Study on the Modification Effect and Mechanism of a Compound Mineral Additive and Basalt Fiber on Coal Gangue Concrete
Abstract
:1. Introduction
2. Materials and Methods
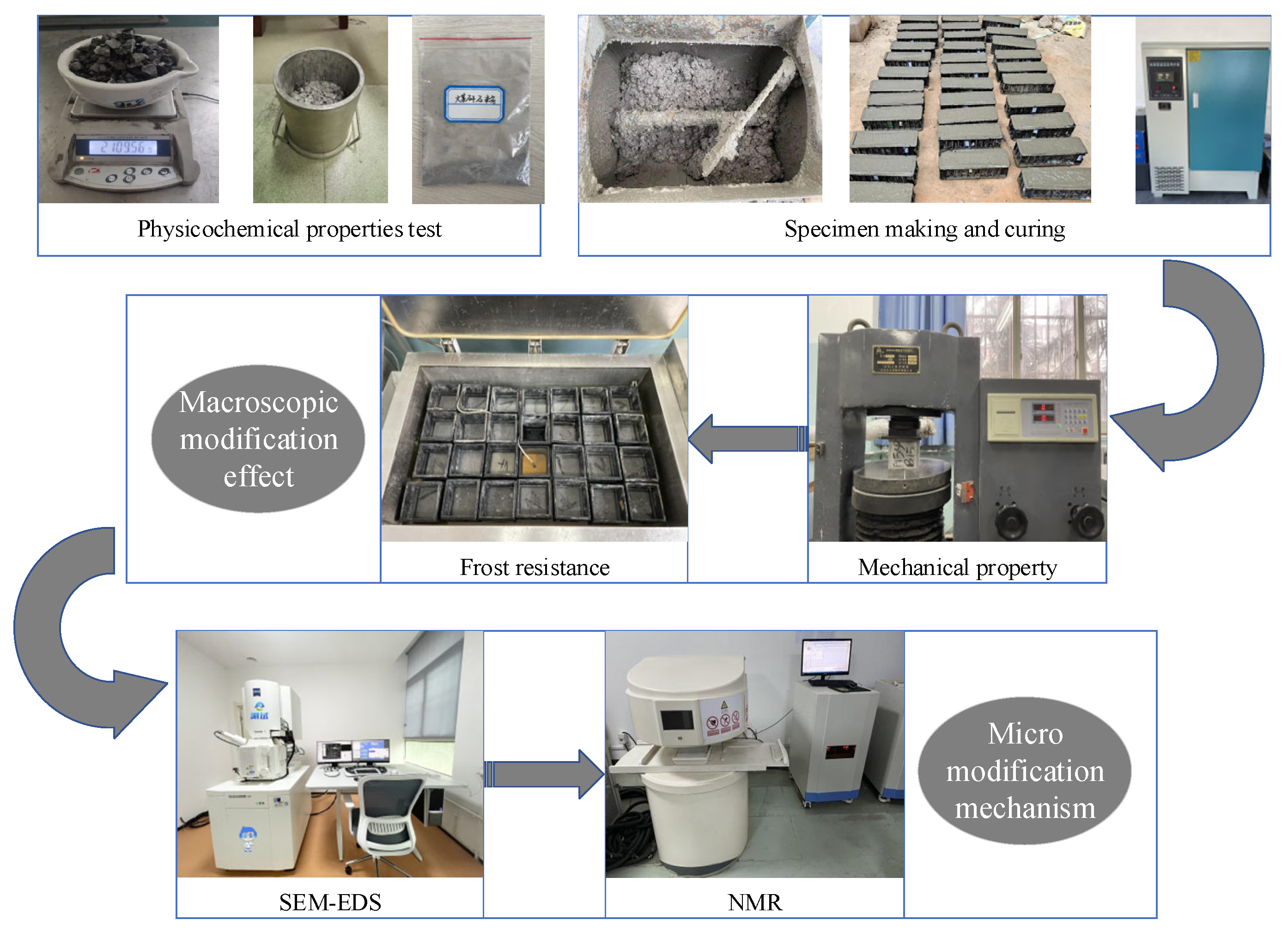
2.1. Experimental Research Plan
2.2. Raw Materials
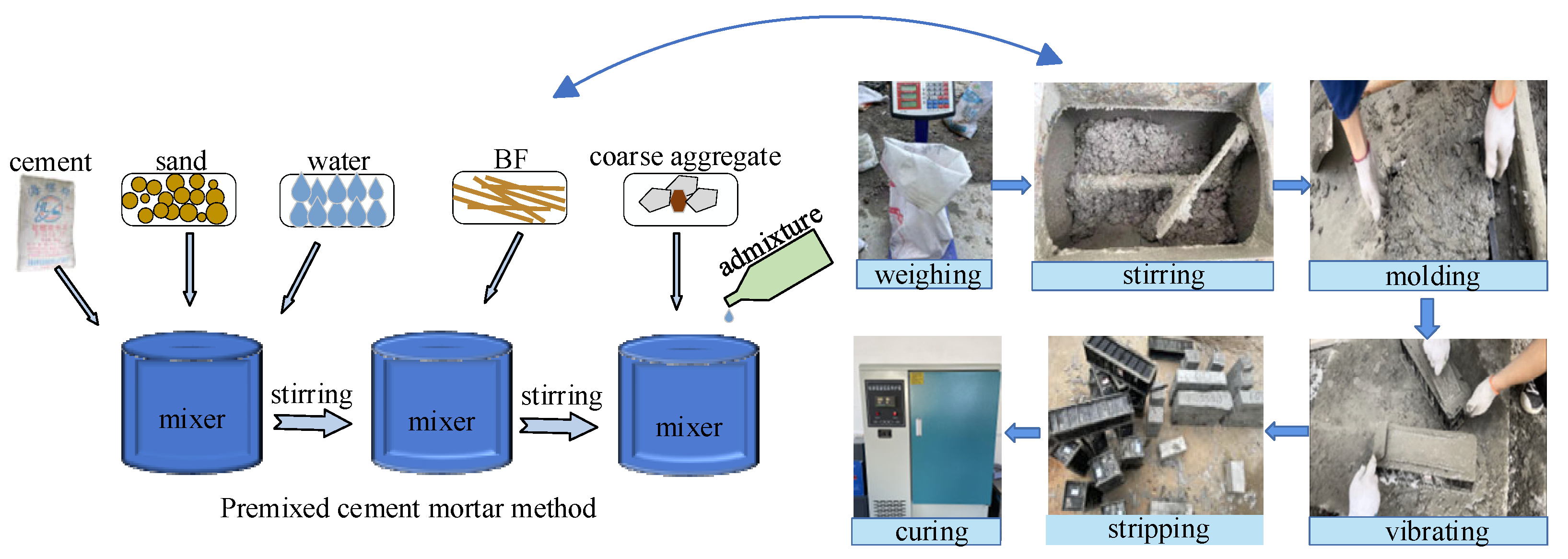
2.3. Mix Proportion
2.4. Specimen Preparation
2.5. Main Test Instruments and Methods
2.5.1. Mechanical Properties Test
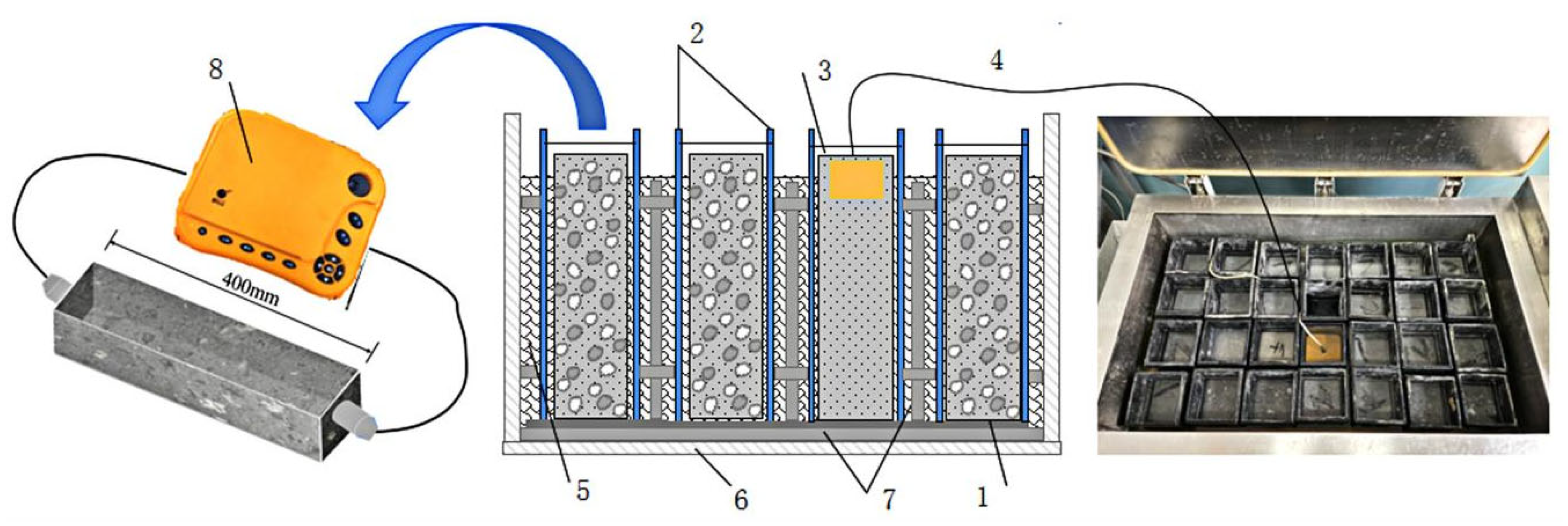
2.5.2. Freeze–Thaw Cycle Test
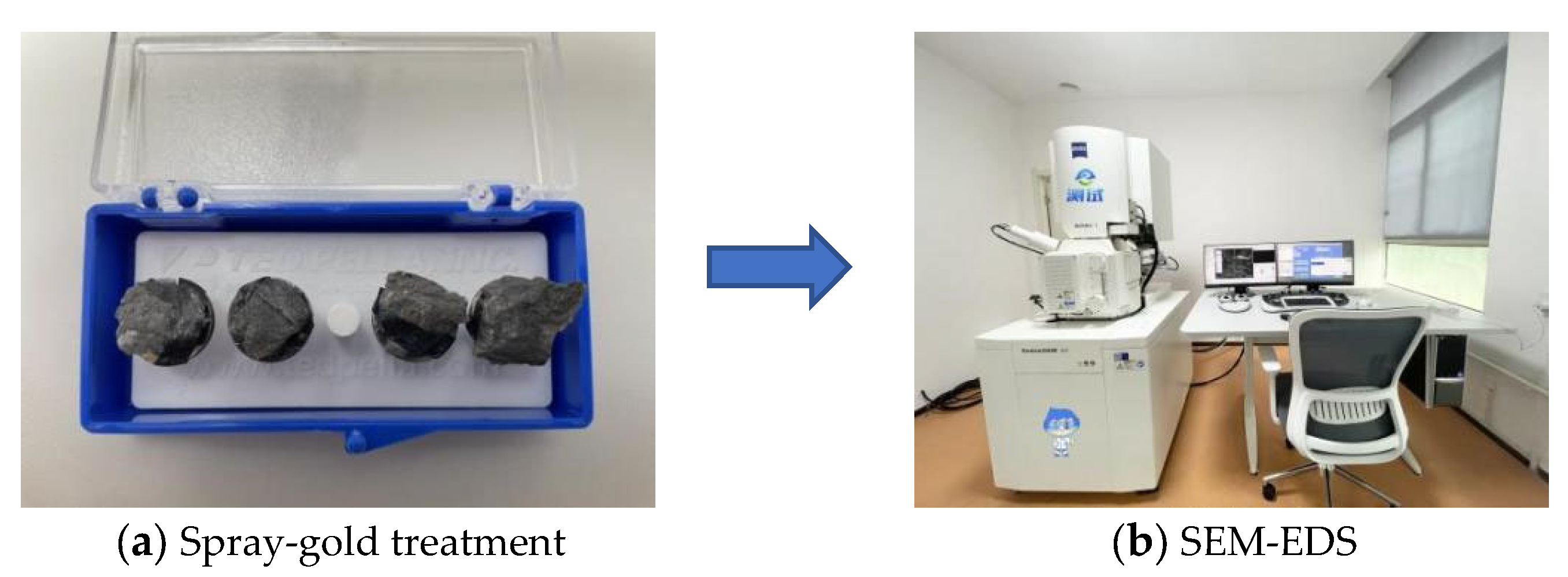
2.5.3. Microscopic Test
3. Test Results and Analysis
