Influence of the Concentration of Seawater on the Early Hydration Properties of Calcium Sulphoaluminate (CSA) Cement: A Preliminary Study
Abstract
1. Introduction
2. Experiments
2.1. Experiment Materials
2.2. Specimen Preparation and Curing
2.3. Test Method
2.3.1. Setting Time
2.3.2. Compressive Strength Tests
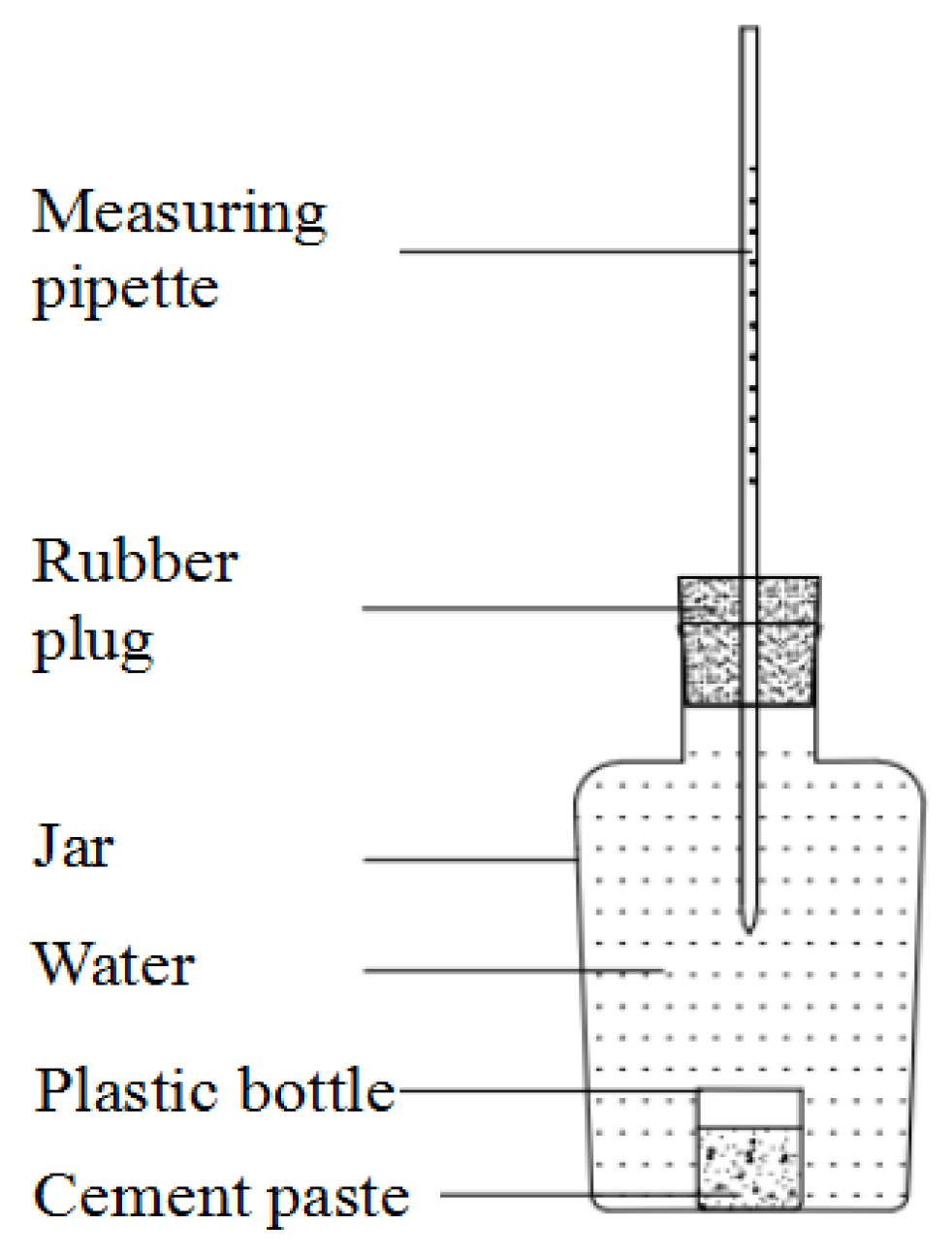
2.3.3. Chemical Shrinkage
2.3.4. X-ray Diffraction (XRD) Analysis
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Thermogravimetric Analysis (TGA)
3. Results and Discussion
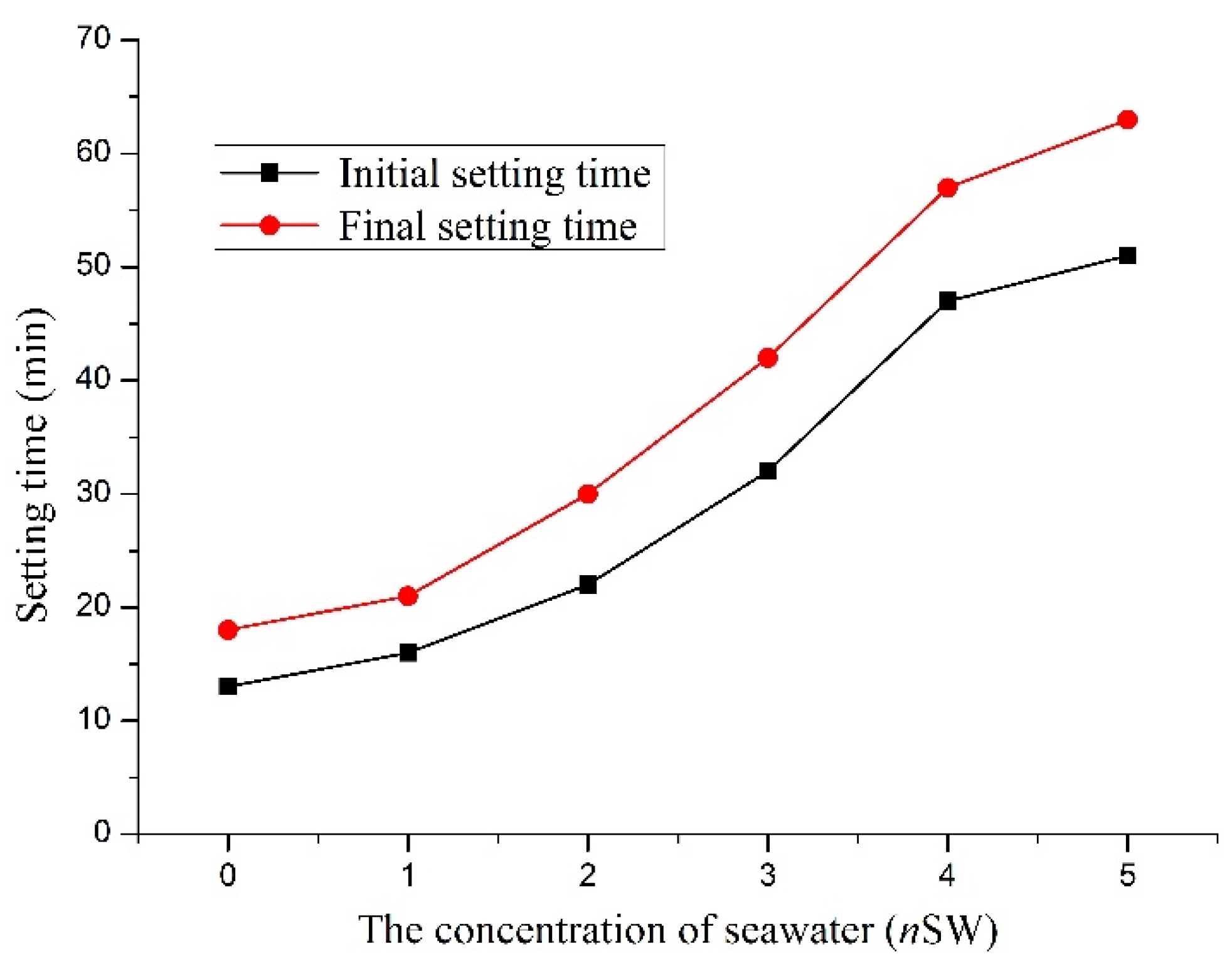
3.1. Influence on the Initial and Final Setting Times
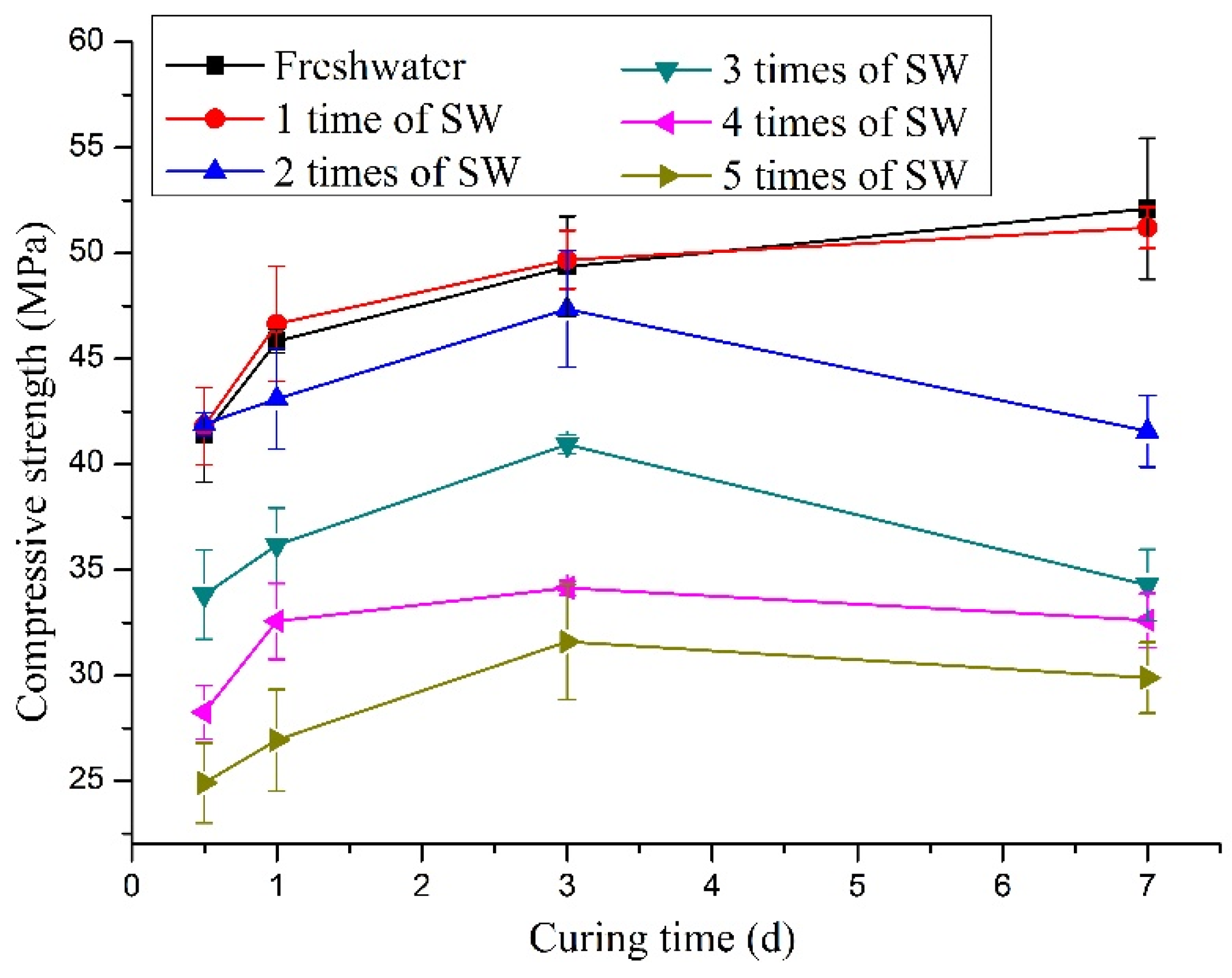
3.2. Affect on the Compressive Strength
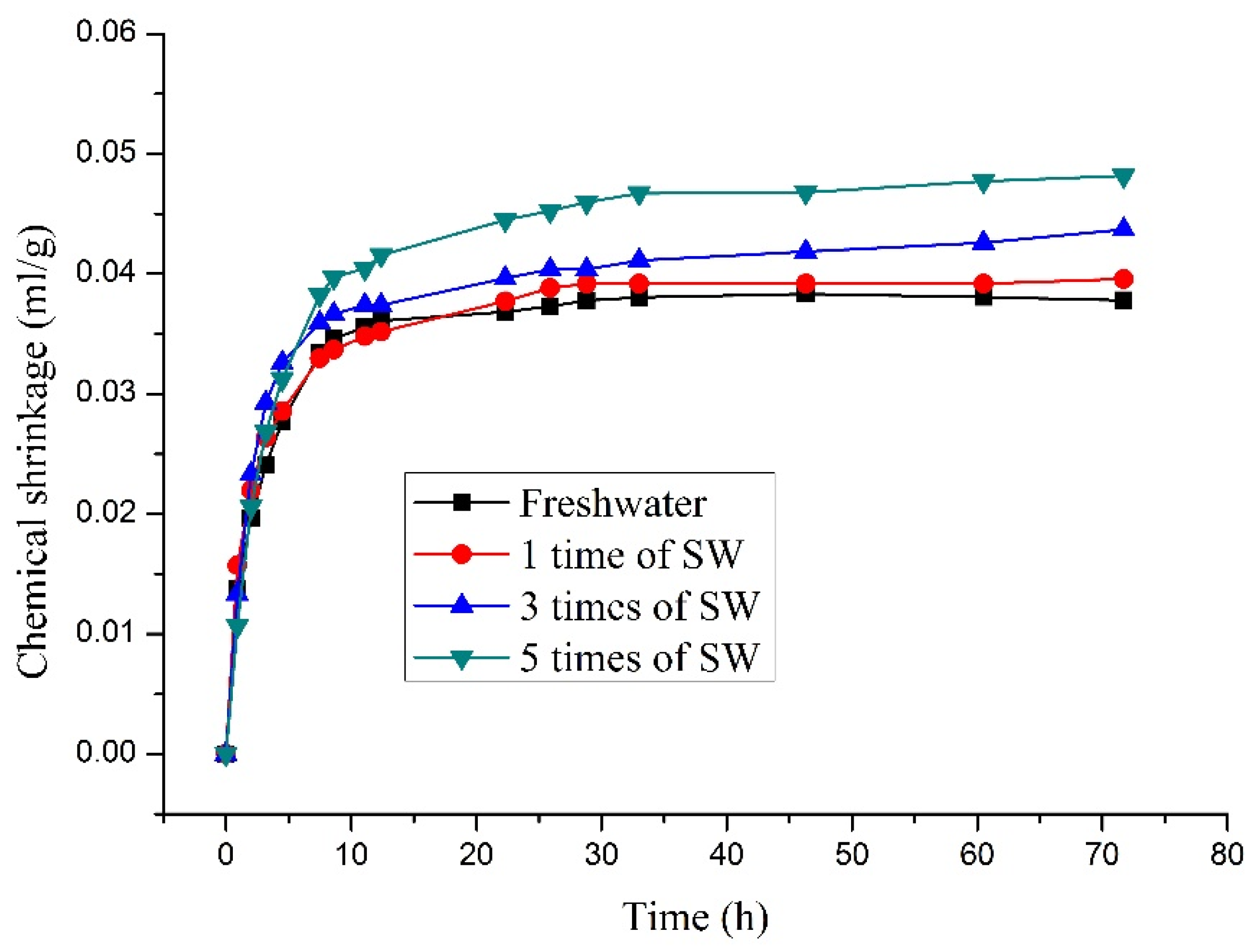
3.3. Influence on Chemical Shrinkage
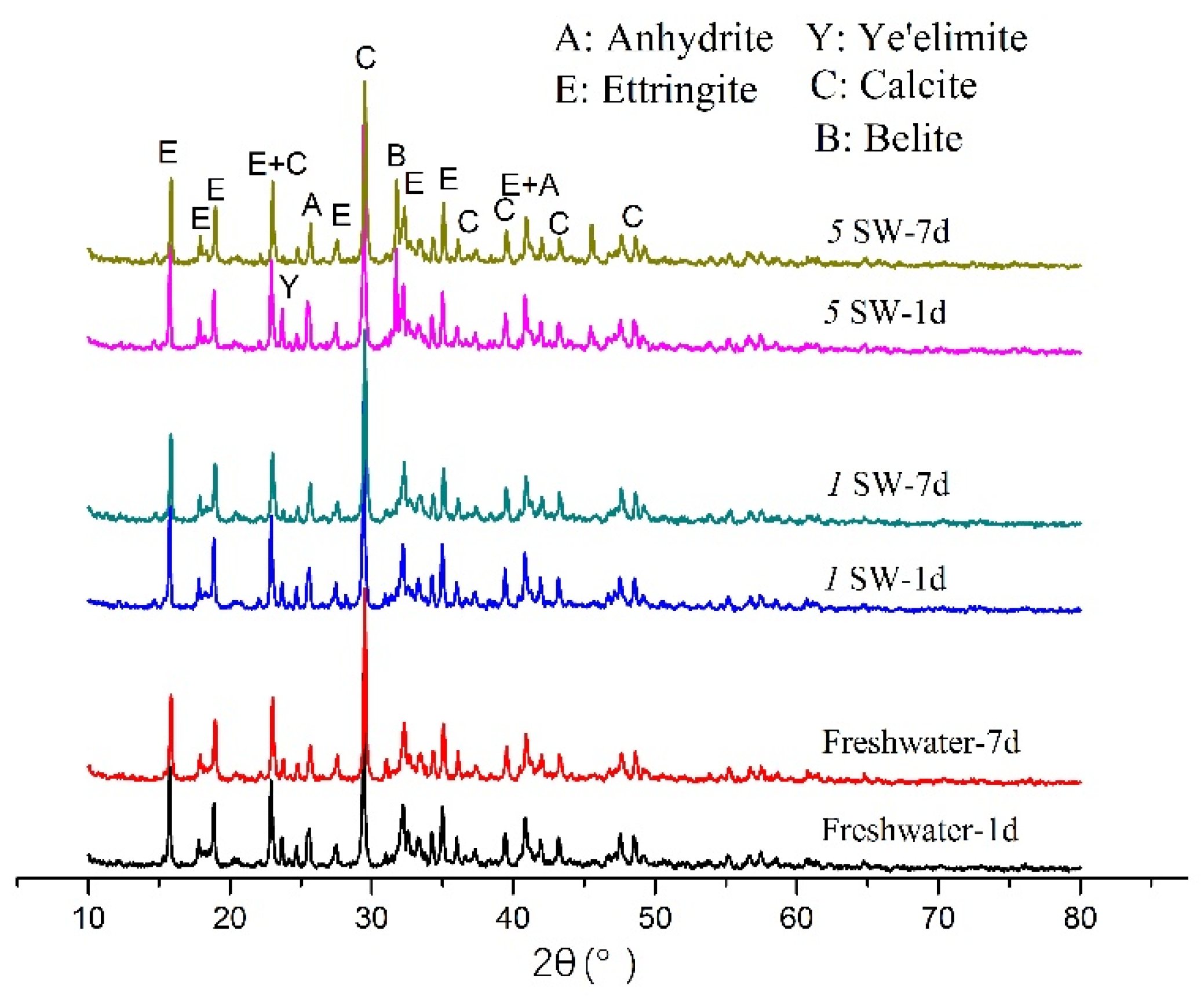
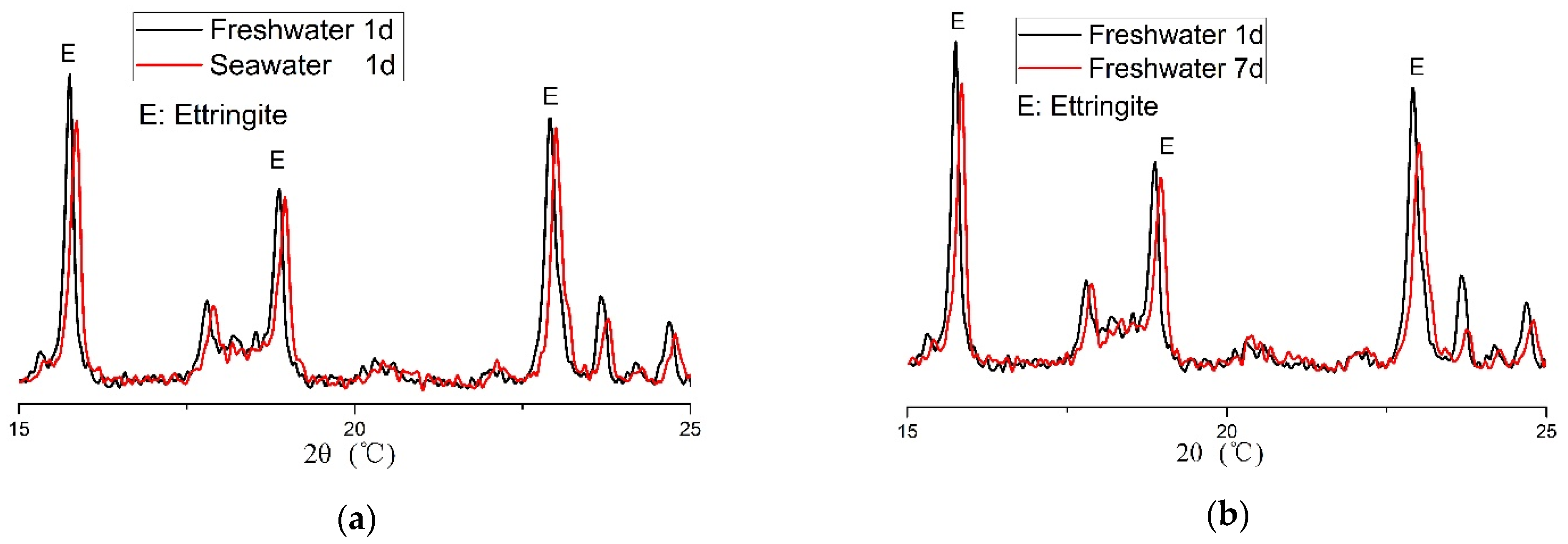
3.4. XRD Analysis
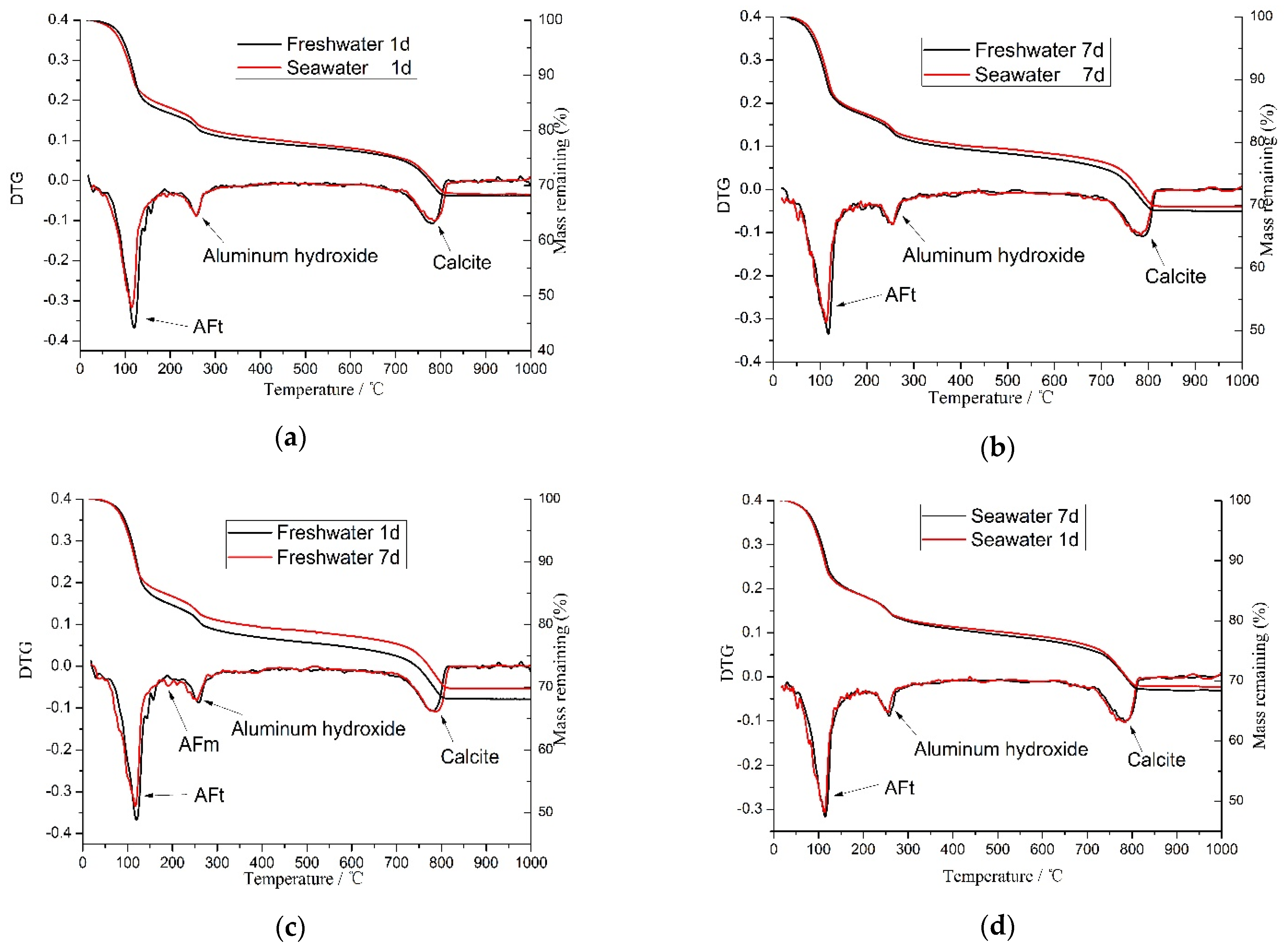
3.5. DTA-TG Analysis
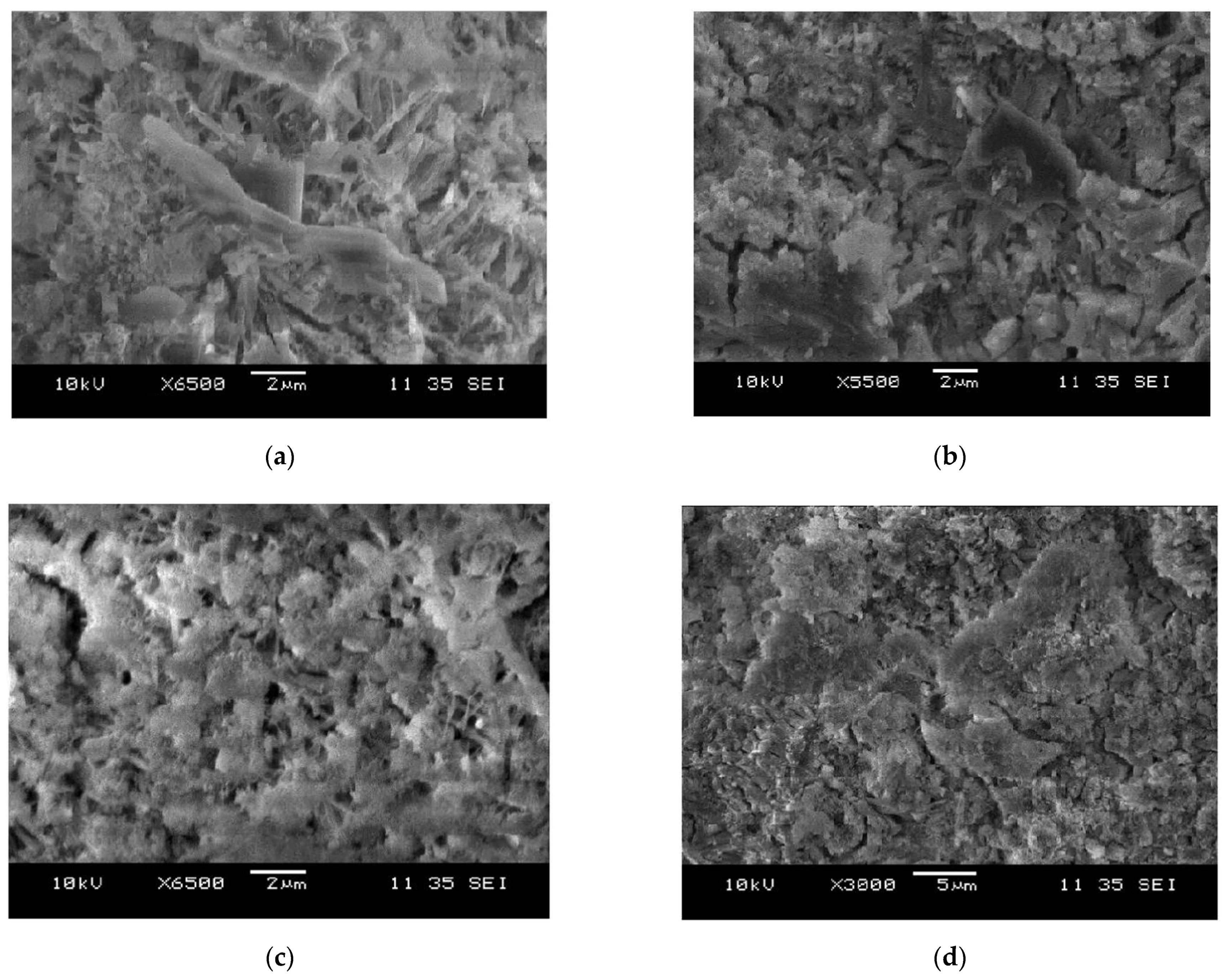
3.6. SEM Analysis
4. Conclusions
- (1)
- The hydration reaction of CSA cement occurs quickly. The primary hydration products are AFt and aluminium hydroxide, while a small amount of ettringite (AFt) becomes monosulfate (AFm) in the freshwater-mixed CSA cement.
- (2)
- A low concentration of seawater can promote the hydration of CSA cement. Sulfate ions can accelerate the hydration reaction to produce AFt. The early strength of the CSA cement mixed with seawater of a lower concentration is higher than that of freshwater-mixed CSA cement. However, the strength of the samples mixed with high concentrations seawater will decrease significantly, and the higher the concentration is, the greater the decrease will be.
