The Effect of Different Modifying Methods on Physical, Mechanical and Thermal Performance of Cellular Geopolymers as Thermal Insulation Materials for Building Structures
Abstract
:1. Introduction
- (1)
- Natural curing in laboratory conditions at temperature 22 ± 3 °C and relative humidity ≈ 16% before testing;
- (2)
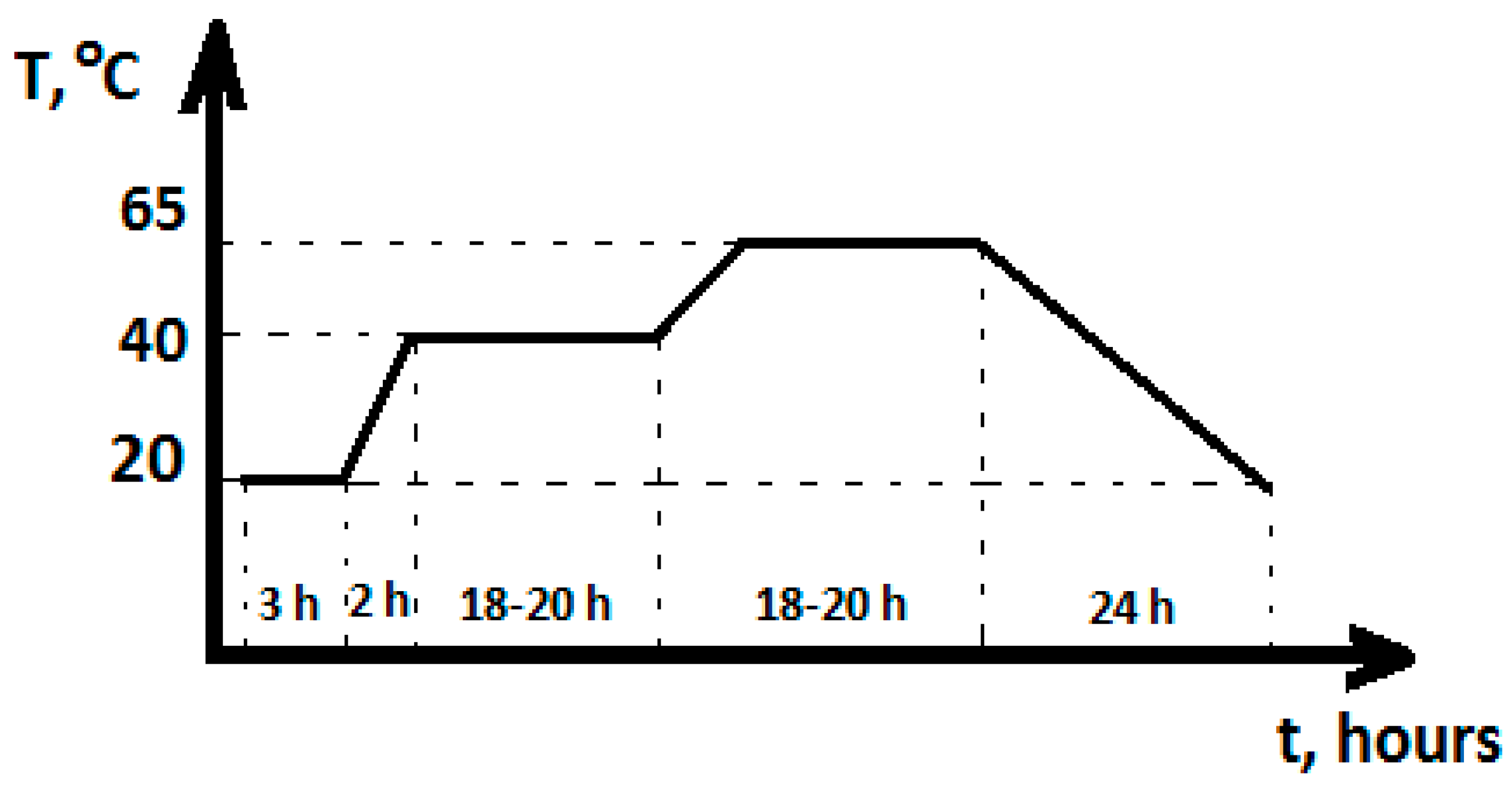
- Natural curing in laboratory conditions at temperature 22 ± 3 °C and relative humidity ≈ 16% for 24 h, followed by heat treatment in an oven according to the following temperature regime: heating to 70 °C—2 h → isothermal curing at 70 °C—18–20 h → cooling to a temperature of 22 ± 3 °C—2 h → natural laboratory conditions until testing (Figure 2).
