Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Theoretical Calculation
2.3. Experimental Methods
3. Results
3.1. Chemical Composition of Ferrotitanium
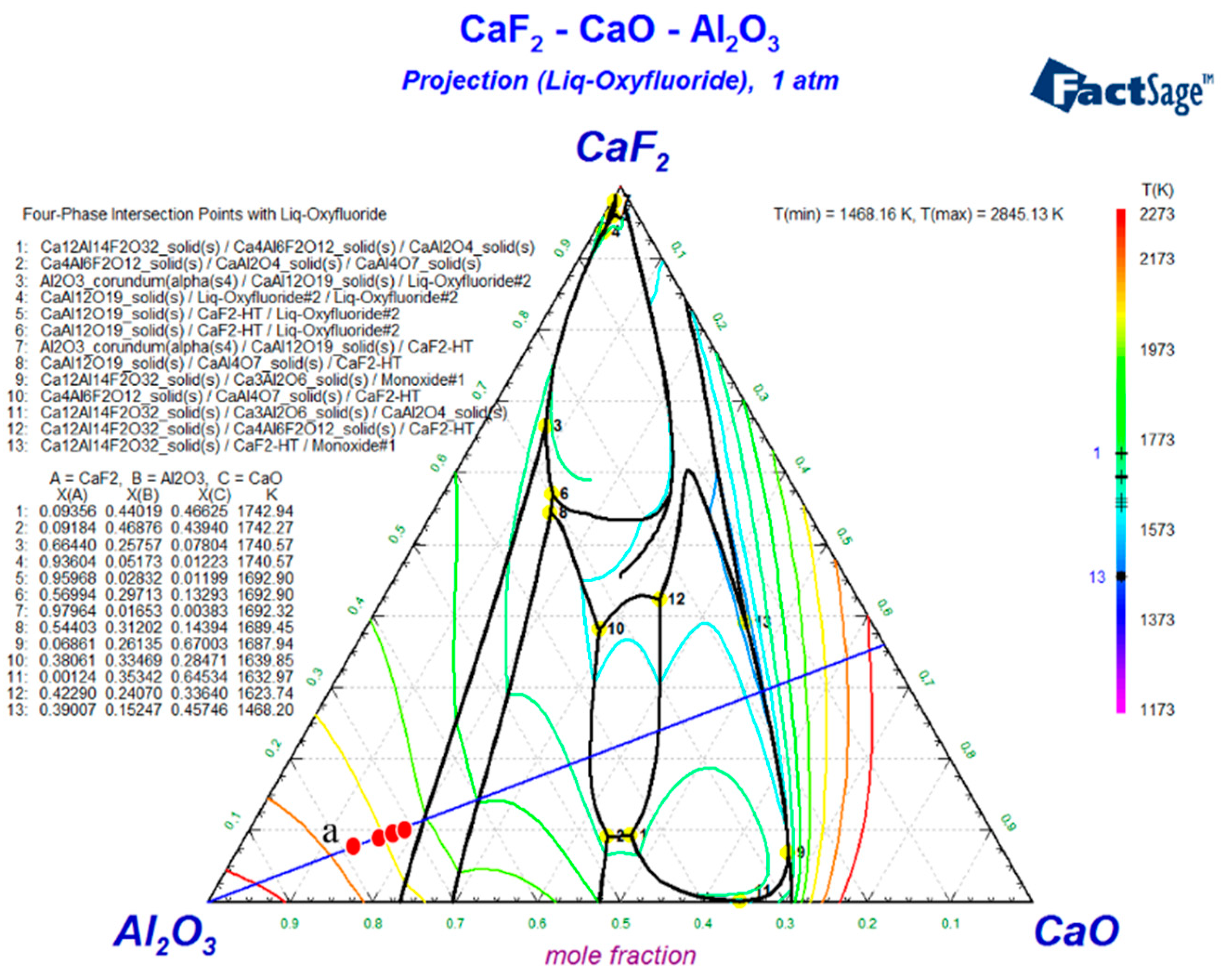
3.2. Phase Transitions
3.3. Morphology of Ferrotitanium and Slag
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Tang, A.-T.; Xiao, X. Effect of milling time on carbothermic reduction of ilmenite. Trans. Nonferrous Met. Soc. China 2015, 25, 4201–4206. [Google Scholar] [CrossRef]
- Panigrahi, M.; Shibata, E.; Iizuka, A.; Nakamura, T. Production of Fe-Ti alloy from mixed ilmenite and titanium dioxide by direct electrochemical reduction in molten calcium chloride. Electrochim. Acta 2013, 93, 143–151. [Google Scholar] [CrossRef]
- Panigrahi, M.; Paramguru, R.K.; Gupta, R.C.; Shibata, E.; Nakamura, T. An overview of production of titanium and an attempt to titanium production with ferro-titanium. High Temp. Mater. Processes 2010, 9, 495–514. [Google Scholar] [CrossRef]