- (3)
- Seawater retards the setting time of CSA cement, and the higher the concentration, the more pronounced the delay. Seawater can increase the chemical shrinkage, and the higher the concentration of seawater, the greater the chemical shrinkage.
- (4)
- According to the analysis of SEM, XRD and TGA, SO42− in seawater can accelerate the hydration reaction and produce insoluble AFt to precipitate on the surface of CSA cement particles and prevent their further hydration. Moreover, the higher the concentration is, the faster ettringite is formed and the more non-hydrated the sulphoaluminate cement is. Aside from that, a lot of AFt is generated in a very short time, which makes the cement matrix expand and produces microcracks in the cement matrix, resulting in the lower compressive strength of the cement paste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fei, S.; Yu, Z.; Yang, F.; Liu, Y.; Lu, Y. Strätlingite and calcium hemicarboaluminate hydrate in belite-calcium sulphoaluminate cement. Ceram. Silikáty 2014, 58, 269–274. [Google Scholar]
- Guo, X.; Shi, H.; Hu, W.; Wu, K. Durability and microstructure of CSA cement based materials from MSWI fly ash. Cem. Concr. Compos. 2014, 46, 26–31. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Z.; Liu, S.; Wang, S.; Cheng, X. Durability of alite-calcium barium sulphoaluminate cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 982. [Google Scholar] [CrossRef]
- Ioannou, S.; Paine, K.; Reig, L.; Quillin, K. Performance characteristics of concrete based on a ternary calcium sulfoaluminate–anhydrite–fly ash cement. Cem. Concr. Compos. 2015, 55, 196–204. [Google Scholar] [CrossRef]