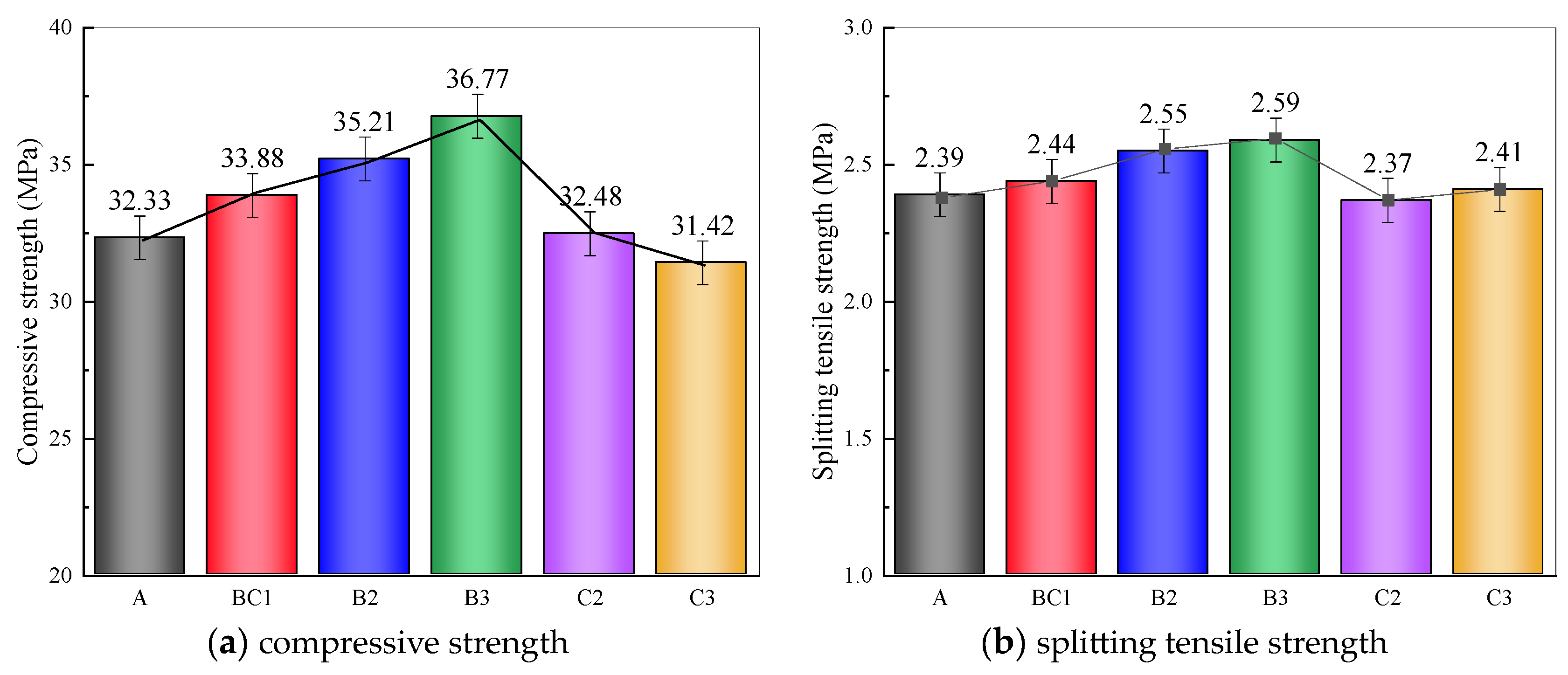
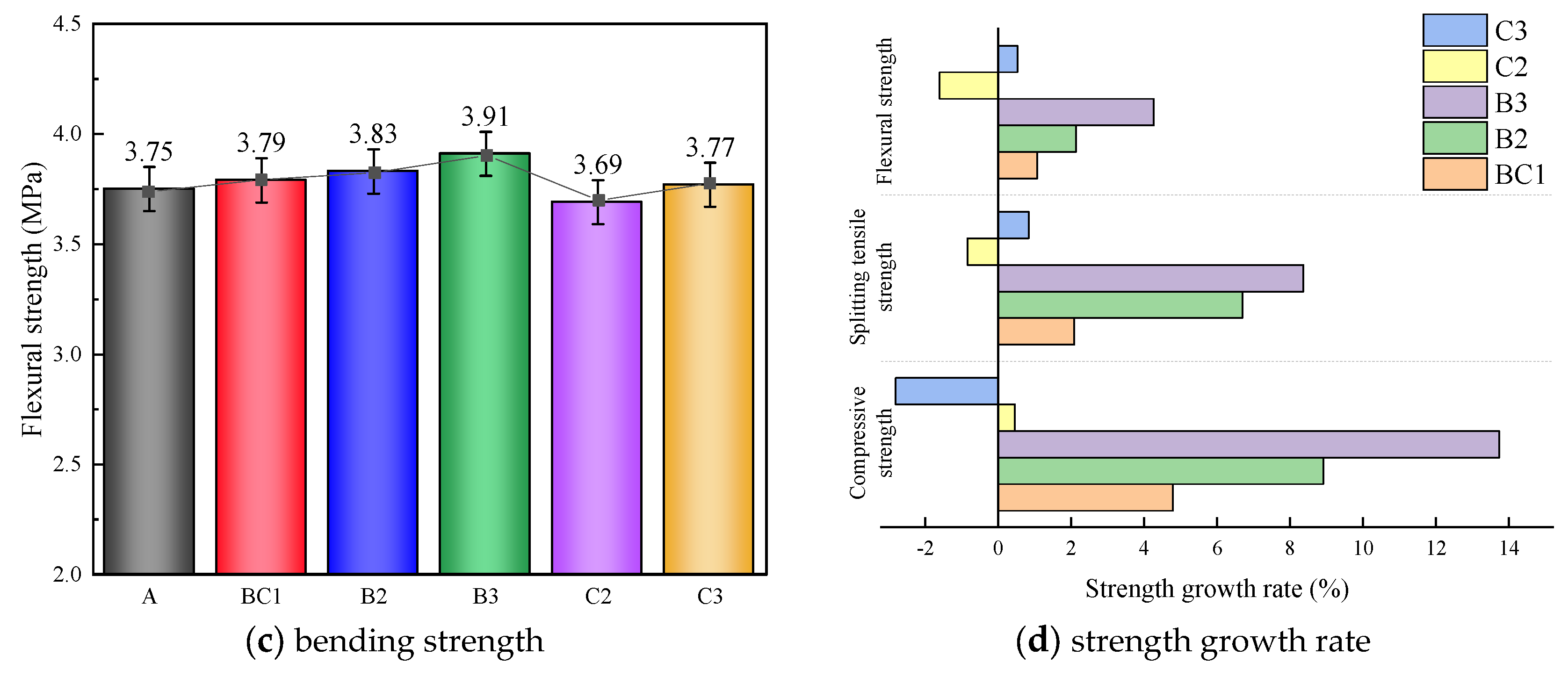
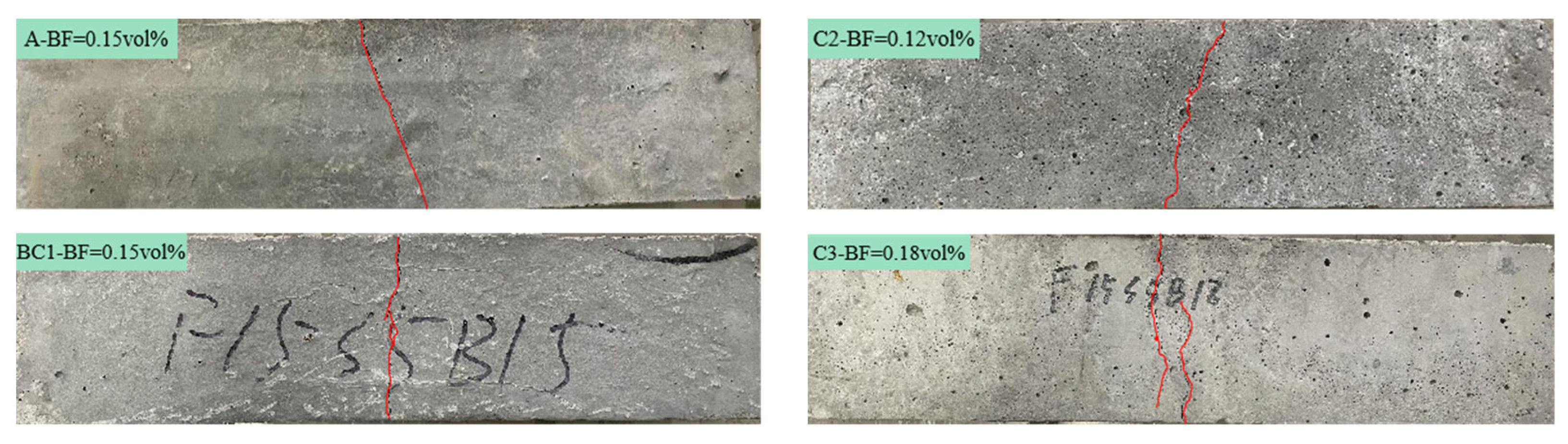
3.1. The Mechanical Properties and Fracture Surface Analysis
3.1.1. Mechanical Properties
3.1.2. Fracture Surface Analysis
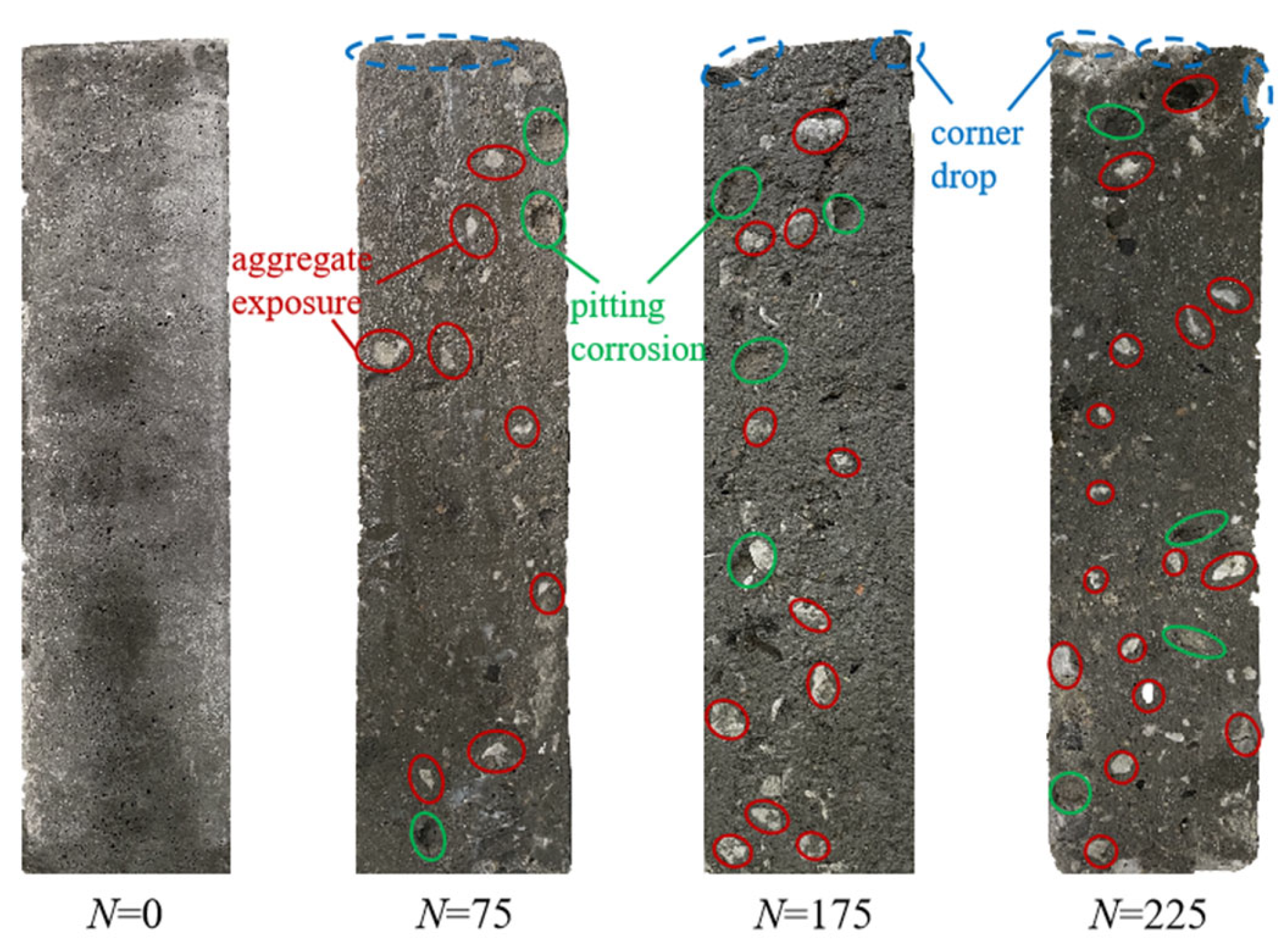
3.2. Analysis of Frost Resistance
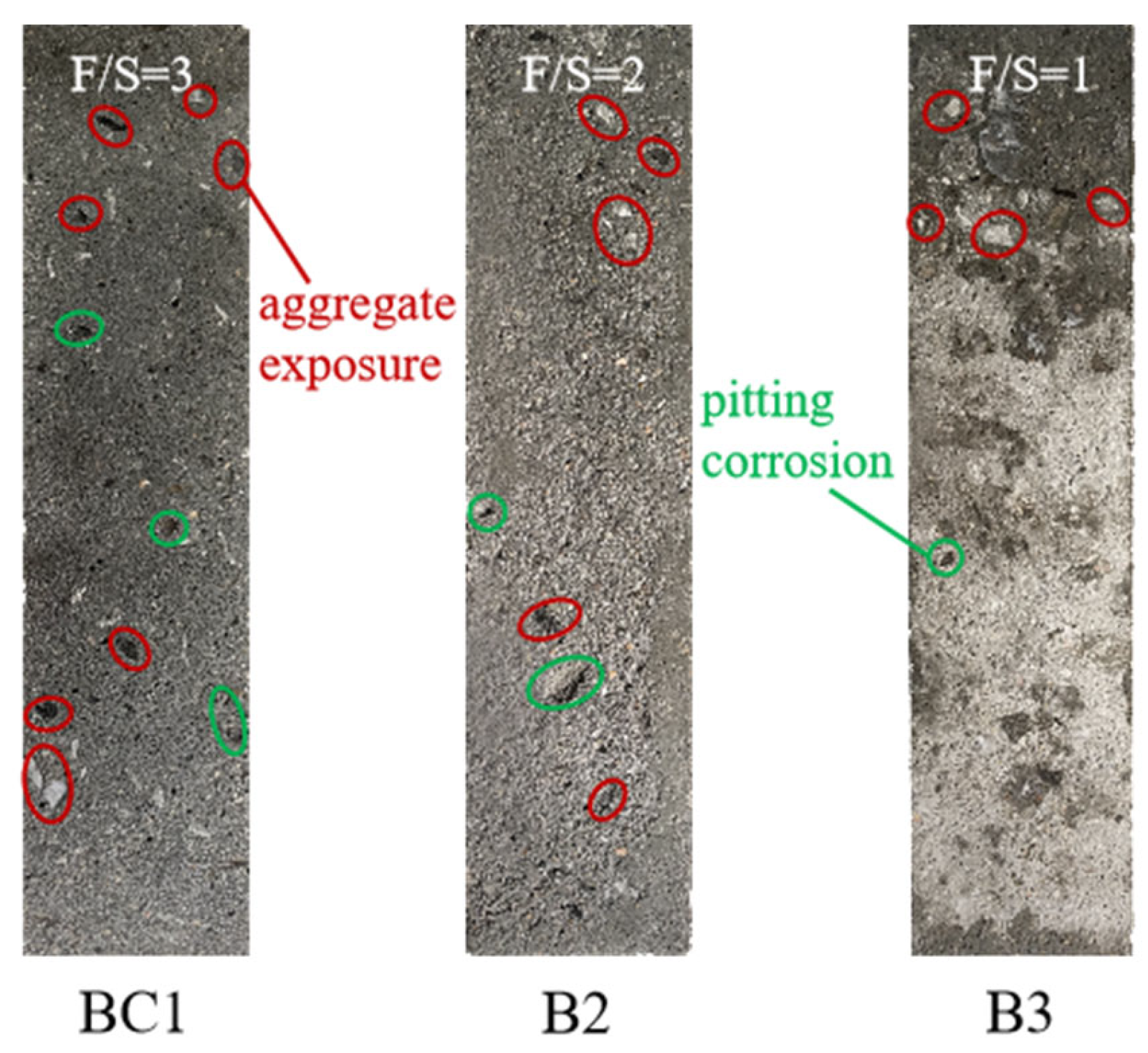
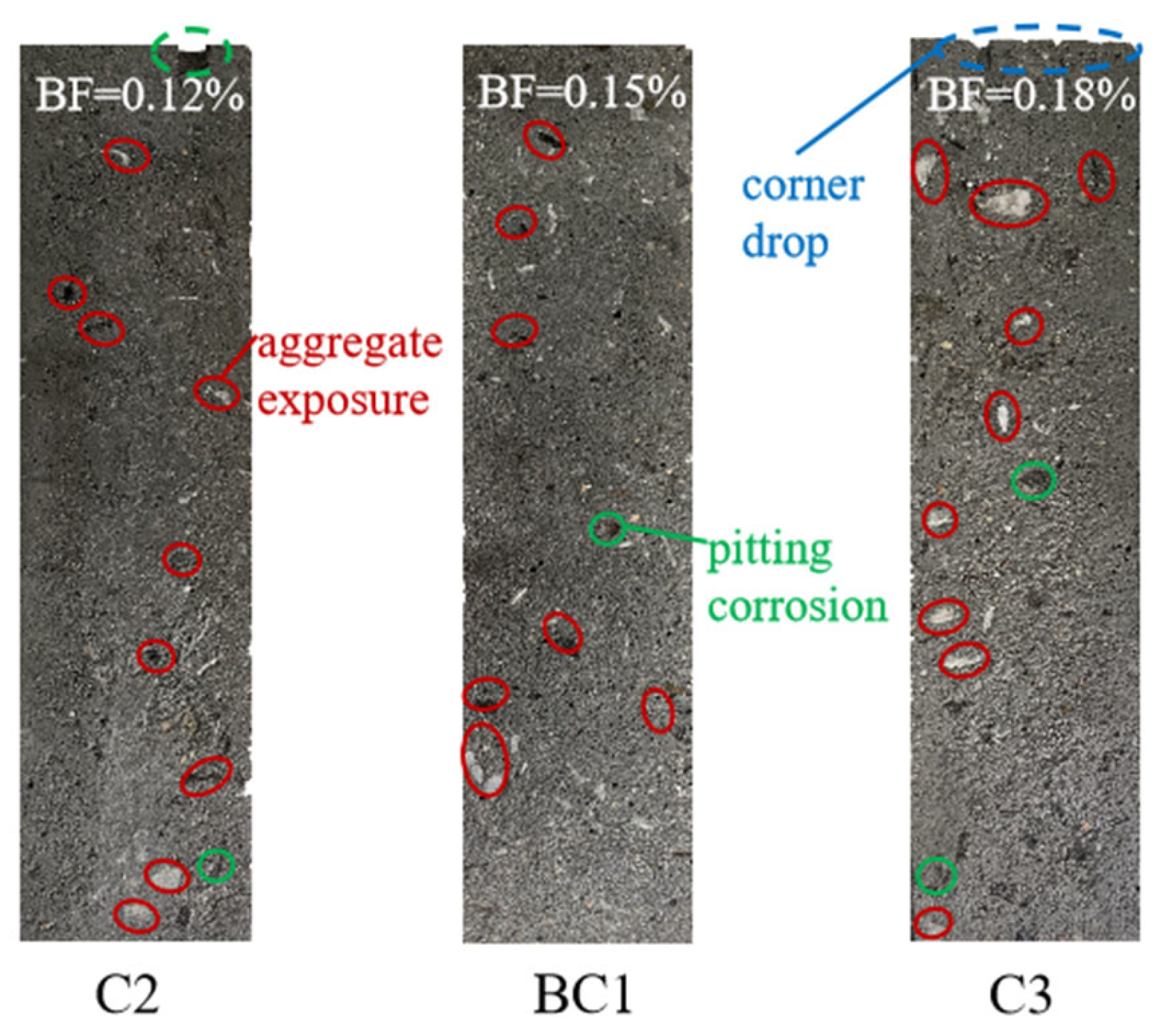
3.2.1. Apparent Morphology
3.2.2. Mass Loss
3.2.3. Relative Dynamic Elastic Modulus
3.3. Microstructure Analysis
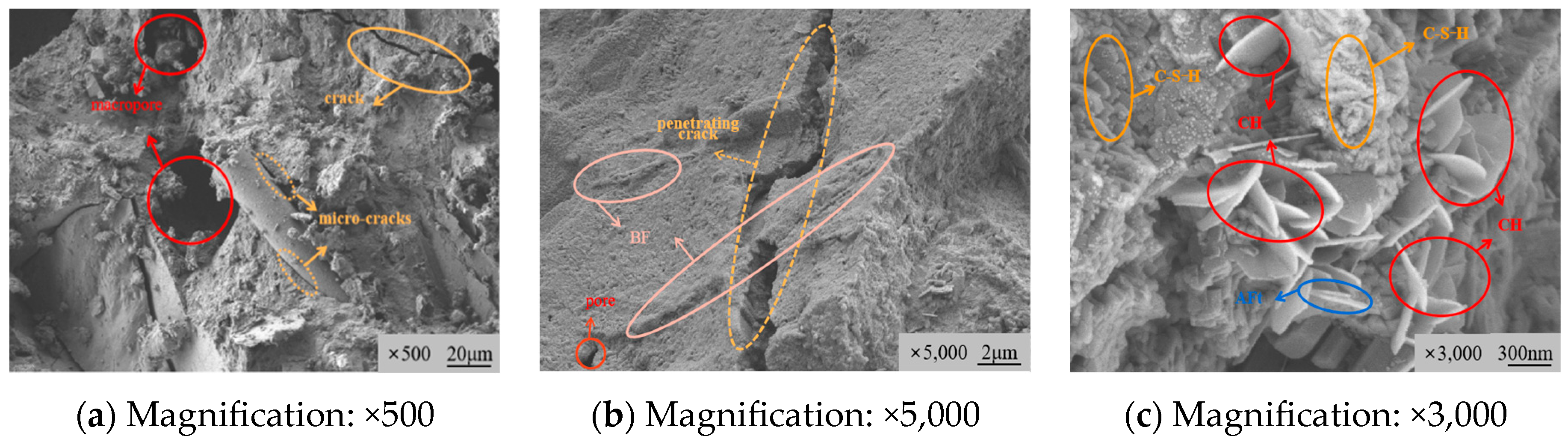
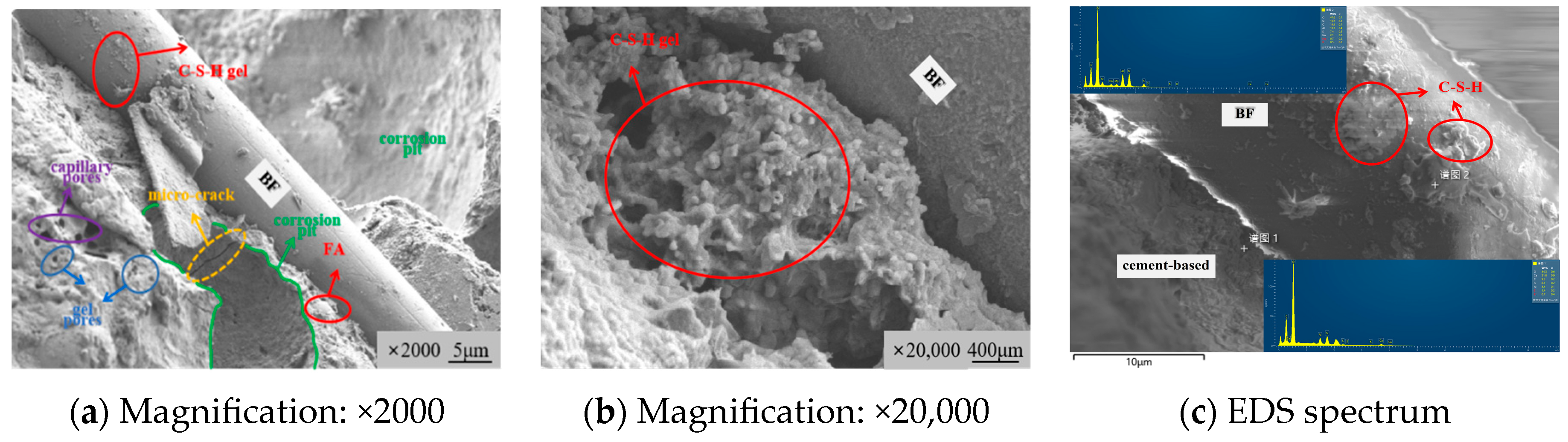
