The Effect of Vibro-Activation Time on the Properties of Highly Active Calcium Hydroxide
Abstract
1. Introduction
2. Materials and Methods
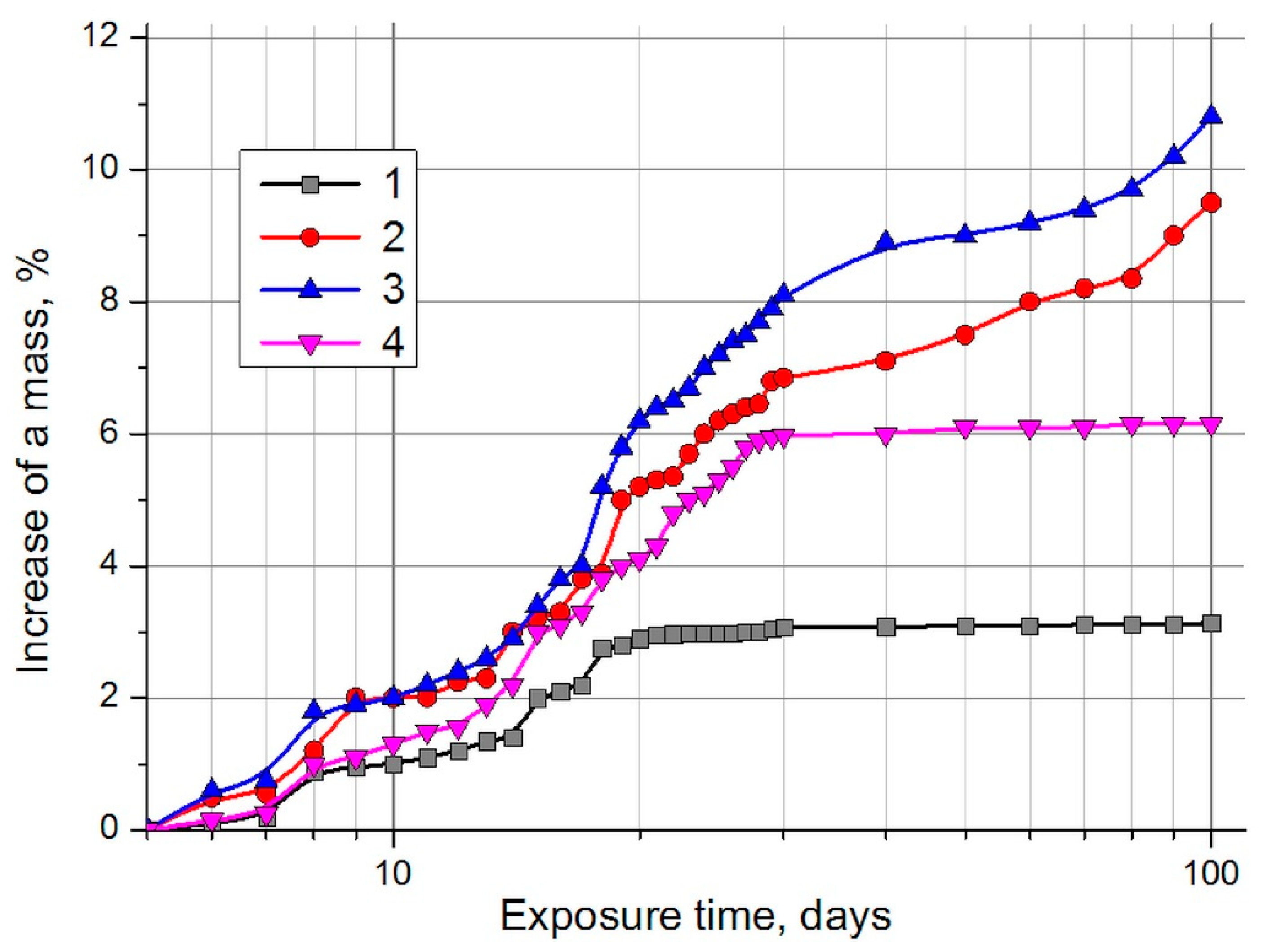
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Macchia, A.; Bettucci, O.; Gravagna, E.; Ferro, D.; Albini, R.; Mazzei, B.; Campanella, L. Calcium hydroxide nanoparticles and hypogeum environment: Test to understand the best way of application. J. Nanomater. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Samanta, A.; Chanda, D.K.; Das, P.S.; Ghosh, J.; Mukhopadhyay, A.K.; Dey, A. Synthesis of nano calcium hydroxide in aqueous medium. J. Am. Ceram. Soc. 2016, 99, 787–795. [Google Scholar] [CrossRef]
- Qin, C.; Yin, J.; An, H.; Liu, W.; Feng, B. Performance of extruded particles from calcium hydroxide and cement for CO2 capture. Energy Fuels 2012, 26, 154–161. [Google Scholar] [CrossRef]
- Yakymechko, Y.; Jaskulski, R.; Lutsyuk, I. New ways of utilizing lime in modern building technology. Mater. Struct. Technol. 2019, 2, 61–69. [Google Scholar] [CrossRef]
- Cazalla, O.; Rodriguez-Navarro, C.; Sebastian, E.; Cultrone, G.; Torre, M.J. Aging of lime putty: Effects on traditional lime mortar carbonation. J. Am. Ceram. Soc. 2004, 83, 1070–1076. [Google Scholar] [CrossRef]
- Lanzón, M.; Madrid, J.A.; Martínez-Arredondo, A.; Mónaco, S. Use of diluted Ca(OH)2 suspensions and their transformation into nanostructured CaCO3 coatings: A case study in strengthening heritage materials (stucco, adobe and stone). Appl. Surf. Sci. 2017, 424, 20–27. [Google Scholar] [CrossRef]
- Bhargavaramireddy, C.; Subramanian, K.S. Synthesis and properties of nano liming materials for reclamation of acid soils. J. Res. Angrau 2015, 43, 122–129. [Google Scholar]
- Bhargavaramireddy, C.; Subramanian, K.S. Nano-lime for remediation of soil acidity: Synthesis and characterization. Curr. Adv. Agric. Sci. Int. J. 2016, 8, 39. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; van Hees, R.; Veiga, R.; Santos Silva, A. Evaluation of the effectiveness and compatibility of nanolime consolidants with improved properties. Constr. Build. Mater. 2017, 142, 385–394. [Google Scholar] [CrossRef]
- Otero, J.; Starinieri, V.; Charola, A.E. Nanolime for the consolidation of lime mortars: A comparison of three available products. Constr. Build. Mater. 2018, 181, 394–407. [Google Scholar] [CrossRef]
