Kinetics of MgO Reduction in CaO-Al2O3-MgO Slag by Al in Liquid Fe
Abstract
:1. Introduction
2. Model Description
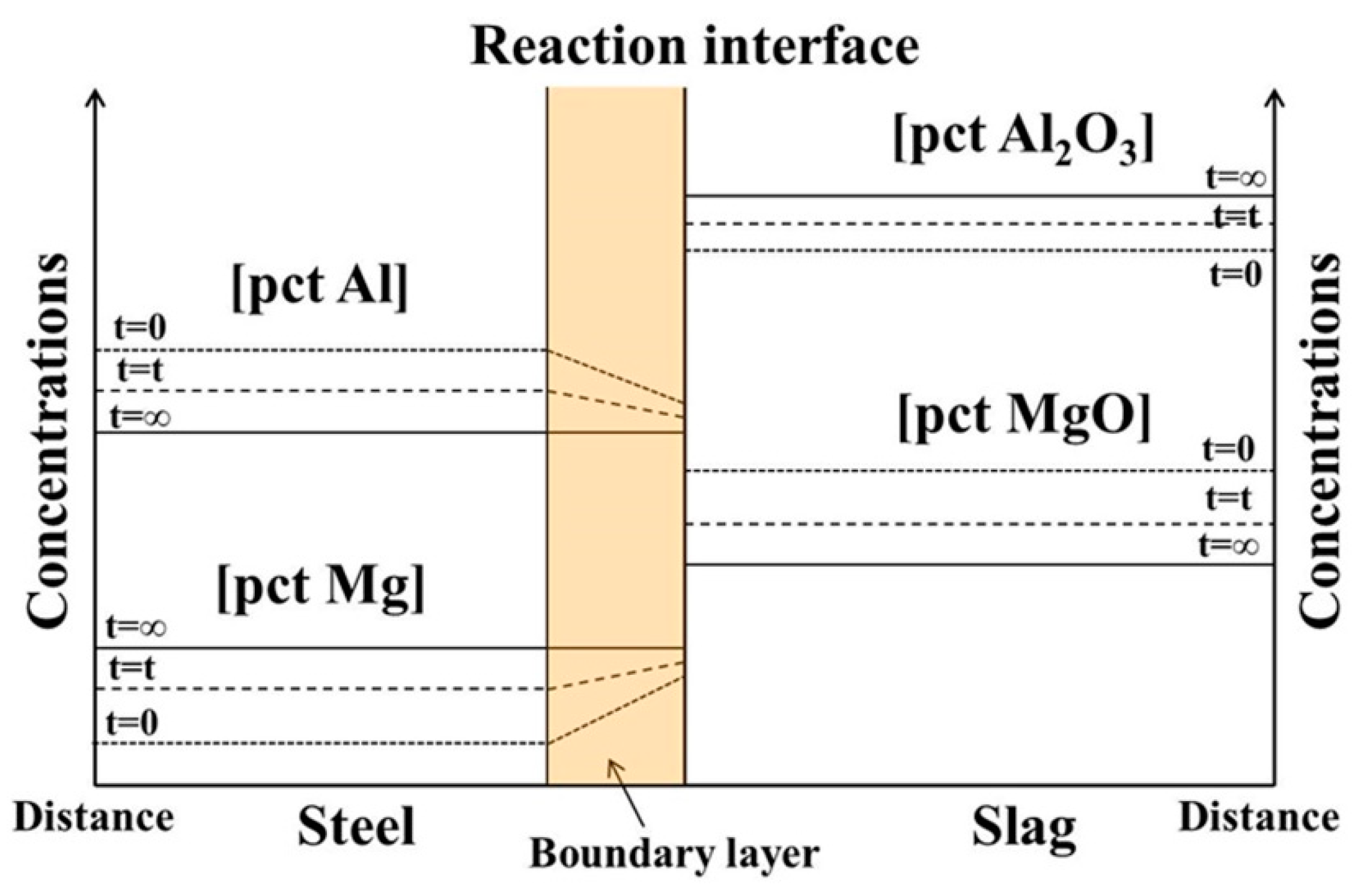