- Jen, G.; Stompinis, N.; Jones, R. Chloride ingress in a belite-calcium sulfoaluminate cement matrix. Cem. Concr. Res. 2017, 98, 130–135. [Google Scholar] [CrossRef]
- Ye, Z.; Zheng, L.; Wan, L.; Cheng, X. Application of second law of Fick in NaCl attack of sulphoaluminate cement. Adv. Mater. Res. 2011, 306–307, 1038–1041. [Google Scholar] [CrossRef]
- He, F. Measurement of Chloride Migration in Cement-Based Materials Using AgNO3 Colorimetric Method. Ph.D. Thesis, Central South University, Changsha, China, 2010. [Google Scholar]
- Wang, Y.; Su, M.; Zhang, L. Sulphoaluminate Cement; Beijing University of Technology Press: Beijing, China, 1999. [Google Scholar]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, G.; Liu, Y. Experimental study on shrinkage reduction of calcium sulphoaluminate cement concrete with addition of pre-wetted lightweight aggregate. Constr. Build. Mater. 2020, 253, 119149. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Haneina, T.; Galvez-Martos, J.L.; Bannerman, N.M. Carbon footprint of calcium sulfoaluminate clinker production. J. Clean. Prod. 2018, 172, 2278–2287. [Google Scholar] [CrossRef]
- García-Maté, M.; Santacruz, I.; Torre, Á.G.D.; León-Reina, L.; Aranda, M.A.G. Rheological and hydration characterization of calcium sulfoaluminate cement pastes. Cem. Concr. Compos. 2012, 34, 684–691. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of calcium sulfoaluminate mortars. Cem. Concr. Compos. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- Pace, M.L.; Telesca, A.; Marroccoli, M.; Valenti, G.L. Use of industrial byproducts as alumina sources for the synthesis of calcium sulfoaluminate cements. Environ. Sci. Technol. 