2. Materials and Methods
2.1. Materials
2.2. Testing Methods and Procedures
2.2.1. Chemical and Microstructural Characterization
2.2.2. Porosity and Density Characteristics
- Destruction of samples integrity, which takes place during sample preparation or directly during analysis. This issue is typical, for example, for the BET method, where the crushing/breaking the test sample into pieces of 4–7 mm is a required preparation protocol. This leads to a violation of the integrity of the internal pores. In addition, the study of porosity on samples of such small dimensions does not provide a complete picture of the porosity cellular material in bulk;
- Testing limitations related to the type and origin of the studied materials. These limitations apply to the subject of testing. For example, resistance to external conditions, such as heating, immersion in a liquid medium, degassing and other conditions are important to consider for each specific material tested. For example, the standard method for determining porosity by immersing a sample in water is not suitable for gypsum materials that are not water-resistant and easily degrade in water;
- Features of pore structure of the tested material. The impossibility of determining the total porosity if there are closed pores in the structure is one common drawbacks for existing analytical methods for porosity evaluation. Below provides the definitions used in this article:
2.2.3. Compressive Strength Testing
2.2.4. Thermal Conductivity Testing
2.3. Sample Preparation
3. Results
3.1. Effect of High-Temperature Treatment on the Properties of Cellular Geopolymer Concrete
3.2. Calculated and Measured Porosity Characteristics
- -
- Porosity determined by calculation method;
- -
- Porosity determined using analytical equipment according to the BET method.
