Cooling Behavior and Microstructure of Semisolid A201 Aluminum Alloy Prepared by the SEED Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Thermodynamic Analyses
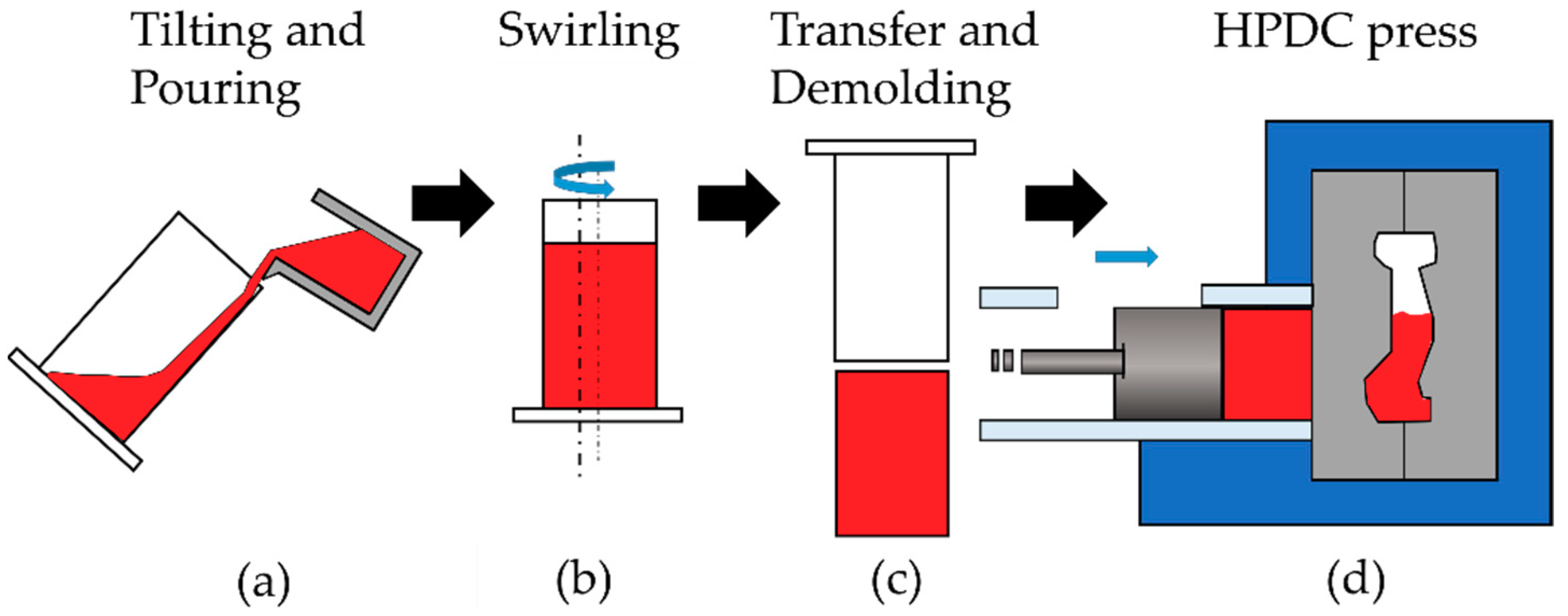
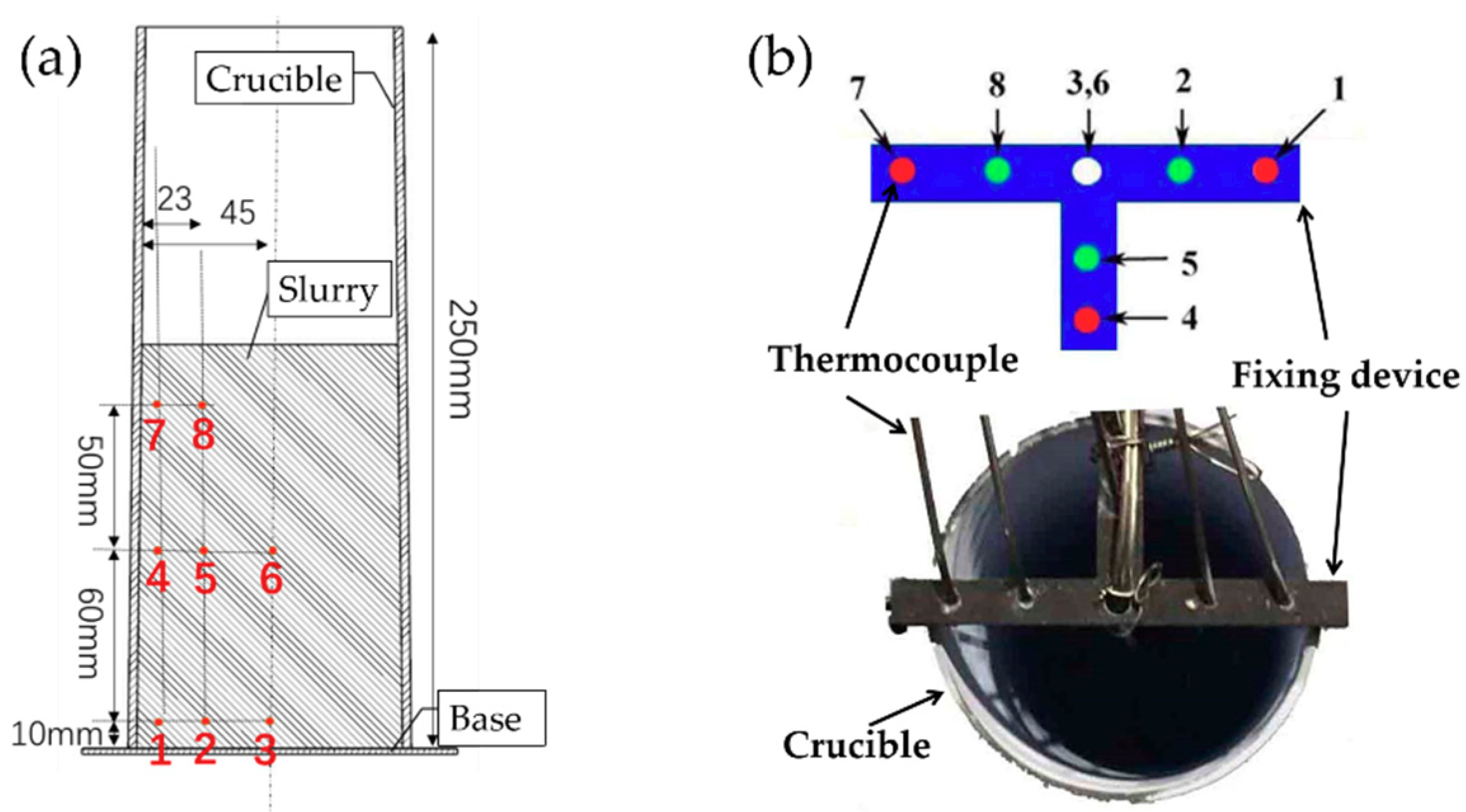
2.2. Slurry Preparation and Temperature Data Collection
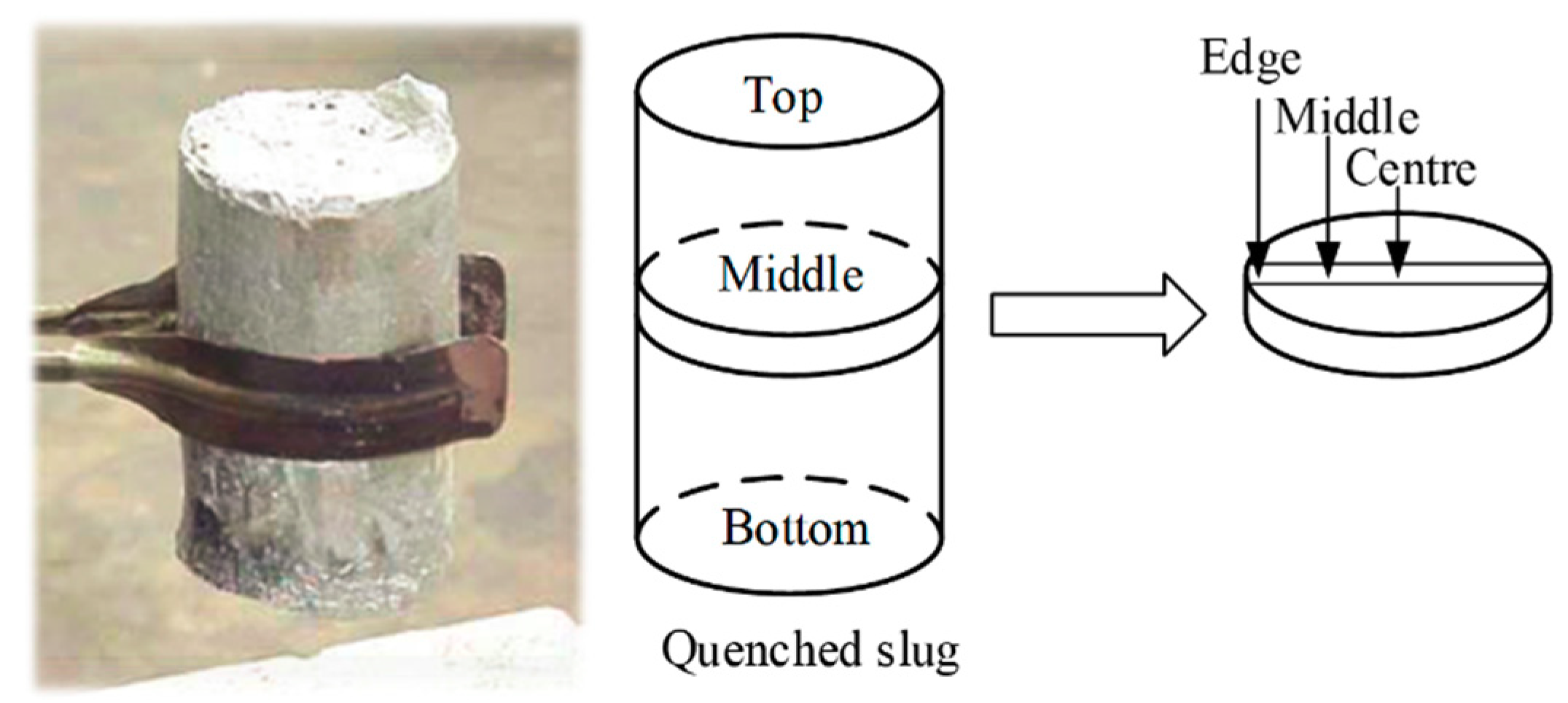
2.3. Microstrucutral Observations
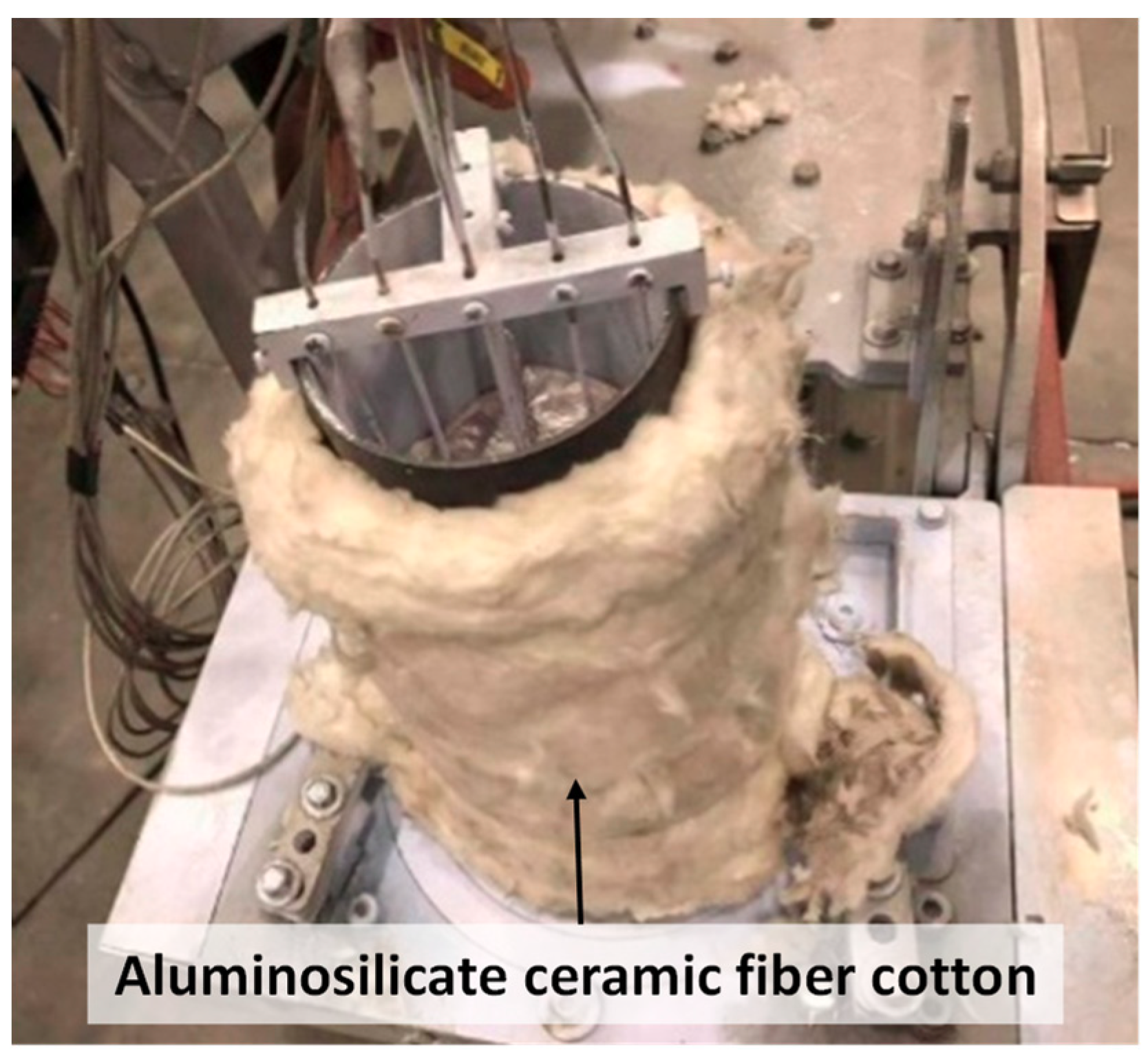
2.4. Modification of the SEED Process
3. Results and Discussion
3.1. Process Window of A201 Alloy
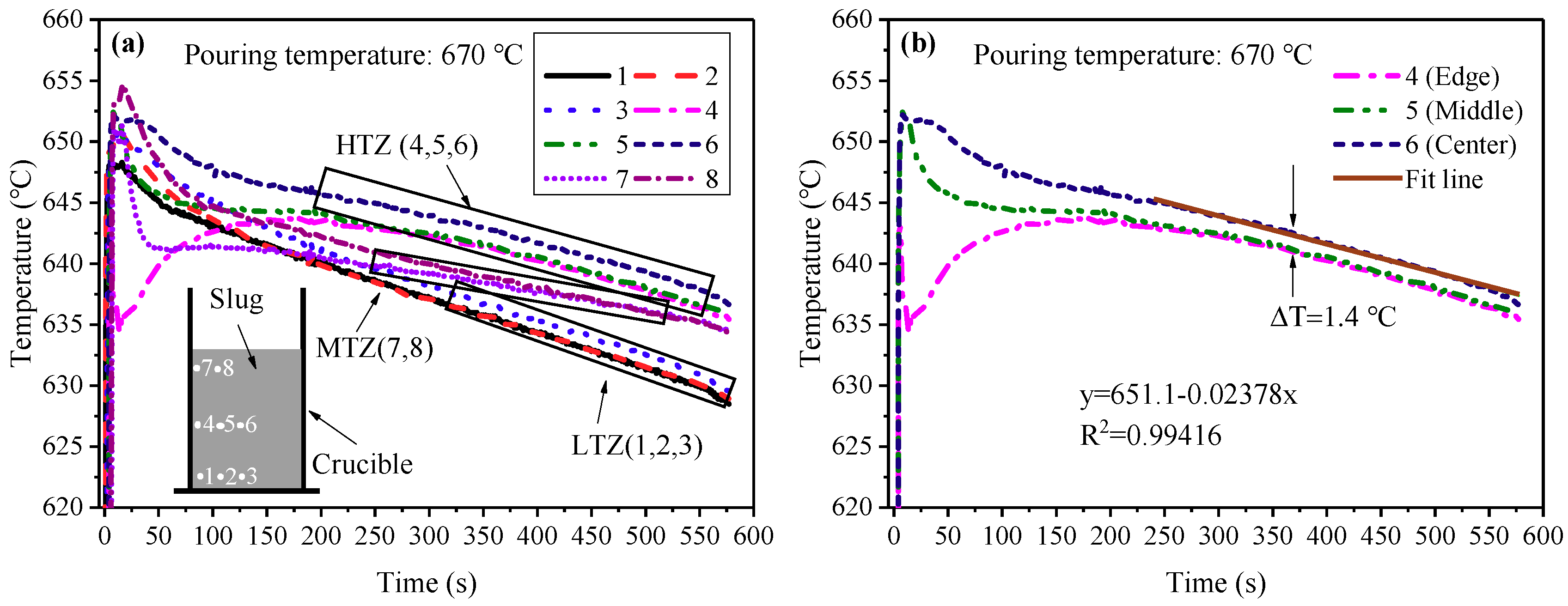
3.2. Cooling Behavior of the Slurry duing the SEED Process
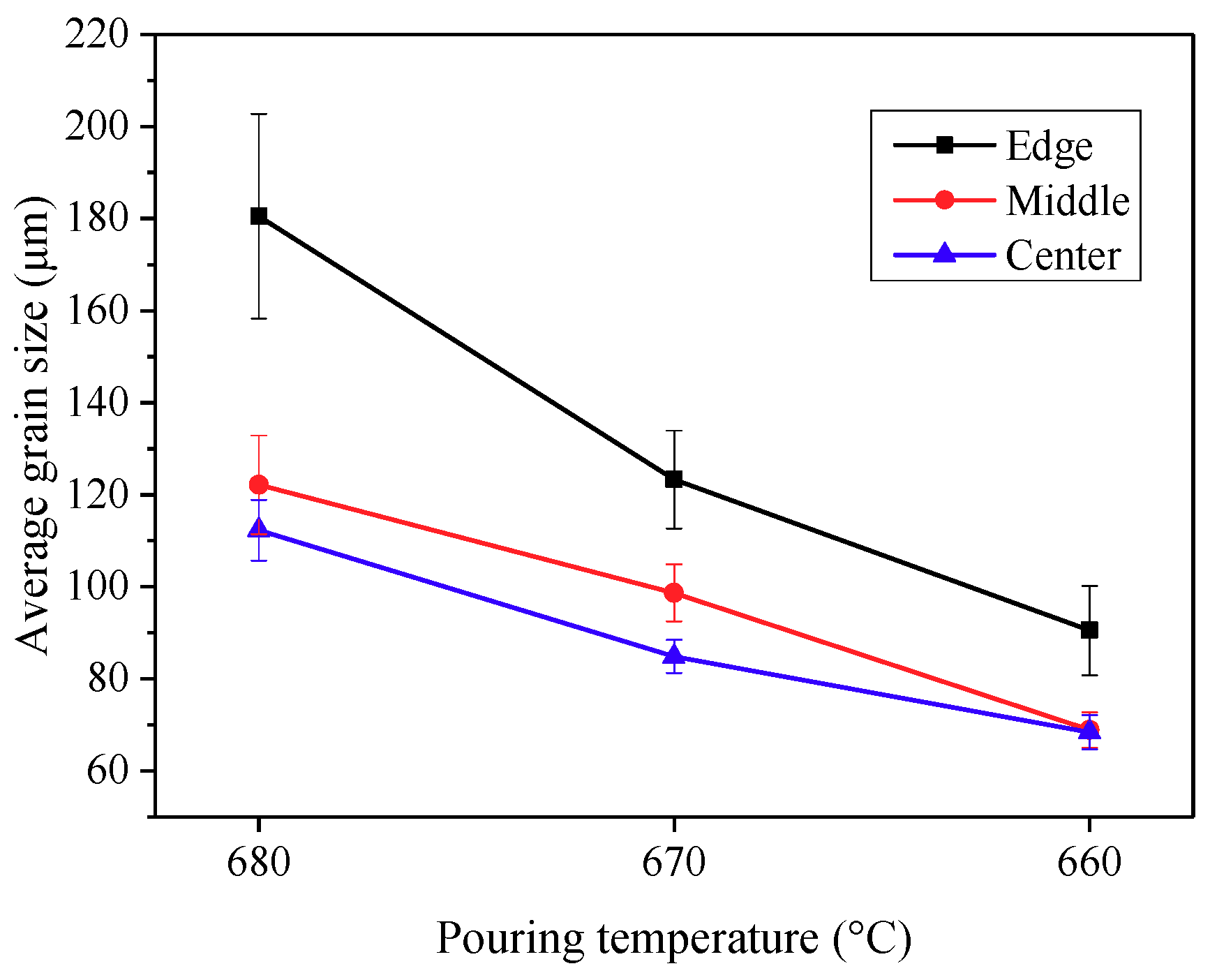
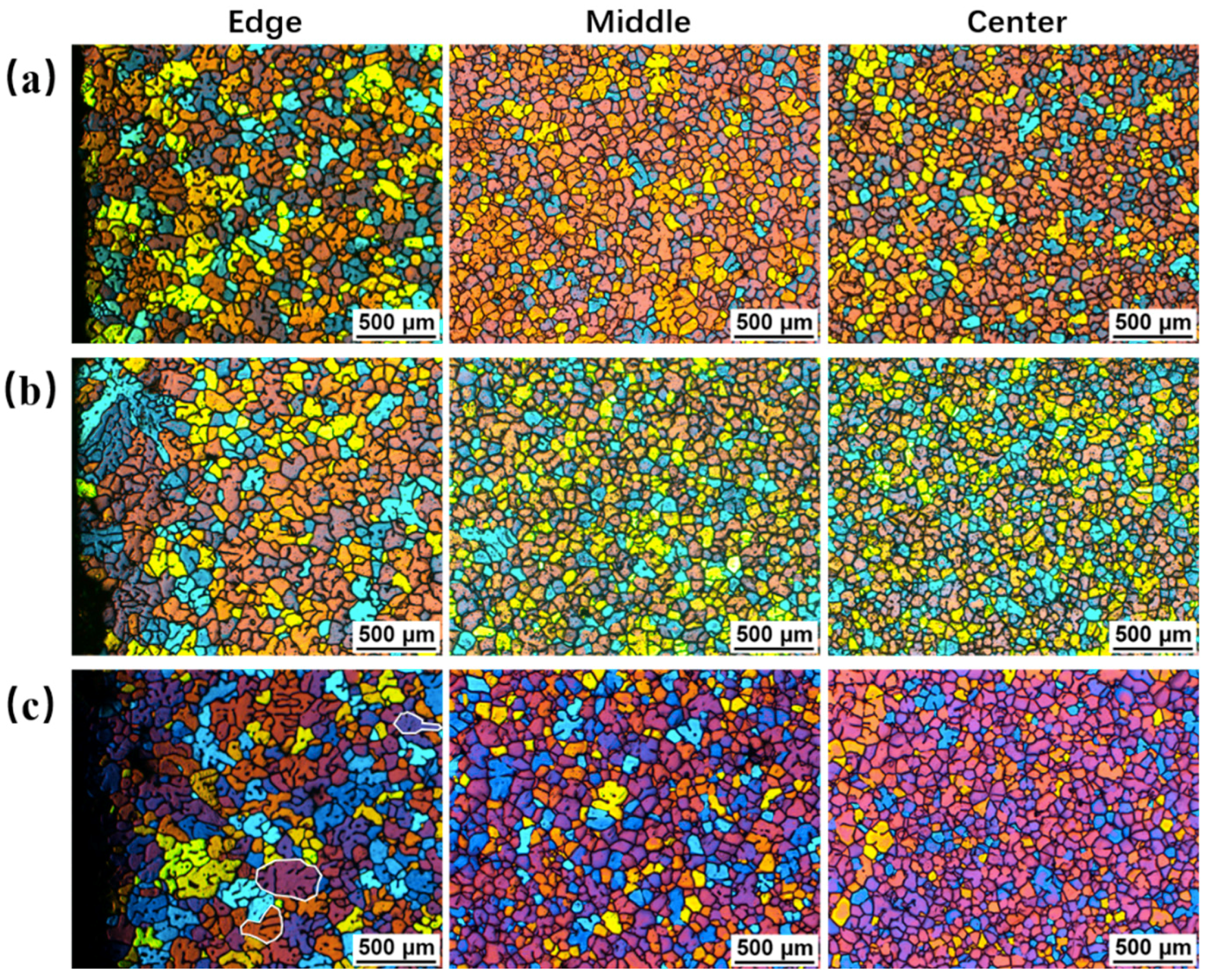
3.3. Effects of Pouring Temperature on Semisolid Microstructure
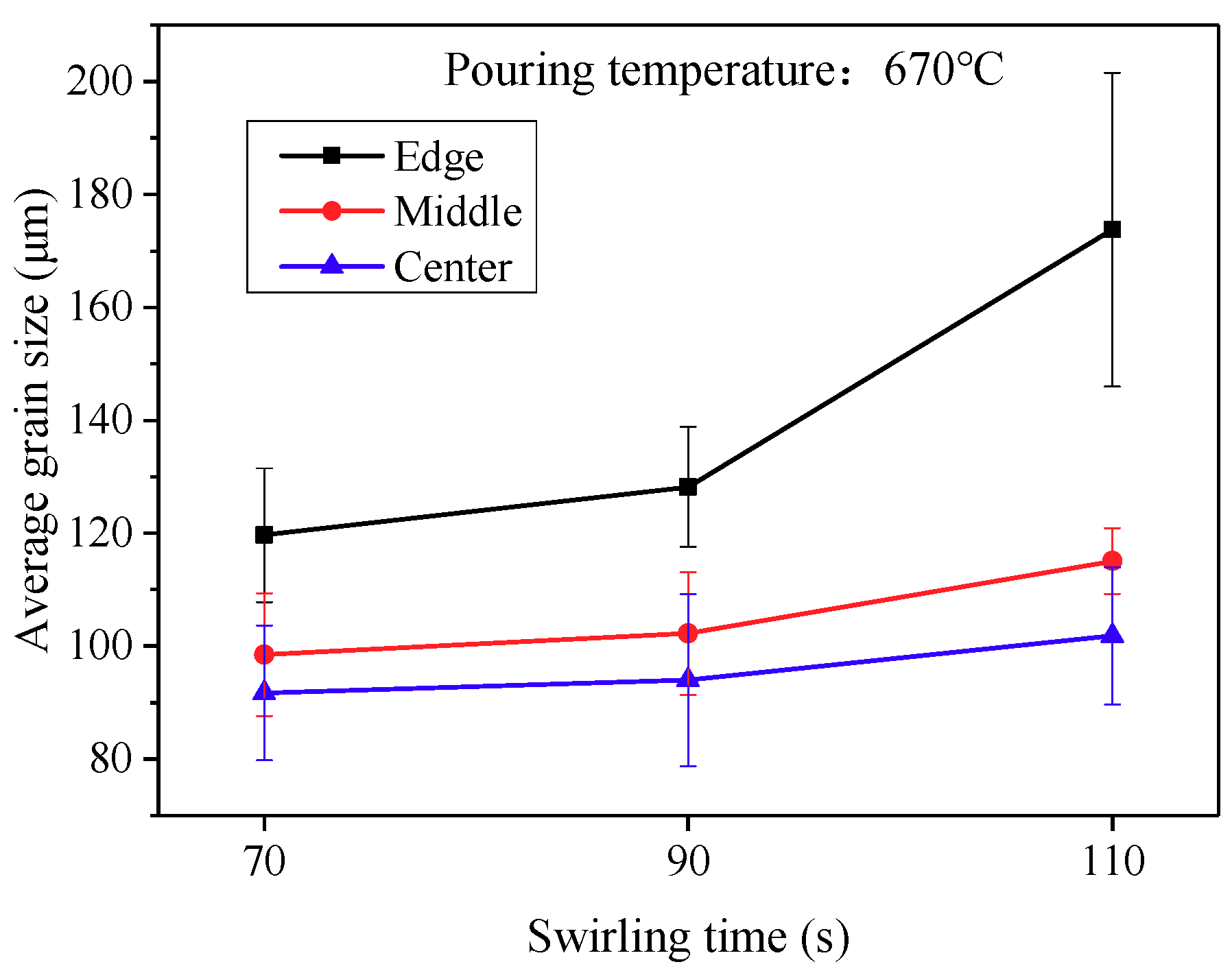
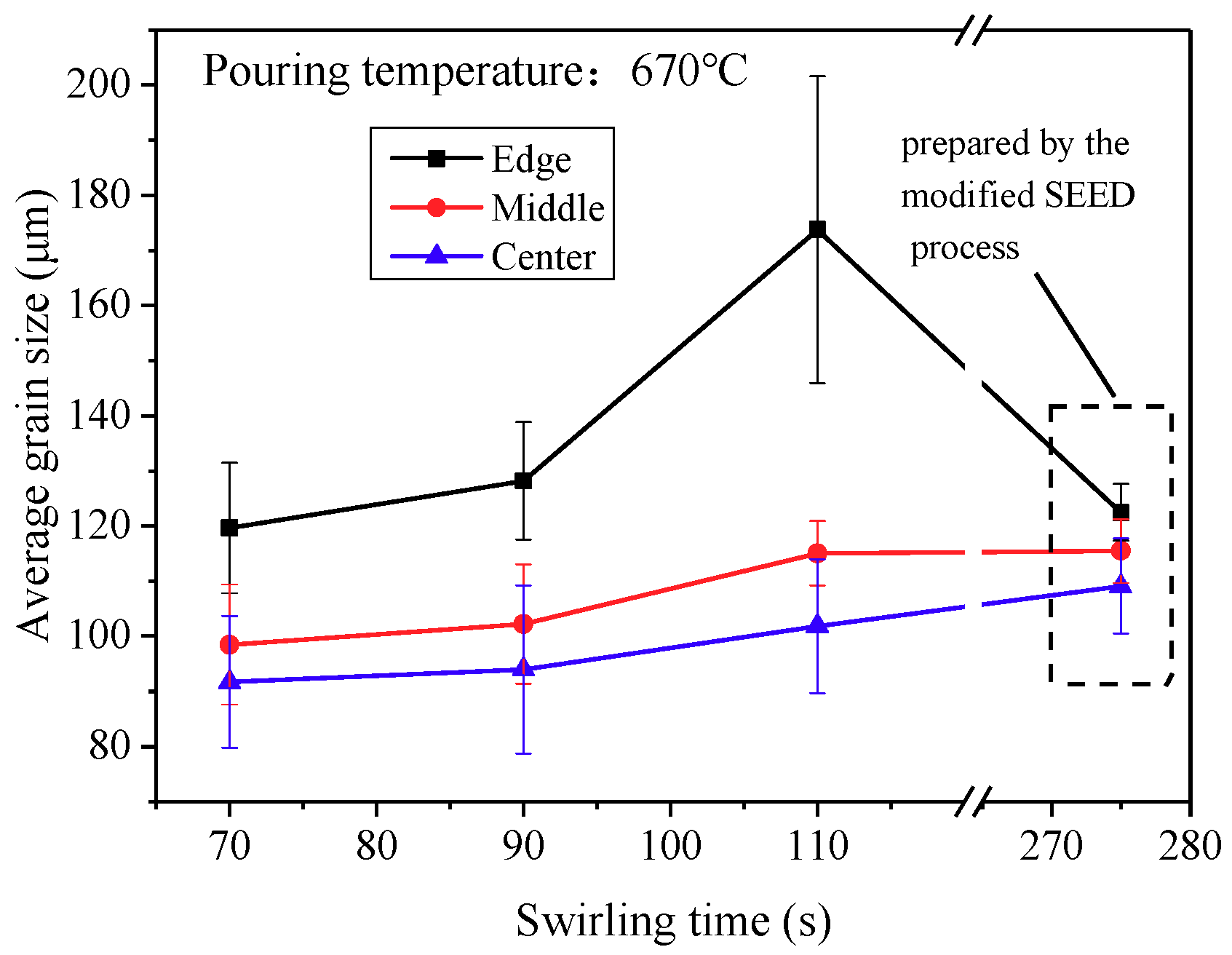
3.4. Effects of Processing Time on Semisolid Microstrutures
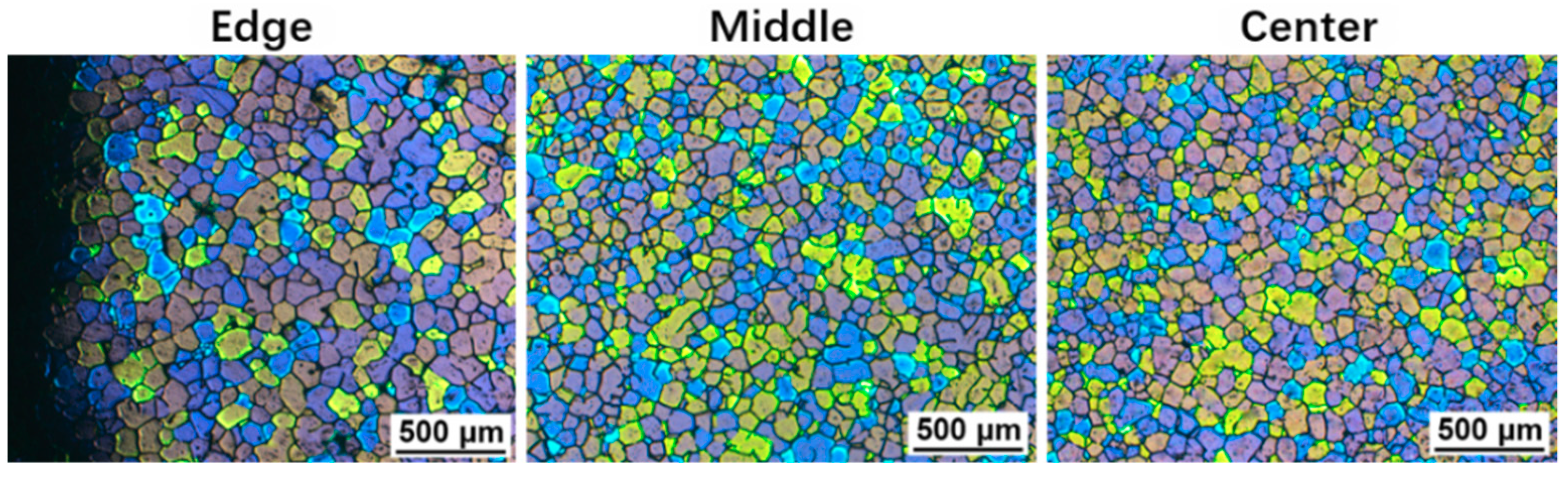
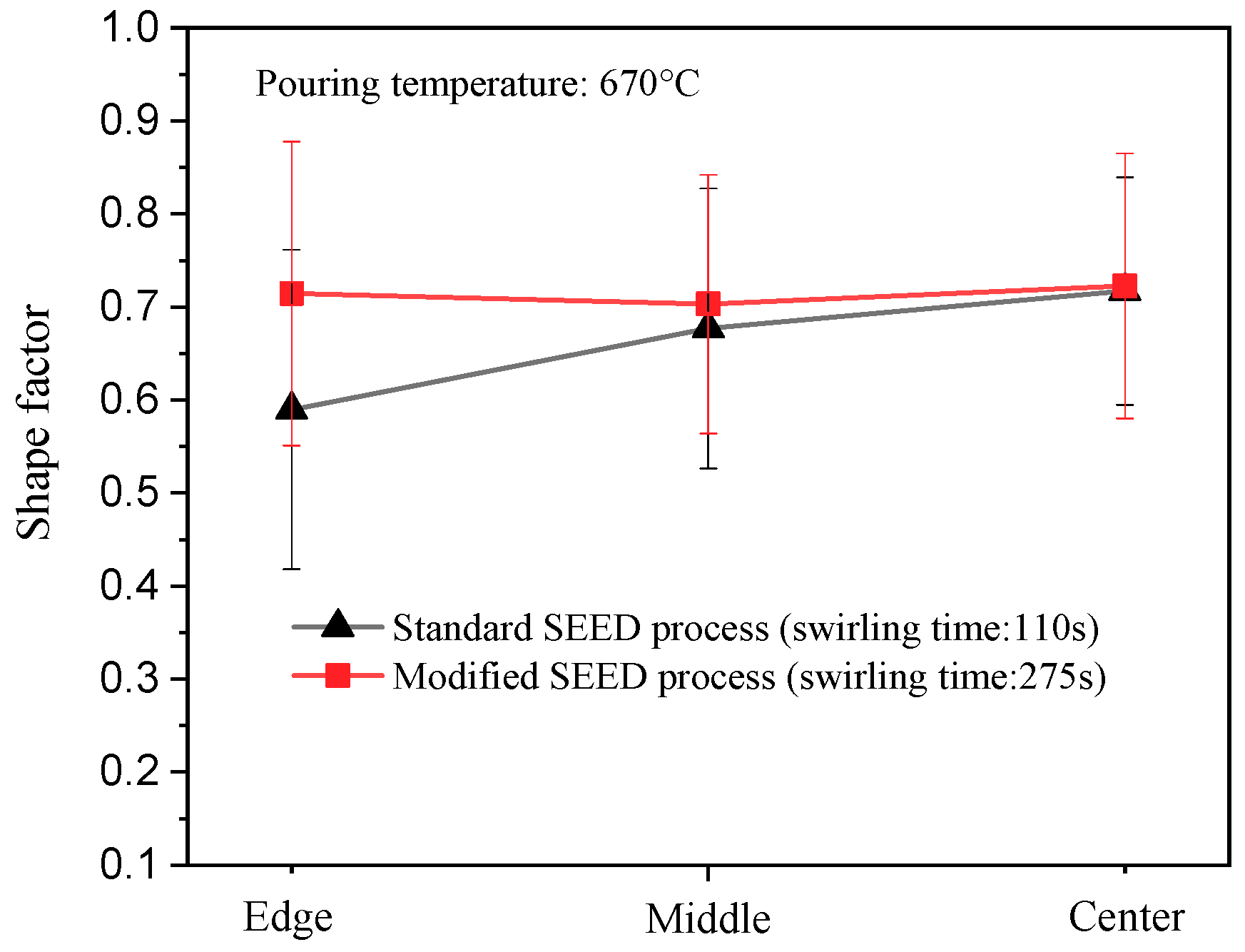
3.5. Cooling Behavior and Microstructures in the Modified SEED Process
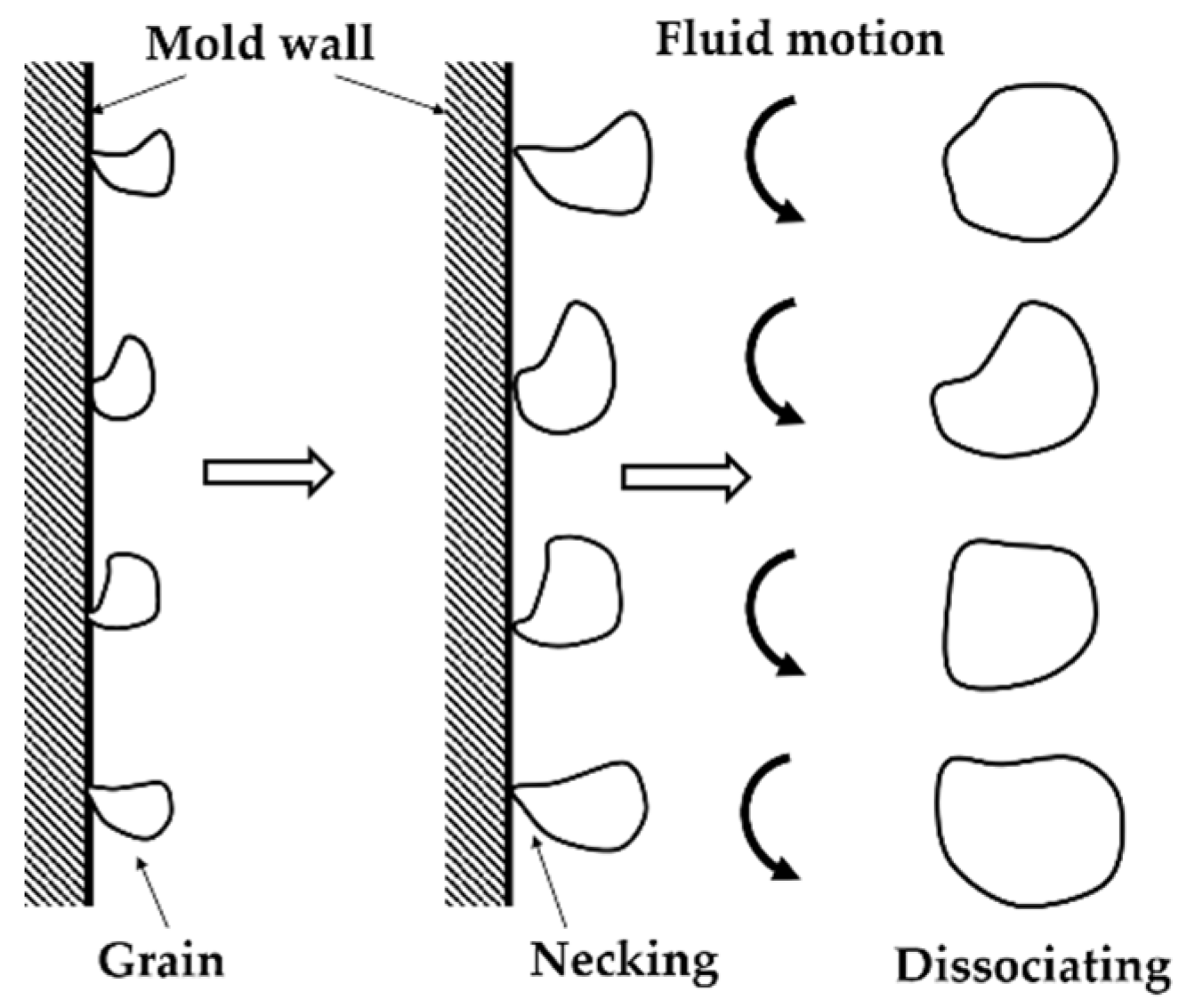
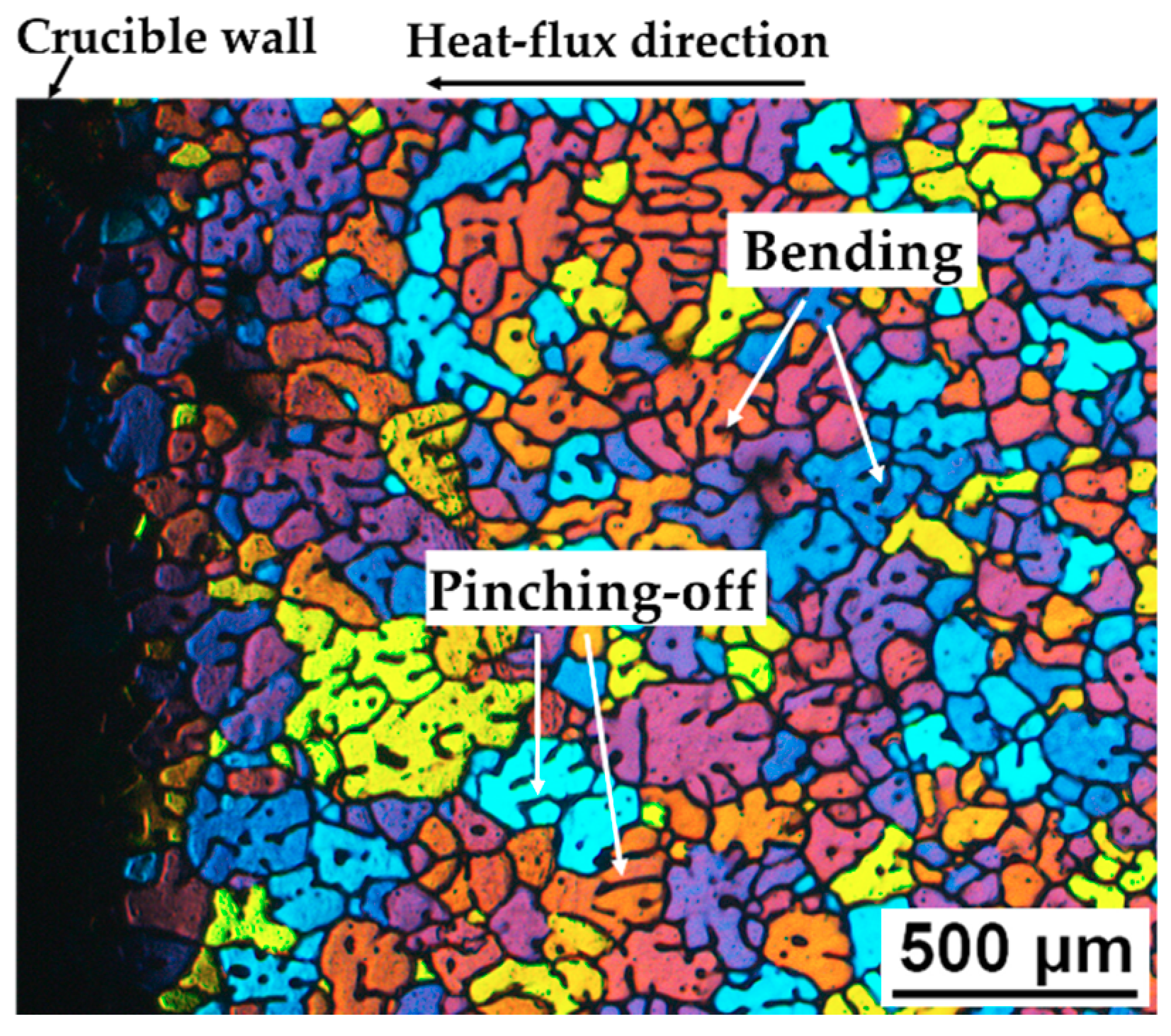
3.6. Microstructure Formation Mechanism of the SEED Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flemings, M.C.; Riek, R.G.; Young, K.P. Rheocasting. Mater. Sci. Eng. 1976, 25, 103–117. [Google Scholar] [CrossRef]