2.1. Single Control Model
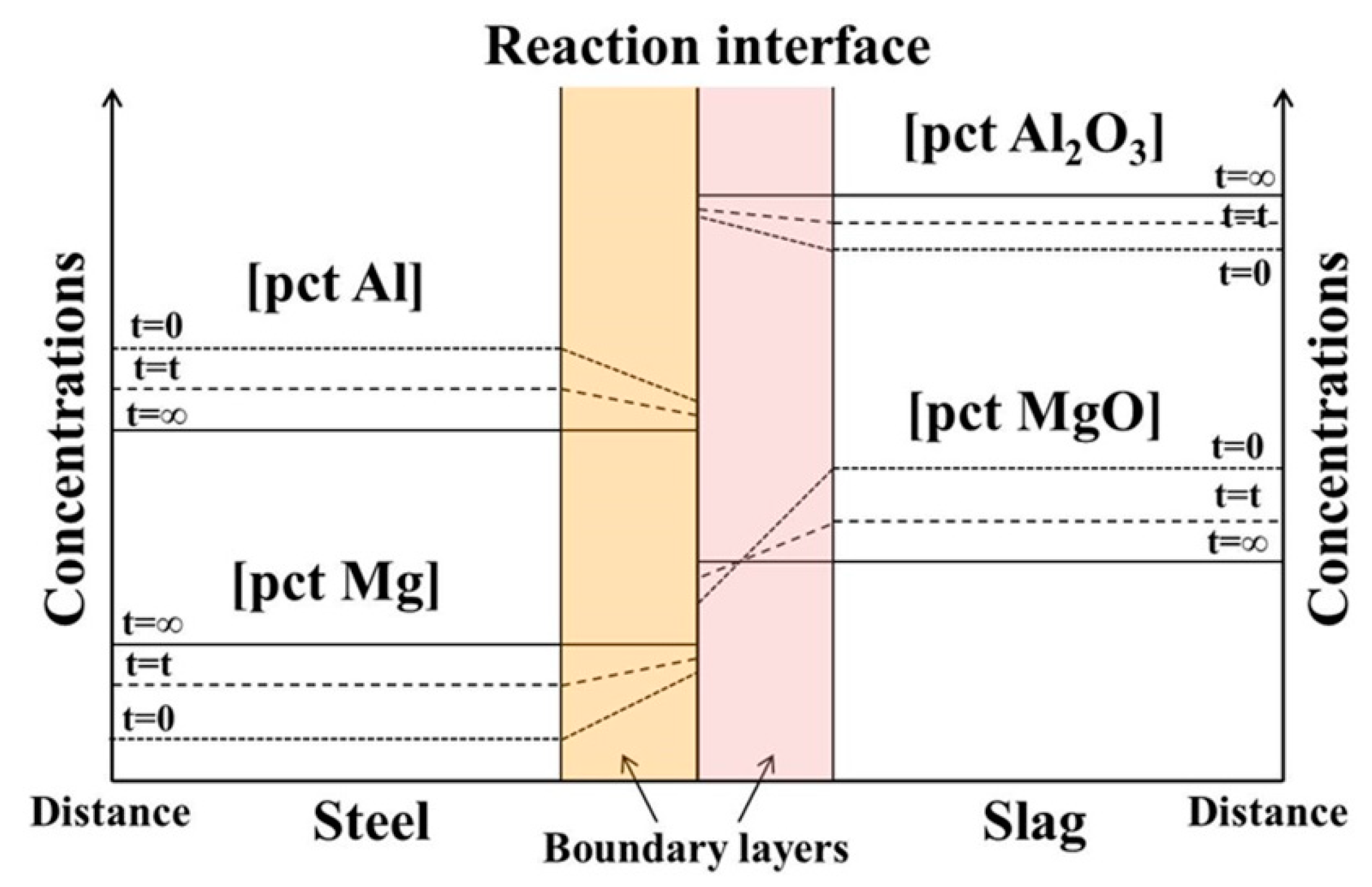
2.2. Mixed Control Model
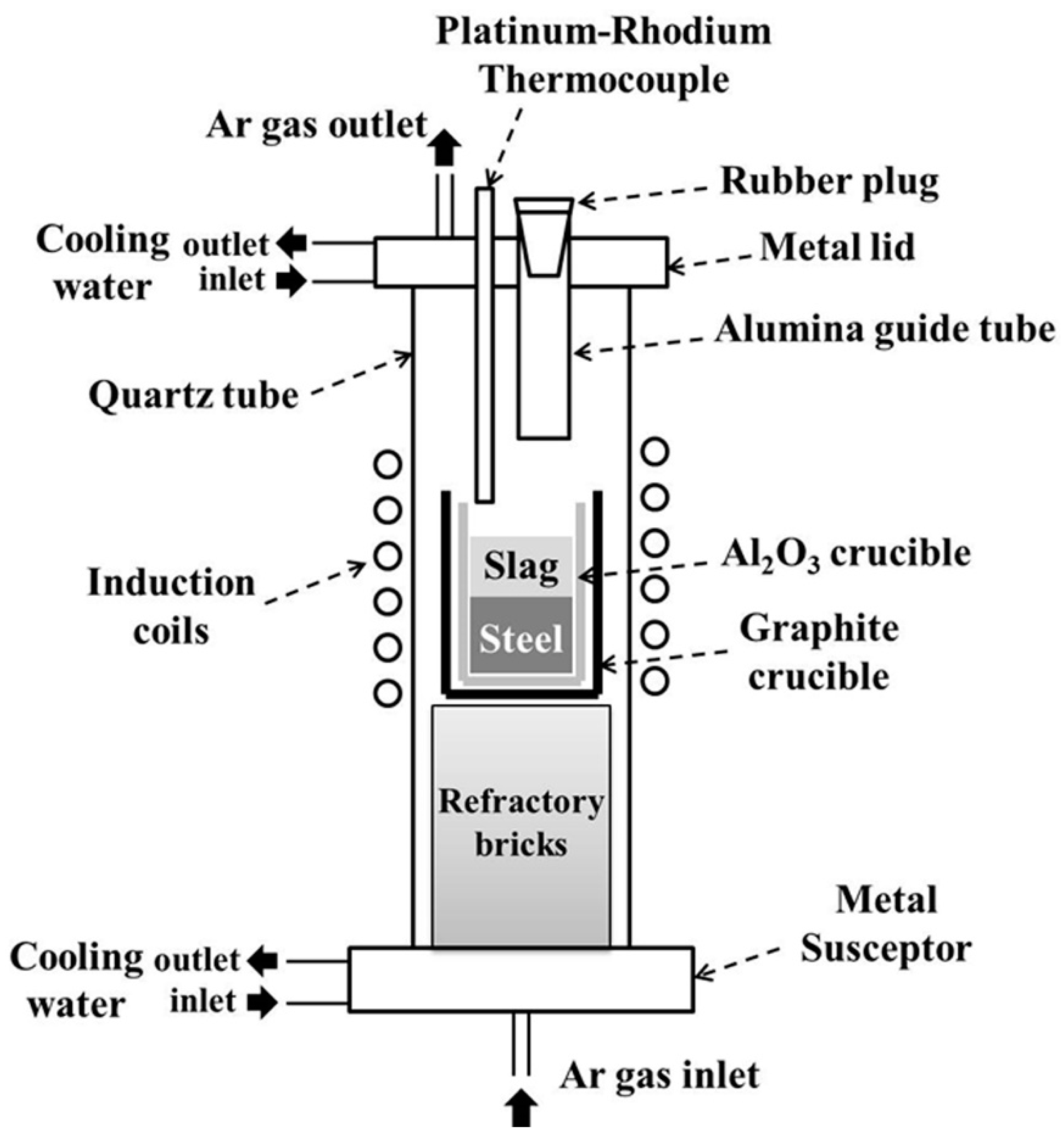
3. Experimental Method