- Xiong, L.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Zhang, Q.; Zhou, Z.; Zhang, Y.; Ru, J. Effect of CaO addition on preparation of ferrotitanium from ilmenite by electrochemical reduction in CaCl 2 NaCl molten salt. J. Alloys Compd. 2016, 676, 383–389. [Google Scholar] [CrossRef]
- Qi, C.-c.; Hua, Y.-x.; Chen, K.-h.; Jie, Y.-f.; Zhou, Z.-r.; Ru, J.-j.; Xiong, L.; Gong, K. Preparation of ferrotitanium alloy from ilmenite by electrochemical reduction in chloride molten salts. JOM 2015, 68, 668–674. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Gong, K.; Ru, J.; Xiong, L. Preparation of Ferrotitanium from Ilmenite by Electrolysis-Assisted Calciothermic Reduction in CaCl2-NaCl Molten Salt. JOM 2015, 68, 532–539. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.; Zhang, Y.; Li, S.; Tang, K.; Song, B. Isothermal oxidation kinetics of ilmenite concentrate powder from Panzhihua in air. Powder Technol. 2017, 320, 239–248. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Li, G. Preparation of nano-titanium dioxide from ilmenite using sulfuric acid-decomposition by liquid phase method. Powder Technol. 2016, 287, 256–263. [Google Scholar] [CrossRef]