2011, 45, 6124–6128. [Google Scholar] [CrossRef]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Marroccoli, M.; Pace, M.L.; Telesca, A.; Valenti, G.L. Synthesis of calcium sulfoaluminate cements from Al2O3-rich by-products from aluminium manufacture. In Proceedings of the 2th International Congress on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010. [Google Scholar]
- Chen, C.; Ji, T.; Zhuang, Y.; Lin, X. Workability, mechanical properties and affinity of artificial reef concrete. Constr. Build. Mater. 2015, 98, 227–236. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Yang, Z.; Ye, Y. Steel rebar corrosion in artificial reef concrete with sulphoaluminate cement, sea water and marine sand. Constr. Build. Mater. 2019, 227, 116685. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Z.; He, F.; Huang, J.; Zhou, J. Sulfate diffusion in calcium sulphoaluminate mortar. Constr. Build. Mater. 2020, 234, 117312. [Google Scholar] [CrossRef]
- Cai, G.; Zhao, J. Application of sulphoaluminate cement to repair deteriorated concrete members in chloride ion rich environment-A basic experimental investigation of durability properties. KSCE J. Civ. Eng. 2016, 20, 2832–2841. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Shi, H.; Wu, Q.; Yu, Z.; Ma, J.; Shen, X. Properties of eco-friendly coral sand powder- Calcium sulfoaluminate cement binary system. Constr. Build. Mater. 2020, 263, 120181. [Google Scholar] [CrossRef]
- Lu, B.; Liang, Y. Experimental study of concrete prepared with coral reef and sea water I. Mater. Sci. Bull. 1993, 5, 69–74. [Google Scholar]