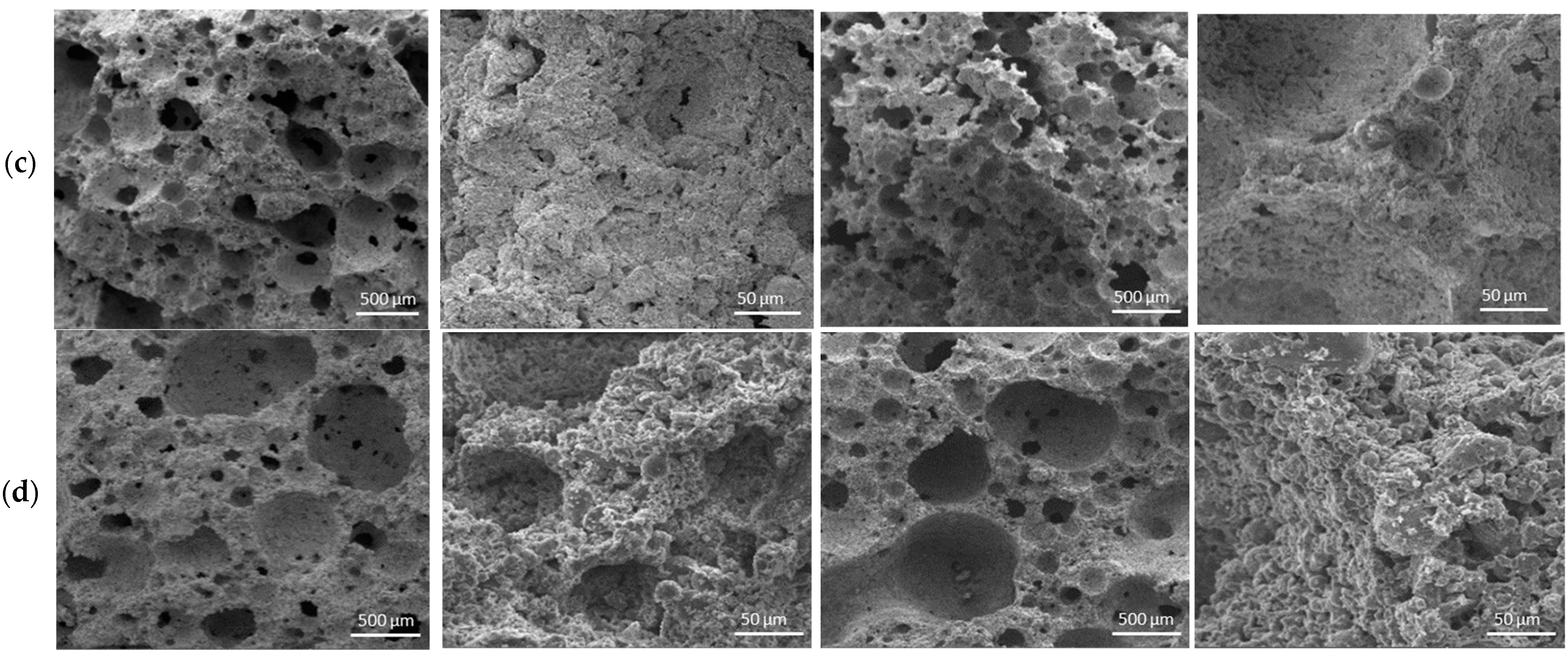
3.3. Microstructure of Cellular Geopolymers
- -
- The effect of high-temperature treatment on the PC-containing CGP shows a negative effect on the formation of the pore structure, regardless of the way the alkaline activator is used. This is because the hydrated PC is not a thermal resistant material. Therefore, the destruction of the cement structure at 600 °C inevitably initiates the degradation of the integrity of the geopolymer cellular structure as a whole. The 24 h aged e NaOH solution leads to the formation of a less-strong structure before high-temperature treatment, but provides higher thermal resistance;
- -
- In the mix containing kaolinite, the 24 h aged NaOH solution has a positive effect on the implementation of structure-forming processes, but negatively affects the formation of a correct uniform pore structure. In this case, high-temperature treatment helps to strengthen the structure and increase its thermal resistance;
- -
- For the mixes using metakaolin, the introduction method of alkaline activator and the high-temperature treatment have no significant effect on the pore morphology. However, these two factors significantly intensify chemical reactions, which leads to the formation of a denser and more-monolithic structure of the inter-pore space and, as a consequence, to the strengthening of the framework CGP;
- -
- Such as in the case of metakaolin, in the mix without a MMC, the introduction method of alkaline activator and high-temperature treatment does not have a noticeable effect on the morphology of the pore space but contributes to the formation of a more-monolithic structure of the inter-pore space and the strengthening of the structure. However, 24 h aged NaOH solution promotes the formation of a more developed pore morphology during high-temperature treatment.