- Sakellariou, K.G.; Criado, Y.A.; Tsongidis, N.I.; Karagiannakis, G.; Konstandopoulos, A.G. Multi-cyclic evaluation of composite CaO-based structured bodies for thermochemical heat storage via the CaO/Ca(OH)2 reaction scheme. Sol. Energy 2017, 146, 65–78. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Fiorucci, M.P.; Ramil, A.; López, A.J.; Rivas, T. Evaluation of the effectiveness of laser crust removal on granites by means of hyperspectral imaging techniques. Appl. Surf. Sci. 2015, 347, 832–838. [Google Scholar] [CrossRef]
- López-Arce, P.; Gomez-Villalba, L.S.; Pinho, L.; Fernández-Valle, M.E.; de Buergo, M.Á.; Fort, R. Influence of porosity and relative humidity on consolidation of dolostone with calcium hydroxide nanoparticles: Effectiveness assessment with non-destructive techniques. Mater. Charact. 2010, 61, 168–184. [Google Scholar] [CrossRef]
- Bastone, S.; Chillura Martino, D.F.; Renda, V.; Saladino, M.L.; Poggi, G.; Caponetti, E. Alcoholic nanolime dispersion obtained by the insolubilisation-precipitation method and its application for the deacidification of ancient paper. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 241–249. [Google Scholar] [CrossRef]
- Taglieri, G.; Mondelli, C.; Daniele, V.; Pusceddu, E.; Trapananti, A. Synthesis and X-ray diffraction analyses of calcium hydroxide nanoparticles in aqueous suspension. Adv. Mater. Phys. Chem. 2013, 3, 108–112. [Google Scholar] [CrossRef]
- Taglieri, G.; Mondelli, C.; Daniele, V.; Pusceddu, E.; Scoccia, G. Synthesis, textural and structural properties of calcium hydroxide nanoparticles in hydro-alcoholic suspension. Adv. Mater. Phys. Chem. 2014, 4, 50–59. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, Y.; Zhang, X.; Zhang, T.; Zhang, T.; Li, X. Synthesis and characterization of calcium hydroxide nanoparticles by hydrogen plasma-metal reaction method. Mater. Lett. 2010, 64, 2575–2577. [Google Scholar] [CrossRef]
- Madrid, J.A.; Lanzón, M. Synthesis and morphological examination of high-purity Ca(OH)2 nanoparticles suitable to consolidate porous surfaces. Appl. Surf. Sci. 2017, 424, 2–8. [Google Scholar] [CrossRef]
- Martínez-Ramírez, S.; Higueruela, L.R.; Cascales, I.; Martín-Garrido, M.; Blanco-Varela, M.T. New approach to nanolime synthesis at ambient temperature. SN Appl. Sci. 2019, 1, 105. [Google Scholar] [CrossRef]
