Effects of Solid Content and Substrate Concentration on Bioleaching of Heavy Metals from Sewage Sludge Using Aspergillus niger
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sludge Sampling
2.2. Fungal Strains and Inoculum Preparation
2.3. Fungal Bioleaching Experiments
3. Results and Discussion
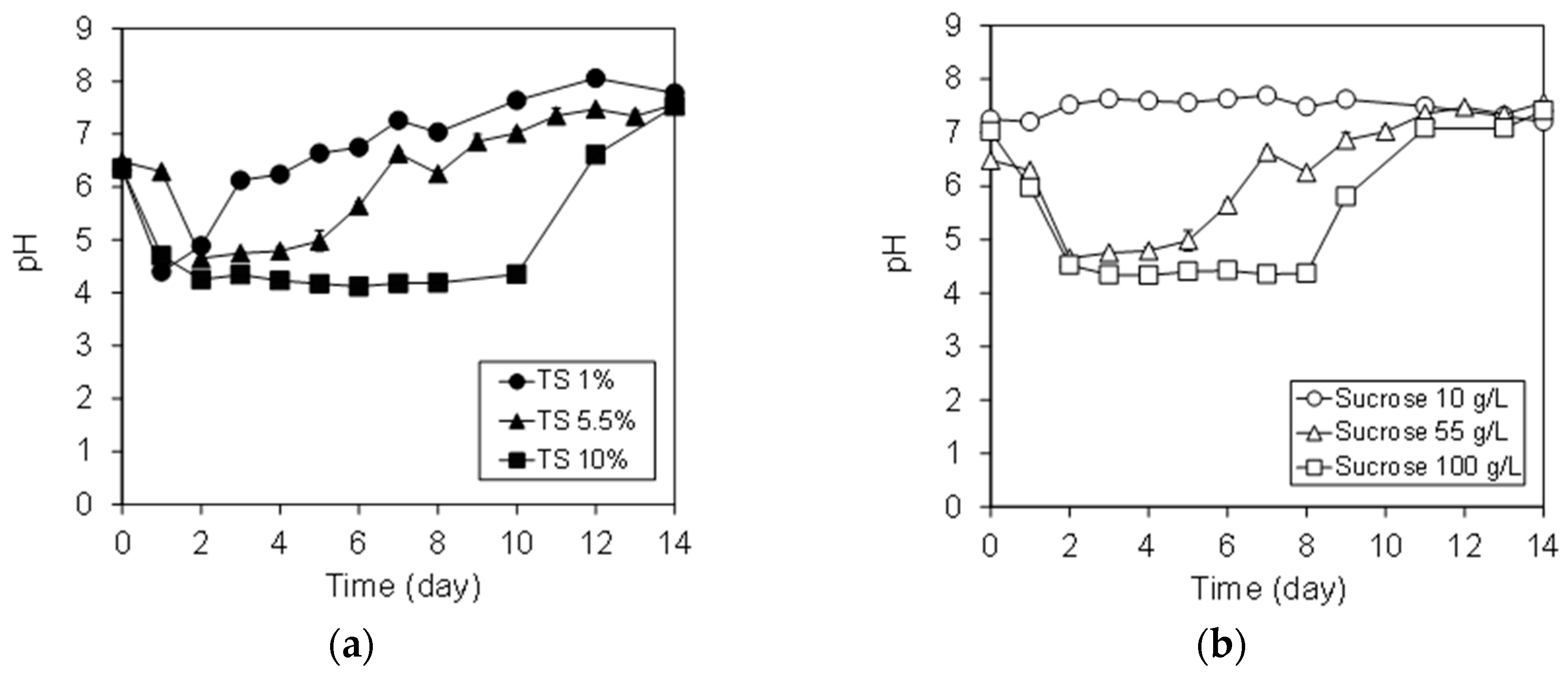
3.1. Variation of pH
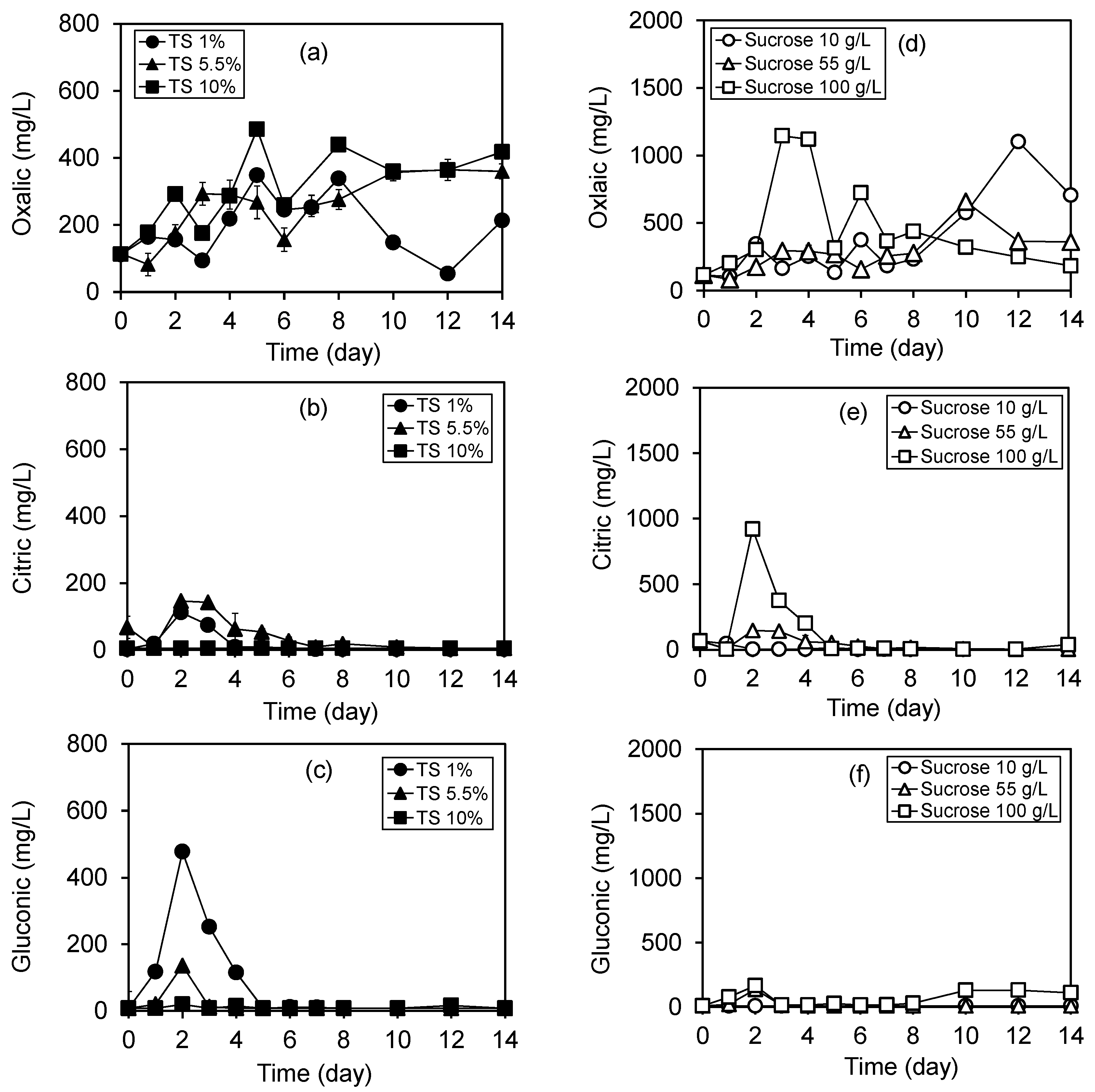
3.2. Variation of Organic Acids
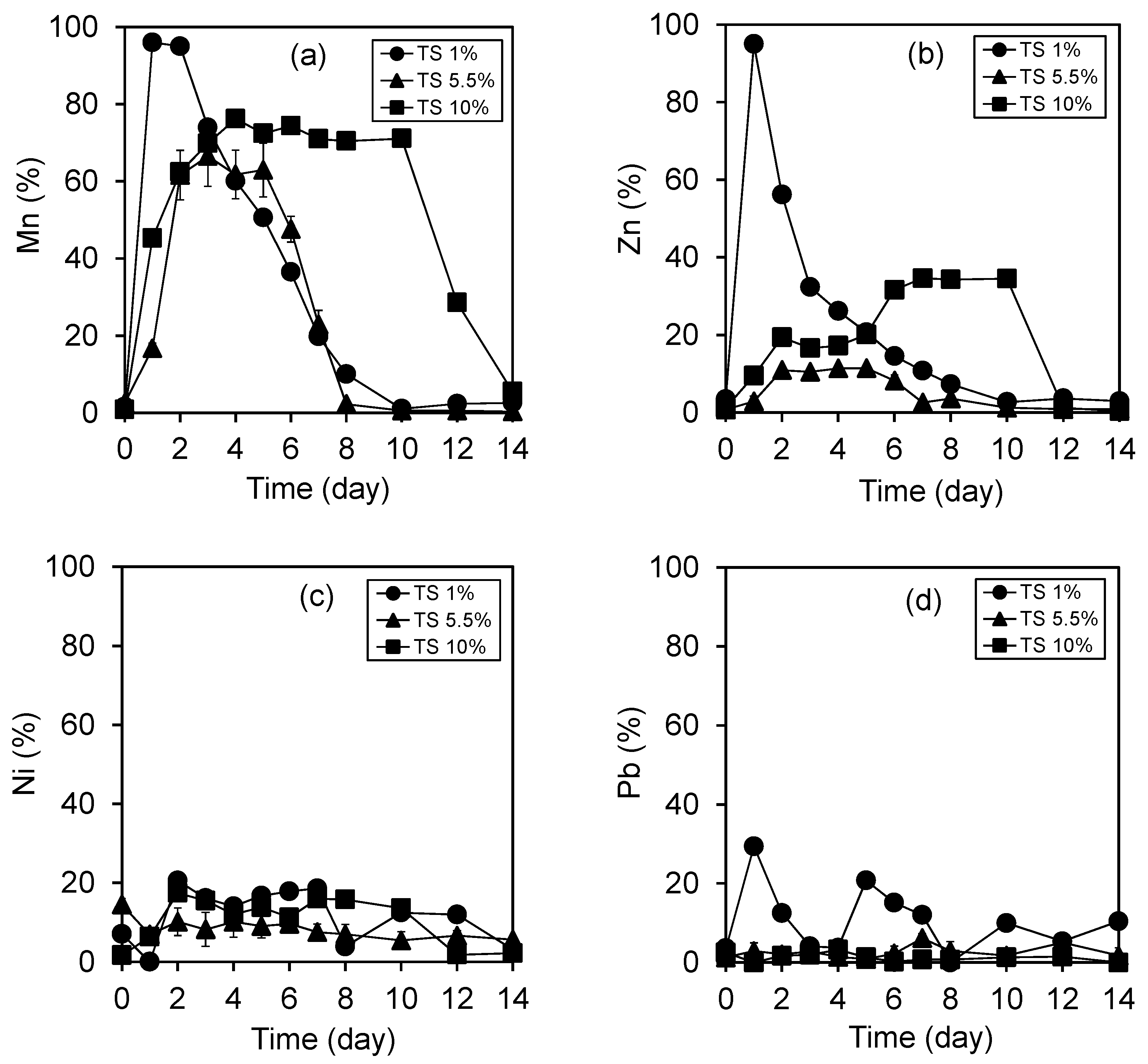
3.3. Removal of Heavy Metals
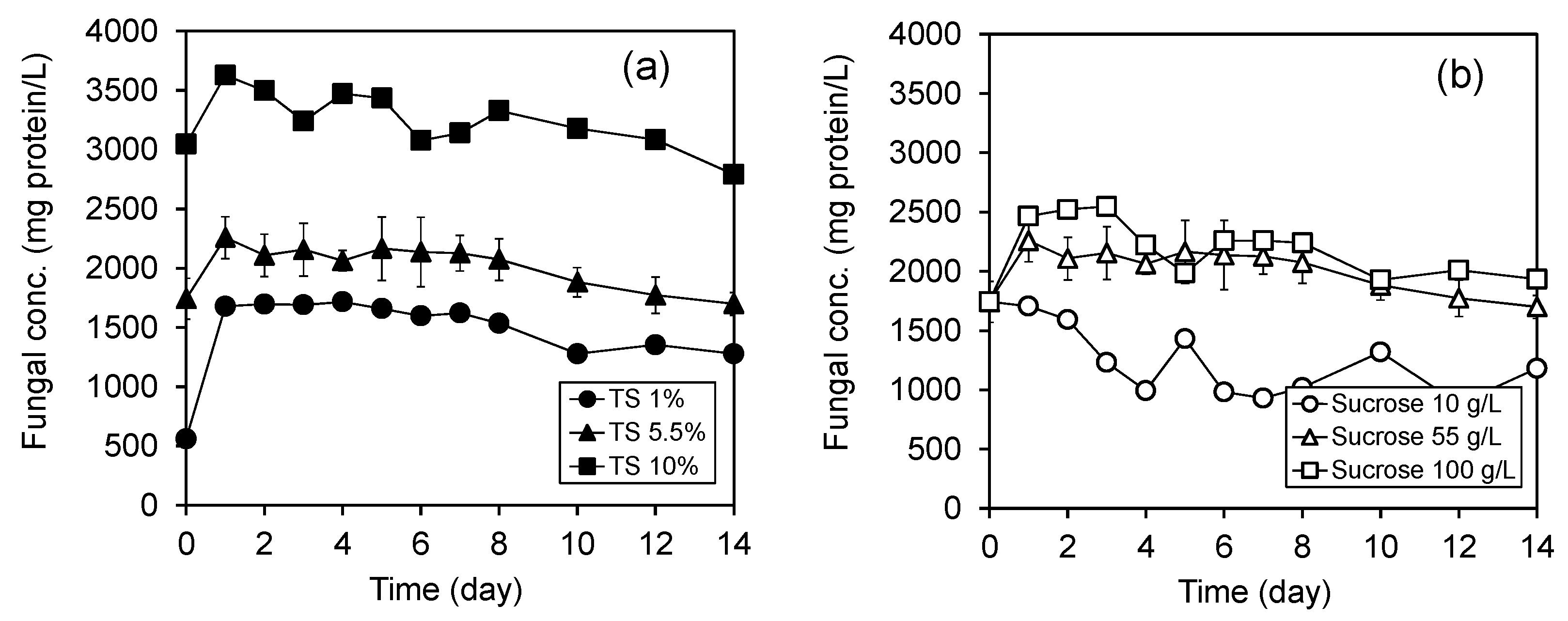
3.4. Variation of Biomass Concentration
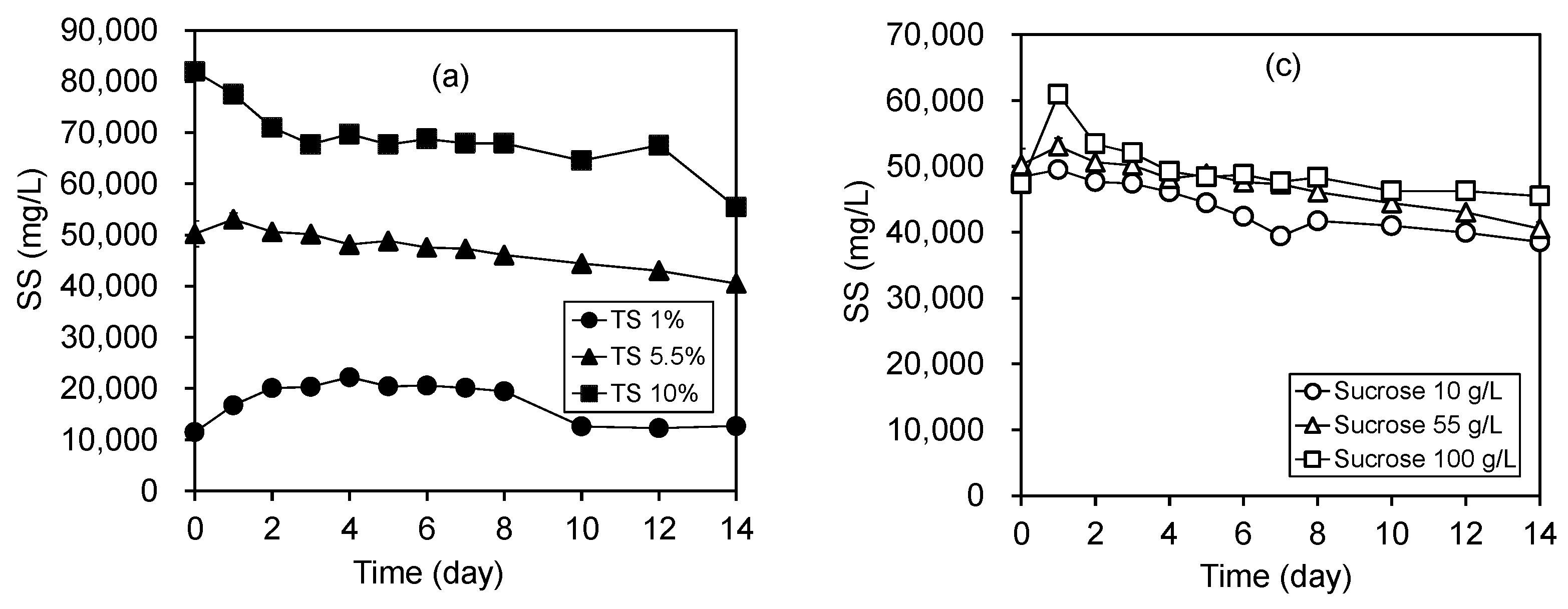
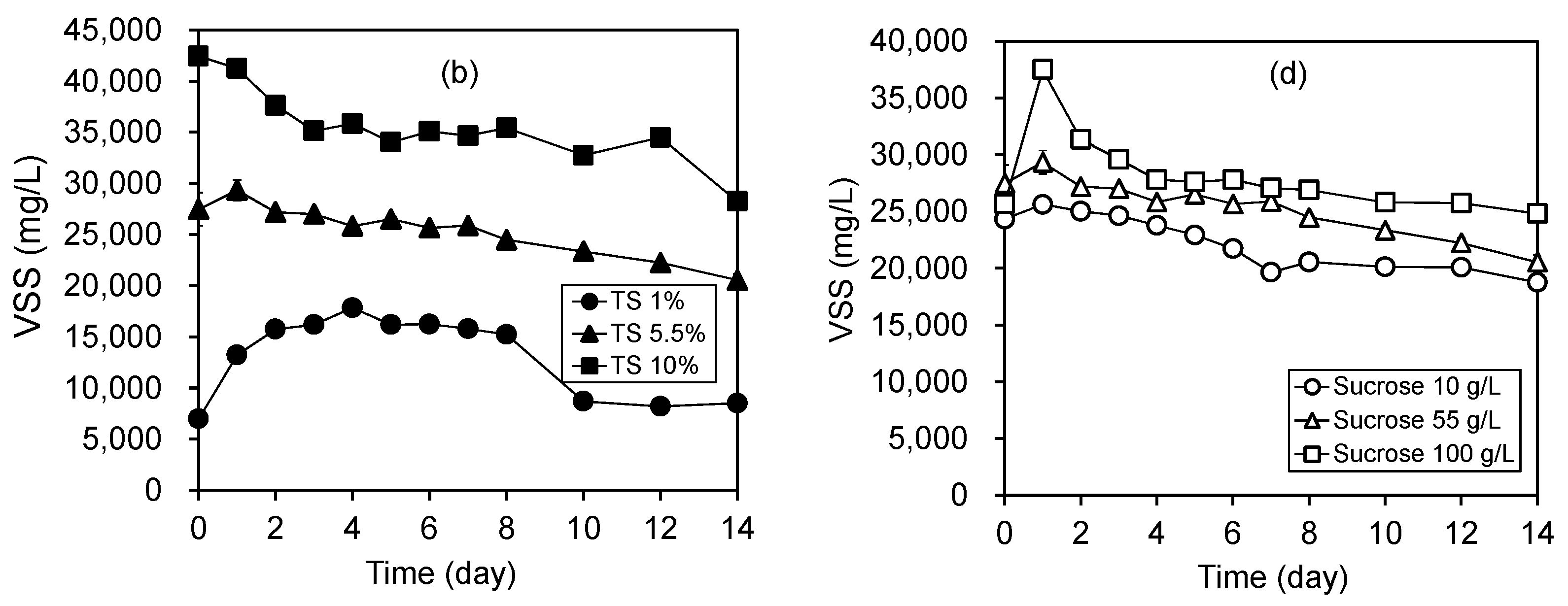
3.5. Degradation of Solids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Cui, Y.; Li, Q.; Sun, J. Effective removal of heavy metals from industrial sludge with the aid of a biodegradable chelating ligand GLDA. J. Hazard. Mater. 2015, 283, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M.; Pode, R.; Manea, F. Comparative heavy metal chemical extraction from anaerobically digested biosolids. Hydrometallurgy 2011, 108, 115–121. [Google Scholar] [CrossRef]
- de La Rochebrochard, S.; Naffrechoux, E.; Drogui, P.; Mercier, G.; Blais, J.F. Low frequency ultrasound-assisted leaching of sewage sludge for toxic metal removal, dewatering and fertilizing properties preservation. Ultrason. Sonochem. 2013, 20, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, Q.S.; Zhang, C.B.; Li, B.Z.; Meng, L. Enhanced electrokinetic removal of cadmium from sludge using a coupled catholyte circulation system with multilayer of anion exchange resin. Chem. Eng. J. 2013, 234, 1–8. [Google Scholar] [CrossRef]
