Influence of the Processing Method on the Properties of Ti-23 at.% Mo Alloy
Abstract
:1. Introduction
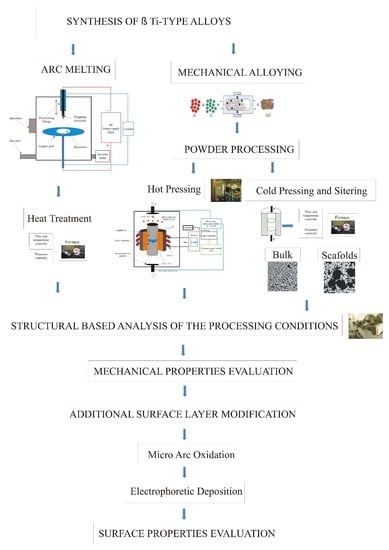
2. Materials and Methods
2.1. Sample Preparation
2.2. Materials Characterization
- W—weight loss [g∙s−1];
- Icorr—corrosion current [µA∙cm−2];
- EW—equivalent weight [g∙mol−1];
- A—surface [cm2];
- F—Faraday constant [A∙s∙mol−1]
3. Results and Discussion
4. Conclusions
- (1)
- -sintering of MA powder leads to the formation of the Ti(β) based type alloys,
- (2)
- -the HP process at a low temperature (800 °C/5 min) of the Ti23Mo alloy in comparison to the cold pressing and sintering (800 °C/0.5 h) approach allows an obtainment of a low porosity high compactness pure Ti(β) phase,
- (3)
- -the low-temperature sintering (below α→β transus) allows the synthesizing of the bulk materials,
- (4)
- -the obtained microhardness test results favoured the samples with high compactness and low porosity,
- (5)
- -the indentation modulus and estimated sinters parameters obtained in this work confirm a relationship between the material phase and the internal structure,
- (6)
- -the potentiodynamic corrosion resistance analysis indicates a heavy dependence of the obtained results on the material’s porosity and their chemical composition,
- (7)
- -the results obtained for surface modified MAO and MAO + EPD treatments confirms that the substrate has a crucial meaning for wetting and corrosion resistance characteristics
- (8)
- -the SFE, as the analysis confirms, stays strongly dependent on structural and internal material characteristics as dictated by different processing approaches.
Author Contributions
Funding
Conflicts of Interest
References
- Okuno, O. Titanium alloys for dental applications. Soc. Mater. Sci. 1996, 14, 267–273. [Google Scholar]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Huang, Q.X.; Shi, X.H.; Shuai, M.R.; Zeng, W.D.; Zhao, Y.Q.; Huang, Z.Q.; Ma, L.F. Precipitation location of secondary phase and microstructural evolution during static recrystallization of as-cast Ti-25V-15Cr-0.3Si titanium alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1521–1529. [Google Scholar] [CrossRef]
