31 |
| 6 | 2 | October | 14.8 | 6 | 19,159 | 1446 | 14,731 | 14.7 | 3.1 | 0.74 | 0.49 | 0.31 |
| 7 | 3 | February | 8.1 | 6 | 11,955 | 902 | 9191 | 9.2 | 2.3 | 0.46 | 0.31 | 0.23 |
| 8 | 3 | February | 8.1 | 6 | 11,955 | 902 | 9191 | 9.2 | 2.3 | 0.46 | 0.31 | 0.23 |
| Phase | Task | Year | Month | Duration (Days) | Days between Works | Temp. (°C) | Working Hours (h/day) | C10 (ng/m3) | Ceq (ng/m3) | Ceq/OELV |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | October | 11 | 104 | 12.8 | 6 | 15,000 | 11,875 | 0.59 |
| 1 | 2 | 2 | February | 5 | - | 8.1 | 5, 5 | 10,000 | 8125 | 0.41 |
| 1 | 3 | 2 | February | 5 | - | 11.1 | 6 | 15,000 | 11,875 | 0.59 |
| 1 | 4 | 2 | March | 11 | >365 | 10.1 | 5, 5 | 10,000 | 8125 | 0.41 |
| 1 | 5 | 2 | October | 2 | - | 11.8 | 6 | 15,000 | 11,875 | 0.59 |
| 1 | 6 | 2 | October | 3 | - | 13.1 | 6 | 15,000 | 11,875 | 0.59 |
| 2 | 1 | 3 | November | 12 | 36 | 8.5 | 6 | 10,000 | 8125 | 0.41 |
| 2 | 3 | 3 | December | 3 | - | 7.1 | 6 | 10,000 | 8125 | 0.41 |
| 2 | 2 | 3 | December | 4 | - | 14.2 | 6 | 15,000 | 11,875 | 0.59 |
| 2 | 4 | 3 | December | 3 | 42 | 14.0 | 6 | 15,000 | 11,875 | 0.59 |
| 2 | 5 | 4 | February | 3 | - | 7.5 | 6 | 10,000 | 8125 | 0.41 |
| 2 | 6 | 4 | February | 3 | - | 8.5 | 6 | 10,000 | 8125 | 0.41 |
| 1/2 | 7 | 4 | February | 5 | - | 9.4 | 4 | 10,000 | 8125 | 0.41 |
| 1/2 | 8 | 4 | February | 5 | >365 | 9.7 | 6 | 10,000 | 8125 | 0.41 |
| Environmental (E) | Social (S) | ||||
|---|---|---|---|---|---|
| Reference | Source | Dimension | Factor | Dimension | Factor |
| [60] | BlackRock FMA analysis | Natural Resources and Pollution | Waste Management/Toxic Emissions | Internal Stakeholder Management | Worker’s rights: safe working conditions |
| [61] | EBA report on ESG risk management and supervision | Environmental | Waste production and management (water, solid, hazardous) | Social | Workplace health and safety |
| [62] | Thomson Reuters | Environmental | Emissions | Social | Workforce |
| [63] | Refinitiv | Emissions reduction | Emissions; Waste; Environmental management systems | Workforce | Working conditions; Health and safety |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, R.; Garcia-Gonzalez, H.; Pastrana, Á.; Hernández, Z. Health and Safety Protocol for the Management of Building Demolition Waste with High Mercury Contamination. Buildings 2023, 13, 1310. https://doi.org/10.3390/buildings13051310
Rodríguez R, Garcia-Gonzalez H, Pastrana Á, Hernández Z. Health and Safety Protocol for the Management of Building Demolition Waste with High Mercury Contamination. Buildings. 2023; 13(5):1310. https://doi.org/10.3390/buildings13051310
Chicago/Turabian StyleRodríguez, Rafael, Hector Garcia-Gonzalez, Ángel Pastrana, and Zenaida Hernández. 2023. "Health and Safety Protocol for the Management of Building Demolition Waste with High Mercury Contamination" Buildings 13, no. 5: 1310. https://doi.org/10.3390/buildings13051310
APA StyleRodríguez, R., Garcia-Gonzalez, H., Pastrana, Á., & Hernández, Z. (2023). Health and Safety Protocol for the Management of Building Demolition Waste with High Mercury Contamination. Buildings, 13(5), 1310. https://doi.org/10.3390/buildings13051310