- Shi, W.; Liu, C.; Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour. Technol. 2013, 137, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbers, B.; Ottosen, L.M.; Jensen, P.E. Electrodialytic treatment of municipal wastewater and sludge for the removal of heavy metals and recovery of phosphorus. Electrochim. Acta 2015, 181, 90–99. [Google Scholar] [CrossRef]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: a review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, W.H. Thermophilic bioleaching of heavy metals from waste sludge using response surface methodology. J. Environ. Sci. Heal. A 2013, 48, 1094–1104. [Google Scholar] [CrossRef]
- Chen, S.Y.; Huang, Q.Y. Heavy metals recovery from wastewater sludge of printed circuit board industry by thermophilic bioleaching process. J. Chem. Technol. Biotechnol. 2014, 89, 158–164. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chou, L.C. Relationship between microbial community dynamics and process performance during thermophilic sludge bioleaching. Environ. Sci. Pollut. Res. 2016, 23, 16006–16014. [Google Scholar] [CrossRef]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Does bioleaching represent a biotechnological strategy for remediation of contaminated sediments? Sci. Total Environ. 2016, 563, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Styriakova, I.; Styriak, I.; Balestrazzi, A. Metal leaching and reductive dissolution of iron from contaminated soil and sediment samples by indigenous bacteria and bacillus isolates. Soil Sediment Contam. 2016, 25, 519–535. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lin, J.G. Enhancement of metal bioleaching from contaminated sediment using silver ion. J. Hazard. Mater. 2009, 161, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chai, L.; Yang, Z.; Tang, C.; Wang, Y.; Shi, Y. Bioleaching mechanisms of heavy metals in the mixture of contaminated soil and slag using indigenous Penicillium chrysogenum strain F1. J. Hazard. Mater. 2013, 248, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Urik, M.; Bujdos, M.; Milova-Ziakova, B.; Mikusova, P.; Slovak, M.; Matus, P. Aluminium leaching from red mud by filamentous fungi. J Inorg. Biochem. 2015, 152, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sour. 2016, 320, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Seo, J.Y.; Choi, Y.S.; Kim, G.H. Bioleaching of spent Zn-Mn or Ni-Cd batteries by Aspergillus species. Waste Manag. 2016, 51, 168–173. [Google Scholar] [CrossRef]
- Mirazimi, S.M.J.; Abbasalipour, Z.; Rashchi, F. Vanadium removal for LD converter slag using bacteria and fungi. J. Environ. Manag. 2015, 153, 144–151. [Google Scholar] [CrossRef]