- Lu, B.; Li, Q.; Huang, S. Research and practice of seawater-coral sand concrete. Guangdong Build. Mater. 1997, 4, 8–10. [Google Scholar]
- Liu, W.; Cui, H.Z.; Dong, Z.J.; Xing, F.; Zhang, H.C.; Lo, T.Y. Carbonation of concrete made with dredged marine sand and its effect on chloride binding. Constr. Build. Mater. 2016, 120, 1–9. [Google Scholar] [CrossRef]
- Xu, C.X.; Ou, Z.W.; Zhou, J.L.; Chen, H.B.; Chen, Q. Investigation on protectional ability on steel bar of compound corrosion inhibitor applied in seawater-andsea sand concrete. In Frontiers of Green Building, Materials and Civil Engineering, Pts 1–8; Sun, D., Sung, W.P., Chen, R., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; pp. 864–870. [Google Scholar]
- Bertola, F.; Gastaldi, D.; Irico, S.; Paul, G.; Canonico, F. Behavior of blends of CSA and Portland cements in high chloride Environment. Constr. Build. Mater. 2020, 262, 120852. [Google Scholar] [CrossRef]
- Nishida, T.; Otsuki, N.; Ohara, H.; Garba-Say, Z.; Nagata, M.T. Some considerations for the applicability of seawater as mixing water in concrete. J. Civ. Eng. 2013, 27, B4014004. [Google Scholar] [CrossRef]
- Kaushik, S.K.; Islam, S. Suitability of sea water for mixing structural concrete exposed to a marine environment. Cem. Concr. Compos. 1995, 17, 177–185. [Google Scholar] [CrossRef]
- Dewar, J.D. The Workability and Compressive Strength of Concrete Made with Sea Water; Technical Report, TRA/374; Cement and Concrete Association: London, UK, 1963. [Google Scholar]
- Mohammed, T.U.; Hamada, H.; Yamaji, T. Performance of seawater-mixed concrete in the tidal environment. Cem. Concr. Res. 2004, 34, 593–601. [Google Scholar] [CrossRef]