- Di, W.U.; Zhang, L.G.; Liu, L.B.; Bai, W.M.; Zeng, L.J. Effect of Fe content on microstructures and properties of Ti6Al4V alloy with combinatorial approach. Trans. Nonferrous Met. Soc. China 2018, 28, 1714–1723. [Google Scholar]
- Li, Y.H.; Wang, F.; Li, J.J. Current developments of biomedical porous Ti–Mo alloys. Int. J. Mater. Res. 2017, 108, 619–624. [Google Scholar] [CrossRef]
- Doi, H.; Yoneyama, T.; Kobayashi, E.; Hamanaka, H. Mechanical properties and corrosion resistance of Ti–5Al–13Ta alloy castings. J. Jpn. Soc. Dent. Mater. Devices 1998, 17, 247–252. [Google Scholar]
- Jones, N.G.; Dashwood, R.J.; Jackson, M.; Dye, D. β Phase decomposition in Ti-5Al-5Mo-5V-3Cr. Acta Mater. 2009, 57, 3830–3839. [Google Scholar] [CrossRef]
- Sen, M.; Suman, S.; Kumar, M.; Banerjee, T.; Bhattacharjee, A.; Kar, S.K. Thermo-mechanical processing window for β phase recrystallization in Ti-5Al-5Mo-5V-3Cr alloy. Mater. Charact. 2018, 146, 55–70. [Google Scholar] [CrossRef]
- Ren, L.; Xiao, W.; Han, W.; Ma, C.; Zhou, L. Influence of duplex ageing on secondary α precipitates and mechanical properties of the near β-Ti alloy Ti-55531. Mater. Charact. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Lin, D.J.; Chuang, C.C.; Chern Lin, J.H.; Lee, J.W.; Ju, C.P.; Yin, H.S. Bone formation at the surface of low modulus Ti–7.5Mo implants in rabbit femur. Biomaterials 2007, 28, 2582–2589. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, Y.; Wu, H.; Song, M.; Zhang, T.Y.; Lan, X.D.; Yao, T.H. Elastic modulus of phases in Ti-Mo alloys. Mater. Charact. 2015, 106, 302–307. [Google Scholar] [CrossRef]