4. Results and Discussion
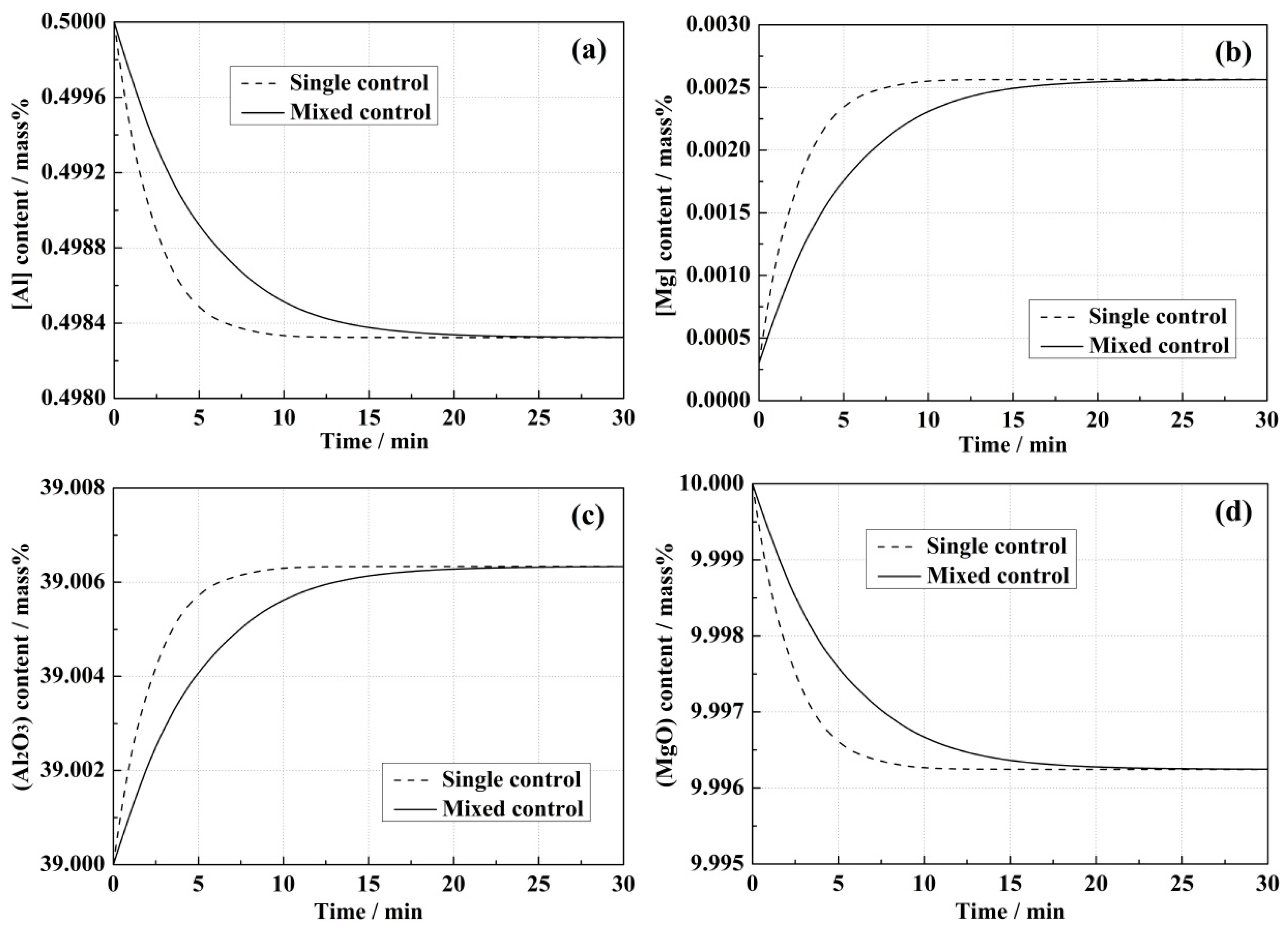
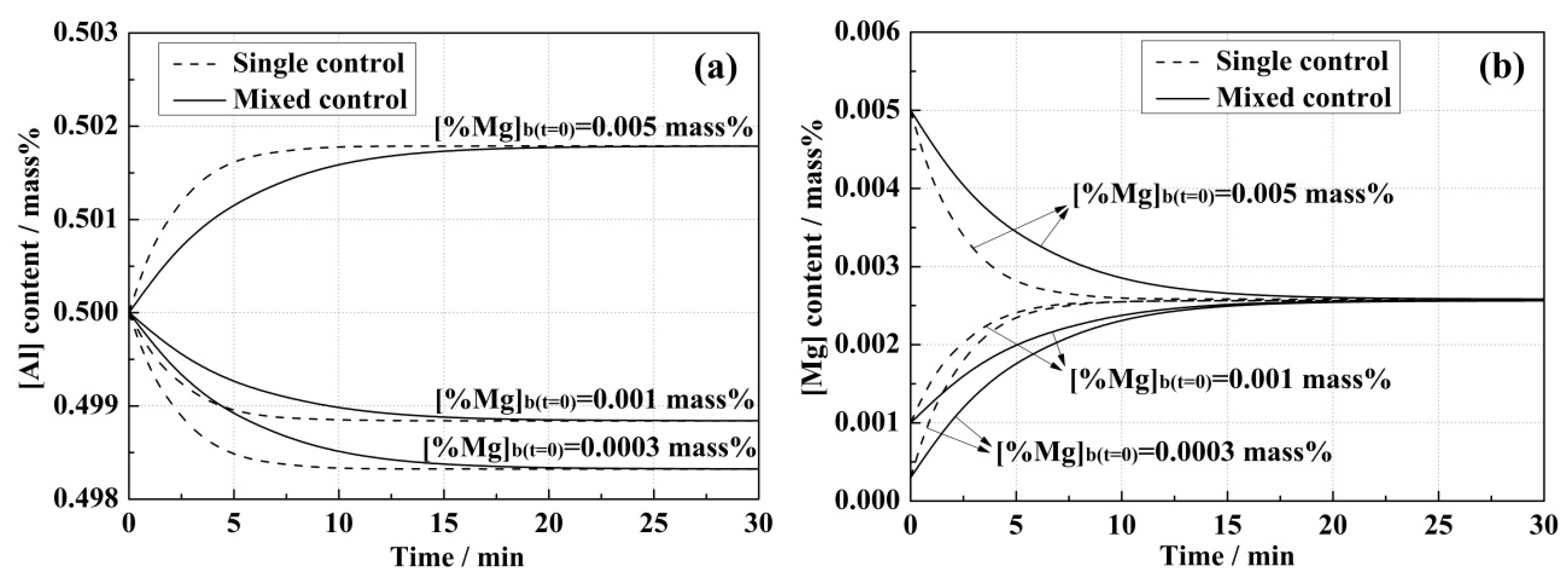
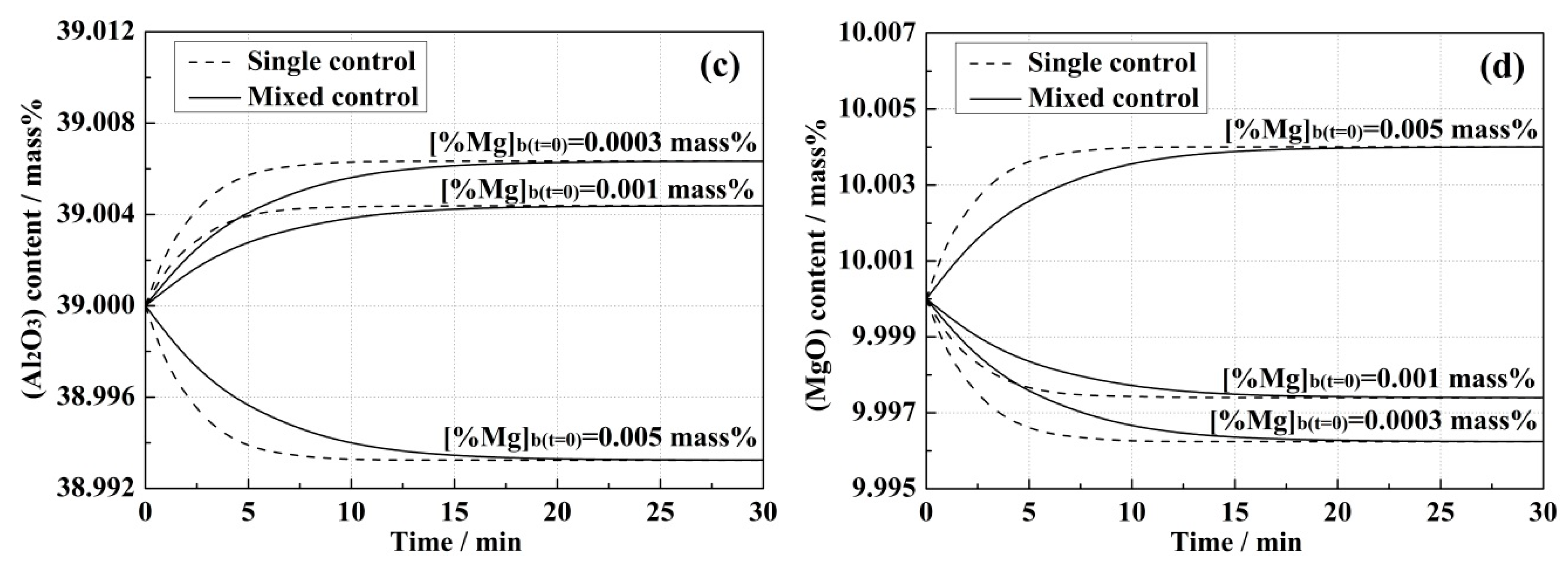