- Neville, A. Seawater in the Mixture. Concr. Intern. 2001, 23, 48–51. [Google Scholar]
- Islam, M.; Islam, S.; Al-Amin, M.I. Suitability of sea water on curing and compressive strength of structural concrete. J. Civ. Eng. 2012, 40, 37–45. [Google Scholar]
- Younis, A.; Ebead, U.; Suraneni, P.; Nanni, A. Fresh and hardened properties of seawater-mixed concrete. Constr. Build. Mater. 2018, 190, 276–286. [Google Scholar] [CrossRef]
- Chang, J.; Cui, K.; Zhang, Y. Effect of hybrid steel fibers on the mechanical performances and microstructure of sulphoaluminate cement-based reactive powder concrete. Constr. Build. Mater. 2020, 261, 120502. [Google Scholar] [CrossRef]
- Li, T.; Huang, F.; Li, L.; Zhu, J.; Jiang, X.; Huang, Y. Preparation and properties of sulphoaluminate cement-based foamed concrete with high performance. Constr. Build. Mater. 2020, 263, 120945. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulphoaluminate cement. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Ma, S.; Shen, X.; Huang, Y.; Zhong, B. Preparation and formation mechanism of calcium sulphoaluminate. J. Chin. Ceram. Soc. 2008, 36, 78–81. [Google Scholar]
- Jiang, X.; Zhong, Z.; Liu, W. Influence of mineral admixture in CSA cement on chloride binding capacity. Earth Environ. Sci. 2019, 283, 012014. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Canonico, F. Behavior of specialty binders mixed with seawater. Adv. Eng. Mater. 2019, 8, 98–109. [Google Scholar] [CrossRef]
- Chang, J.; Yu, X.; Shang, X.; Zhao, J.; Zhang, Y. A reaction range for hydration of calcium sulfoaluminae with calcium sulfate and calcium hydroxide: Theory and experimental validation. Adv. Cem. Res. 2016, 28, 664–674. [Google Scholar] [CrossRef]