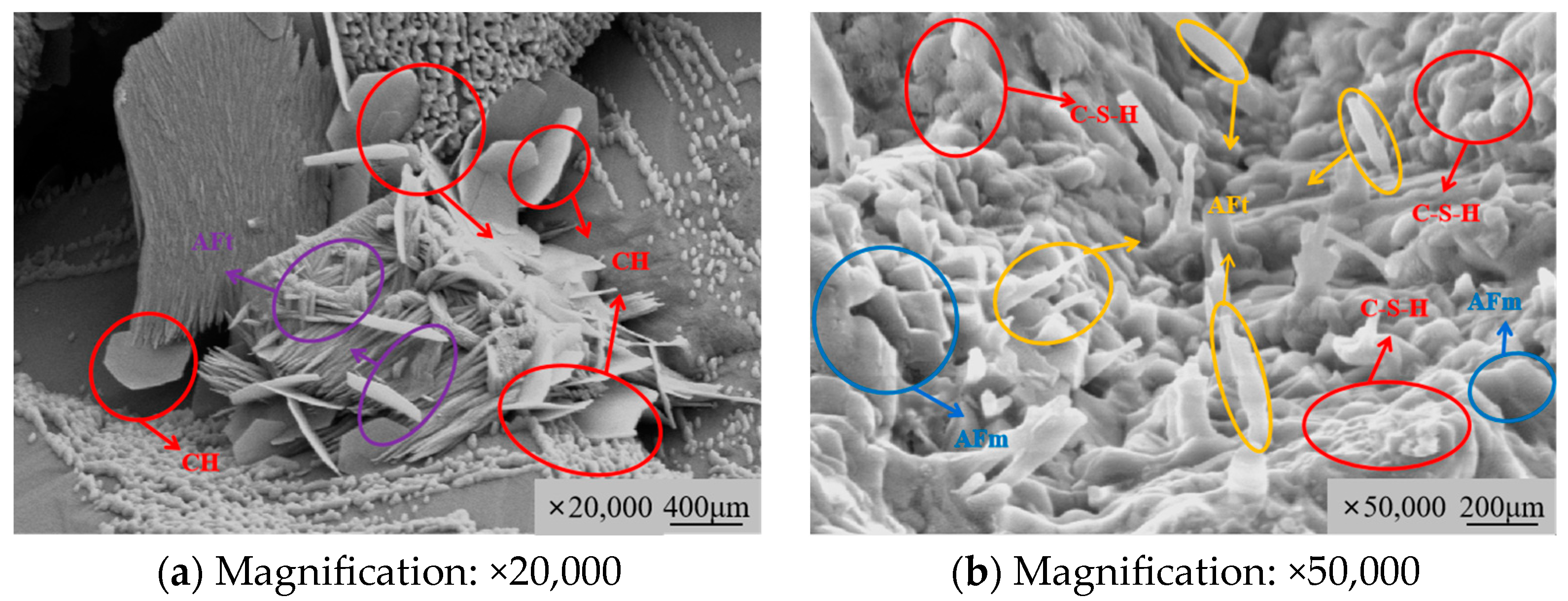
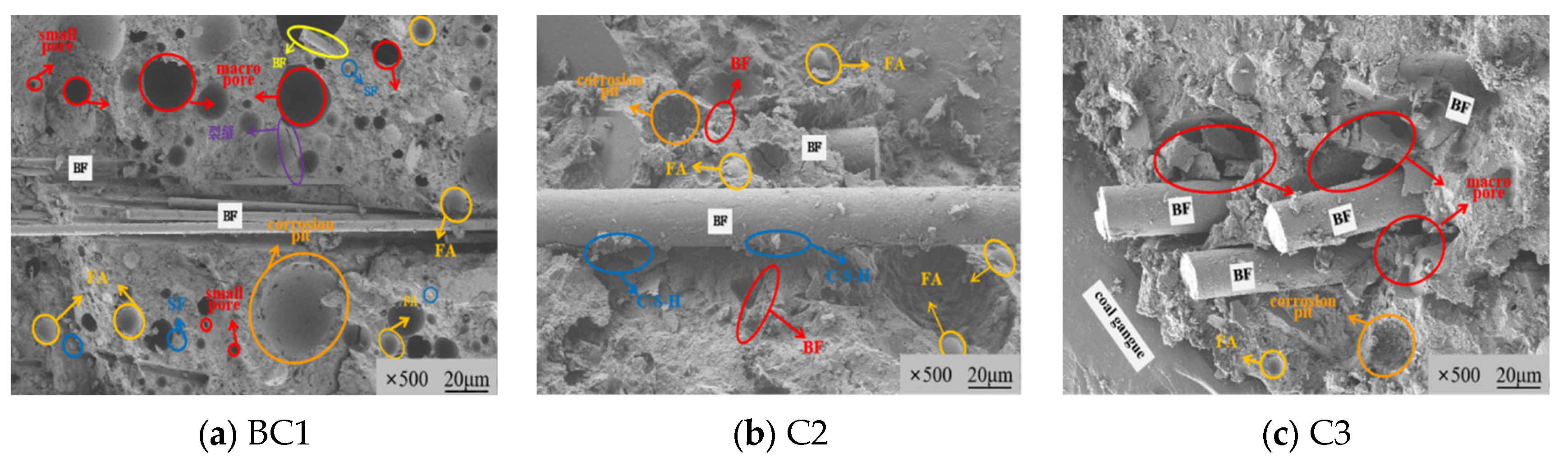
3.3.1. Microstructure Morphology Characteristics
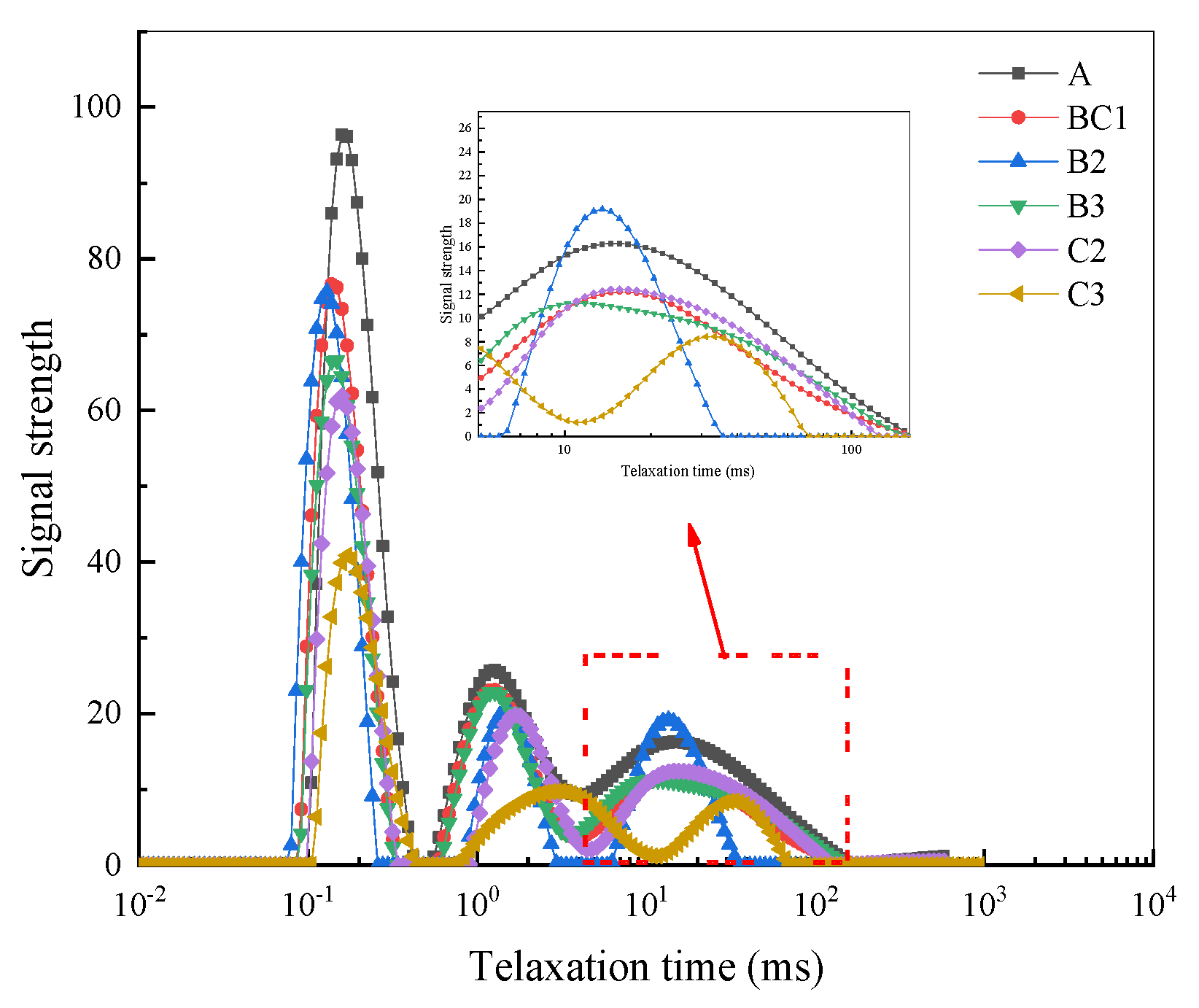
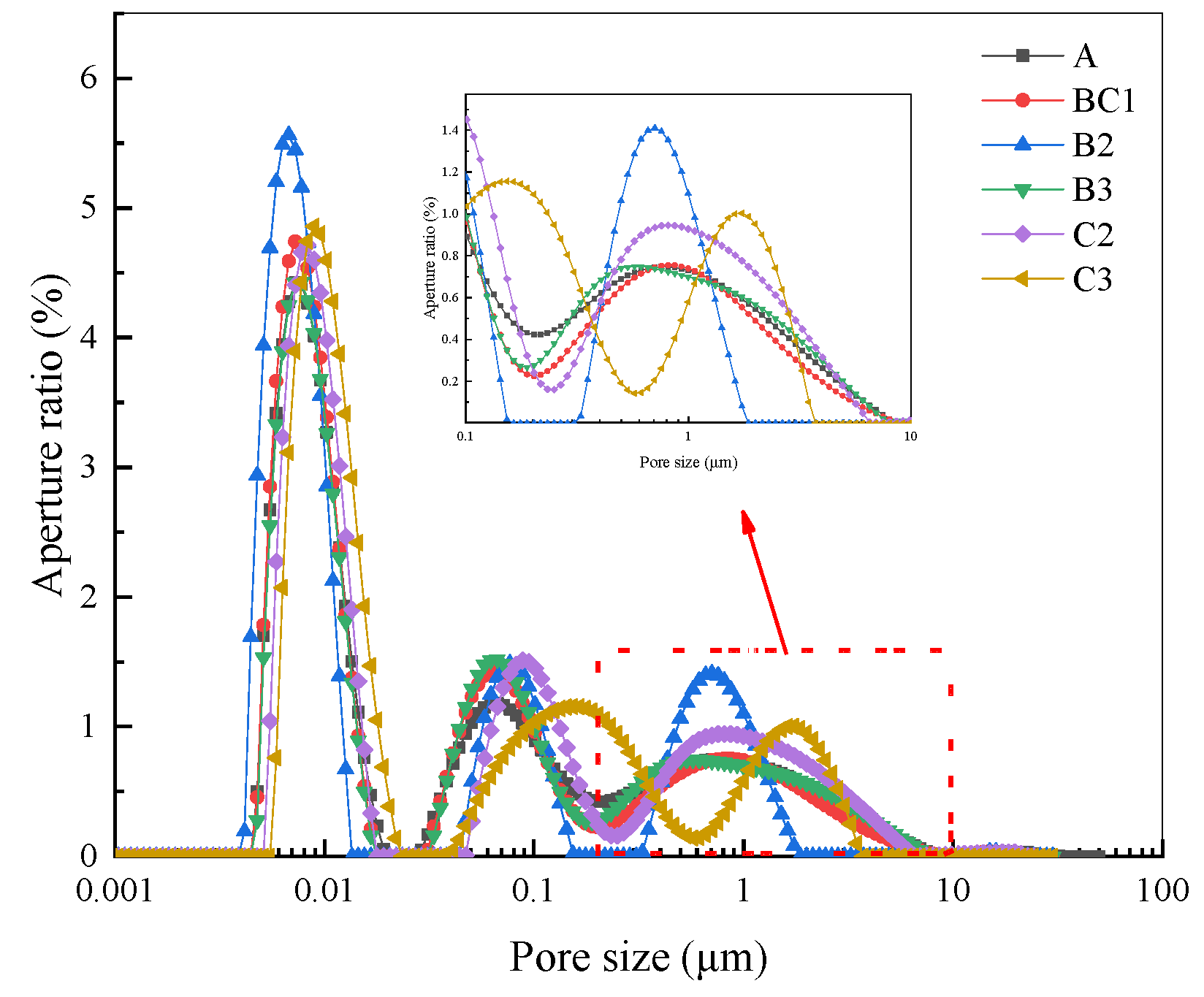
3.3.2. Pore Structure Characteristics
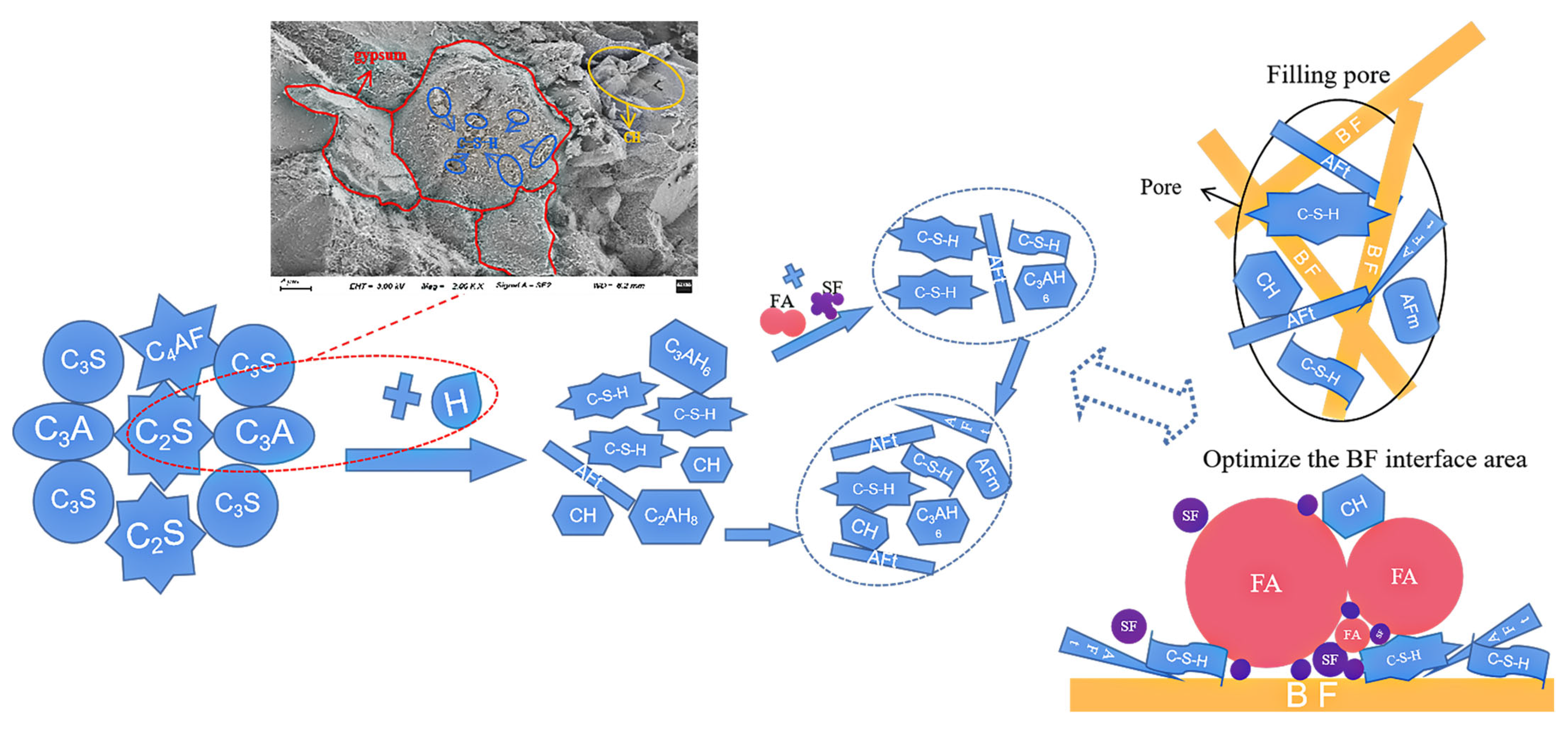
3.4. Modification Mechanism
3.4.1. Chemical Modification Mechanism
- (1)
- 2C3S + 6H→C-S-H + 3CH
- (2)
- 2C2S + 4H→C-S-H + CH
- (3)
- SiO2 + Al2O3 + nCH + mH→nC-S-H
- (4)
- xCa(OH)2 + ySiO2 + zH2O→xCaO·ySiO2·(x + z)H2O
- (5)
- (Secondary hydration reaction)
- (1)
- C3A + 18H→C2AH8 + C4AH13→C3AH6
- (2)