- Ho, W.F.; Ju, C.P.; Chern Lin, J.H. Structure and properties of cast binary Ti–Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Zhao, X.; Niinomi, M.; Nakai, M.; Hieda, J. Beta type Ti–Mo alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2012, 8, 1990–1997. [Google Scholar] [CrossRef]
- Murray, J.L. The Mo-Ti (Molybdenum-Titanium) System. Bull. Alloy Phase Diagrams 1981, 2, 185–192. [Google Scholar] [CrossRef]
- Almeida, A.; Gupta, D.; Loable, C.; Vilar, R. Laser-assisted synthesis of Ti–Mo alloys for biomedical applications. Mater. Sci. Eng. C 2012, 32, 1190–1195. [Google Scholar] [CrossRef]
- Cardoso, F.F.; Ferrandini, P.L.; Lopes, E.S.N.; Cremasco, A.; Caram, R. Ti–Mo alloys employed as biomaterials: Effects of composition and aging heat treatment on microstructure and mechanical behavior. J. Mech. Behav. Biomed. Mater. 2014, 32, 31–38. [Google Scholar] [CrossRef]
- Marczewski, M.; Miklaszewski, A.; Jurczyk, M. Structure evolution analysis in ultrafine-grained Zr and Nb-based beta titanium alloys. J. Alloys Compd. 2018, 765, 459–469. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. Development of <beta> type Ti-x at. % Mo alloys by mechanical alloying and powder metallurgy: Phase evolution and mechanical properties (10 ≤ x ≤ 35). J. Alloys Compd. 2019, 776, 370–378. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2014, 25, 4731–4739. [Google Scholar] [CrossRef]
- Jurczyk, K.; Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, M. Development of β type Ti23Mo-45S5 Bioglass nanocomposites for dental applications. Materials 2018, 8, 5441. [Google Scholar] [CrossRef]
- Estrin, Y.; Kasper, C.; Diederichs, S.; Lapovok, R. Accelerated growth of preosteoblastic cells on ultrafine grained titanium. J. Biomed. Mater. Res. Part A 2008, 90, 1239–1242. [Google Scholar] [CrossRef]
- Adamek, G.; Jakubowicz, J. Mechanoelectrochemical synthesis and properties of porous nano Ti-6Al-4V alloy with hydroxyapatite layer for biomedical applications. Electrochem. Commun. 2010, 12, 653–656. [Google Scholar] [CrossRef]