4. Conclusions
- -
- The nature of mineral modifying agent dramatically affects pore structure formation, compressive strength, average density and porosity of cellular framework, as well as thermal conductivity. Plus, the hardening effect of cellular geopolymers is provided only by PC as a MMC in case of using a freshly prepared NaOH aqueous solution. However, PC-containing CGP are not thermal-resistant composites;
- -
- The way the alkaline activator is used for CGP synthesis plays a significant role in compressive strength development for all experimental mixes. The 24 h aged NaOH solution contributes to the formation of a more durable matrix structure of CGP; the formation of a more regular pore structure, which leads to reduction of average density, yet enables to increase compressive strength of CGP and also contributes to an increase thermal-resistant properties of the studied CGP specimens;
- -
- The 24 h ”ageing” of NaOH aqueous solution contributes to a more-complete realization of chemical processes. This statement is supported by significant areas of a monolithic structure formed within the interpore space. In addition, a more-regular pore volume and size distribution, as well as a higher degree of pore integrity, is a good indicator of lower thermal conductivity for mixes produced with 24 h aged alkaline NaOH solution vs. specimens produced with freshly prepared alkaline solution;
- -
- Lower values of the thermal conductivity coefficient are typical for unmodified CGP and MK-containing CGP composites, as well. This is because FA and MK are characterized by a predominantly vitreous structure, the concentration of which increases during geopolymerization;
- -
- The introduction method of alkaline activator did not have a noticeable effect on thermal conductivity for all studied CGP. However, the high-temperature treatment of CGP at 600 °C led to decrease in thermal conductivity;
- -
- A high degree of correlation was revealed between the average density and the calculated total porosity for the studied CGP before (R2 = 0.967) and after (R2 = 0.969) high-temperature treatment at 600 °C. At the same time, the correlations between the average density and the nanoporosity measured by the BET method were R2 = 0.286 and R2 = 0.179, for the mixes before and after high-temperature treatment at 600 °C, respectively. Thus, the proposed computational method for determining total porosity is more efficient and practical.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.S.; Chen, G.F. Chapter Eight—Applications of Polymer Foams. Porous Mater. Processing Appl. 2014, 383–410. [Google Scholar] [CrossRef]
- Ogabi, R.; Manescau, B.; Chetehouna, K.; Gascoin, N.A. Study of Thermal Degradation and Fire Behaviour of Polymer Composites and Their Gaseous Emission Assessment. Energies 2021, 14, 7070. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.; Flodström, K.; Mao, X.; Guo, J. Cellular silica-based ceramics prepared by direct foaming at high temperature. Ceram. Int. 2010, 36, 923–927. [Google Scholar] [CrossRef]
- Zhernovsky, I.V.; Cherevatova, A.V.; Kozhukhova, N.I.; Osadchaya, M.S.; Ksenofontov, D.A. Low-temperature aluminosilicate nanostructured binder. Characteristics and applicability. Mater. Sci. Forum 2018, 945, 193–198. [Google Scholar] [CrossRef]
- Zhernovsky, I.V.; Cherevatova, A.V.; Voitovich, E.V.; Kozhukhova, N.I.; Evtushenko, E.I. High-temperature phase transformations in CaO-SO3-SiO2-H2O system with nanosized component. Int. J. Appl. Eng. Res. 2016, 11, 7732–7735. [Google Scholar]
- Murri, A.N.; Medri, V.; Papa, E.; Laghi, L.; Mingazzini, C.; Landi, E. Porous Geopolymer Insulating Core from a Metakaolin/Biomass Ash Composite. Environments 2017, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Abdulkareem, O.A.; Al Bakri Abdullah, M.M.; Kamarudin, H.; Khairul, N.I.; Binhussain, M. Mechanical and Microstructural Evaluations of Lightweight Aggregate Geopolymer Concrete before and after Exposed to Elevated Temperatures. Materials 2013, 6, 4450–4461. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Hu, L.; Dong, Z.; Tang, L.; Xing, F.; Liu, J. Effect of silica fume on the mechanical property and hydration characteristic of alkali-activated municipal solid waste incinerator (MSWI) fly ash. J. Clean. Prod. 2021, 295, 126317. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef]