- Middlemas, S.; Fang, Z.Z.; Fan, P. Life cycle assessment comparison of emerging and traditional Titanium dioxide manufacturing processes. J. Cleaner Prod. 2015, 89, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Gou, H.-P.; Zhang, G.-H.; Chou, K.-C. Influence of pre-oxidation on carbothermic reduction process of ilmenite concentrate. ISIJ Int. 2015, 55, 928–933. [Google Scholar] [CrossRef]
- Khoshhal, R.; Soltanieh, M.; Boutorabi, M.A. Formation mechanism and synthesis of Fe–TiC/Al2O3 composite by ilmenite, aluminum and graphite. Int. J. Refract. Met. Hard Mater 2014, 45, 53–57. [Google Scholar] [CrossRef]
- Azizov, S.T.; Kachin, A.R.; Loryan, V.E.; Borovinskaya, I.P.; Mnatsakanyan, A.S. Aluminothermic SHS of ferrotitanium from ilmenite: Influence of Al and KClO4 content of green composition. Int. J. Self Propag. High Temp. Synth. 2014, 23, 161–164. [Google Scholar] [CrossRef]
- Akhgar, B.; Pazouki, M.; Ranjbar, M.; Hosseinnia, A.; Salarian, R. Application of Taguchi method for optimization of synthetic rutile nano powder preparation from ilmenite concentrate. Chem. Eng. Res. Des. 2012, 90, 220–228. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Wang, Z.; Guo, H.; Wang, X.; Wu, F.; Fang, J.; Wang, Z.; Li, L. A novel process for producing synthetic rutile and LiFePO4 cathode material from ilmenite. J. Alloys Compd. 2010, 506, 271–278. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, Z.; Wu, L.; Yue, P.; Guo, H.; Wu, F.; Ma, T. Preparation and characterization of Li4Ti5O12 from ilmenite. Powder Technol. 2010, 204, 198–202. [Google Scholar] [CrossRef]
- Chumarev, V.M.; Dubrovskii, A.Y.; Pazdnikov, I.P.; Shurygin, Y.Y.; Sel’menskikh, N.I. Technological possibilities of manufacturing high-grade ferrotitanium from crude ore. Russ. Metall. 2009, 2008, 459–463. [Google Scholar] [CrossRef]
