Production of the Traditional Organic Mortars of Padmanabhapuram Palace—A Characterization Study on the Simulated Mortars for Their Compatibility
Abstract
:1. Introduction
Background of Padmanabhapuram Palace
2. Materials and Methods
- 0.30 < HI < 0.50—weakly hydraulic
- 0.50 < HI < 0.70—moderately hydraulic
- 0.70 < HI < 1.10—the higher the index, the greater the hydraulic properties
- CI < 0.15—air lime
- 0.15 < CI < 0.30—sub-hydraulic lime
- 0.30 < CI < 0.50—weakly hydraulic
- 0.50 < CI < 0.70—moderately hydraulic
- 0.70 < CI < 1.10—the higher the index, the greater the hydraulic properties
2.1. Preparation of the Organic Lime Mortar
2.1.1. Preparation of the Traditional Organic Herbs
| Algorithm 1. Description of the lime mortar mix proportions. |
|
2.1.2. Organic Lime Preparation
2.1.3. Mix Proportions of the Organic Lime Mortar
2.2. Characterization Study on the Palace Mortars
2.3. Mechanical Strength
2.4. Carbonation Test
2.5. Analytical Investigations
2.5.1. X-ray Diffraction
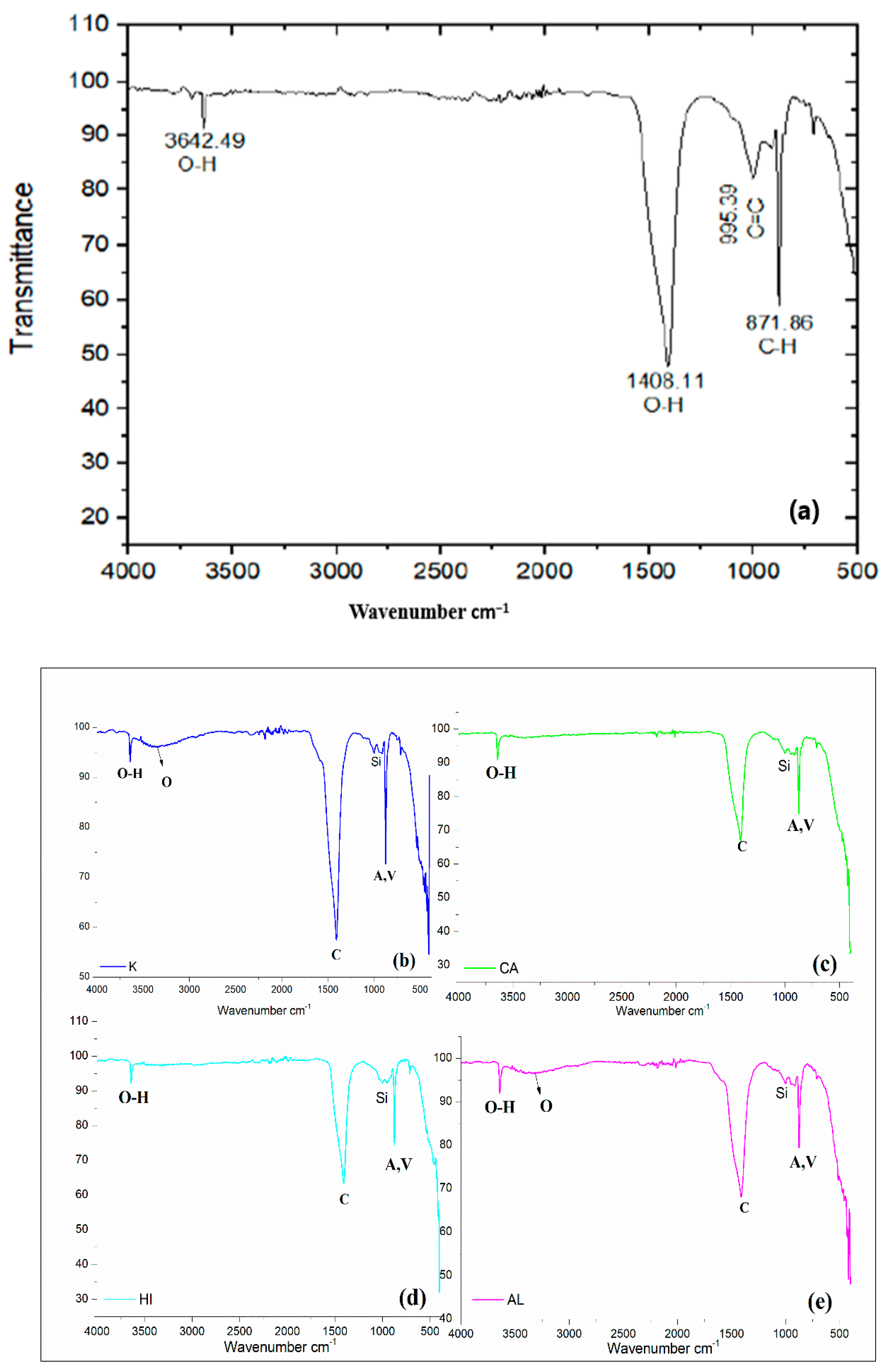
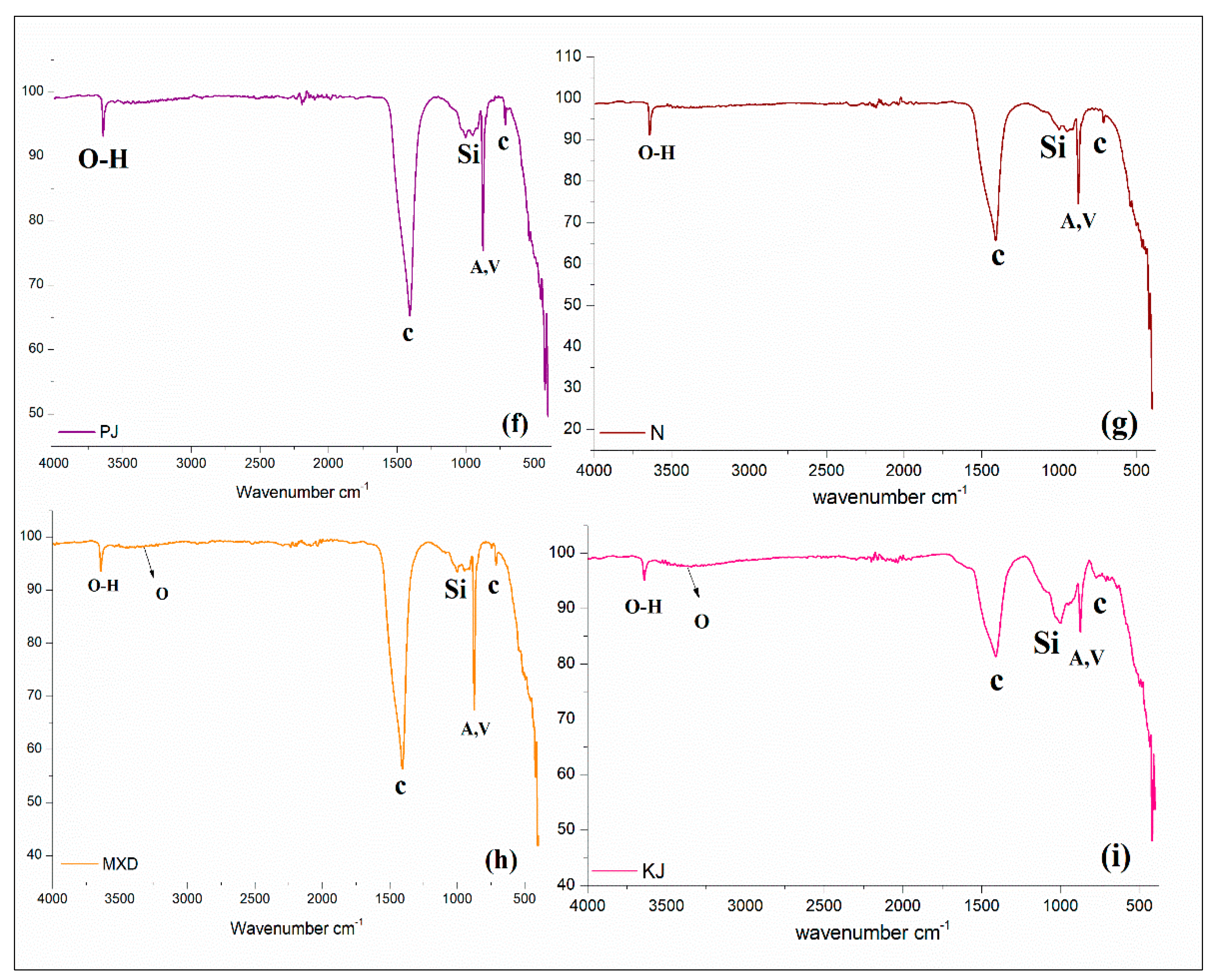
2.5.2. FT-IR
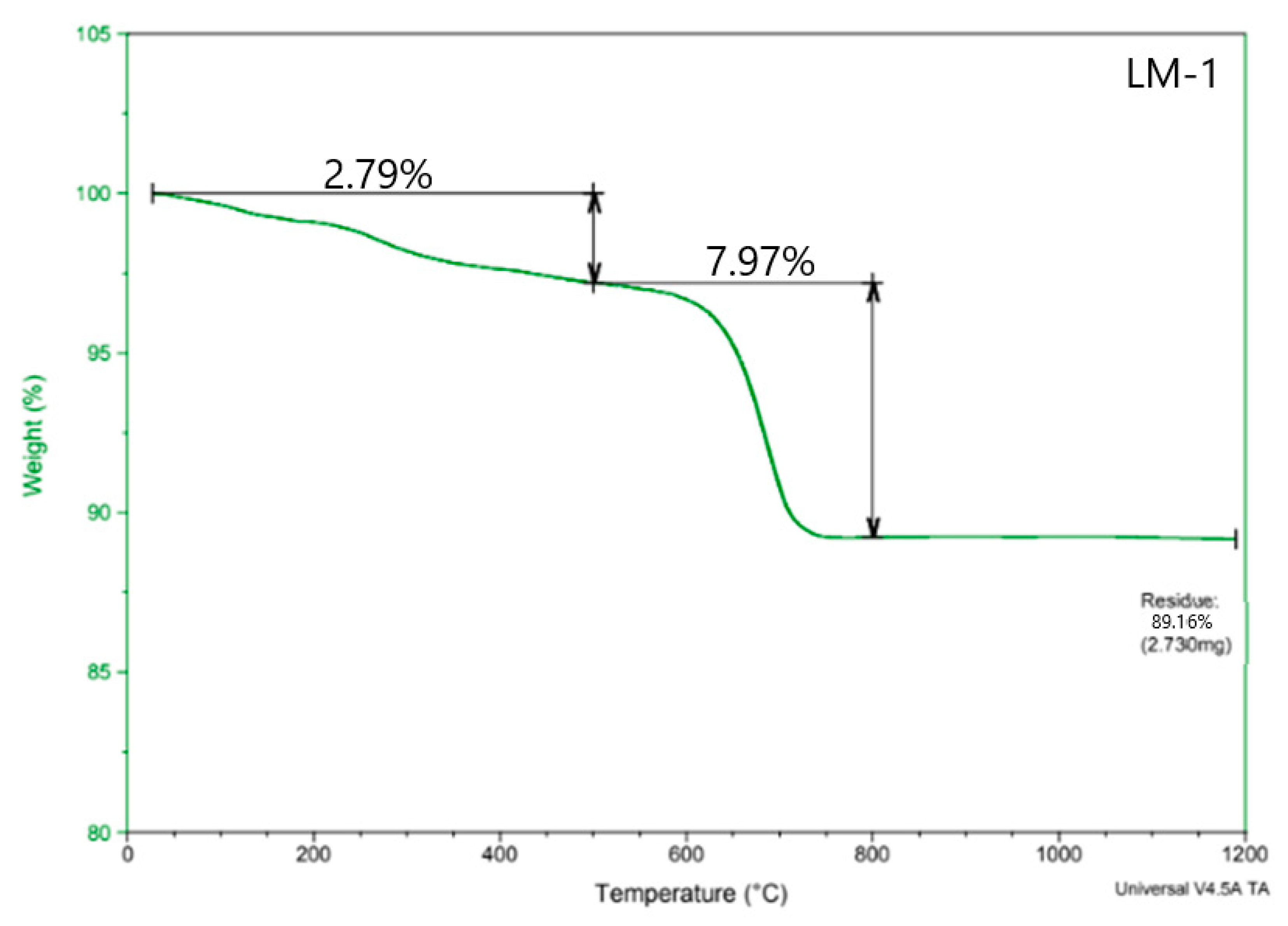
2.5.3. TGA-DTA