- Shekhovtsova, J.; Zhernovsky, I.; Kovtun, M.; Kozhukhova, N.; Zhernovskaya, I.; Kearsley, E.P. Estimation of fly ash reactivity for use in alkali-activated cements—A step towards sustainable building material and waste utilization. J. Clean. Prod. 2018, 178, 22–33. [Google Scholar] [CrossRef]
- Figiela, B.; Korniejenko, K. The possibility of using wastes materials as raw materials for the production of geopolymers. Acta Innov. 2020, 36, 48–56. [Google Scholar] [CrossRef]
- Łach, M. Geopolymer Foams—Will They Ever Become a Viable Alternative to Popular Insulation Materials?—A Critical Opinion. Materials 2021, 14, 3568. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, N.; Zhang, M. Fiber-reinforced geopolymer composites: A review. Cem. Concr. Compos. 2020, 107, 103498. [Google Scholar] [CrossRef]
- Silva, G.; Kim, S.; Bertolotti, B.; Nakamatsu, J.; Aguilar, R. Optimization of a reinforced geopolymer composite using natural fibers and construction wastes. Constr. Build. Mater. 2020, 258, 119697. [Google Scholar] [CrossRef]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel hybrid organic-geopolymer materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Tarallo, O.; Ferone, C.; Colangelo, F.; Roviello, V.; Cioffi, R. Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties. Materials 2016, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Cioffi, R.; Tarallo, O. Hybrid Geopolymers from Fly Ash and Polysiloxanes. Molecules 2019, 24, 3510. [Google Scholar] [CrossRef] [Green Version]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Tarallo, O. Fire resistant melamine based organic-geopolymer hybrid composites. Cem. Concr. Compos. 2015, 59, 89–99. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Messina, F.; Ferone, C.; Asprone, D.; Cioffi, R. Lightweight geopolymer-based hybrid materials. Compos. Part B Eng. 2017, 128, 225–237. [Google Scholar] [CrossRef]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A. Effect of adding mineral additives to alkali-activated natural pozzolan paste. Constr. Build. Mater. 2011, 6, 2906–2910. [Google Scholar] [CrossRef]
- Awais Ashfaq Alvi, M.; Khalifeh Mesfin, M.; Agonafir, B. Effect of nanoparticles on properties of geopolymers designed for well cementing applications. J. Petrol. Sci. Eng. 2020, 191, 107128. [Google Scholar] [CrossRef]
- Le, C.H.; Louda, P.; Ewa Buczkowska, K.; Dufkova, I. Investigation on Flexural Behavior of Geopolymer-Based Carbon Textile/Basalt Fiber Hybrid Composite. Polymers 2021, 13, 751. [Google Scholar] [CrossRef]
- Fadhil Nuruddin, M.; Demie, S.; Fareed Ahmed, M.; Shafiq, N. Effect of Superplasticizer and NaOH Molarity on workability, Compressive Strength and micro Structure Properties of Self-Compacting Geopolymer concrete. World Acad. Sci. Eng. Technol. 2011, 5, 187–194. [Google Scholar]
- Kozhukhova, N.I.; Teslya, A.Y.; Alfimova, N.I.; Kozhukhova, M.I. Effect of mixing procedure and chemical composition on physical and mechanical performance of geopolymers. Mater. Sci. Forum 2021, 1017, 41–50. [Google Scholar] [CrossRef]
- AinJaya, N.; Yun-Ming, L.; Yong, H.C.; Al Bakri Abdullah, M.M.; Hussin, K. Correlation between pore structure, compressive strength and thermal conductivity of porous metakaolin geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar]
- Poston, R.W.; A.C. Institute. Building Code Requirements for Structural Concrete (ACI 318M–11) and Commentary, Farmington Hills Michigan. A.C. Institute: New York, NY, USA, 2011. [Google Scholar]
- Decathlon, S.; Snell, C.; Tempest, B.; Gentry, T. Comparison of the Thermal Characteristics of Portland Cement and Geopolymer Cement Concrete Mixes. J. Archit. Eng. 2017, 23, 04017002. [Google Scholar] [CrossRef]
- Denissen, J.; Kriskova, L.; Pontikes, Y. Kinetics of pore formation and resulting properties of lightweight inorganic polymers. J. Am. Ceram. Soc. 2019, 102, 3940–3950. [Google Scholar] [CrossRef]
- Jacops, E.; Tri Phung, Q.; Frederickx, L.; Levasseur, S. Diffusive transport of dissolved gases in potential concretes for nuclear waste disposal. Sustainability 2021, 13, 10007. [Google Scholar] [CrossRef]
- Herbert Sinduja, J.; Sakthieswaran, N.; Shiny Brintha, G. Review On Geopolymer Concerte With Different Additives. Int. J. Eng. Res. IJOER 2015, 1, 21–31. Available online: https://www.academia.edu/12745734/Review_On_Geopolymer_Concerte_With_Different_Additives?email_work_card=reading-history (accessed on 26 December 2021).