- Wang, C.; Song, M. Influence of water-cement ratio and type of mixing water on the early hydration performance of calcium sulphoaluminate (CSA) cement. Adv. Mater. Sci. Eng. 2021, 20, 5557763. [Google Scholar]
- Xu, L.; Liu, S.; Li, N.; Peng, Y.; Wu, K.; Wang, P. Retardation effect of elevated temperature on the setting of calcium sulfoaluminate cement clinker. Constr. Build. Mater. 2018, 178, 112–119. [Google Scholar] [CrossRef]
- Szelag, M. Mechano-physical properties and microstructure of carbon nanotube reinforced cement paste after thermal load. Nanomaterials 2017, 7, 267. [Google Scholar] [CrossRef]
- Pinson, M.B.; Masoero, E.; Bonnaud, P.A.; Manzano, H.; Ji, Q.; Yip, S.; Thomas, J.J.; Bazant, M.Z.; Van Vliet, K.J.; Jennings, H.M. Hysteresis from multiscale porosity: Modeling water sorption and shrinkage in cement paste. Phys. Rev. Appl. 2015, 3, 064009. [Google Scholar] [CrossRef]




| MgO | SiO2 | Al2O3 | SO3 | CaO | Fe2O3 | TiO2 |
|---|---|---|---|---|---|---|
| 1.85 | 6.71 | 25.52 | 12.02 | 45.96 | 4.35 | 1.36 |
| Fineness Gravity (m2/kg) | Setting Time (min) | Strength in 1 d (MPa) | Strength in 3 d (MPa) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Flexural | Compressive | Flexural | Compressive | |
| 408 | 15 | 30 | 6.3 | 35.8 | 6.6 | 45.8 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl |
|---|---|---|---|---|
| 24.36 | 5.12 | 4.02 | 1.16 | 0.69 |
| The Concentration of Seawater (nSW) | Initial Setting Time (min) | Final Setting Time (min) | (Final-Initial) Setting Time (min) | Improvement on Initial Setting Time (%) | Improvement on Final Setting time (%) |