- Kirkwood, D.H.; Suéry, M.; Kapranos, P.; Atkinson, H.V.; Young, K.P. Semi-Solid Processing of Alloys, 1st ed.; Springer: London, UK, 2010. [Google Scholar] [CrossRef]
- Zoqui, E.J. 5.09—Alloys for Semisolid Processing. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 163–190. [Google Scholar] [CrossRef]
- Fan, Z. Semisolid metal processing. Int. Mater. Rev. 2002, 47, 49–85. [Google Scholar] [CrossRef]
- Li, D.Q.; Zhang, F.; Midson, S.P.; Liang, X.K.; Yao, H. Recent Developments of Rheo-Diecast Components for Transportation Markets. Solid State Phenom. 2019, 285, 417–422. [Google Scholar] [CrossRef]
- Wannasin, J.; Fuchs, M.; Lee, J.Y.; Lee, C.U.; Narasimha Rao, T.V.L.; Flemings, M.C. GISS Technology: Principle and Applications in Die Casting. Solid State Phenom. 2019, 285, 470–475. [Google Scholar] [CrossRef]
- UBE Industries Ltd. Method and Apparatus of Shaping Semisolid Metals. European Patent EP0745694A1, 4 December 1996. [Google Scholar]
- Qi, M.F.; Kang, Y.L.; Zhu, G.M. Microstructure and properties of rheo-HPDC Al-8Si alloy prepared by air-cooled stirring rod process. Trans. Nonferrous Met. Soc. China 2017, 27, 1939–1946. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, Y.L.; Qi, M.F.; Zhang, H.H.; Zhu, G.M. R-HPDC Process with Forced Convection Mixing Device for Automotive Part of A380 Aluminum Alloy. Materials 2014, 7, 3084–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.F.; Kang, Y.L.; Zhou, B.; Liao, W.N.; Zhu, G.M.; Li, Y.D.; Li, W.R. A forced convection stirring process for Rheo-HPDC aluminum and magnesium alloys. J. Mater. Process. Technol. 2016, 234, 353–367. [Google Scholar] [CrossRef]
- Janudom, S.; Wannasin, J.; Basem, J.; Wisutmethangoon, S. Characterization of flow behavior of semi-solid slurries containing low solid fractions in high-pressure die casting. Acta Materialia 2013, 61, 6267–6275. [Google Scholar] [CrossRef]
- Hu, X.G.; Zhu, Q.; Midson, S.P.; Atkinson, H.V.; Dong, H.B.; Zhang, F.; Kang, Y.L. Blistering in semi-solid die casting of aluminium alloys and its avoidance. Acta Mater. 2017, 124, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Midson, S.P. Semi-solid moulding: Competition to cast and machine from forging in making automotive complex components. Trans. Nonferrous Met. Soc. China 2010, 20, 1042–1047. [Google Scholar] [CrossRef]
- Wallace, G.; Jackson, A.P.; Midson, S.P.; Zhu, Q. High-quality aluminum turbocharger impellers produced by thixocasting. Trans. Nonferrous Met. Soc. China 2010, 20, 1786–1791. [Google Scholar] [CrossRef]