- Andi Arham, A.; Horianto, X.X.X. The effect of temperature and duration of curing on the strength of fly ash based geopolymer mortar. Procedia Eng. 2014, 95, 410–414. [Google Scholar]
- Kozhukhova, N.I.; Kozhukhova, M.I.; Zhernovskaya, I.V.; Promakhov, V.V. The Correlation of Temperature-Mineral Phase Transformation as a Controlling Factor of Thermal and Mechanical Performance of Fly Ash-Based Alkali-Activated Binders. Materials 2020, 13, 5181. [Google Scholar] [CrossRef] [PubMed]
- Łach, M.; Pławecka, K.; Bąk, A.; Lichocka, K.; Korniejenko, K.; Cheng, A.; Lin, W.-T. Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams. Materials 2021, 14, 5090. [Google Scholar] [CrossRef]
- Samsona, G.; Cyra, M.; Xiao Gao, X. Thermomechanical performance of blended metakaolin-GGBS alkali-activated foam concrete. Constr. Build. Mater. 2017, 157, 982–993. [Google Scholar] [CrossRef]
- Hájková, P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals 2018, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Riahi, S.; Nemati, A.; Khodabandeh, A.R.; Baghshahi, S. The effect of mixing molar ratios and sand particles on microstructure and mechanical properties of metakaolin-based geopolymers. Mater. Chem. Phys. 2020, 240, 122223. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]







| MMC | Oxides Content, wt.% | Real Density, g/cm3 | SSA, m2/kg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | TiO2 | K2O | MgO | CaO | P2O5 | SO3 | N2O | LOI | SiO2/Al2O3 | |||
| FA | 58.9 | 28.3 | 4.63 | 0.97 | 0.65 | 1 | 3.74 | 0.36 | - | 0.63 | 6.07 | 2.08 | 1.87 | 290 |
| PC | 19.1 | 5.21 | 3.58 | 0.32 | 0.6 | 1.28 | 65.4 | - | 3.47 | - | 0.23 | 3.67 | 3.05 | 320 |
| K | 53.8 | 43.4 | 1.02 | 0.58 | 0.56 | 0.21 | 0.01 | 0.06 | - | 0.03 | 4.2 | 1.23 | 2.61 | 2610 |
| MK | 53.1 | 42.8 | 0.7 | 0.3 | 0.9 | - | 0.15 | - | - | 0.02 | 0.4 | 1.24 | 2.52 | 2520 |
| Mix ID | Component Composition,% wt. | ||||||