- Salvadori, B.; Dei, L. Synthesis of Ca(OH)2 nanoparticles from diols. Langmuir 2001, 17, 2371–2374. [Google Scholar] [CrossRef]
- Darroudi, M.; Bagherpour, M.; Hosseini, H.A.; Ebrahimi, M. Biopolymer-assisted green synthesis and characterization of calcium hydroxide nanoparticles. Ceram. Int. 2016, 42, 3816–3819. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Taufiq-Yap, Y.H.; Lee, H.V. Synthesis of clamshell derived Ca(OH) 2 nano-particles via simple surfactant-hydration treatment. Chem. Eng. J. 2015, 262, 1043–1051. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Claveau, D.; Corcuff, R.; Belkacemi, K.; Arul, J. Preparation of nano-CaO using thermal-decomposition method. Mater. Lett. 2008, 62, 2096–2098. [Google Scholar] [CrossRef]
- Amin Alavi, M.; Morsali, A. Ultrasonic-assisted synthesis of Ca(OH)2 and CaO nanostructures. J. Exp. Nanosci. 2010, 5, 93–105. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Synthesis of Ca(OH)2 nanoparticles by wet chemical method. Micro Nano Lett. 2010, 5, 131. [Google Scholar] [CrossRef]
- Saoud, K.M.; Ibala, I.; El Ladki, D.; Ezzeldeen, O.; Saeed, S. Microwave assisted preparation of calcium hydroxide and barium hydroxide nanoparticles and their application for conservation of cultural heritage. In Digital Heritage. Progress in Cultural Heritage: Documentation, Preservation, and Protection; Lecture Notes in Computer Science; Ioannides, M., Magnenat-Thalmann, N., Fink, E., Žarnić, R., Yen, A.-Y., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 342–352. ISBN 978-3-319-13694-3. [Google Scholar]
- Kilic, S.; Toprak, G.; Ozdemir, E. Stability of CaCO3 in Ca(OH)2 solution. Int. J. Miner. Process. 2016, 147, 1–9. [Google Scholar] [CrossRef]
- Nicoleau, L.; Nonat, A. A new view on the kinetics of tricalcium silicate hydration. Cem. Concr. Res. 2016, 86, 1–11. [Google Scholar] [CrossRef]
- Galmarini, S.; Bowen, P. Atomistic simulation of the adsorption of calcium and hydroxyl ions onto portlandite surfaces—Towards crystal growth mechanisms. Cem. Concr. Res. 2016, 81, 16–23. [Google Scholar] [CrossRef]
- Van Balen, K.; Van Gemert, D. Modelling lime mortar carbonation. Mater. Struct. 1994, 27, 393–398. [Google Scholar] [CrossRef]