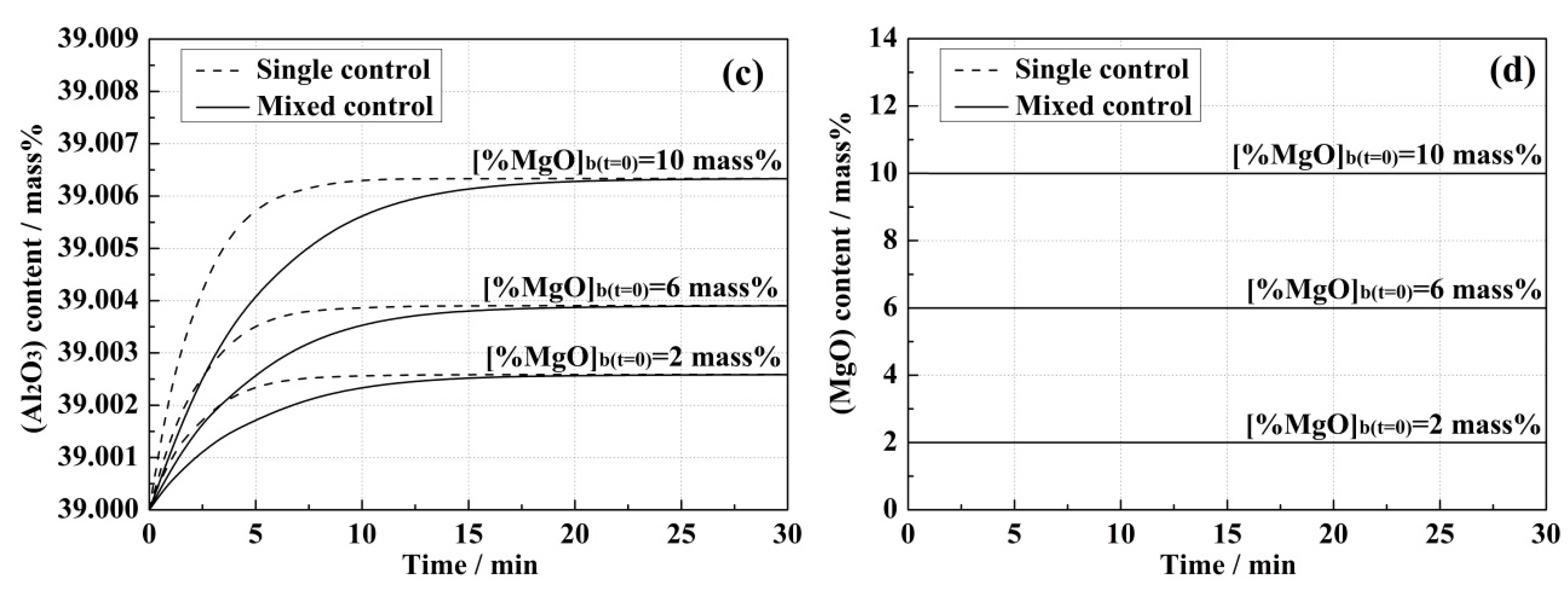
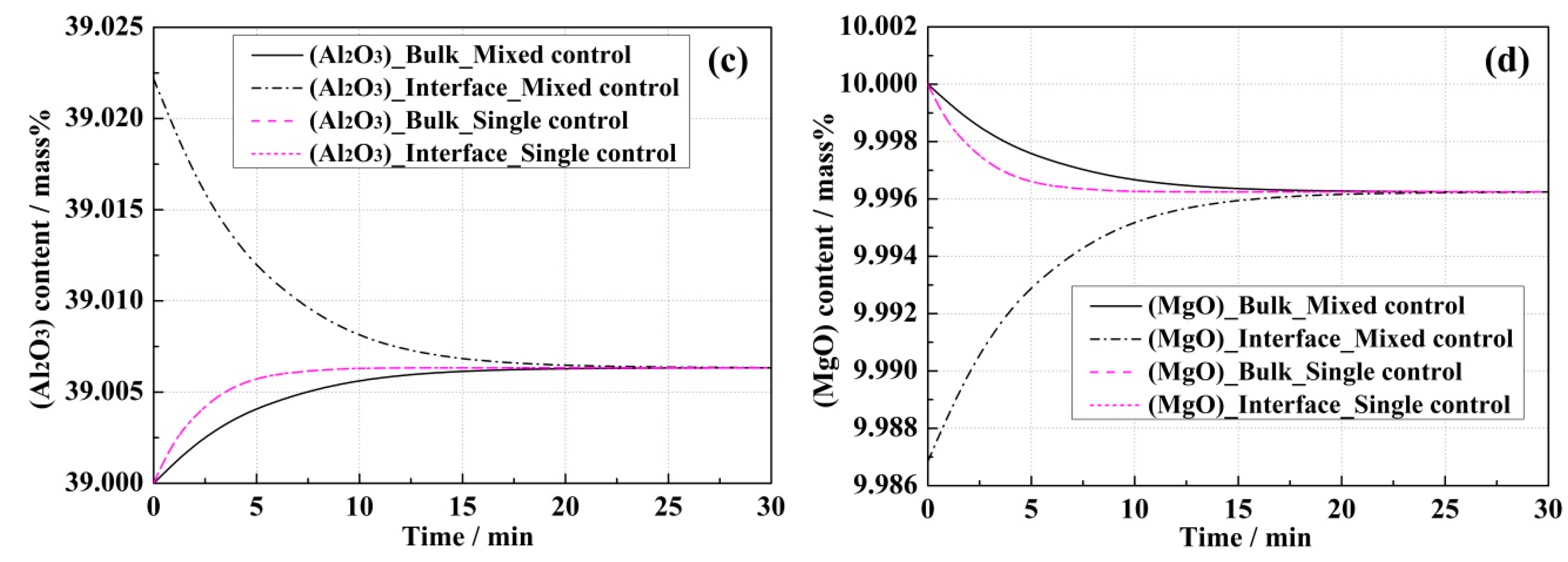
4.1. Model Predictions and Effect of Correlative Condition