3. Results and Discussions
3.1. Influence of Organic Additives on the Mechanical Strength of the Lime Mortar
3.2. TGA-DTA
3.3. FT-IR
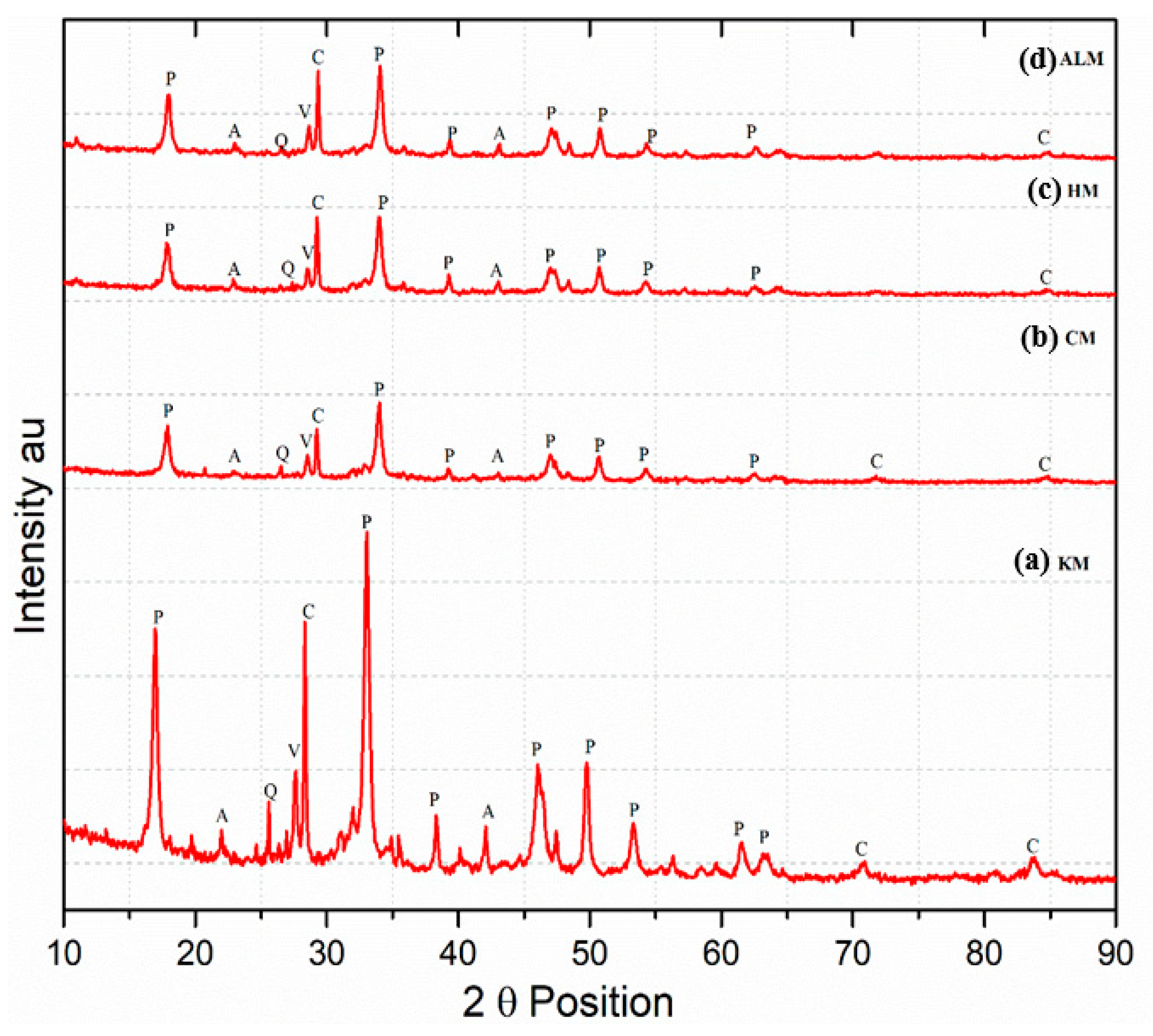
3.4. Mineralogical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carran, D.; Hughes, J.; Leslie, A.; Kennedy, C. A short history of the use of lime as a building material beyond Europe and North America. Int. J. Archit. Herit. 2012, 6, 117–146. [Google Scholar] [CrossRef]
- Thirumalini, S. Heritage Lime Mortar Characterization and Simulation. Ph.D. Thesis, School of Mechanical and Building Science, VIT University, Vellore, India, 2015. [Google Scholar]
- Ventolà, L.; Vendrell, M.; Giraldez, P.; Merino, L. Traditional organic additives improve lime mortars: New old materials for restoration and building natural stone fabrics. Constr. Build. Mater. 2011, 25, 3313–3318. [Google Scholar]
- Thirumalini, P.; Ravi, R.; Sekar, S.K.; Nambirajan, M. Study on the performance enhancement of lime mortar used in ancient temples and monuments in India. Indian J. Sci. Technol. 2011, 4, 1484–1487. [Google Scholar] [CrossRef]