- Gu, X.Y.; Wong, J.W.C. Degradation of inhibitory substances by heterotrophic microorganisms during bioleaching of heavy metals from anaerobically digested sewage sludge. Chemosphere 2007, 69, 311–318. [Google Scholar] [CrossRef]
- Wu, H.Y.; Ting, Y.P. Metal extraction from municipal solid waste (MSW) incinerator fly ash-chemical leaching and fungal bioleaching. Enzyme Microb. Technol. 2006, 38, 839–847. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Sreekrishnan, T.R. A two-stage process for simultaneous thermophilic sludge digestion, pathogen control and metal leaching. Environ. Technol. 2009, 30, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Pan, S.H. Simultaneous metal leaching and sludge digestion by thermophilic microorganisms: effect of solids content. J. Hazard. Mater. 2010, 15, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lian, B.; Mo, B.; Liu, C. Bioleaching of heavy metals from red mud using Aspergillus niger. Hydrometallurgy 2013, 136, 71–77. [Google Scholar] [CrossRef]
- Gharehbagheri, H.; Safdari, J.; Roostaazad, R.; Rashidi, A. Two-stage fungal leaching of vanadium from uranium ore residue of the leaching stage using statistical experimental design. Ann. Nucl. Energy 2013, 56, 48–52. [Google Scholar] [CrossRef]
- Amin, M.M.; Elaassy, I.E.; El-Feky, M.G.; Sallam, A.S.M.; Talaat, M.S.; Kawady, N.A. Effect of mineral constituents in the bioleaching of uranium from uraniferous sedimentary rock samples, Southwestern Sinai, Egypt. J. Environ. Radioact. 2014, 134, 76–82. [Google Scholar] [CrossRef]