- Lasheen, T.A. Soda ash roasting of titania slag product from Rosetta ilmenite. Hydrometallurgy 2008, 93, 124–128. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Zhang, L.-f.; Duan, H.-j.; Wang, L. Population evolution of oxide inclusions in ti-stabilized ultra-low carbon steels after deoxidation. J. Iron. Steel Res. Int. 2015, 22, 1069–1077. [Google Scholar] [CrossRef]
- Pande, M.M.; Guo, M.; Blanpain, B. Inclusion formation and interfacial reactions between FeTi alloys and liquid steel at an early stage. ISIJ Int. 2013, 53, 629–638. [Google Scholar] [CrossRef]
- Chen, L. Study on the effects of Ti micro-addition on the characteristics of MnS inclusions in rail steel. Ironmaking Steelmaking 2017, 46, 508–512. [Google Scholar] [CrossRef]
- Shatokhin, I.M.; Shaimardanov, K.R.; Bigeev, V.A.; Ziatdinov, M.K.; Shchegoleva, E.A.; Manashev, I.R. Production of ferrosilicotitanium for smelting pipe steels. Metallurgist 2016, 60, 524–529. [Google Scholar] [CrossRef]
- Shaimardanov, K.R.; Shatokhin, I.M.; Ziatdinov, M.K. Production and use of ferrosilicotitanium produced by self-propagating high-temperature synthesis. Steel Transl. 2014, 44, 215–220. [Google Scholar] [CrossRef]