- C4AF + 13H→C4(A,F)H13
3.4.2. Physical Modification Mechanism
3.4.3. The Influence Mechanism of Excess BF on the Performance of CGC
4. Conclusions
- (1)
- The addition of mineral additives can improve the mechanical properties and frost resistance of CGC, and with the decrease in F/S, the improvement effect is better. When F/S = 1 and BF = 0.15 vol%, the performance is the best.
- (2)
- The modification mechanism of FA and SF on CGC is mainly reflected in the improvement of pore structure and pore gradation. Chemically, the hydration products generated by the volcanic ash reaction can compact the cement mortar and improve the adhesion between BF and mortar interface. Physically, FA and SF particles exert a filling effect and overlapping effect, which can complement each other, reduce shrinkage and improve performance.
- (3)
- The appropriate amount of BF can split and fill the large pores, optimize the pore structure, and reduce the frost heaving force during the freeze–thaw cycle. However, excessive BF will not only produce an agglomeration effect, but also hinder the hydration of cement.
- (4)
- Considering the economic benefits and modification effects, it is recommended to use the ratio of F/S = 2 in practical engineering applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, Y.; Liu, X.; Zhang, M. Research Status and Prospect of Microbial Promotion of Coal Gangue Reclamation and Utilization. Silic. Notif. 2015, 34, 2529–2533. [Google Scholar] [CrossRef]
- Zhang, B.; Tong, H.; Long, X.; Su, P.; He, X.; Zhang, Y. Research Status and Progress of High Value Utilization of Coal Gangue Solid Waste [J/OL]. Clean Coal Technology: 1–15. Available online: http://kns.cnki.net/kcms/detail/11.3676.td.20230524.1257.002.html (accessed on 17 September 2023).