- Ghosh, S.; Paul, A.K. Bioleaching of nickel by Aspergillus humicola SKP102 isolated from Indian lateritic overburden. J. Sustain. Min. 2016, 15, 108–114. [Google Scholar] [CrossRef]
- Amiri, F.; Mousavi, S.M.; Yaghmaei, S.; Barati, M. Bioleaching kinetics of a spent refinery catalyst using Aspergillus niger at optimal conditions. Biochem. Eng. J. 2012, 67, 208–217. [Google Scholar] [CrossRef]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Yim, M.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; et al. Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
- Ghazala, R.A.; Fathy, W.M.; Salem, F.H. Application of the produced microbial citric acid as a leachate for uranium from El-Sebaiya phosphate rock. J. Radiat. Res. Appl. Sci. 2019, 12, 78–86. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for Examination of Water and Wastewater, 21th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Method 9045D, USEPA. Physical/Chemical Methods: Soil and Waste pH. Test Methods for Evaluating Solid Waste; U.S Environmental Protection Agency: Washington, DC, USA, 2004.
- Method 3051A, USEPA. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils and Oils. Test Methods for Evaluating Solid Waste; U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, P.; Zhang, G.; Wang, Y.; Yang, A. Degradation properties of protein and carbohydrate during sludge anaerobic digestion. Bioresour. Technol. 2015, 192, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ding, L.; Lin, R.; Liu, M.; Zhou, J.; Cen, K. Physicochemical characterization of typical municipal solid wastes for fermentative hydrogen and methane co-production. Energy Convers. Manag. 2016, 117, 297–304. [Google Scholar] [CrossRef]