- Rampazzi, L.; Colombini, M.P.; Conti, C.; Corti, C.; Lluveras-Tenorio, A.; Sansonetti, A.; Zanaboni, M. Technology of Medieval mortars: An investigation into the use of organic additives. Archaeometry 2016, 58, 115–130. [Google Scholar] [CrossRef]
- Arcolao, C. The deterioration of stone materials: A tool for its identification and characterization. In Proceedings of the 6th International Symposium on the Conservation of Monuments in the Mediterranean Basin, Lisbon, Portugal, 7–10 April 2004; 2004; pp. 128–132. [Google Scholar]
- Carbonara, G.; Carbonara, G. Trattato di Restauro Architettonico: Restauro Architettonico e Impianti; Unione Tipografica Editrice Torinese, UTET: Torino, Italy, 2001. [Google Scholar]
- Bugini, R.; Della Torre, S.; Pozzi, A.; Rampazzi, L.; Sansonetti, A. Classification of multi-layered plaster in St. Abbondio Cloister at Como, Italy: An analytical tool for architectural archaeology. Mater. Construcción 2006, 56, 5–16. [Google Scholar] [CrossRef]
- Bugini, R.; Corti, C.; Folli, L.; Rampazzi, L. Unveiling the use of creta in Roman plasters: Analysis of clay wall paintings from Brixia (Italy). Archaeometry 2017, 59, 84–95. [Google Scholar] [CrossRef]