- Jurczyk, M.U.; Jurczyk, K.; Miklaszewski, A.; Jurczyk, M. Nanostructured titanium-45S5 Bioglass scaffold composites for medical applications. Mater. Des. 2011, 32, 4882–4889. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.U.; Kaczmarek, M.; Paszel-Jaworska, A.; Romaniuk, A.; Llipinska, N.; Zurawski, J.; Urbaniak, P.; Jurczyk, M. Nanoscale size effect in in situ titanium based composites with cell viability and cytocompatibility studies. Mater. Sci. Eng. C 2017, 73, 525–536. [Google Scholar] [CrossRef]
- Van De Voorde, M. Nanoscience and Nanotechnology—Advances and Developments in Nano-sized Materials; De Guyter: Berlin, Germany, 2018; pp. 57–74. ISBN 978-3-11-054722-1. [Google Scholar]
- Pathan, D.S.; Doshi, S.B.; Muglikar, S.D. Nanotechnology in implants: The future is small. Uni. Res. J. Dent. 2015, 5, 8–13. [Google Scholar]
- Valiev, R.Z.; Semenova, I.P.; Jakushina, E.; Latysh, V.V.; Rack, H.; Lowe, R.C.; Petruzelka, J.; Dluhos, L.; Hrusak, D.; Sochova, J. Nanostructured SPD processed titanium for medical implants. Mater. Sci. Forum 2008, 584, 49–54. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. The influence of Mo content on phase transformation in Ti-Mo alloys. Arch. Metall. Mater. 2017, 62, 2051–2056. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Q.; Yu, H.; Wang, X.; Wang, D. Microstructure of porous TiO2 coating on pure Ti by micro-arc oxidation. Adv. Eng. Mater. 2006, 8, 754–759. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Liu, J.; Yao, J. Influence of the Ti alloy substrate on the anodic oxidation in an environmentally-friendly electrolyte. Surf. Coat. and Technol. 2018, 344, 680–688. [Google Scholar] [CrossRef]