- Das, S.; Deshavath, N.N.; Goud, V.V.; Dasu, V.V. Bioleaching of Al from spent fluid catalytic cracking catalyst using Aspergillus species. Biotechnol. Rep. 2019, 23, e00349. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv. Microb. Physiol. 1999, 41, 47–92. [Google Scholar] [PubMed]
- Yang, J.; Wang, Q.; Wang, Q.; Wu, T. Comparisons of one-step and two-step bioleaching for heavy metals removed from municipal solid waste incineration fly ash. Environ. Eng. Sci. 2008, 25, 783–789. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Schreferl-Kunar, G.; Wohrer, W.; Rohr, M. Evidence for a cytoplasmic pathway of oxalate biosynthesis in Aspergillus niger. Appl. Environ. Microbiol. 1988, 54, 633–637. [Google Scholar] [Green Version]
- Max, B.; Salgado, J.M.; Rodríguez, N.; Cortés, S.; Converti, A.; Domínguez, J.M. Biotechnological production of citric acid. Braz. J. Microbiol. 2010, 41, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Amiri, F.; Yaghmaei, S.; Mousavi, S.M. Bioleaching of tungsten-rich spent hydrocracking catalyst using Penicillium simplicissimum. Bioresour. Technol. 2011, 102, 1567–1573. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lin, J.G. Bioleaching of heavy metals from sediment: significance of pH. Chemosphere 2001, 44, 1093–1102. [Google Scholar] [CrossRef]