- Rampazzi, L.; Rizzo, B.; Colombo, C.; Conti, C.; Realini, M.; Bartolucci, U.; Facchin, L. The stucco decorations from St. Lorenzo in Laino (Como, Italy): The materials and the techniques employed by the “Magistri Comacini”. Anal. Chim. Acta 2008, 630, 91–100. [Google Scholar] [CrossRef]
- Centauro, I.; Cantisani, E.; Grandin, C.; Salvini, A.; Vettori, S. The influence of natural organic materials on the properties of traditional lime-based mortars. Int. J. Archit. Herit. 2017, 11, 670–684. [Google Scholar] [CrossRef]
- Amaran, R.; Ravi, R. Effect of Cactus on The Rheological Properties Of Cement. Int. J. Chem. Sci. 2016, 14, 203–210. [Google Scholar]
- Shivakumar, M.; Selvaraj, T. A scientific study on the role of organic lime mortars of Padmanabhapuram Palace, Thuckalay, Tamilnadu, India. Eur. Phys. J. Plus 2020, 135, 923. [Google Scholar] [CrossRef]
- Toguyeni, D.Y.; Lawane, A.; Zoma, F.; Khamis, G. Formulation of Compressed Earth Blocks Stabilized with Lime and Hibiscus sabdariffa Fibres Showcasing Good Thermal and Mechanical Properties. J. Mater. Sci. Surf. Eng. 2018, 6, 817–824. [Google Scholar]