- Pola, A. Correlation between Microstructure and Properties of Semi-Solid Products. Solid State Phenom. 2019, 285, 12–23. [Google Scholar] [CrossRef]
- Hu, X.G.; Zhu, Q.; Lu, H.X.; Zhang, F.; Li, D.Q.; Midson, S.P. Microstructural evolution and thixoformability of semi-solid aluminum 319s alloy during re-melting. J. Alloys Compd. 2015, 649, 204–210. [Google Scholar] [CrossRef]
- Winklhofer, J. Semi-Solid Casting of Aluminium from an Industrial Point of View. Solid State Phenom. 2019, 285, 24–30. [Google Scholar] [CrossRef]
- Kapranos, P. Thixoforming of Aluminum A201-Expectations and Fulfilment. Solid State Phenom. 2019, 285, 476–488. [Google Scholar] [CrossRef]
- Langlais, J.; Lemieux, A. The SEED Technology for Semi-Solid Processing of Aluminum Alloys: A Metallurgical and Process Overview. Solid State Phenom. 2006, 116–117, 472–477. [Google Scholar] [CrossRef]
- Côté, P.; Larouche, M.E.; Chen, X.G. New Developments with the SEED Technology. Solid State Phenom. 2013, 192–193, 373–378. [Google Scholar] [CrossRef]
- Lemieux, A.; Langlais, J.; Bouchard, D.; Chen, X.G. Effect of Si, Cu and Fe on mechanical properties of cast semi-solid 206 alloys. Trans. Nonferrous Met. Soc. China 2010, 20, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.N.; Omar, M.Z.; Salleh, M.S.; Alhawari, K.S.; Kapranos, P. Semisolid Metal Processing Techniques for Nondendritic Feedstock Production. Sci. World J. 2013, 2013, 752175. [Google Scholar] [CrossRef]
- Chayong, S.; Atkinson, H.V.; Kapranos, P. Multistep induction heating regimes for thixoforming 7075 aluminium alloy. Mater. Sci. Technol. 2004, 20, 490–496. [Google Scholar] [CrossRef]
- Liu, D.; Atkinson, H.V.; Kapranos, P.; Jirattiticharoean, W.; Jones, H. Microstructural evolution and tensile mechanical properties of thixoformed high performance aluminium alloys. Mater. Sci. Eng. A 2003, 361, 213–224. [Google Scholar] [CrossRef]
- Liu, D.; Atkinson, H.V.; Kapranos, P.; Jones, H. Effect of heat treatment on properties of thixoformed high performance 2014 and 201 aluminium alloys. J. Mater. Sci. 2004, 39, 99–105. [Google Scholar] [CrossRef]
- Langlais, J.; Andrade, N.; Lemieux, A.; Chen, X.G.; Bucher, L. The Semi-Solid Forming of an Improved AA6061 Wrought Aluminum Alloy Composition. Solid State Phenom. 2008, 141–143, 511–516. [Google Scholar] [CrossRef]
- Hu, X.G.; Zhu, Q.; Atkinson, H.V.; Lu, H.X.; Zhang, F.; Dong, H.B.; Kang, Y.L. A time-dependent power law viscosity model and its application in modelling semi-solid die casting of 319s alloy. Acta Mater. 2017, 124, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Kazakov, A. Alloy Compositions for Semisolid Forming. Adv. Mater. Process. 2000, 157, 31–34. [Google Scholar]