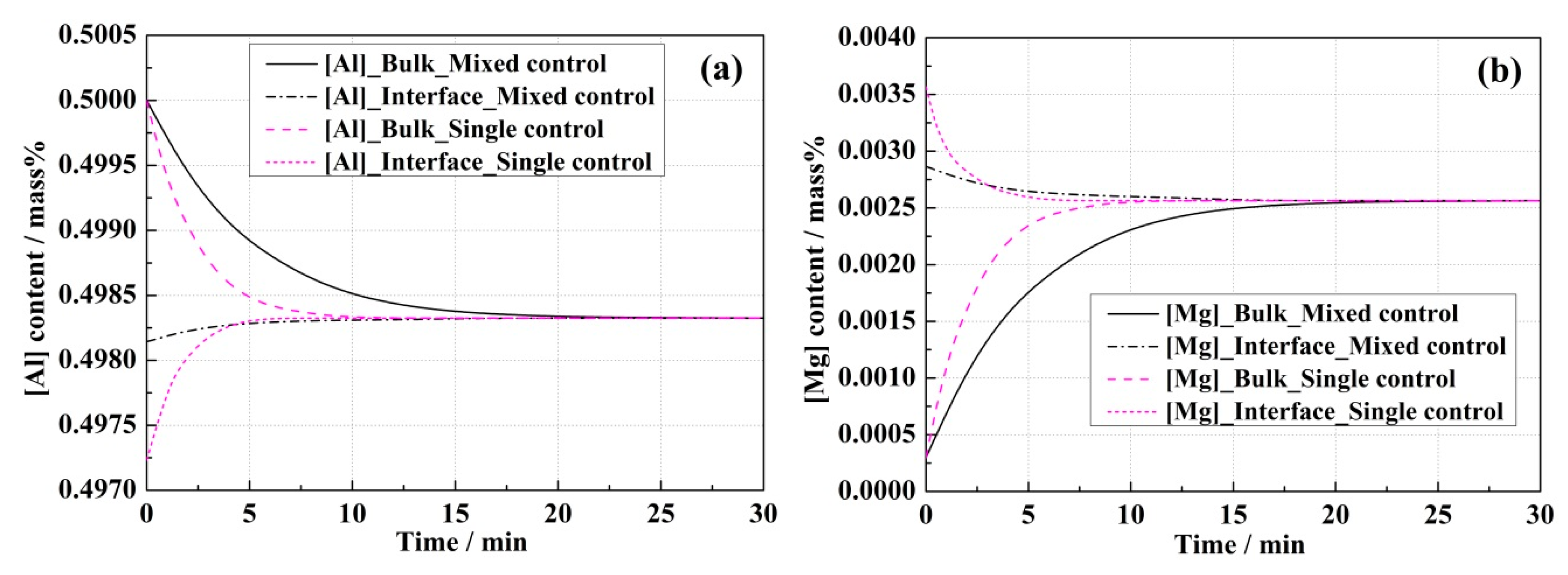
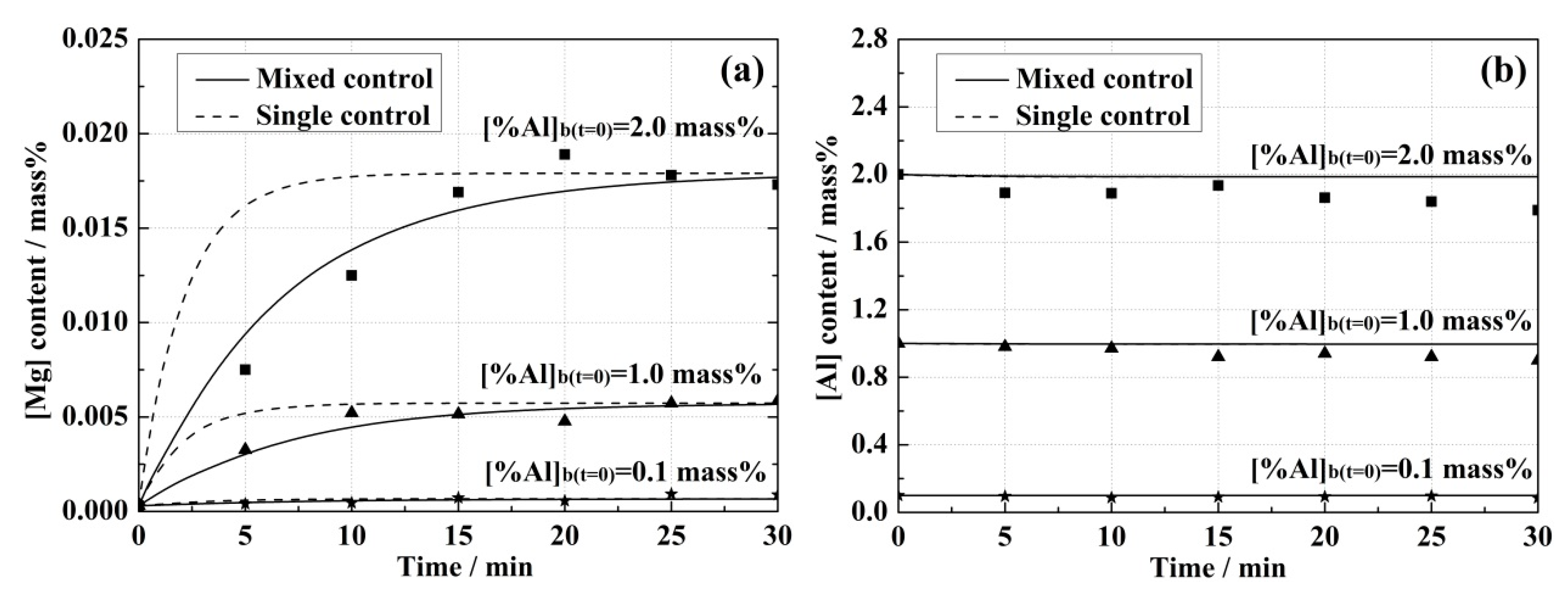
4.2. Comparison Analysis of Experiment and Model Results
5. Conclusions
- Due to the slow mass transport in the slag, the reaction rate in the mixed control model was always lower than that in the single control model.