- Alisi, C.; Bacchetta, L.; Bojorquez, E.; Falconieri, M.; Gagliardi, S.; Insaurralde, M.; Martinez, M.F.F.; Orozco, A.M.; Persia, F.; Sprocati, A.R.; et al. Mucilages from Different Plant Species Affect the Characteristics of Bio-Mortars for Restoration. Coatings 2021, 11, 75. [Google Scholar] [CrossRef]
- Procacci, S.; Bojórquez-Quintal, E.; Platamone, G.; Maccioni, O.; Vecchio, V.L.; Morreale, V.; Bacchetta, L. Opuntia ficus-indica pruning waste recycling: Recovery and characterization of mucilage from cladodes. Nat. Resour. 2021, 12, 91–107. [Google Scholar]
- Fang, S.Q.; Zhang, H.; Zhang, B.J.; Zheng, Y. The identification of organic additives in traditional lime mortar. J. Cult. Herit. 2014, 15, 144–150. [Google Scholar] [CrossRef]
- Cárdenas, A.; Arguelles, W.M.; Goycoolea, F.M. On the possible role of Opuntia ficus-indica mucilage in lime mortar performance in the protection of historical buildings. J. Prof. Assoc. Cactus Dev. 1998. [Google Scholar]
- Oates, J.A. Lime and Limestone: Chemistry and Technology, Production and Uses; John Wiley & Sons:: Hoboken, NJ, USA, 2008. [Google Scholar]
- Standard, I. ISO 712: 1984; Specification for Building Limes. BIS: New Delhi, India, 1989.