- Babilas, D.; Służalska, K.; Krząkała, A.; Maciej, A.; Socha, R.; Dercz, G.; Tylko, G.; Michalska, J.; Osyczka, A.; Simka, W. Plasma electrolytic oxidation of a Ti–15Mo alloy in silicate solutions. Mater. Lett. 2013, 100, 252–256. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, D.; Li, D.; Zhu, J.; Li, G.; Xie, J. Controllable synthesis of fluorapatite nanocrystals with various morphologies: Effects of pH value and chelating reagent. J. Alloys Compd. 2009, 485, 396–401. [Google Scholar] [CrossRef]
- Mudali, U.K.; Sridhar, T.M.; Rajendran, N. Electrophoretic deposition of TiO2 and TiO2 + CeO2 coatings on type 304L stainless steel. Surf. Eng. 2007, 23, 267–272. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. Nanoindentation, 3nd ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4757-5943-3. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic-modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kowalski, K.; Jurczyk, M.U.; Wirstlein, P.K.; Jakubowicz, J.; Jurczyk, M. Influence of 45S5 Bioglass addition on microstructure and properties of ultrafine grained (Mg-4Y-5.5Dy-0.5Zr) alloy. Mater. Sci. Eng. B 2017, 219, 28–36. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Kowalski, K.; Jurczyk, M. Hydrothermal Surface Treatment of Biodegradable Mg-Materials. Metals 2018, 8, 894. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Guo, S.; Meng, Q.; Chenga, X.; Zhao, X. α’ martensite Ti–10Nb–2Mo–4Sn alloy with ultra low elastic modulus and high strength. Mater. Lett. 2014, 133, 236–239. [Google Scholar] [CrossRef]
- Wang, C.H.; Liu, M.; Hu, P.F.; Peng, J.C.; Wang, J.A.; Ren, Z.M.; Cao, G.H. The effects of α” and ω phases on the superelasticity and shape memory effect of binary Ti-Mo alloys. J. of Alloys and Compd. 2017, 720, 488–496. [Google Scholar] [CrossRef]
- Grosdidier, T.; Philippe, M.J. Deformation induced martensite and superelasticity in a β-metastable titanium alloy. Mater. Sci. Eng. A. 2000, 291, 218–233. [Google Scholar] [CrossRef]
- Lewis, G. Properties of open-cell porous metals and alloys for orthopaedic applications. J. Mater. Sci. Mater. Med. 2013, 24, 2293–2325. [Google Scholar] [CrossRef]