- Jiang, X. Study on Preparation of High Titanium Ferroalloy by Thermite Method and Its Deoxidizing Performance in Molten Steel. Master’s Thesis, Northeastern University, Shenyang, China, June 2012. [Google Scholar]
- Misra, S.B.; Kamble, A.; Yadav, S.; Ranganathan, S. Influence of charge segregation on specific aluminium consumption in production of ferro-titanium. Can. Metall. Q. 2014, 54, 101–109. [Google Scholar] [CrossRef]
- Gao, Z.; Cheng, G.; Xue, X.; Yang, H.; Duan, P. Property investigations of low-grade vanadium-titanium magnetite pellets with different MgO contents. Steel Res. Int. 2018, 89, 1700543. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xiao, X.H. Development of 65%~75% high grade ferrotitanium with material rutile. Ferroalloys 2001, 158, 15. [Google Scholar]
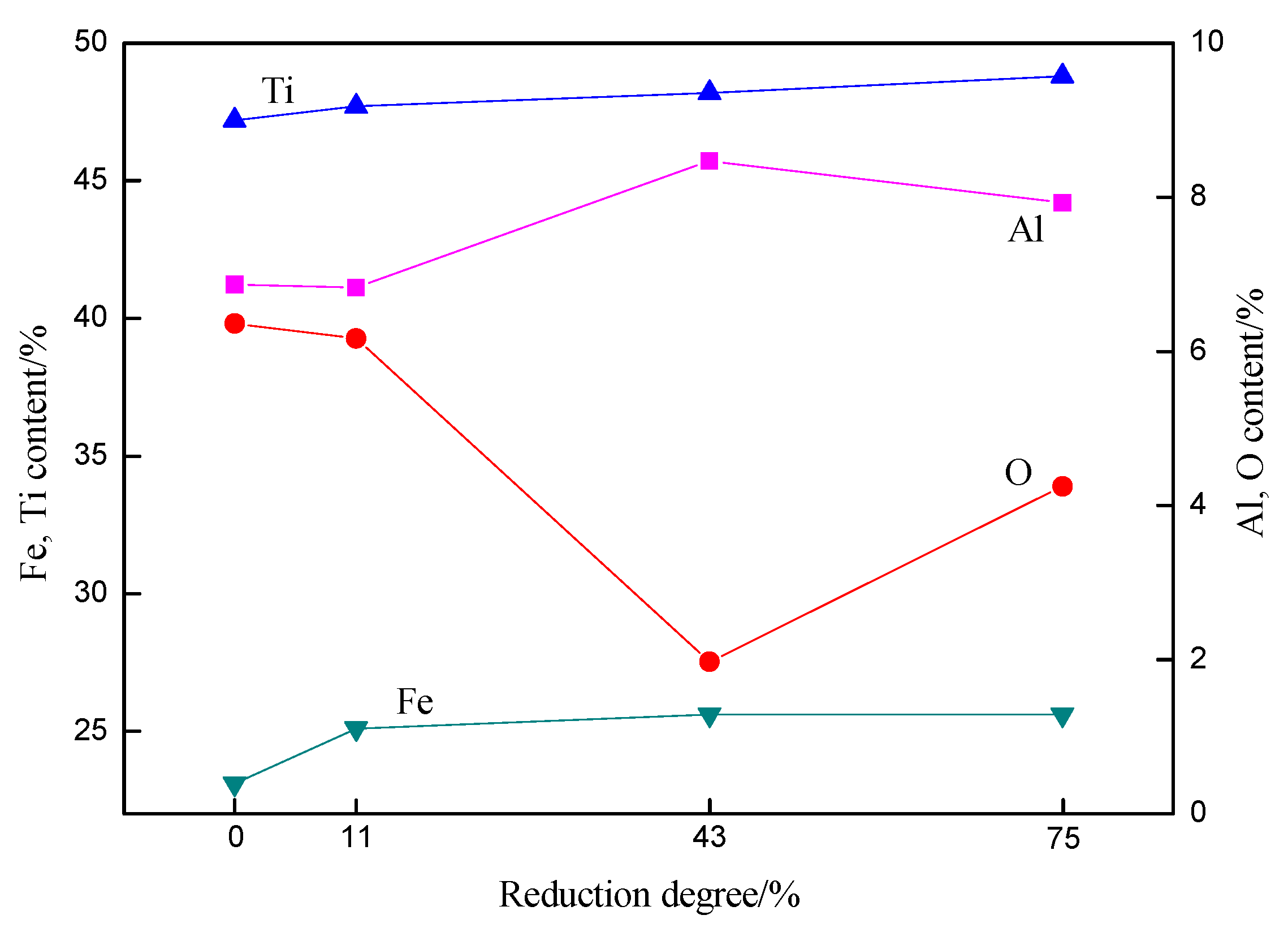
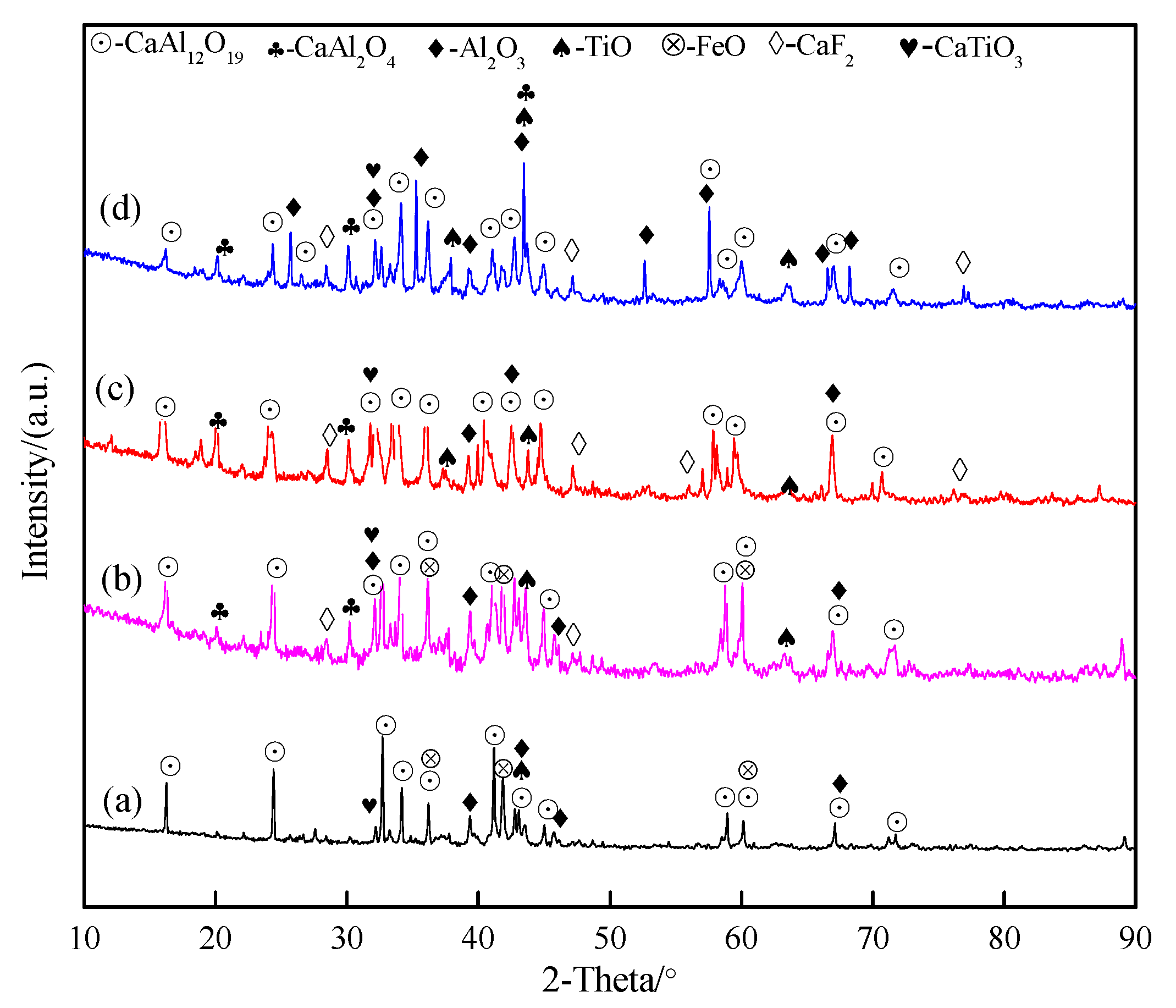


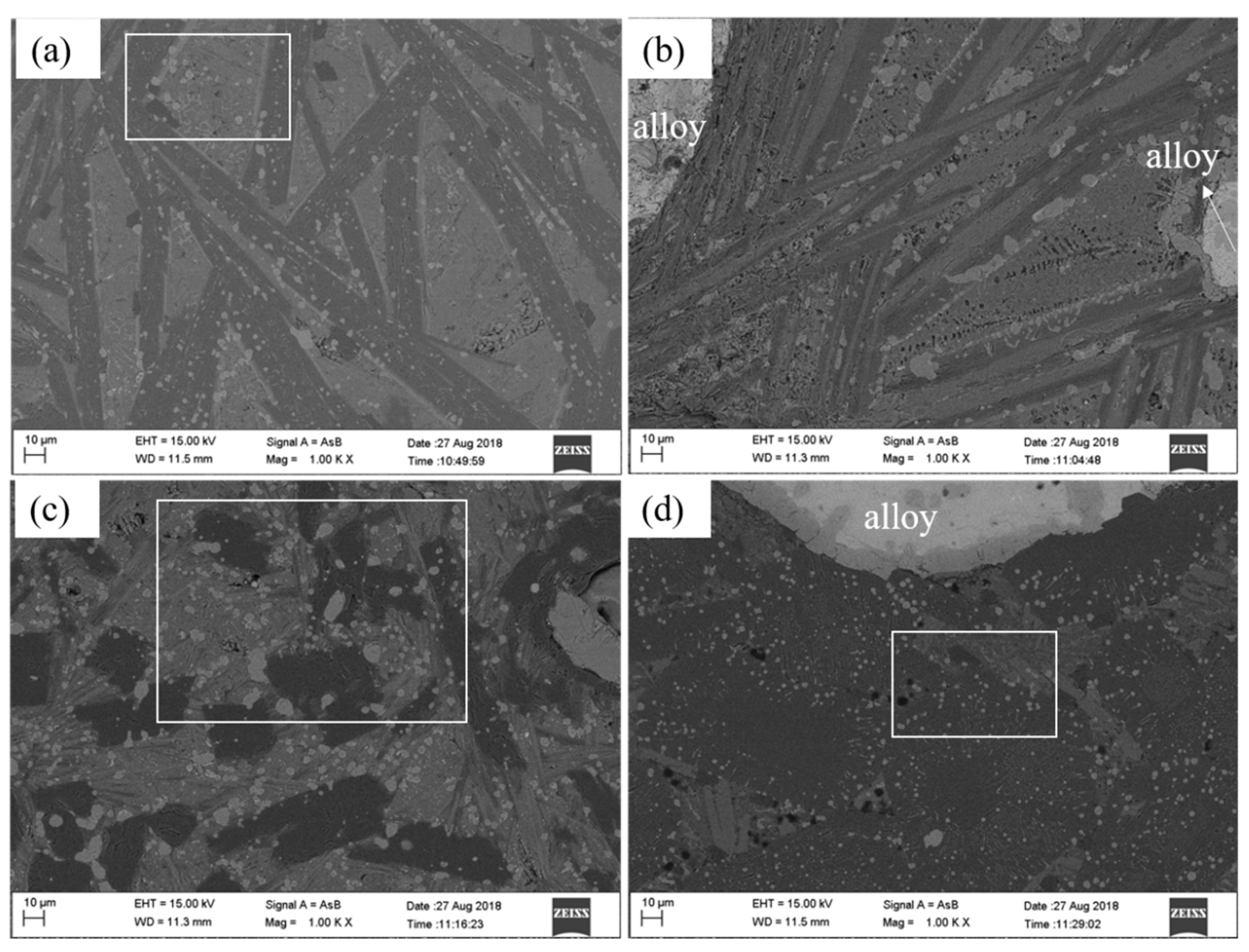
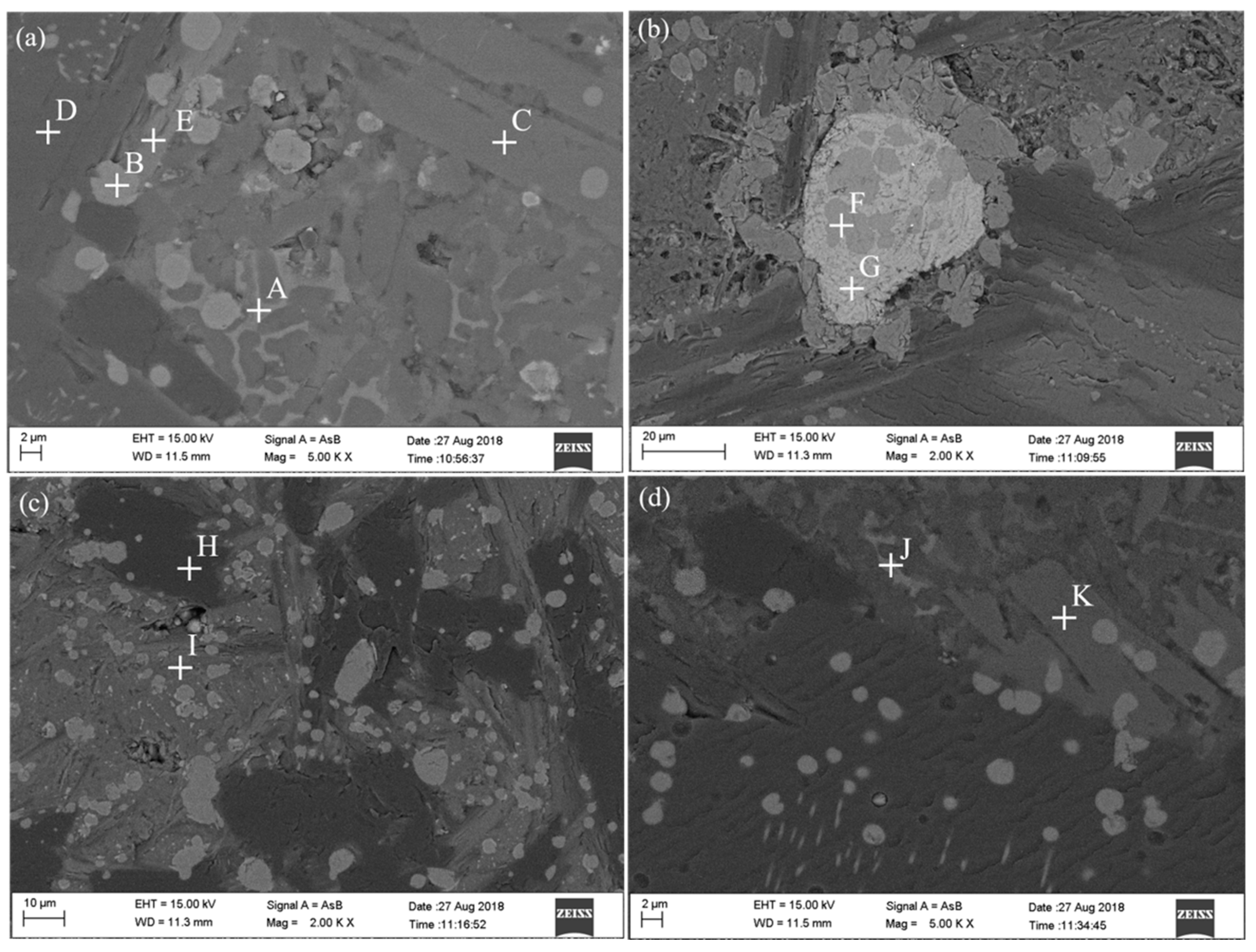
| Reduction Degree/% | Ilmenite/g | TiO2/g | Al/g | NaClO3/g | CaO/g | CaF2/g |