- Higher initial [Al] content in the steel and (MgO) content in the slag led to the increase of equilibrium concentrations of [Mg] in the steel and (Al2O3) in the slag, and also improved the reaction rates in both kinetic models.
- In the single control model, (Al2O3) and (MgO) contents in the bulk slag were the same as those at the steel–slag interface, while differences between the bulk and interface concentrations of (Al2O3) and (MgO) were observed in the mixed control model, which resulted in the mass transportation of the slag components being an extra rate-limiting step.
- The mass transfer coefficient of [Mg] in the steel was computed to be 6.2 × 10−5 m·s−1 according to the experimental equilibrium results between an Fe-1.0 mass% Al steel and 51 mass% CaO-39 mass% Al2O3-10 mass% MgO slag at 1873 K (1600 °C). Using this value, the mixed control model was validated at different initial Al levels in the steels.
- Experimental results with the model predictions confirmed that the mass transport in the slag should be given full consideration during the kinetics analysis of MgO reduction reaction.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aydin, H.; Essadiqi, E.; Jung, I.H.; Yue, S. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A 2013, 564, 501–508. [Google Scholar] [CrossRef]
- Khan, M.I.; Kuntz, M.L.; Biro, E.; Zhou, Y. Microstructure and mechanical properties of resistance spot welded advanced high strength steels. Mater. Trans. 2008, 49, 1629–1637. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Tian, J.Y.; Hu, H.J.; Yuan, Q. Bainitic transformation and properties of low carbon carbide-free bainitic steels with Cr addition. Metals 2017, 7, 263. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, K.M.; Zhong, L.; Zhang, C.; Hou, T.P.; Misra, R.D.K. A comparative study on the dynamic tensile behavior of nanostructured bainitic and quenched-tempered martensitic steels. Metals 2018, 8, 728. [Google Scholar] [CrossRef]
- Guo, J.; Seo, M.D.; Shi, C.B.; Cho, J.W.; Kim, S.H. Control of crystal morphology for mold flux during high-aluminum AHSS continuous casting process. Metall. Mater. Trans. B 2016, 47, 2211–2221. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking, 1st ed.; Tohoku University Press: Sendai, Japan, 2010; pp. 10–24, 74, 82. [Google Scholar]
- Liu, C.; Huang, F.; Suo, J.; Wang, X. Effect of magnesia-carbon refractory on the kinetics of MgO·Al2O3 spinel inclusion generation in extra-low oxygen steels. Metall. Mater. Trans. B 2016, 47, 989–998. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, M.; Sichen, D. Effect of refractory on nonmetallic inclusions in Al-killed steel. Metall. Mater. Trans. B 2016, 47, 3158–3167. [Google Scholar] [CrossRef]
- Mu, H.Y.; Zhang, T.S.; Fruehan, R.J.; Webler, B.A. Reduction of CaO and MgO slag components by Al in liquid Fe. Metall. Mater. Trans. B 2018, 49, 1665–1674. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M. Modification of spinel inclusions by calcium in liquid steel. Iron Steel Technol. 2010, 7, 189–197. [Google Scholar]
- Okuyama, G.; Yamaguchi, K.; Takeuchi, S.; Sorimachi, K. Effect of slag composition on the kinetics of formation of Al2O3–MgO inclusions in aluminum killed ferritic stainless steel. ISIJ Int. 2000, 40, 121–128. [Google Scholar] [CrossRef]
- Kumar, D.; Pistorius, P.C. Rate of MgO pickup in alumina inclusions in aluminum-killed steel. Metall. Mater. Trans. B 2019, 50, 181–191. [Google Scholar] [CrossRef]
- Robertson, D.G.C.; Deo, B.; Ohguchi, S. A multicomponent mixed transport control theory for the kinetics of coupled slag/metal and slag/metal/gas reactions: Application to de sulphurization of molten iron. Ironmak. Steelmak. 1984, 11, 41–55. [Google Scholar]
- Rhamdhani, M.A.; Brooks, G.A.; Coley, K.S. Kinetics of metal/slag reactions during spontaneous emulsification. Metall. Mater. Trans. B 2005, 36, 219–227. [Google Scholar] [CrossRef]
- Harada, A.; Maruoka, N.; Shibata, H.; Kitamura, S.Y. A kinetic model to predict the compositions of metal, slag and inclusions during ladle refining: Part 1. Basic concept and application. ISIJ Int. 2013, 53, 2110–2117. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban-ya, S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Metall. Mater. Trans. B 1997, 28, 953–956. [Google Scholar] [CrossRef]