- Jin, X.; Wang, S.; Xiao, Y.; Guo, Y.; Song, H. Research on resource characteristics and synergistic utilization of red mud and fly ash. J. Guizhou Univ. (Nat. Sci. Ed.) 2022, 39, 18–26. [Google Scholar]
- Yi, L.; Wang, H.; Wang, X. Research progress of resource utilization of fly ash building materials. Silic. Bull. 2012, 31, 88–91. [Google Scholar] [CrossRef]
- Sun, D.; Gu, Z.; Sun, S.; Gong, X.; Tian, Q. Performance and characterization of alkali-activated fly ash-silica fume based geopolymer. Silic. Bull. 2020, 39, 1533–1539. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, L.; Ma, D.; Chai, X.; Lin, C.; Zhang, F. Road Performance Evaluation of Unburned Coal Gangue in Cold Regions. Sustainability 2023, 15, 13915. [Google Scholar] [CrossRef]
- Bai, G.; Liu, H.; Wang, J.; Zhu, K.; Dai, M. Drying shrinkage model of coal gangue concrete based on the difference of aggregate characteristics. J. Civ. Eng. 2023. [Google Scholar] [CrossRef]
- Li, Y.; Xing, Y.; Zhang, X.; Yan, X. Experimental Study on Durability of Coal Gangue Aggregate Concrete. Coal J. 2013, 38, 1215–1219. [Google Scholar] [CrossRef]
- Ge, J.; Zhu, H.; Li, Z.; Li, W.; Shen, Z.; Hou, J.; Yang, S. Study on the mechanical and durability properties of coal gangue coarse aggregate-geopolymer concrete. Mater. Bull. 2021, 35, 218–223. [Google Scholar]
- Guan, X.; Qiu, J.; Song, H.; Qin, Q.; Zhang, C. Stress-strain behaviour and acoustic emission characteristic of gangue concrete under axial compression in frost environment. Constr. Build. Mater. 2019, 220, 476–488. [Google Scholar] [CrossRef]
- Su, L. Experimental Study on Basic Mechanical Properties of Coal Gangue Concrete; Xi’an University of Architecture and Technology: Xi’an, China, 2022. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Jin, S.; Chen, C.; Yang, R. Study on the durability of coal gangue aggregate concrete. J. Beijing Univ. Technol. 2011, 37, 116–125. [Google Scholar]
- Guan, X.; Qiu, J.; Dai, J.; Xiao, Q.; Zhang, C. Study on acoustic emission characteristics and damage model of coal gangue concrete under axial compression. J. Build. Mater. 2019, 22, 459–466. [Google Scholar]
- Huang, H.; Zhu, L.; Huang, M.; Wang, Y.; Ding, Z. Performance research and current situation analysis of modified concrete with different materials. Silic. Bull. 2018, 37, 1887–1896. [Google Scholar]
- Li, W.; Long, C.; Luo, Z.; Huang, Z.; Long, B.; Ren, J. Review on failure mechanism and modification of recycled aggregate concrete. J. Build. Sci. Eng. 2016, 33, 60–72. [Google Scholar]
- Jiao, C.; Zhang, X.; Chen, W.; Chen, X. Mechanical Property and Dimensional Stability of Chopped Basalt Fiber-Reinforced Recycled Concrete and Modeling with Fuzzy Inference System. Buildings 2022, 13, 97. [Google Scholar] [CrossRef]
- Yao, X.; Pei, Z.; Zheng, H.; Guan, Q.; Wang, F.; Wang, S.; Ji, Y. Review of Mechanical and Temperature Properties of Fiber Reinforced Recycled Aggregate Concrete. Buildings 2022, 12, 1224. [Google Scholar] [CrossRef]
- Zhu, M.; Qiu, J.; Chen, J. Effect and mechanism of coal gangue concrete modification by basalt fiber. Constr. Build. Mater. 2022, 328, 126601. [Google Scholar] [CrossRef]
- Guo, W.; Wen, H. Experimental Study on the Strength of Coal Gangue Aggregate Concrete with Basalt Fiber. J. Phys. Conf. Ser. 2022, 2185, 012059. [Google Scholar]
- Yao, X.; Guo, X.; Han, R.; Guan, J.; Li, H. Effect of nano-SiO2 and polypropylene fiber on mechanical properties and microstructure of coal gangue aggregate concrete. J. Compos. Materials. 2023. [Google Scholar] [CrossRef]
- Cai, B.; Bai, B.; Duan, W.; Wang, L.; Wang, S. Study on the Eccentric Compressive Performance of Steel Fibre Reinforced Coal Gangue Concrete Columns. Buildings 2023, 13, 1290. [Google Scholar] [CrossRef]
- Qiu, J.; Zhu, M.; Zhou, Y.; Guan, X. Effect and mechanism of coal gangue concrete modification by fly ash. Constr. Build. Mater. 2021, 294, 123563. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, Y.; Guan, X.; Zhu, M. The influence of fly ash content on ITZ microstructure of coal gangue concrete. Constr. Build. Mater. 2021, 298, 123562. [Google Scholar] [CrossRef]
- Meng, Y.; Tan, Y.; Li, R. Strength Studies of High Performance Concrete with Fly-Ash and Gangue. Adv. Mater. Res. 2012, 1615, 3544–3553. [Google Scholar] [CrossRef]
- Zhang, X.; Kuang, C.; Fang, Z.; Liu, X.; Wang, G.; Lai, S. Orthogonal experimental study on strength of steel fiber fly ash recycled concrete. J. Build. Mater. 2014, 17, 677–684 + 694. [Google Scholar]
- Xin, Z.; Zhu, Y.; Xu, P.; Ren, Y.; Zhang, Q. Effect of fly ash and polypropylene fiber on the performance of recycled pervious concrete. Concrete 2023, 73–77 + 81. [Google Scholar]
- Li, W. Experimental Study on Strength and Crack Resistance of Glass Fiber Fly Ash Coal Gangue Aggregate Concrete. Build. Struct. 2020, 50, 49–53. [Google Scholar] [CrossRef]
- Yang, Q.; Jing, Y.; Zhang, D. Study on the modification of mechanical properties of coal gangue concrete by fiber and mineral admixtures. Funct. Mater. 2022, 53, 7150–7156. [Google Scholar]
- Wu, Z. High performance concrete and its fine mineral admixture. Build. Technol. 1999, 30, 160–163. [Google Scholar]
- JGJ/55-2011; Specification for Mix Proportion Design of Ordinary Concrete. China Academy of Building Research: Beijing, China, 1 December 2011.
- Qiu, J.; Zhu, M.; Zhou, Y.; Gao, X.; Li, L. Modification effect of fly ash on interfacial transition zone of coal gangue concrete. Mater. Introd. 2023, 37, 75–81. [Google Scholar]
- Qiu, J.; Zhou, Y.; Nikolay, I.; Gao, X.; Shukhrat, S.; Khon, K. Damage constitutive model of coal gangue concrete under freeze-thaw cycles. Constr. Build. Mater. 2023, 264, 120720. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Luo, J.; Zhu, W.; Fan, X.; Zhu, Y.; Zhang, Z. Mechanical properties and microscopic characteristics of steel fiber coal gangue concrete. Front. Mater. 2023, 10. [Google Scholar] [CrossRef]
- GB/T50080-2002; Standard for Performance Test Method of Ordinary Concrete Mixture. China Academy of Building Research: Beijing, China, 2007.
- GB/T50081-2019; Standard for Test Methods of Mechanical Properties of Ordinary Concrete. China Academy of Building Research: Beijing, China, 2019.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Academy of Building Research: Beijing, China, 2007.