- ISO: 6932 (Part V)−1973; Methods of Tests for Building Limes, Determination of Unhydrated Oxide. Bureau of Indian Standards: New Delhi, India, 1973.
- Rowland, I.D.; Howe, T.N. Vitruvius: Ten Books on Architecture; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Gotti, E.; Oleson, J.P.; Bottalico, L.; Brandon, C.; Cucitore, R.; Hohlfelder, R.L. A comparison of the chemical and engineering characteristics of ancient Roman hydraulic concrete with a modern reproduction of Vitruvian hydraulic concrete. Archaeometry 2008, 50, 576–590. [Google Scholar] [CrossRef]
- UNE EN. 14630: 2007; Products and Systems for the Protection and Repair of Concrete Structures-Test Methods—Determination of Carbonation Depth in Hardened Concrete by the Phenolphthalein Method. CEN: Brussels, Belgium, 2007.
- Lawrence, R.M.H. A Study of Carbonation in Non-Hydraulic Lime Mortars. Ph.D. Thesis, University of Bath, Claverton Down, UK, 2006. [Google Scholar]
- Genestar, C.; Pons, C.; March, J.G.; Sanz, D. Historical Mortars from the Fortified Upper Town of Eivissa (Balearic Islands, Spain). Int. J. Archit. Herit. 2015, 9, 201–213. [Google Scholar] [CrossRef]
- Nunes, C.; Mácová, P.; Frankeová, D.; Ševčík, R.; Viani, A. Influence of linseed oil on the microstructure and composition of lime and lime-metakaolin pastes after a long curing time. Constr. Build. Mater. 2018, 189, 787–796. [Google Scholar] [CrossRef]
- Santos, A.R.; do Rosário Veiga, M.; Silva, A.S.; de Brito, J.; Álvarez, J.I. Evolution of the microstructure of lime-based mortars and influence on the mechanical behaviour: The role of the aggregates. Constr. Build. Mater. 2018, 187, 907–922. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Burgos-Cara, A.; Elert, K.; Hansen, E.F. Crystallization and colloidal stabilization of Ca (OH) 2 in the presence of nopal juice (Opuntia Ficus indica): Implications in architectural heritage conservation. Langmuir 2017, 33, 10936–10950. [Google Scholar] [CrossRef]