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 spinel inclusions in stainless steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef]
- Richardson, F.D.; Jeffes, J.H.E. The thermodynamics of substances of interest in iron and steel making. III–sulphides. J. Iron Steel Inst. 1952, 171, 165–175. [Google Scholar]
- Alcock, J.C.B.; Easterbrook, E. Aluminum and Aluminum Alloy. In Corrosion, 1st ed.; Butterworth-Heinemann: London, UK, 1994; pp. 3–37. [Google Scholar]
- Ohta, H.; Suito, H. Activities in CaO-MgO-Al2O3 slags and deoxidation equilibria of Al, Mg, and Ca. ISIJ Int. 1996, 36, 983–990. [Google Scholar] [CrossRef]
- Harada, A.; Miyano, G.; Maruoka, N.; Shibata, H.; Kitamura, S. Dissolution behavior of Mg from MgO into molten steel deoxidized by Al. ISIJ Int. 2014, 54, 2230–2238. [Google Scholar] [CrossRef]
- Kitamura, S.; Kitamura, T.; Shibata, K.; Mizukami, Y.; Mukawa, S.; Nagakawa, J. Effect of stirring energy, temperature and flux composition on hot metal dephosphorization kinetics. ISIJ Int. 1991, 31, 1322–1328. [Google Scholar] [CrossRef]
- Park, J.W.; Sridhar, S.; Fruehan, R.J. Kinetics of reduction of SiO2 in SiO2-Al2O3-CaO slags by Al in Fe-Al(-Si) melts. Metall. Mater. Trans. B 2014, 45, 1380–1388. [Google Scholar] [CrossRef]
- Monaghan, B.J.; Nightingale, S.A.; Chen, L.; Brooks, G.A. The Dissolution Behavior of Selected Oxides in CaO-SiO2-Al2O3 Liquid Oxides. In Proceedings of the VII International Conference Molten Slags, Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004; The South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2004. [Google Scholar]
- Elizaveta, C. Dissolution Kinetics of CaO and MgO in Converter Steelmaking Slags. Ph.D. Thesis, University of Leoben, Leoben, Styria, Austria, 2016. [Google Scholar]
- Huang, X.H. Mechanism of Ferrous Metallurgy, 4th ed.; Metallurgical Industry Press: Beijing, China, 2013; pp. 120–122. [Google Scholar]
- Vu-Bac, N.; Lahmer, T.; Zhuang, X.; Nguyen-Thoi, T.; Rabczuk, T. A software framework for probabilistic sensitivity analysis for computationally expensive models. Adv. Eng. Softw. 2016, 100, 19–31. [Google Scholar] [CrossRef]
- Hamdia, M.K.; Ghasemi, H.; Zhuang, X.Y.; Alajlan, N.; Rabczuk, T. Sensitivity and uncertainty analysis for flexoelectric nanostructures. Comput. Methods Appl. Mech. Eng. 2018, 337, 95–109. [Google Scholar] [CrossRef]


| No. | Chemical Compositions of the Steels/Mass% | Slag Composition/Mass% | |||||
|---|---|---|---|---|---|---|---|
| C | Al | S/ppm | Mg/ppm | CaO | Al2O3 | MgO | |
| 1 | 5 | 0.1 | 10 | 3 | 51 | 39 | 10 |
| 2 | 5 | 1 | 10 | 3 | |||
| 3 | 5 | 2 | 10 | 3 | |||
| Model Parameter | Value | Model Parameter | Value |
|---|---|---|---|
| [%Al]b (t = 0) | 0.5 mass% | Mass transfer coefficient of Al in steel, | 1 × 10−4 m·s−1 [21] |
| [%Mg]b (t = 0) | 3 × 10−4 mass% | Mass transfer coefficient of Mg in steel, | 1 × 10−4 m·s−1 [21] |
| [%Al2O3]b (t = 0) | 39.0 mass% | Mass transfer coefficient of Al2O3 in slag, | 1 × 10−5 m·s−1 [13,22] |
| [%MgO]b (t = 0) | 10.0 mass% | Mass transfer coefficient of MgO in slag, | 1 × 10−5 m·s−1 [13,22] |
| [%CaO]b (t = 0) | Balanced | Experiment temperature, | 1873 K (1600 °C) |
| −0.13 [6] | Reaction area between the steel and slag, A | π·(0.03)2 m2 | |
| 0.043 [6] | Density of steel, | 7000 kg·m−3 | |
| 0.27 [6] | Density of slag, | 3000 kg·m−3 | |
| 0 [6] | Weight of steel, | 500 g | |
| K (1873 K) | 9.74 × 10−11 [6] | Weight of slag, | 75 g |
| Time/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|---|
| [%Mg]b/ppm | 3 | 32.6 | 52.2 | 51.5 | 47.7 | 57.3 | 58.5 |
| [%Al]b/mass% | 1.0 | 0.98 | 0.97 | 0.92 | 0.94 | 0.92 | 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, X.; Yang, X.; Zhang, H.; Zhong, M. Kinetics of MgO Reduction in CaO-Al2O3-MgO Slag by Al in Liquid Fe. Metals 2019, 9, 998. https://doi.org/10.3390/met9090998
Liu C, Liu X, Yang X, Zhang H, Zhong M. Kinetics of MgO Reduction in CaO-Al2O3-MgO Slag by Al in Liquid Fe. Metals. 2019; 9(9):998. https://doi.org/10.3390/met9090998
Chicago/Turabian StyleLiu, Chengsong, Xiaoqin Liu, Xiaoliu Yang, Hua Zhang, and Ming Zhong. 2019. "Kinetics of MgO Reduction in CaO-Al2O3-MgO Slag by Al in Liquid Fe" Metals 9, no. 9: 998. https://doi.org/10.3390/met9090998
APA StyleLiu, C., Liu, X., Yang, X., Zhang, H., & Zhong, M. (2019). Kinetics of MgO Reduction in CaO-Al2O3-MgO Slag by Al in Liquid Fe. Metals, 9(9), 998. https://doi.org/10.3390/met9090998