|---|---|---|---|---|---|---|
| 0 | 500 | 822 | 652 | 217 | 130 | 65 |
| 11 | 482 | 822 | 643 | 229 | 129 | 64 |
| 43 | 471 | 822 | 633 | 244 | 127 | 63 |
| 75 | 450 | 822 | 619 | 264 | 124 | 62 |
| Point | Fe | Ti | Al | O | Ca | Si | F | Mg | Na |
|---|---|---|---|---|---|---|---|---|---|
| A | 12.75 | 11.48 | 57.00 | 15.67 | 0.13 | 2.39 | 0.32 | 0.26 | |
| B | 41.44 | 2.47 | 54.43 | 0.48 | 1.19 | ||||
| C | 15.30 | 20.45 | 57.46 | 5.88 | 0.91 | ||||
| D | 3.22 | 36.57 | 55.97 | 3.00 | 1.23 | ||||
| E | 3.04 | 32.51 | 60.75 | 0.31 | 2.76 | 0.65 | |||
| F | 4.12 | 55.26 | 11.54 | 28.60 | |||||
| G | 30.60 | 38.36 | 1.28 | 10.84 | 13.57 | ||||
| H | 0.88 | 43.69 | 55.43 | ||||||
| I | 2.33 | 28.46 | 55.59 | 13.62 | |||||
| J | 15.26 | 6.70 | 56.26 | 16.81 | 4.48 | 0.48 | |||
| K | 13.66 | 22.90 | 56.13 | 6.24 | 1.06 |
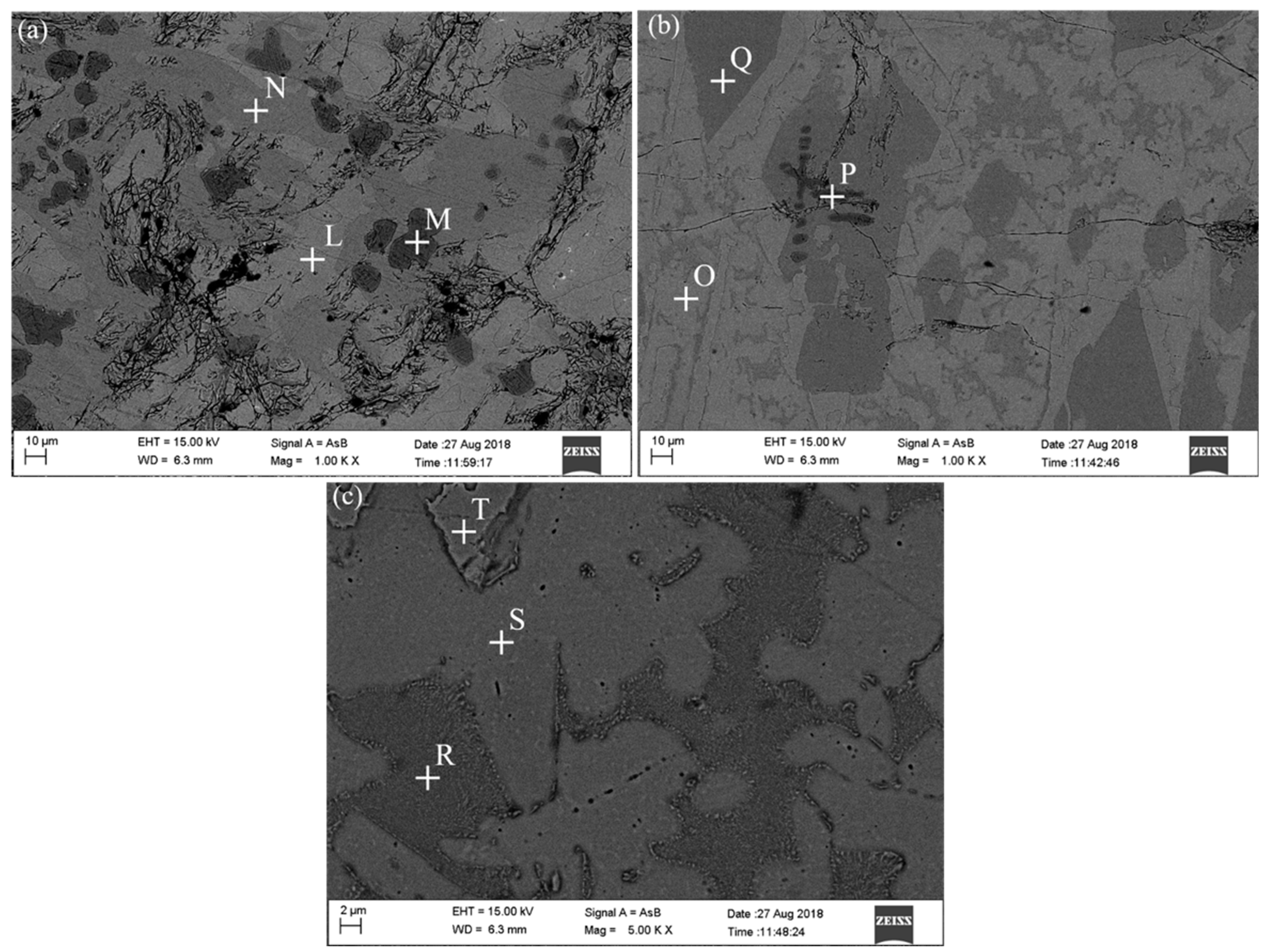
| Point | Fe | Ti | Al | O | Si |
|---|---|---|---|---|---|
| L | 21.34 | 46.83 | 17.84 | 11.29 | 2.70 |
| M | 64.17 | 35.64 | |||
| N | 12.31 | 55.35 | 5.62 | 22.87 | 3.85 |
| O | 24.53 | 46.77 | 8.08 | 15.35 | 4.82 |
| P | 62.48 | 37.52 | |||
| Q | 11.44 | 54.14 | 6.02 | 20.70 | 3.68 |
| R | 20.16 | 56.20 | 19.15 | 4.50 | |
| S | 33.87 | 41.41 | 20.17 | 4.55 | |
| T | 25.59 | 46.48 | 8.00 | 14.61 | 5.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Cheng, G.; Yang, H.; Xue, X.; Ri, J. Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees. Metals 2019, 9, 962. https://doi.org/10.3390/met9090962
Gao Z, Cheng G, Yang H, Xue X, Ri J. Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees. Metals. 2019; 9(9):962. https://doi.org/10.3390/met9090962
Chicago/Turabian StyleGao, Zixian, Gongjin Cheng, He Yang, Xiangxin Xue, and Jongchol Ri. 2019. "Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees" Metals 9, no. 9: 962. https://doi.org/10.3390/met9090962
APA StyleGao, Z., Cheng, G., Yang, H., Xue, X., & Ri, J. (2019). Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees. Metals, 9(9), 962. https://doi.org/10.3390/met9090962