- Liu, D.; Atkinson, H.V.; Jones, H. Thermodynamic prediction of thixoformability in alloys based on the Al–Si–Cu and Al–Si–Cu–Mg systems. Acta Mater. 2005, 53, 3807–3819. [Google Scholar] [CrossRef]
- Gao, J.Z.; Zhu, Q.; Li, D.Q.; Hu, X.G.; Luo, M.; Kang, Y.L. Slurry Preparation and Hot Tearing Susceptibility of A201 Aluminum Alloy in Rheological Die Casting. Solid State Phenom. 2019, 285, 311–317. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 4th revised ed.; Trans Tech Publications: Zürich, Switzerland, 1998; pp. 5–11. [Google Scholar]
- Kang, C.G.; Seo, P.K.; Kim, B.M. Determination of Die Design Rules for Semi-Solid Die Casting Process and Its Experimental Investigation. Mater. Sci. Forum 2005, 475–479, 2533–2538. [Google Scholar] [CrossRef]
- Uggowitzer, P.J.; Kaufmann, H. Evolution of globular microstructure in new rheocasting and super rheocasting semi-solid slurries. Steel Res. Int. 2004, 75, 525–530. [Google Scholar] [CrossRef]
- Chalmers, B. Principles of Solidification. In Applied Solid State Physics; Low, W., Schieber, M., Eds.; Springer: Boston, MA, USA, 1970; pp. 161–170. [Google Scholar] [CrossRef]