- Van Balen, K. Carbonation reaction of lime, kinetics at ambient temperature. Cem. Concr. Res. 2005, 35, 647–657. [Google Scholar] [CrossRef]


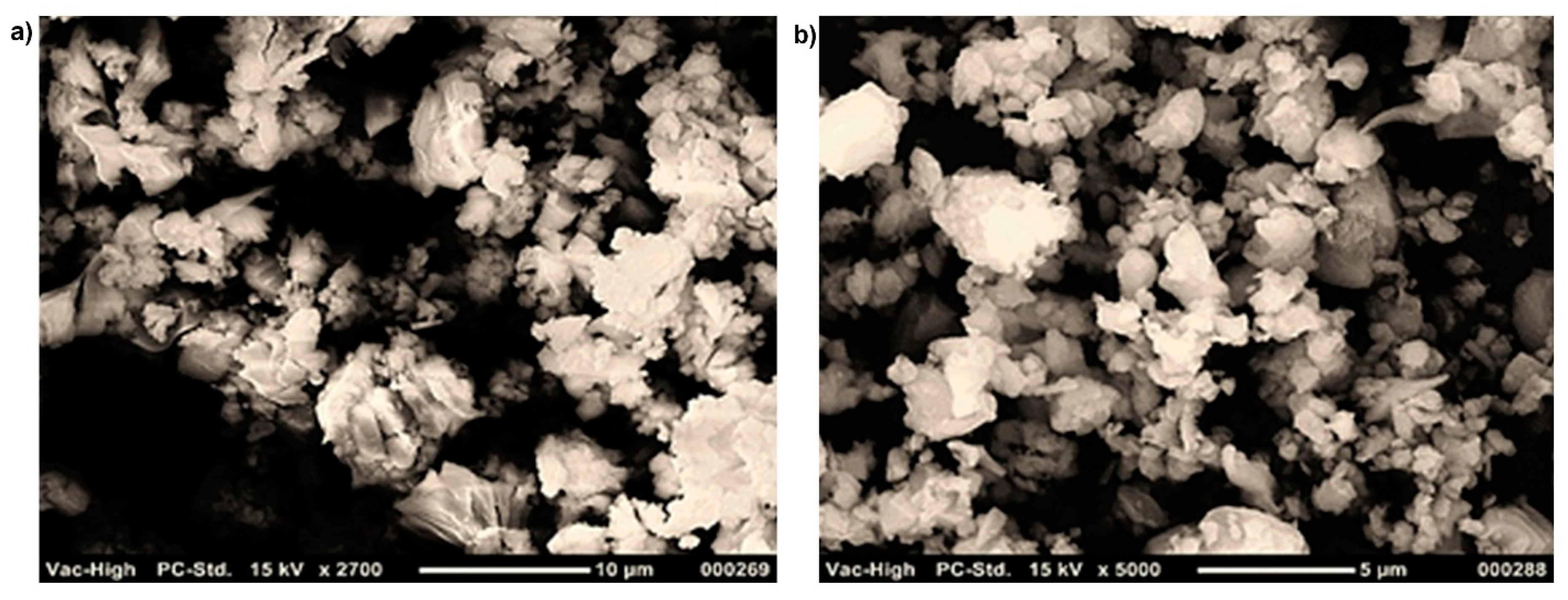
| Material | Specific Surface, (m2/kg) | Particle Content, %, in Range (µm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 500–1000 | 200–500 | 90–200 | 63–90 | 45–63 | 32–45 | 0.1–32 | ||
| Quicklime | 282.0 | 0.01 | 9.76 | 31.93 | 13.42 | 9.59 | 7.14 | 28.15 |
| Hydrated lime | 522.5 | 0.00 | 0.00 | 5.28 | 8.59 | 8.84 | 7.76 | 69.53 |
| Type of Lime | Fraction Content (%) | Estimated Volume of Average Diameter (µm) | Effective Diameters (µm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.5 µm | <1 µm | <5 µm | <10 µm | <20 µm | <60 µm | D [3; 2] | D [4;3] | d10 | d50 | d90 | |
| Quicklime | 1.95 | 2.99 | 9.58 | 14.6 | 21.4 | 46.6 | 6.63 | 89.7 | 5.36 | 73.0 | 198 |
| Hydrated | - | 1.26 | 39.4 | 52.5 | 63.67 | 89.5 | 4.95 | 25.4 | 1.88 | 9.31 | 73.1 |
| Activated for 10 min | - | 2.94 | 46.4 | 59.4 | 70.3 | 93.7 | 3.93 | 18.6 | 1.56 | 6.27 | 55.6 |
| Activated for 15 min | - | 3.23 | 40.7 | 54.0 | 65.8 | 86.4 | 3.80 | 18.6 | 1.53 | 5.86 | 53.7 |
| Activated for 20 min | 0.23 | 7.57 | 48.3 | 64.1 | 78.6 | 100 | 3.24 | 11.7 | 1.27 | 5.61 | 31.9 |
| Activated for 45 min | 0.14 | 6.06 | 50.1 | 60.5 | 70.6 | 91.8 | 3.38 | 20.3 | 1.35 | 5.18 | 59.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakymechko, Y.; Lutsyuk, I.; Jaskulski, R.; Dulnik, J.; Kropyvnytska, T. The Effect of Vibro-Activation Time on the Properties of Highly Active Calcium Hydroxide. Buildings 2020, 10, 111. https://doi.org/10.3390/buildings10060111
Yakymechko Y, Lutsyuk I, Jaskulski R, Dulnik J, Kropyvnytska T. The Effect of Vibro-Activation Time on the Properties of Highly Active Calcium Hydroxide. Buildings. 2020; 10(6):111. https://doi.org/10.3390/buildings10060111
Chicago/Turabian StyleYakymechko, Yaroslav, Iryna Lutsyuk, Roman Jaskulski, Judyta Dulnik, and Tetyana Kropyvnytska. 2020. "The Effect of Vibro-Activation Time on the Properties of Highly Active Calcium Hydroxide" Buildings 10, no. 6: 111. https://doi.org/10.3390/buildings10060111
APA StyleYakymechko, Y., Lutsyuk, I., Jaskulski, R., Dulnik, J., & Kropyvnytska, T. (2020). The Effect of Vibro-Activation Time on the Properties of Highly Active Calcium Hydroxide. Buildings, 10(6), 111. https://doi.org/10.3390/buildings10060111