|---|---|---|---|---|---|
| 0 | 13 | 18 | 5 | - | - |
| 1 | 16 | 21 | 5 | 23.0 | 16.6 |
| 2 | 22 | 30 | 8 | 69.2 | 66.6 |
| 3 | 32 | 42 | 10 | 146.1 | 133.3 |
| 4 | 47 | 57 | 10 | 261.5 | 216.6 |
| 5 | 51 | 63 | 12 | 292.3 | 250.0 |
| Specimen | Ettringite | Calcite | Belite | Ye’elimite | Anhydrite |
|---|---|---|---|---|---|
| Freshwater, 1 d | 45.5 | 25.4 | 26.4 | 2.7 | 0.0 |
| 1SW, 1 d | 46.4 | 26.1 | 23.6 | 2.5 | 1.4 |
| 5SW, 1 d | 42.0 | 27.2 | 25.7 | 3.2 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, Z.; Zhang, T.; Zhang, Y.; Liu, Z.; Zhao, X. Influence of the Concentration of Seawater on the Early Hydration Properties of Calcium Sulphoaluminate (CSA) Cement: A Preliminary Study. Buildings 2021, 11, 243. https://doi.org/10.3390/buildings11060243
Wang C, Liu Z, Zhang T, Zhang Y, Liu Z, Zhao X. Influence of the Concentration of Seawater on the Early Hydration Properties of Calcium Sulphoaluminate (CSA) Cement: A Preliminary Study. Buildings. 2021; 11(6):243. https://doi.org/10.3390/buildings11060243
Chicago/Turabian StyleWang, Chuanlin, Zeping Liu, Tengteng Zhang, Yuxuan Zhang, Zehong Liu, and Xianbo Zhao. 2021. "Influence of the Concentration of Seawater on the Early Hydration Properties of Calcium Sulphoaluminate (CSA) Cement: A Preliminary Study" Buildings 11, no. 6: 243. https://doi.org/10.3390/buildings11060243
APA StyleWang, C., Liu, Z., Zhang, T., Zhang, Y., Liu, Z., & Zhao, X. (2021). Influence of the Concentration of Seawater on the Early Hydration Properties of Calcium Sulphoaluminate (CSA) Cement: A Preliminary Study. Buildings, 11(6), 243. https://doi.org/10.3390/buildings11060243