- Yamamuro, T.; Hench, L.; Wilson, J. Handbook of Bioactive Ceramics: Vol. 1 and Vol. 2; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive glass and glass–ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef]
- He, G.; Hagiwara, M. Ti alloy design strategy for biomedical applications. Mater. Sci. Eng. C 2006, 26, 14–19. [Google Scholar] [CrossRef]
- Zhao, X.; Niinomi, M.; Nakai, M. Relationship between various deformation-induced products and mechanical properties in metastable Ti–30Zr–Mo alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2011, 4, 2009–2016. [Google Scholar] [CrossRef]
- Wu, X.; Cai, C.; Yang, L.; Liu, W.; Li, W.; Li, M.; Liu, J.; Zhou, K.; Shi, Y. Enhanced mechanical properties of Ti-6Al-2Zr-1Mo-1V with ultrafine crystallites and nano-scale twins fabricated by selective laser melting. Mater. Sci. Eng. A 2018, 738, 10–14. [Google Scholar] [CrossRef]
- Collings, E.; Gegel, H. A physical basis for solid solution strengthening and phase stability in alloys of titanium. Scr. Metall. 1973, 7, 437–443. [Google Scholar] [CrossRef]
- NIiinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Dercz, G.; Kalemba, I.; Suchanek, K.; Kukharenko, A.; Korotin, D.; Michalska, J.; Krząkała, A.; Piotrowski, J.; Kurmaev, E.; et al. Surface characterisation of Ti–15Mo alloy modified by a PEO process in various suspensions. Mater. Sci. Eng. C 2014, 39, 259–272. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, J.; Wang, C.; Ma, W.; Lee, M.H. Effect of solvent composition on the bioactive coating fabricated by micro-arc oxidation combined with electrophoretic deposition. Mater. Res. Innovations 2016, 20, 451–457. [Google Scholar] [CrossRef]
- Eraković, S.; Janković, A.; Veljović, D.; Palcevskis, E.; Mitrić, M.; Stevanović, T.; Janaćković, D.; Mišković-Stanković, V. Corrosion stability and bioactivity in Simulated Body Fluid of silver/hydroxyapatite and silver/hydroxyapatite/lignin coatings on titanium obtained by electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1633–1643. [Google Scholar] [CrossRef]