- Nandagopalan, V.; Gritto, M.J.; Doss, A. GC-MS analysis of bioactive components of the methanol extract of Hibiscus tiliaceus Linn. Asian J. Plant Sci. Res. 2015, 5, 6–10. [Google Scholar]
- Jayasingh, S.; Selvaraj, T.; Raneri, S. Evaluating the Impact of Organic Addition and Aggregate Gradation on Air Lime Mortar: New Compatible Green Material for Heritage Application. Int. J. Archit. Herit. 2022, 16, 681–691. [Google Scholar] [CrossRef]

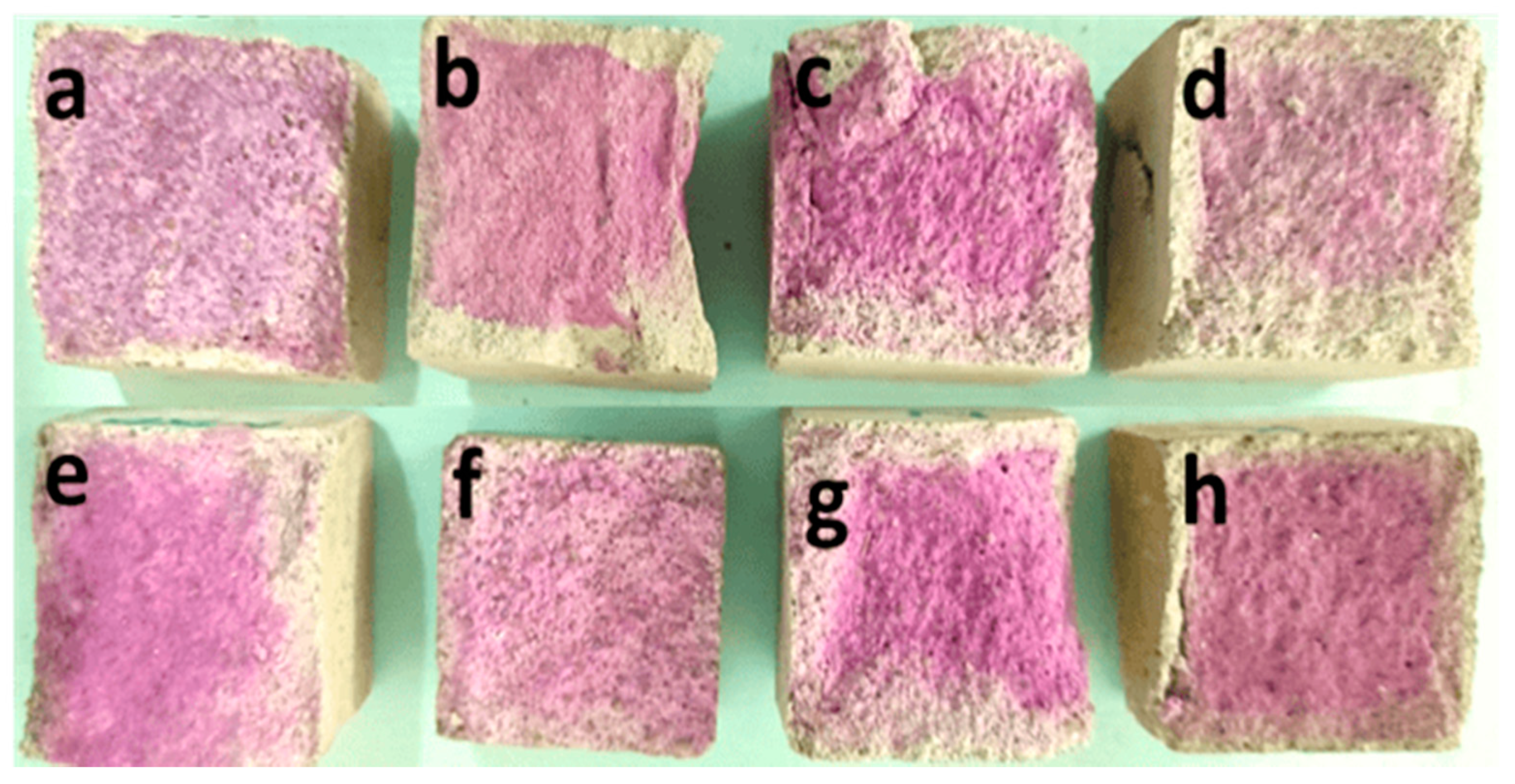






| Sl No. | Oxides | Percentage (%) |
|---|---|---|
| 1 | CaO | 55.5 |
| 2 | SiO2 | 30.2 |
| 3 | MgO | 0.98 |
| 4 | Al2O3 | 6.26 |
| 5 | Fe2O3 | 3.89 |
| 6 | K2O | 1.97 |
| 7 | Hydraulic Index | 0.71 |
| 8 | Cementation Index | 1.66 |
| Type of Mortars | Samples | Initial Weight | Acid Loss | Weight Retained after Acid Loss | Sand Retained | Binder Retained | B/Agg Ratio | Ranges |
|---|---|---|---|---|---|---|---|---|
| (gm) | (gm) | (gm) | (gm) | (gm) | ||||
| Wall | S1 | 30.5 | 7.75 | 22.75 | 15.5 | 7.25 | 1:2.5 | 1:3 |
| S2 | 25.8 | 3 | 22.8 | 16.45 | 6.35 | 1:3 | ||
| S3 | 25.2 | 5.87 | 19.33 | 14.35 | 4.98 | 1:3 | ||
| Bedding | S4 | 28.4 | 2.34 | 26.06 | 18.56 | 7.5 | 1:2 | 1:2 |
| S5 | 15.3 | 4 | 11.3 | 6.62 | 4.68 | 1:2.5 | ||
| Flooring | S6 | 10.3 | 1.7 | 8.6 | 4.35 | 4.25 | 1:1 | 1:1 |
| Sl No. | Organic Lime Mortar/Specimens | (28D) N/mm2 | (56D) N/mm2 |
|---|---|---|---|
| 1 | Reference lime mortar | 0.895 | 1.81 |
| 2 | Kadukkai lime mortar | 1.141 | 1.68 |
| 3 | Cactus lime mortar | 0.38 | 1.62 |
| 4 | Hibiscus lime mortar | 0.96 | 1.42 |
| 5 | Aloe vera lime mortar | 0.65 | 1.85 |
| 6 | Jaggery lime mortar | 1.91 | 2.76 |
| 7 | Neelamari lime mortar | 0.387 | 0.62 |
| 8 | Kadukkai + p. jaggery | 2.1 | 2.6 |
| 9 | All mixed (organics) | 0.839 | 1.55 |
| Days | Temperature–Weight Loss (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0–200 °C | 200–400 °C | 400–600 °C | 600–800 °C | 800–1000 °C | Total (%) | ||
| Reference lime mortar | 28/56 Days | 2.42 | 5.51 | 7.51 | 9.76 | 0.65 | 25.85 |
| 2.2 | 4 | 4.34 | 16.18 | 0.69 | 27.41 | ||
| Kadukkai L.M | 4.49 | 5.15 | 5.75 | 13.85 | 0.88 | 30.12 | |