|---|---|---|---|---|---|---|---|
| FA | MMC | NaOH | Water | Foaming Agent | |||
| PC | MK | K | |||||
| NaOH freshly prepared aqueous NaOH solution | |||||||
| R | 53.5 | - | - | - | 8.02 | 38.3 | 0.17 |
| GPC | 32.09 | 21.4 | - | - | 8.02 | 38.3 | 0.17 |
| GMK | 32.09 | - | 21.4 | - | 8.02 | 38.3 | 0.17 |
| GK | 32.09 | - | - | 21.4 | 8.02 | 38.3 | 0.17 |
| 24-h aged aqueous NaOH solution | |||||||
| R-24 | 53.5 | - | - | - | 8.02 | 38.3 | 0.17 |
| GPC-24 | 32.09 | 21.4 | - | - | 8.02 | 38.3 | 0.17 |
| GMK-24 | 32.09 | - | 21.4 | - | 8.02 | 38.3 | 0.17 |
| GK-24 | 32.09 | - | - | 21.4 | 8.02 | 38.3 | 0.17 |
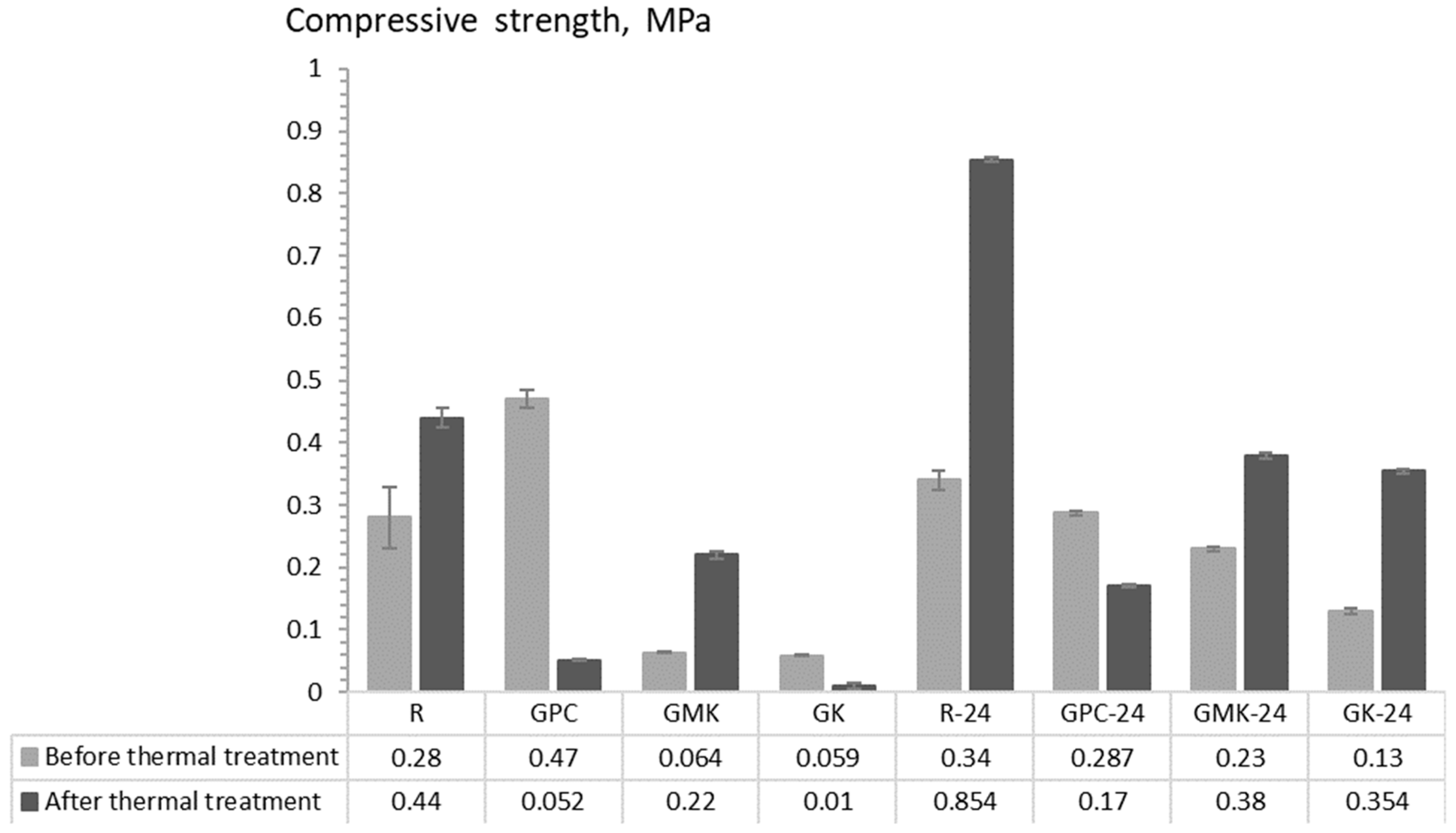
| Mix ID | Average Density, g/cm3 | Compressive Strength, MPa | Thermal Conductivity, W∙m⁻2·K⁻1 | |||
|---|---|---|---|---|---|---|
| Before TT | After TT | Before TT | After TT | Before TT | After TT | |
| R | 0.732 | 0.585 | 0.28 | 0.44 | 0.049 | 0.044 |
| GPC | 0.963 | 0.695 | 0.67 | 0.052 | 0.154 | 0.071 |
| GMK | 0.496 | 0.564 | 0.064 | 0.22 | 0.043 | 0.041 |
| GK | 0.456 | 0 | 0.059 | 0 | 0.088 | 0.001 |
| R-24 | 0.788 | 0.726 | 0.34 | 0.854 | 0.047 | 0.042 |
| GPC-24 | 0.74 | 0.682 | 0.287 | 0.17 | 0.096 | 0.054 |
| GMK-24 | 0.71 | 0.63 | 0.23 | 0.38 | 0.045 | 0.044 |
| GK-24 | 0.832 | 0.629 | 0.13 | 0.354 | 0.074 | 0.055 |
| Raw Materials | ρtrue, g/cm3 | Ratios, % |
|---|---|---|
| FA | 1.87 | 53.5 |
| NaOH | 2.13 | 8.02 |
| Water | 1.00 | 38.3 |
| Foaming agent | 1.10 | 0.17 |
| Mix ID | Pore Volume in the Specimen at a Relative Pressure P/Po = 0.984699778 (VP/P0) | Specimen Weight (m0), g | ||
|---|---|---|---|---|
| Before TT | After TT | Before TT | After TT | |
| R | 0.002097 | 0.000052 | 1.1506 | 1.2142 |
| GPC | 0.011405 | 0.002097 | 1.0848 | 1.1620 |
| GMK | 0.005102 | 0.001563 | 1.0436 | 1.0876 |
| GK | 0.009027 | 0.004900 | 1.1244 | 1.1239 |
| R-24 | 0.000790 | - | 0.9977 | - |