- Ohno, A. Solidification—The Separation Theory and Its Practical Applications, 1st ed.; Springer: Berlin, Germany, 1987. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Morphological Stability of a Particle Growing by Diffusion or Heat Flow. J. Appl. Phys. 1963, 34, 323–329. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Stability of a Planar Interface During Solidification of a Dilute Binary Alloy. J. Appl. Phys. 1964, 35, 444–451. [Google Scholar] [CrossRef]
- Molenaar, J.M.M.; Katgerman, L.; Kool, W.H.; Smeulders, R.J. On the formation of the stircast structure. J. Mater. Sci. 1986, 21, 389–394. [Google Scholar] [CrossRef]
- Doherty, R.D.; Lee, H.-I.; Feest, E.A. Microstructure of stir-cast metals. Mater. Sci. Eng. 1984, 65, 181–189. [Google Scholar] [CrossRef]
- Vogel, A.; Doherty, R.D.; Cantor, B. Solidification and Casting of Metals; The Metals Society: London, UK, 1979; pp. 518–525. [Google Scholar]
- George, S.L.; Knutsen, R.D. Composition segregation in semi-solid metal cast AA7075 aluminium alloy. J. Mater. Sci. 2012, 47, 4716–4725. [Google Scholar] [CrossRef]
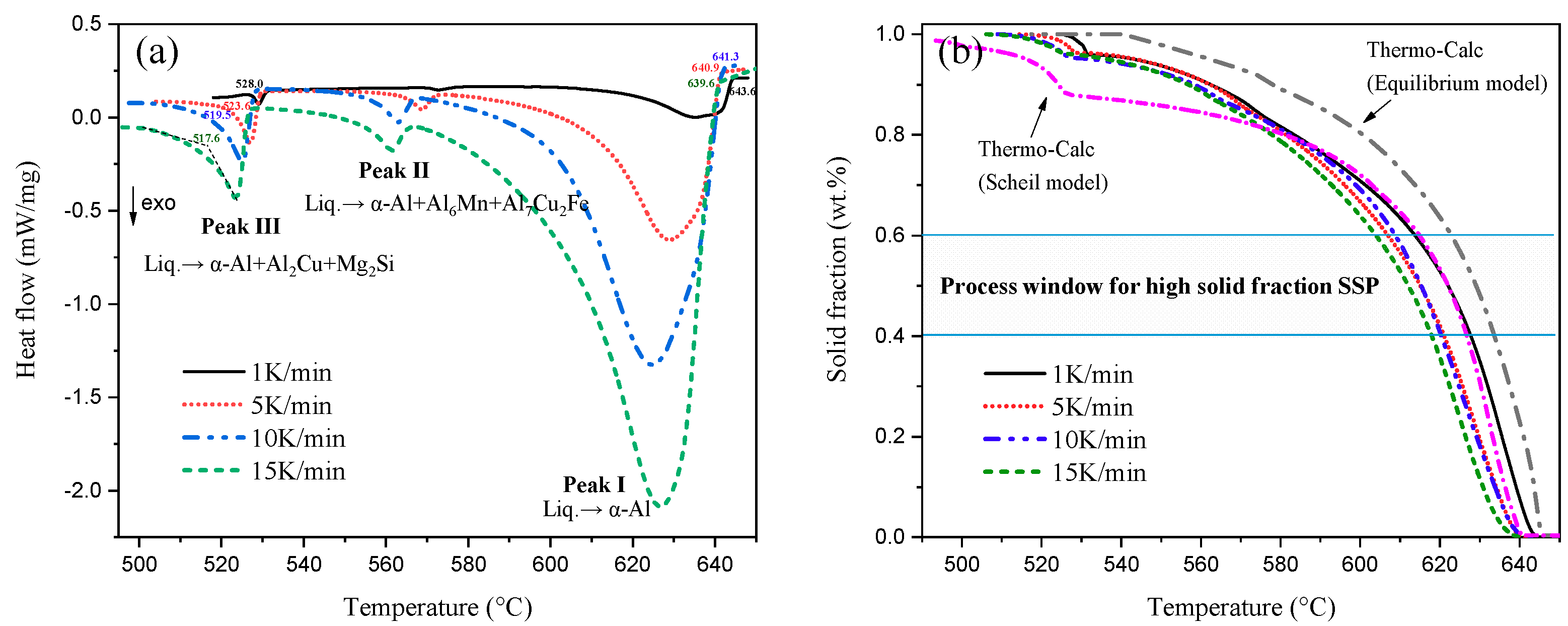
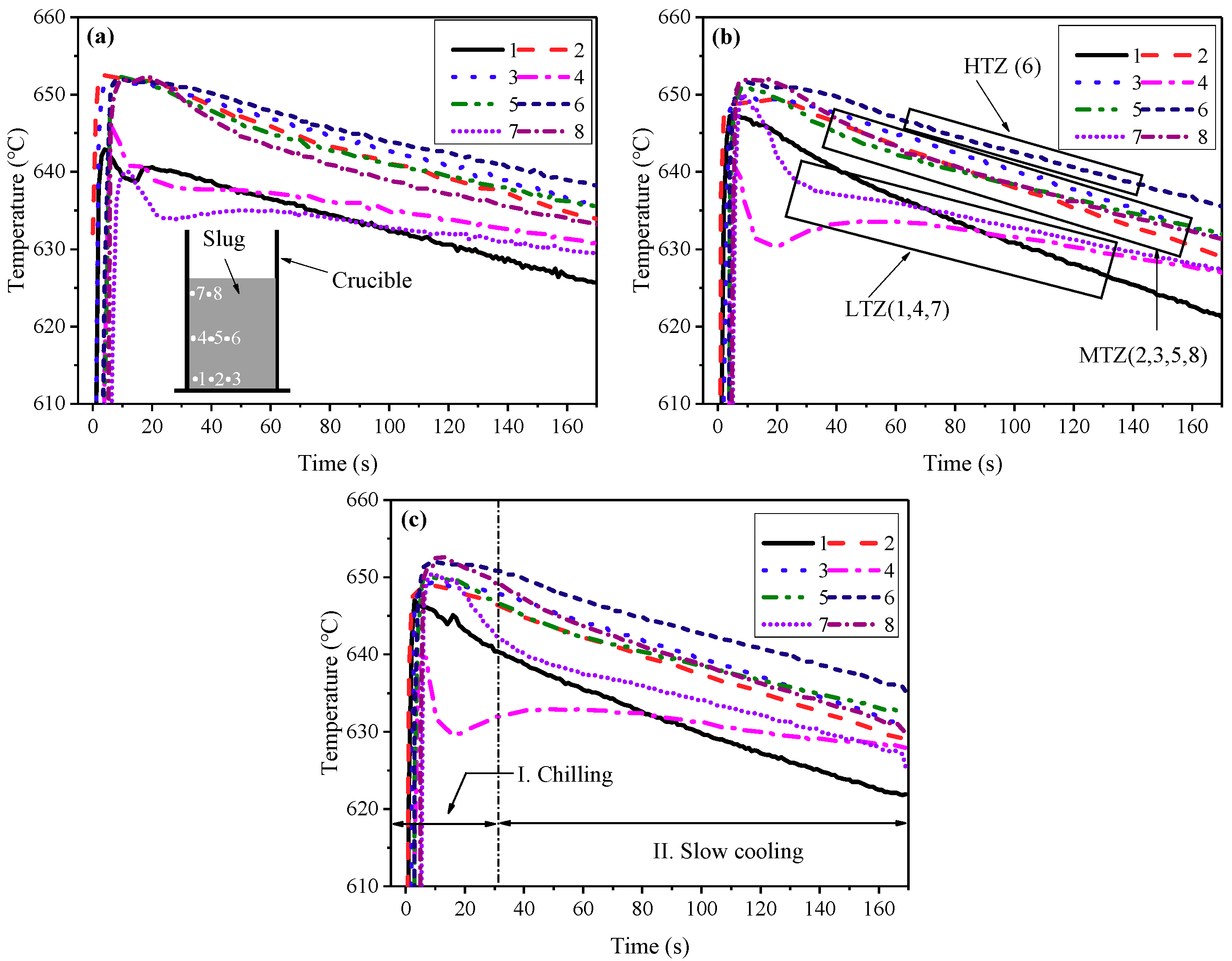
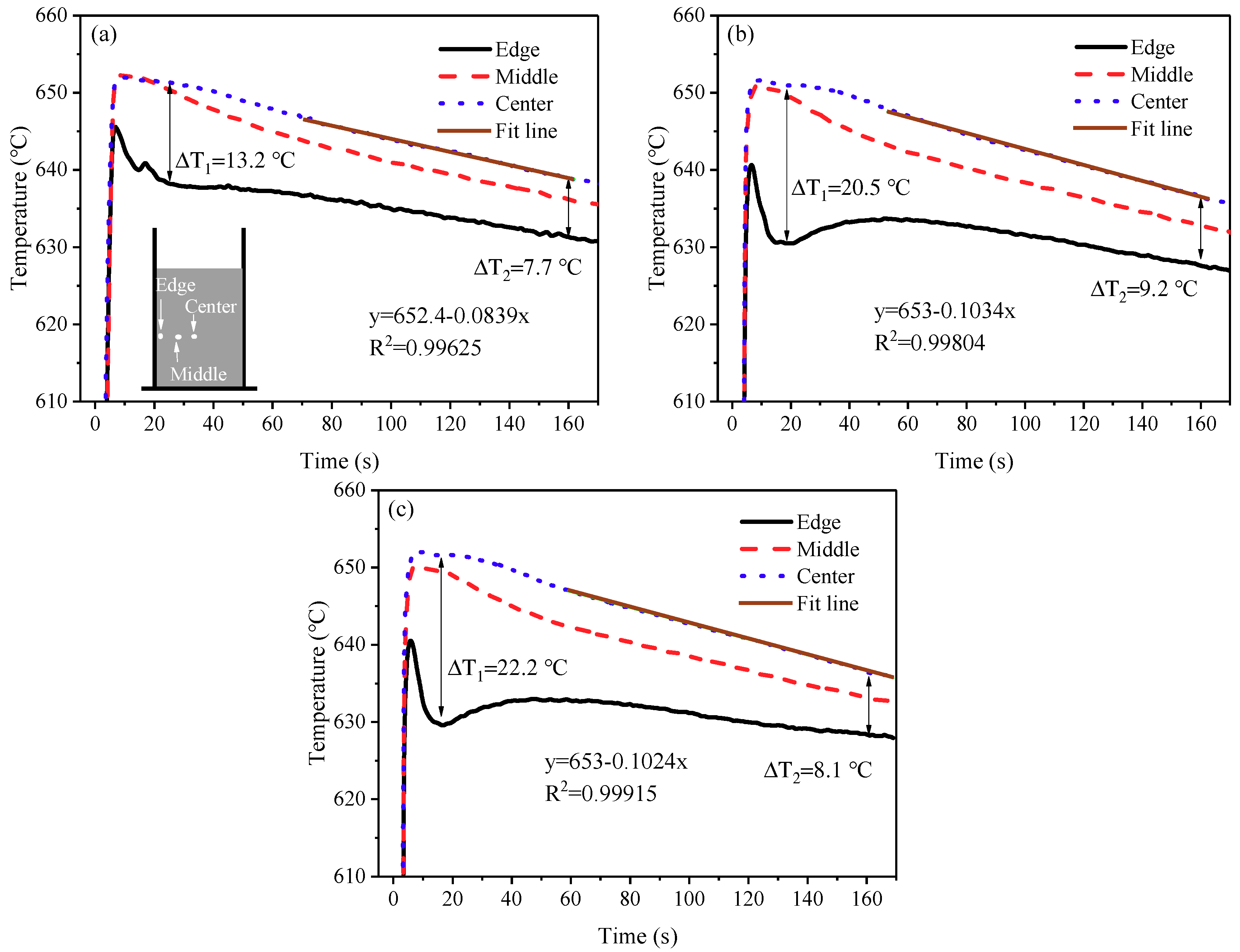
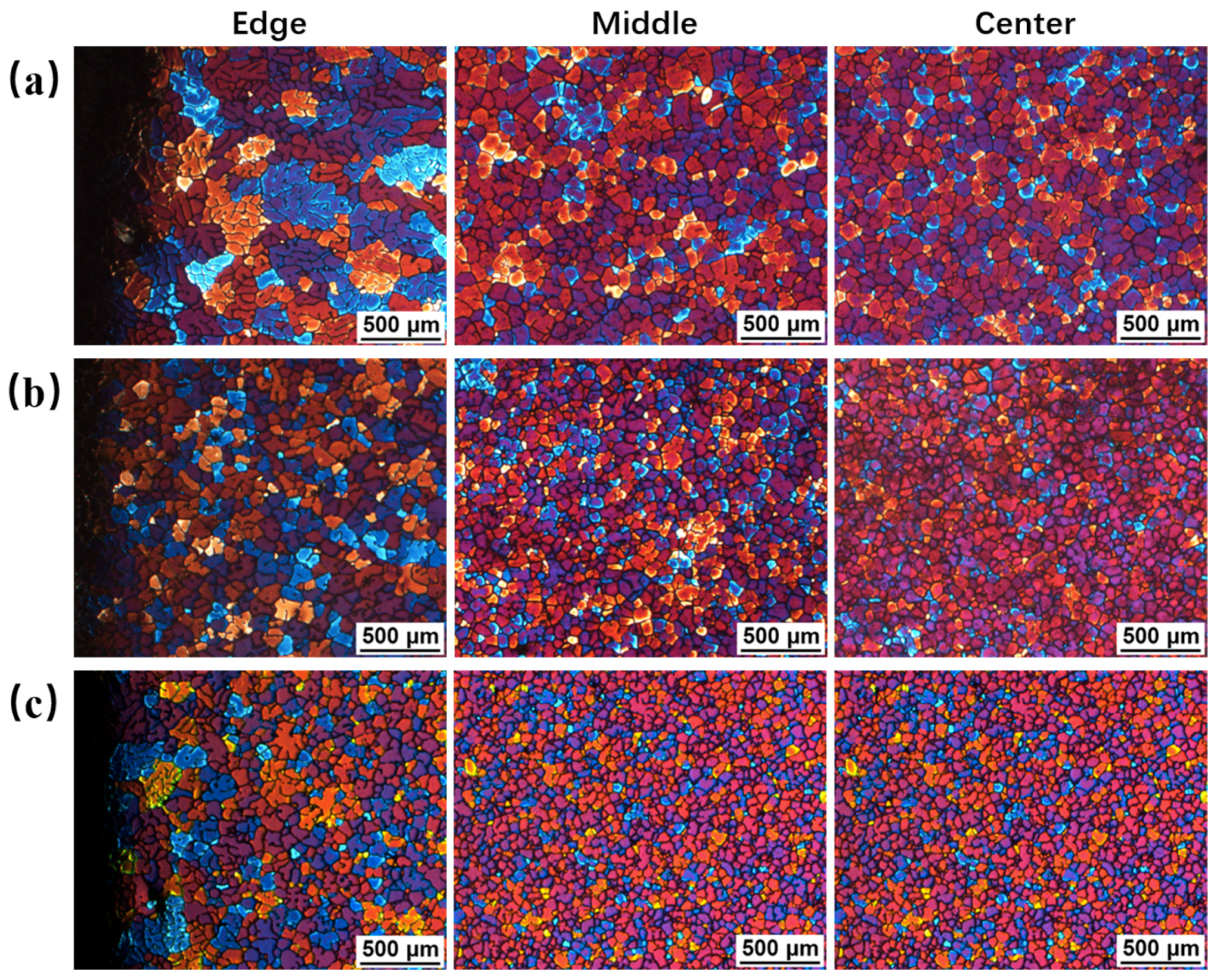