| 4.1 | 4.3 | 3.56 | 17.55 | 0.89 | 30.4 | ||
| Cactus L.M | 2.72 | 4.85 | 10.45 | 11.25 | 1.51 | 30.78 | |
| 2.3 | 4.12 | 7.63 | 16.25 | 1.75 | 32.05 | ||
| Hibiscus L.M | 2.44 | 5.69 | 7.93 | 9.83 | 0.81 | 26.7 | |
| 2.1 | 4.22 | 4.25 | 14.28 | 0.89 | 25.74 | ||
| Aloe vera L.M | 2.72 | 4.85 | 14.21 | 12.03 | 1.51 | 35.32 | |
| 2.3 | 3.95 | 10.62 | 18.63 | 1.98 | 37.48 | ||
| Jaggery L.M | 2.62 | 4.95 | 7.52 | 11.25 | 0.67 | 27.01 | |
| 2.22 | 4.15 | 4.56 | 16.26 | 0.81 | 28 | ||
| Neelamari L.M | 2.54 | 3.76 | 7.13 | 16.5 | 0.78 | 30.71 | |
| 2.12 | 2.85 | 4.66 | 20.55 | 0.82 | 31 | ||
| Kadukkai + P. Jaggery L.M | 2.71 | 5.25 | 8.1 | 11.1 | 0.67 | 27.83 | |
| 1.98 | 4.65 | 5.6 | 15.45 | 0.85 | 28.53 | ||
| All mixed (organics) | 2.39 | 5.54 | 7.49 | 10.01 | 0.65 | 26.08 | |
| 2.2 | 4.1 | 4.44 | 16.18 | 0.69 | 27.61 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivakumar, M.; Singh, A.; Selvaraj, T.; Thangaraj, S. Production of the Traditional Organic Mortars of Padmanabhapuram Palace—A Characterization Study on the Simulated Mortars for Their Compatibility. Buildings 2022, 12, 1466. https://doi.org/10.3390/buildings12091466
Shivakumar M, Singh A, Selvaraj T, Thangaraj S. Production of the Traditional Organic Mortars of Padmanabhapuram Palace—A Characterization Study on the Simulated Mortars for Their Compatibility. Buildings. 2022; 12(9):1466. https://doi.org/10.3390/buildings12091466
Chicago/Turabian StyleShivakumar, Mani, Aruna Singh, Thirumalini Selvaraj, and Shanmugapriya Thangaraj. 2022. "Production of the Traditional Organic Mortars of Padmanabhapuram Palace—A Characterization Study on the Simulated Mortars for Their Compatibility" Buildings 12, no. 9: 1466. https://doi.org/10.3390/buildings12091466
APA StyleShivakumar, M., Singh, A., Selvaraj, T., & Thangaraj, S. (2022). Production of the Traditional Organic Mortars of Padmanabhapuram Palace—A Characterization Study on the Simulated Mortars for Their Compatibility. Buildings, 12(9), 1466. https://doi.org/10.3390/buildings12091466