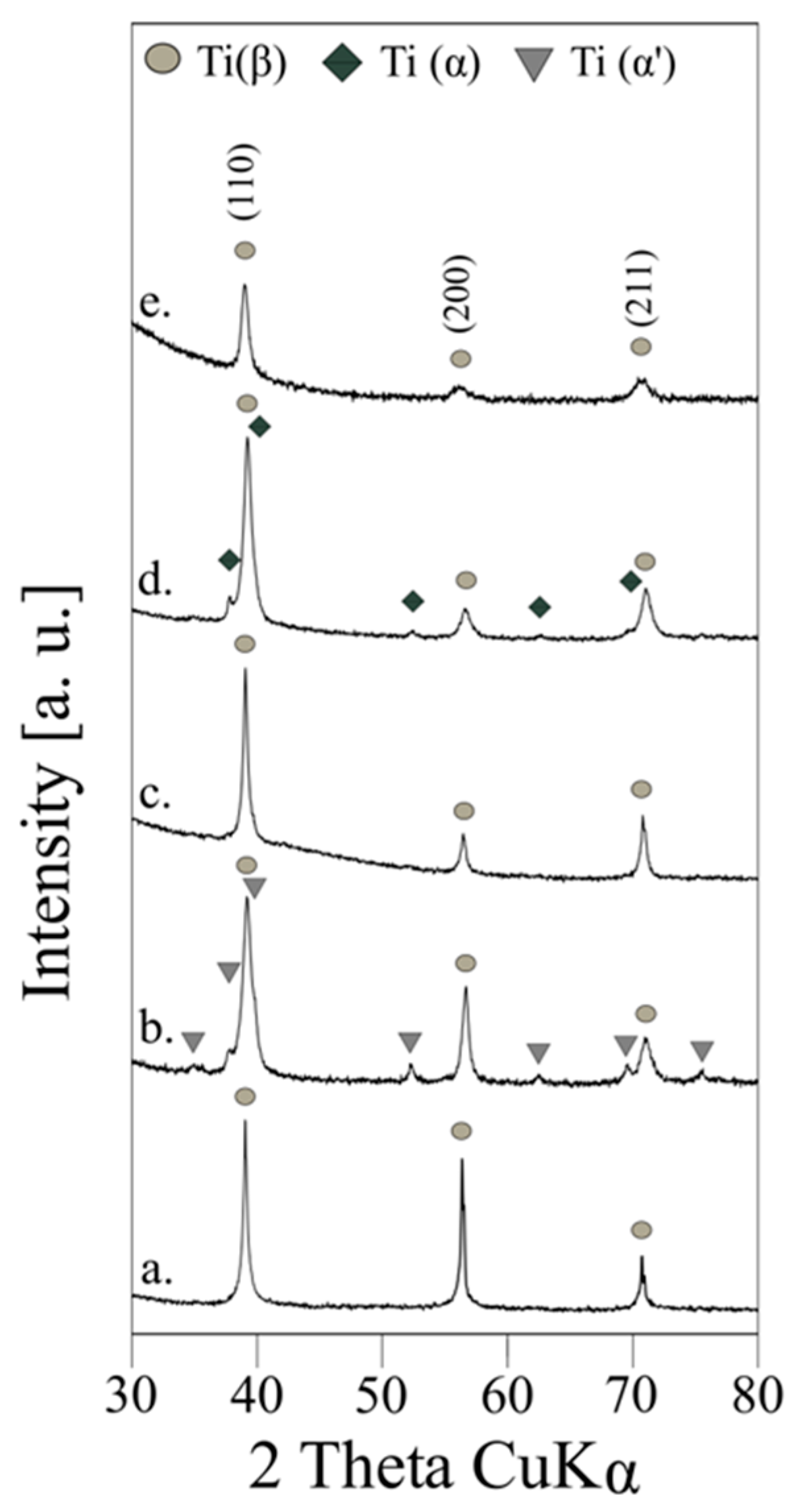

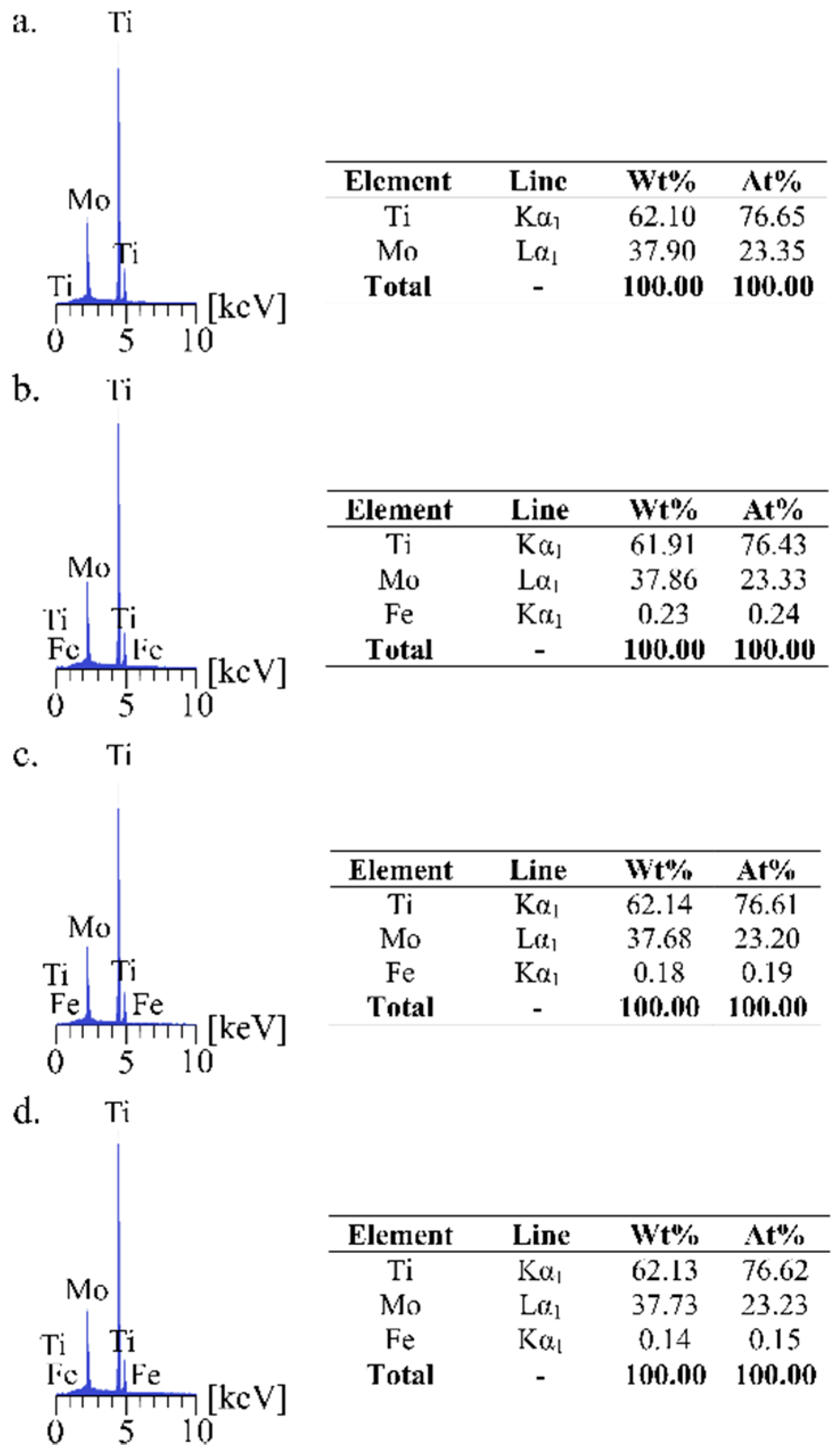
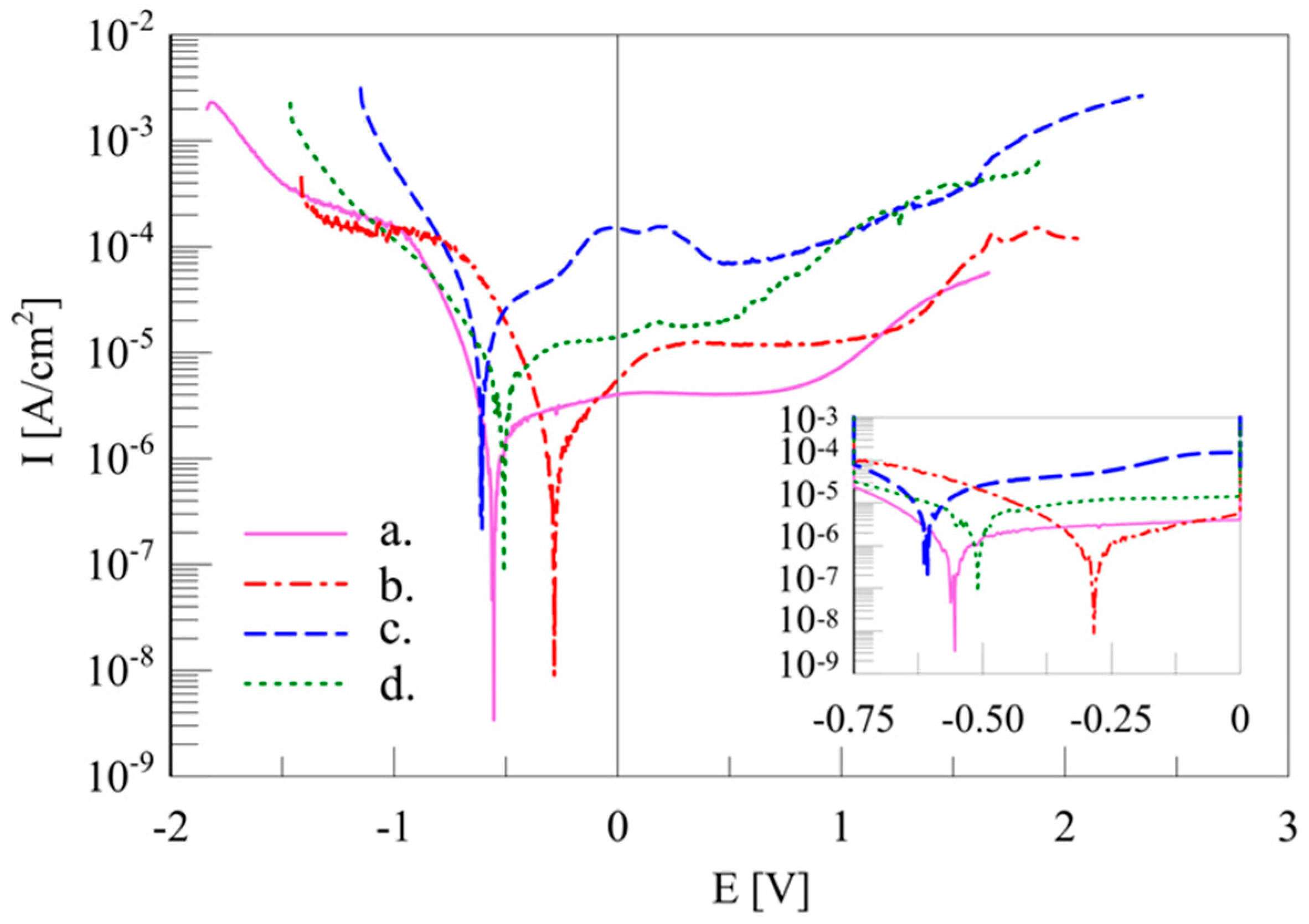


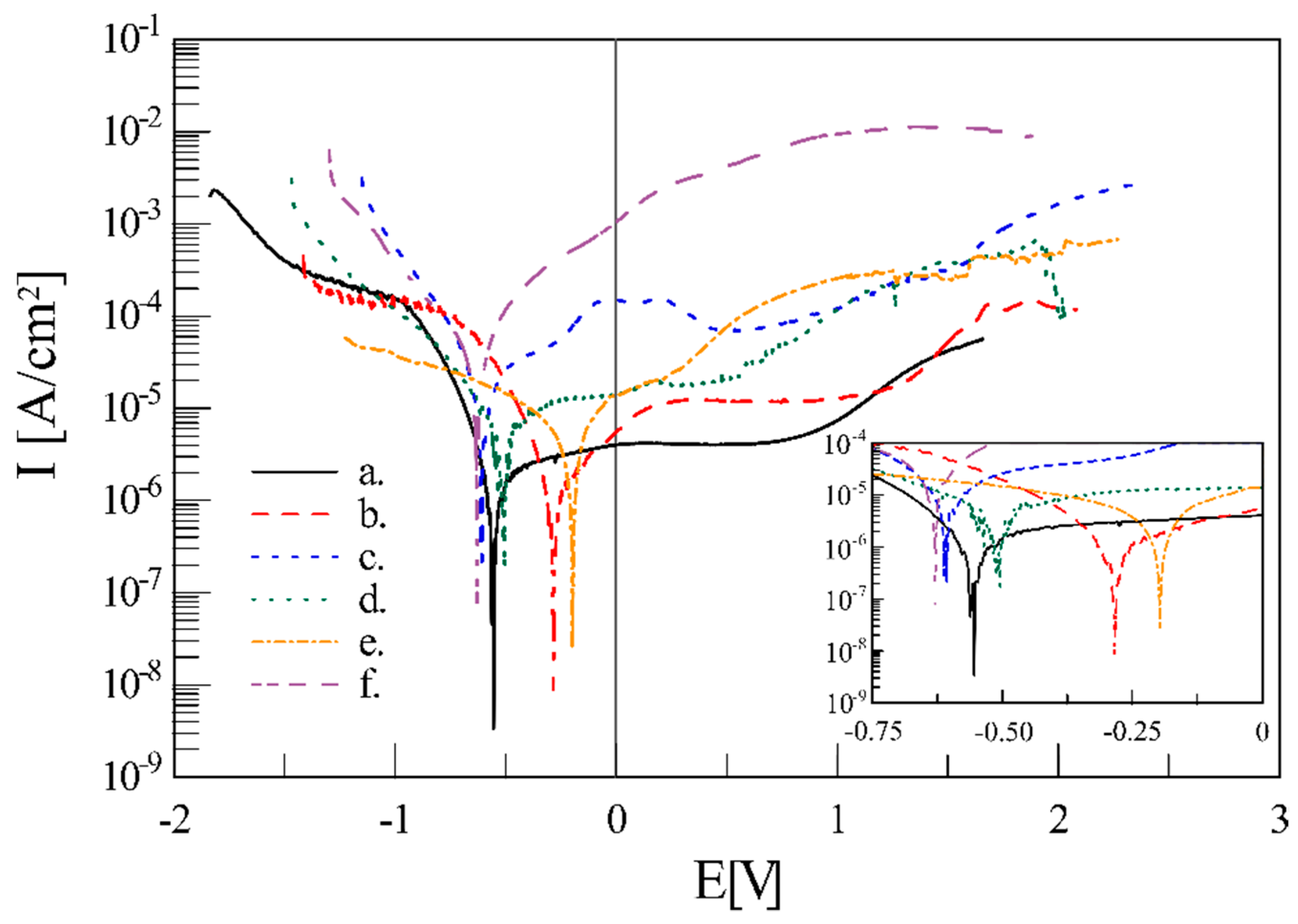
| Material | Sig | Rwp (%) | Rexp (%) | Phase Type | A (%) | Structural Parameters | ||
|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | V (Å3) | ||||||
| AM | 2.666797 | 10.484024 | 3.9313207 | Ti(β) | 100.0 | 3.2619(1) | - | 34.707(3) |
| AMA800 | 1.573098 | 6.3340626 | 4.0264897 | Ti(β) | 82.98 | 3.2475(1) | - | 34.250(3) |
| Ti(α’) | 17.02 | 2.9712(11) | 4.7592(18) | 36.385(41) | ||||
| HP | 2.3671894 | 8.234684 | 3.4786754 | Ti(β) | 100.0 | 3.2570(0) | - | 34.550(1) |
| CP | 1.7763894 | 5.885216 | 3.3130217 | Ti(β) | 94.80 | 3.2453(0) | - | 34.178(1) |
| Ti(α) | 5.20 | 2.9700(10) | 4.7540(28) | 36.316(45) | ||||
| CP + NH4HCO3 | 1.1901675 | 5.851319 | 4.9163833 | Ti(β) | 100.0 | 3.2615(2) | - | 34.659(7) |
| Material | ρth(g/cm3) | ρcal(g/cm3) | P (%) |
|---|---|---|---|
| AM | 6.695 ± 0.164 | 6.674 ± 0.180 | 0.31 ± 0.06 |
| AMA800 | 6.695 ± 0.164 | 6.691 ± 0.167 | 0.06 ± 0.01 |
| HP | 6.700 ± 0.177 | 6.684 ± 0.195 | 0.24 ± 0.08 |
| CP | 6.688 ± 0.219 | 5.040 ± 0.671 | 24.64 ± 0.45 |
| CP + NH4HCO3 | 6.690 ± 0.095 | 3.046 ± 0.578 | 54.47 ± 0.67 |
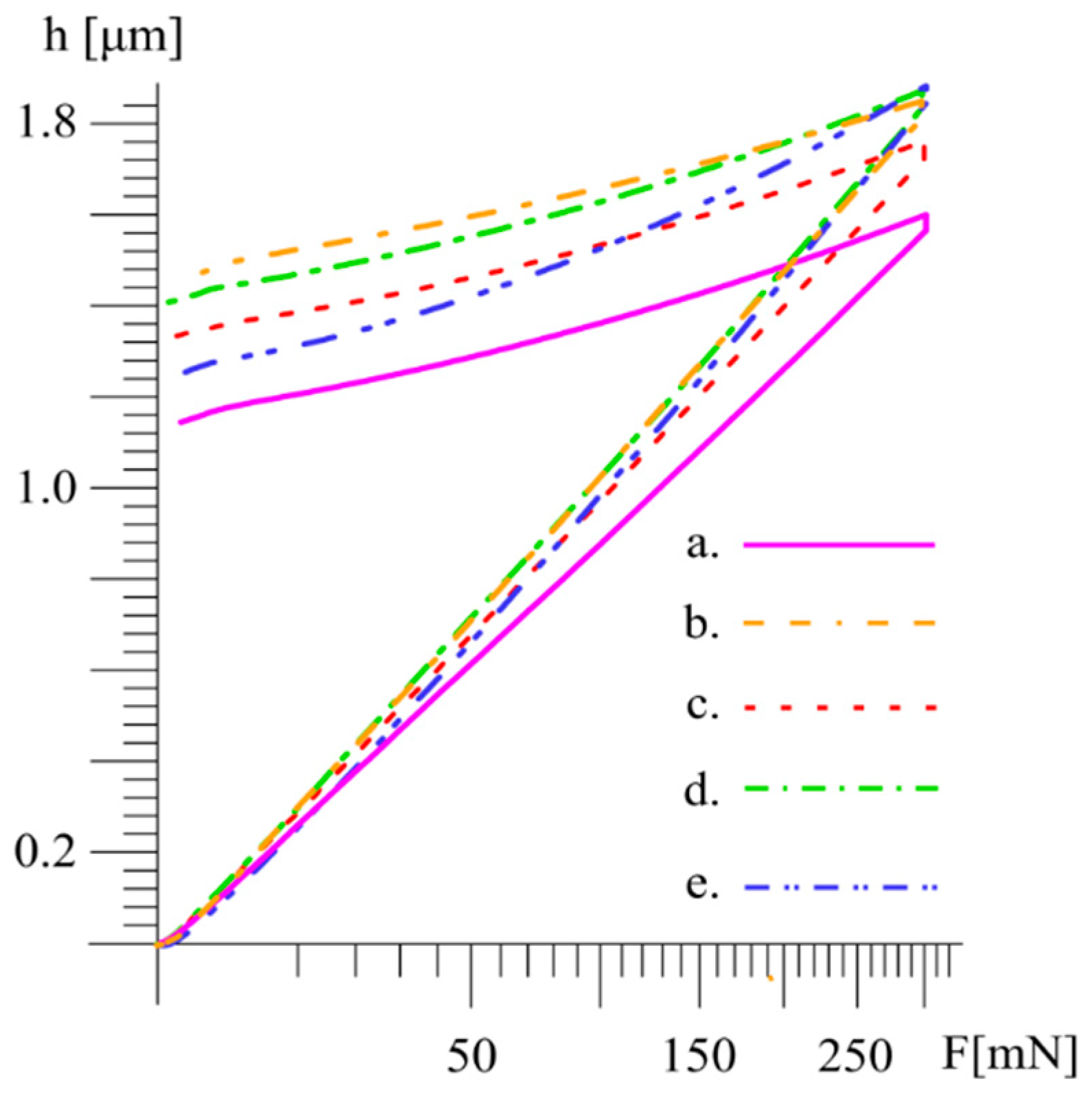
| Material | HV0.3 ± σ | HM ± σ (N/mm2) | EIT ± σ (GPa) |
|---|---|---|---|
| AM | 547 ± 7 | 4289.4 ± 28.2 | 141.2 ± 2.6 |
| AMA800 | 366 ± 6 | 3270.7 ± 47.9 | 142.8 ± 4.3 |
| HP | 454 ± 6 | 3531.2 ± 32.7 | 127.3 ± 1.2 |
| CP | 366 ± 19 | 3093.1 ± 111.4 | 104.9 ± 10.5 |
| CP + NH4HCO3 | 397 ± 17 | 2880.7 ± 184.3 | 69.5 ± 8.9 |
| Material | Ecorr | Icorr | W by Polarization | W by Weight Loss |
|---|---|---|---|---|
| (V) | (µA·cm−2) | (µg·day−1) | (µg·day−1) | |
| AMA800 | −0.556(2) | 0.3913(66) | 4.7(1) | 3.5(5) |
| HP | −0.276(6) | 0.3333(355) | 4.0(4) | 1.7(2) |