- Del Mundo Dacera, D.; Babel, S. Removal of heavy metals from contaminated sewage sludge using Aspergillus niger fermented raw liquid from pineapple wastes. Bioresour. Technol. 2008, 99, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, Y. Feasibility of sewage sludge leached by Aspergillus niger in land Utilization. Pol. J. Environ. Stud. 2016, 25, 405–412. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Kamali, M. Bioleaching of copper and other metals from low-grade oxidized mining ores by Aspergillus niger. J. Chem. Technol. Biotechonl. 2003, 78, 497–503. [Google Scholar] [CrossRef]
- Bayat, B.; Sari, B. Comparative evaluation of microbial and chemical leaching processes for heavy metal removal from dewatered metal plating sludge. J. Hazard. Mater. 2010, 174, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, H.; Tian, W.; Wang, X.; Wang, X.; Jia, X.; Shi, B.; Song, G.; Tang, Y. Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner. Eng. 2015, 81, 1–4. [Google Scholar] [CrossRef]
- Aung, K.M.; Ting, Y.P. Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger. J. Biotechnol. 2005, 116, 159–170. [Google Scholar] [CrossRef]
- Castro, I.M.; Fietto, J.L.R.; Vieira, R.X.; Tropia, M.J.M.; Campos, L.M.M.; Paniage, E.B.; Brandao, R.L. Bioleaching of zinc and nickel from silicate using Aspergillus niger culture. Hydrometallurgy 2000, 57, 39–49. [Google Scholar] [CrossRef]