| Alloy | Cu | Mg | Ag | Si | Fe | Ti | Mn | Al |
|---|---|---|---|---|---|---|---|---|
| A201 | 4.0–5.2 | 0.15–0.55 | 0.4–1.0 | <0.1 | <0.15 | 0.15–0.35 | 0.20–0.50 | Bal. |
| This study | 4.8 | 0.25 | 0.56 | 0.09 | 0.15 | 0.25 | 0.29 | Bal. |
| Cooling Rate (K/min) | Solidification Interval (°C) | Temperature Window (°C) | Temperature Sensitivity | Time Window (s) |
|---|---|---|---|---|
| 1 | 115.6 | 14.2 | 0.014 | 852 |
| 5 | 117.3 | 14.0 | 0.014 | 168 |
| 10 | 121.8 | 13.7 | 0.017 | 70.2 |
| 15 | 122.4 | 14.1 | 0.014 | 56.4 |
| Equilibrium | 104.8 | 10.7 | 0.019 | / |
| Scheil model | 146.0 | 11.9 | 0.017 | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Hu, X.; Zhu, Q.; Li, D.; Kang, Y. Cooling Behavior and Microstructure of Semisolid A201 Aluminum Alloy Prepared by the SEED Process. Metals 2019, 9, 922. https://doi.org/10.3390/met9090922
Gao J, Hu X, Zhu Q, Li D, Kang Y. Cooling Behavior and Microstructure of Semisolid A201 Aluminum Alloy Prepared by the SEED Process. Metals. 2019; 9(9):922. https://doi.org/10.3390/met9090922
Chicago/Turabian StyleGao, Junzhen, Xiaogang Hu, Qiang Zhu, Daquan Li, and Yonglin Kang. 2019. "Cooling Behavior and Microstructure of Semisolid A201 Aluminum Alloy Prepared by the SEED Process" Metals 9, no. 9: 922. https://doi.org/10.3390/met9090922