- Guan, X.; Shi, H.; Chen, L.; Warren, A.D. Alkali-activated complex binders from class C fly ash and Ca-containing admixtures. J. Hazard. Mater. 2010, 173, 480–486. [Google Scholar]
- Zhao, X. Synthesis, Composition, Structure and Morphology of Calcium Silicate Hydrate; Wuhan University of Technology: Wuhan, China, 2010. [Google Scholar]
- Dadsetan, S.; Bai, J. Mechanical and microstructural properties of self-compacting concrete blended with metakaolin, ground granulated blast-furnace slag and fly ash. Constr. Build. Mater. 2017, 146, 658–667. [Google Scholar] [CrossRef]
- Heijden DV, G.; Pel, L.; Adan, O. Fire spalling of concrete, as studied by NMR. Cem. Concr. Res. 2012, 42, 265–271. [Google Scholar] [CrossRef]
- Pop, A.; Badea, C.; Ardelean, I. The Effects of Different Superplasticizers and Water-to-Cement Ratios on the Hydration of Gray Cement Using T_2-NMR. Appl. Magn. Reson. 2013, 44, 1223–1234. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Y.; Wei, X.; Jia, H.; Yan, R. Investigation on meso-debonding process of the sandstone–concrete interface induced by freeze–thaw cycles using NMR technology. Constr. Build. Mater. 2020, 252, 118962. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Professional: New York, NY, USA, 2014. [Google Scholar]
- Wu, Z. Discussion on the recent development direction of concrete science and technology. J. Silic. 1979, 3, 262–270. [Google Scholar]
- Cheng, H.; Wu, A.; Wang, Y.; Wang, H.; Wang, S. Fractal characterization and dynamic characteristics of microstructure of fly ash-cement paste. J. Rock Mech. Eng. 2016, 35, 4241–4248. [Google Scholar] [CrossRef]
- Zhang, Q. The Mechanism of Fly Ash in the Hydration Process of Composite Cementitious Materials; Tsinghua University: Beijing, China, 2007. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Edition Published: London, UK, 1997. [Google Scholar]
- Win, T.T.; Panwisawas, C.; Jongvivatsakul, P.; Pansuk, W.; Prasittisopin, L. Effects of Fly Ash Composition to Mitigate Conversion of Calcium Aluminate Cement Composites. Buildings 2023, 13, 2453. [Google Scholar] [CrossRef]
- Wu, J.; Liao, H.; Ma, Z.; Song, H. Effect of different initial CaO/SiO2 molar ratios and curing times on the preparation and formation mechanism of calcium silicate hydrate. Materials 2023, 16, 717. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, Z.; Guan, X.; Xing, M.; Zhang, C.; Qin, Q. Microscopic pore structure characteristics and compressive strength of coal gangue ceramsite concrete. J. Xi’an Univ. Sci. Technol. 2020, 40, 110–117. [Google Scholar] [CrossRef]







| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | LOI |
|---|---|---|---|---|---|---|---|
| Cement | 18.30–21.5 | 4.80–5.15 | 2.90–5.15 | 64.73–68.05 | 0.8–1.16 | 0.00–1.25 | 2.13–3.22 |
| FA | 53.46–69.87 | 23.08–28.06 | 2.39–3.78 | 0.10–2.77 | 0.78–1.87 | 0.81–1.38 | 2.30–2.48 |
| SF | 94.05–97.92 | 0.00–0.45 | 0.05–1.09 | 0.29–1.17 | 0.13–0.89 | 0.03–2.36 | 1.28–2.50 |
| Coarse Aggregate | Apparent Density (kg/m3) | Packing Density (kg/m3) | Water Absorption (%) | Crushing Index (%) |
|---|---|---|---|---|
| Natural aggregate | 2870–2880 | 1469–1550 | 0.5–0.6 | 6.3–7.0 |
| Coal gangue | 2247–2291 | 1350–1397 | 7.14–7.38 | 17.7–18.9 |
| Length (mm) | Diameter (μm) | Linear Density (Tex) | Tensile Strength (MPa) | Elastic Modulus (GPa) | Fracture Strength (N/Tex) |
|---|---|---|---|---|---|
| 30 | 10 | 2392–2399 | 3000–4800 | 62–91 | 0.69–0.71 |
| Composition | SiO2 | MgO | CaO | Al2O3 | Fe4O3+FeO | N2O+K2O | Others |
|---|---|---|---|---|---|---|---|
| Content | 51.6–58.9 | 2.9–5.2 | 5.8–9.2 | 15.1–18.9 | 9.1–13.5 | 3.4–5.3 | 0.08–0.12 |
| Group | Water | Cement | Sand | Stone | Coal Gangue | FA | SF | BF | PCA | AOS |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 176 | 440 | 624 | 696 | 363 | 0 | 0 | 0.15% | 2.20 | 0.13 |
| BC1 | 176 | 352 | 624 | 696 | 363 | 66 | 22 | 0.15% | 2.20 | 0.13 |
| B2 | 176 | 352 | 624 | 696 | 363 | 57 | 31 | 0.15% | 2.20 | 0.13 |
| B3 | 176 | 352 | 624 | 696 | 363 | 44 | 44 | 0.15% | 2.20 | 0.13 |
| C2 | 176 | 352 | 624 | 696 | 363 | 66 | 22 | 0.12% | 2.20 | 0.13 |
| C3 | 176 | 352 | 624 | 696 | 363 | 66 | 22 | 0.18% | 2.20 | 0.13 |
| Group | Peak Area | Proportion of Peak% | ||
|---|---|---|---|---|
| First Peak | Second Peak | Third Peak | ||
| A | 2179.950 | 52.374 | 22.082 | 25.544 |
| BC1 | 1617.748 | 54.139 | 22.698 | 23.162 |
| B2 | 1460.460 | 55.836 | 28.222 | 15.942 |
| B3 | 1504.654 | 62.124 | 21.761 | 16.115 |
| C2 | 1413.404 | 51.130 | 24.659 | 24.211 |
| C3 | 1228.550 | 50.174 | 24.142 | 25.684 |
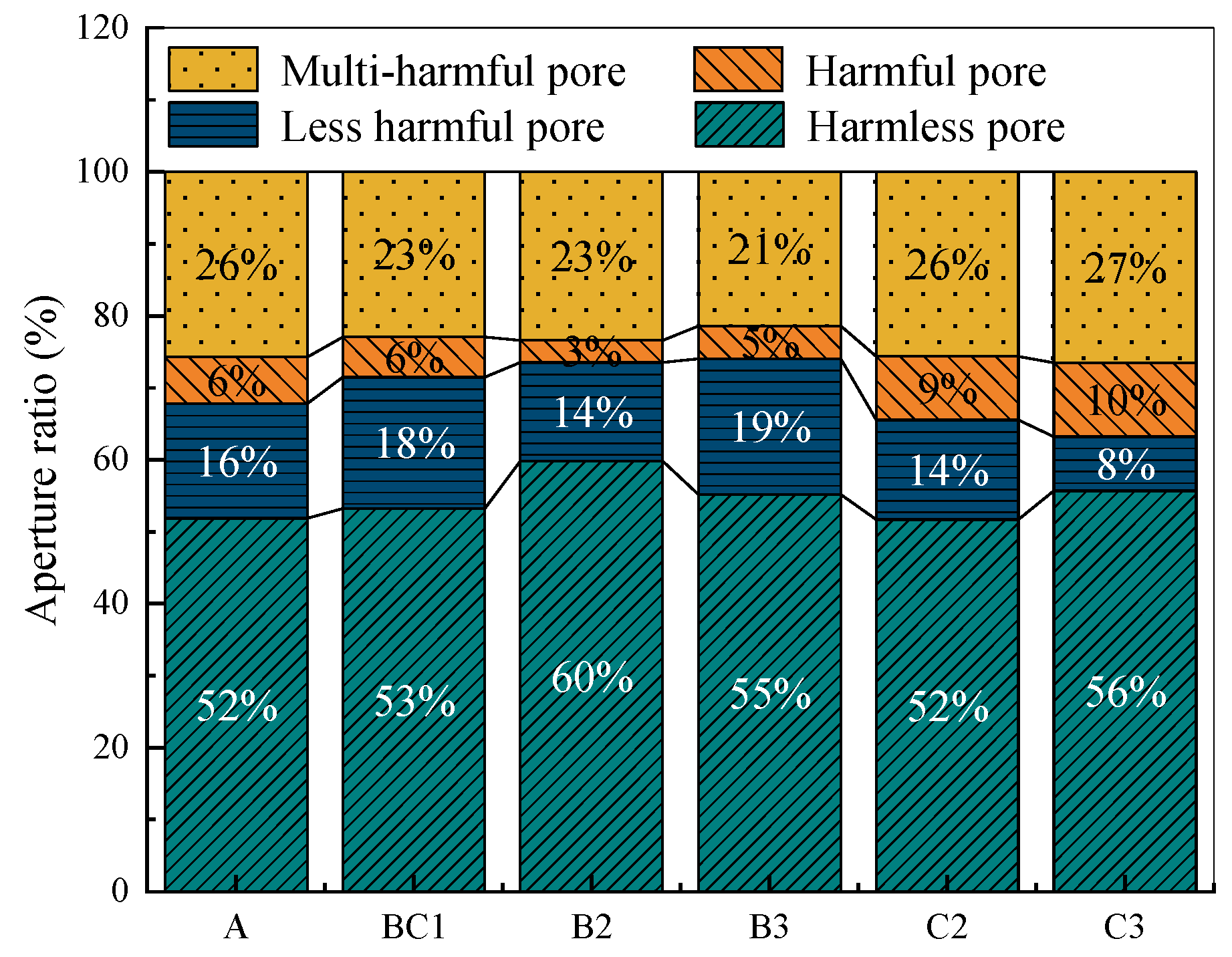
| Classification | A | BC1 | B2 | B3 | C2 | C3 |
|---|---|---|---|---|---|---|
| Harmless pores | 51.84 | 53.22 | 59.82 | 55.17 | 51.69 | 55.70 |
| Less harmful pores | 15.95 | 18.24 | 13.70 | 18.85 | 13.84 | 7.51 |
| Harmful pores | 6.45 | 5.61 | 3.06 | 4.57 | 8.81 | 10.25 |
| Multi-harmful pores | 25.76 | 22.93 | 23.42 | 21.41 | 25.66 | 26.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Huo, Y.; Feng, Z.; Li, L.; Wang, J.; Zhang, Y.; Guan, X. Study on the Modification Effect and Mechanism of a Compound Mineral Additive and Basalt Fiber on Coal Gangue Concrete. Buildings 2023, 13, 2756. https://doi.org/10.3390/buildings13112756
Qiu J, Huo Y, Feng Z, Li L, Wang J, Zhang Y, Guan X. Study on the Modification Effect and Mechanism of a Compound Mineral Additive and Basalt Fiber on Coal Gangue Concrete. Buildings. 2023; 13(11):2756. https://doi.org/10.3390/buildings13112756
Chicago/Turabian StyleQiu, Jisheng, Yong Huo, Zeping Feng, Le Li, Jianwei Wang, Yuqing Zhang, and Xiao Guan. 2023. "Study on the Modification Effect and Mechanism of a Compound Mineral Additive and Basalt Fiber on Coal Gangue Concrete" Buildings 13, no. 11: 2756. https://doi.org/10.3390/buildings13112756
APA StyleQiu, J., Huo, Y., Feng, Z., Li, L., Wang, J., Zhang, Y., & Guan, X. (2023). Study on the Modification Effect and Mechanism of a Compound Mineral Additive and Basalt Fiber on Coal Gangue Concrete. Buildings, 13(11), 2756. https://doi.org/10.3390/buildings13112756