| Affecting Parameter | Sludge Solid Content (%) | Sucrose Concentration (g/L) |
|---|---|---|
| Sludge Solid Content | 1 | 55 |
| 5.5 1 | 55 | |
| 10 | 55 | |
| Sucrose Concentration | 5.5 | 10 |
| 5.5 | 55 | |
| 5.5 | 100 |
| Factor | pH | Oxalic acid | Critic Acid | Gluconic Acid | Organic Acids |
|---|---|---|---|---|---|
| Organic Acids | −0.45 1 | 0.80 1 | 0.76 1 | 0.28 | - |
| Mn | −0.87 1 | 0.40 1 | 0.26 | 0.41 1 | 0.52 1 |
| Zn | −0.59 1 | 0.31 | 0.17 | 0.50 1 | 0.43 1 |
| Ni | −0.23 | 0.22 | 0.34 | 0.37 1 | 0.41 1 |
| Pb | 0.06 | −0.16 | −0.11 | 0.31 | −0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Wang, S.-Y. Effects of Solid Content and Substrate Concentration on Bioleaching of Heavy Metals from Sewage Sludge Using Aspergillus niger. Metals 2019, 9, 994. https://doi.org/10.3390/met9090994
Chen S-Y, Wang S-Y. Effects of Solid Content and Substrate Concentration on Bioleaching of Heavy Metals from Sewage Sludge Using Aspergillus niger. Metals. 2019; 9(9):994. https://doi.org/10.3390/met9090994
Chicago/Turabian StyleChen, Shen-Yi, and Sheng-Ying Wang. 2019. "Effects of Solid Content and Substrate Concentration on Bioleaching of Heavy Metals from Sewage Sludge Using Aspergillus niger" Metals 9, no. 9: 994. https://doi.org/10.3390/met9090994
APA StyleChen, S.-Y., & Wang, S.-Y. (2019). Effects of Solid Content and Substrate Concentration on Bioleaching of Heavy Metals from Sewage Sludge Using Aspergillus niger. Metals, 9(9), 994. https://doi.org/10.3390/met9090994