| CP | −0.610(3) | 4.506(705) | 53.7(8) | 73.3(6) |
| CP + NH4HCO3 | −0.511(4) | 1.139(313) | 13.6(2) | 42.3(8) |
| Material | Ecorr | Icorr | W by Polarization | W by Weight Loss |
|---|---|---|---|---|
| (V) | (µA·cm−2) | (µg·day−1) | (µg·day−1) | |
| CP | −0.610(3) | 4.506(705) | 53.7(8) | 73.3(6) |
| CP + MAO | −0.194(2) | 0.8367(376) | 4.4(2) | 2.8(5) |
| CP + MAO + EPD | −0.615(6) | 7.519(725) | 39.5(4) | 6.9(3) |
| Material | Diiodomethane CA (°) | Glycerol CA (°) | Surface Free Energy (mN/m) | Disperse (mN/m) | Polar (mN/m) |
|---|---|---|---|---|---|
| AMA800 | 63.62 ± 9.44 | 62.72 ± 11.30 | 35.56 ± 6.31 | 27.84 ± 1.71 | 7.56 ± 5.26 |
| HP | 60.47 ± 5.68 | 69.98 ± 6.78 | 31.91 ± 3.16 | 28.14 ± 3.42 | 3.61 ± 1.94 |
| CP | 53.43 ± 13.61 | 28.09 ± 4.38 | 56.34 ± 1.81 | 32.30 ± 7.75 | 27.37 ± 4.00 |
| CP + NH4HCO3 | - | 54.34 ± 10.05 | - | - | - |
| CP + MAO | 64.64 ± 1.66 | 50.54 ± 14.46 | 42.56 ± 9.81 | 25.91 ± 0.94 | 16.65 ± 5.38 |
| CP + MAO + EPD | 54.93 ± 9.31 | 41.58 ± 3.35 | 48.84 ± 1.86 | 31.85 ± 5.31 | 16.99 ± 6.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochacka, P.; Miklaszewski, A.; Kowalski, K.; Jurczyk, M. Influence of the Processing Method on the Properties of Ti-23 at.% Mo Alloy. Metals 2019, 9, 931. https://doi.org/10.3390/met9090931
Sochacka P, Miklaszewski A, Kowalski K, Jurczyk M. Influence of the Processing Method on the Properties of Ti-23 at.% Mo Alloy. Metals. 2019; 9(9):931. https://doi.org/10.3390/met9090931
Chicago/Turabian StyleSochacka, Patrycja, Andrzej Miklaszewski, Kamil Kowalski, and Mieczyslaw Jurczyk. 2019. "Influence of the Processing Method on the Properties of Ti-23 at.% Mo Alloy" Metals 9, no. 9: 931. https://doi.org/10.3390/met9090931
APA StyleSochacka, P., Miklaszewski, A., Kowalski, K., & Jurczyk, M. (2019). Influence of the Processing Method on the Properties of Ti-23 at.% Mo Alloy. Metals, 9(9), 931. https://doi.org/10.3390/met9090931