| GPC-24 | 0.016408 | 0.001655 | 1.0054 | 0.9742 |
| GMK-24 | 0.006274 | 0.001335 | 1.2268 | 0.9295 |
| GK-24 | 0.004460 | 0.004193 | 1.0602 | 1.0391 |
| Mix ID | Calculated Porosity, % | BET Nanoporosity | |||
|---|---|---|---|---|---|
| Before TT | After TT | Before TT | After TT | ||
| R | 1.55 | 52.8 | 62.2 | 0.177 | 0.0037 |
| GPC | 1.84 | 31.3 | 62.2 | 1.19 | 0.169 |
| GMK | 1.69 | 70.6 | 66.6 | 0.263 | 0.096 |
| GK | 1.71 | 73.3 | - | 0.456 | - |
| R-24 | 1.55 | 49.2 | 53.1 | 0.122 | - |
| GPC-24 | 1.84 | 59.8 | 62.9 | 3.02 | 0.3 |
| GMK-24 | 1.69 | 57.9 | 62.7 | 1.06 | 0.225 |
| GK-24 | 1.71 | 51.3 | 63.2 | 0.76 | 0.716 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhova, N.; Kozhukhova, M.; Teslya, A.; Nikulin, I. The Effect of Different Modifying Methods on Physical, Mechanical and Thermal Performance of Cellular Geopolymers as Thermal Insulation Materials for Building Structures. Buildings 2022, 12, 241. https://doi.org/10.3390/buildings12020241
Kozhukhova N, Kozhukhova M, Teslya A, Nikulin I. The Effect of Different Modifying Methods on Physical, Mechanical and Thermal Performance of Cellular Geopolymers as Thermal Insulation Materials for Building Structures. Buildings. 2022; 12(2):241. https://doi.org/10.3390/buildings12020241
Chicago/Turabian StyleKozhukhova, Natalia, Marina Kozhukhova, Anastasia Teslya, and Ivan Nikulin. 2022. "The Effect of Different Modifying Methods on Physical, Mechanical and Thermal Performance of Cellular Geopolymers as Thermal Insulation Materials for Building Structures" Buildings 12, no. 2: 241. https://doi.org/10.3390/buildings12020241
APA StyleKozhukhova, N., Kozhukhova, M., Teslya, A., & Nikulin, I. (2022). The Effect of Different Modifying Methods on Physical, Mechanical and Thermal Performance of Cellular Geopolymers as Thermal Insulation Materials for Building Structures. Buildings, 12(2), 241. https://doi.org/10.3390/buildings12020241







