Composition Optimum Design and Strengthening and Toughening Mechanisms of New Alumina-Forming Austenitic Heat-Resistant Steels
Abstract
:1. Introduction
2. Experiment and Calculation Details
3. Results and Discussion
3.1. Composition Optimum Design
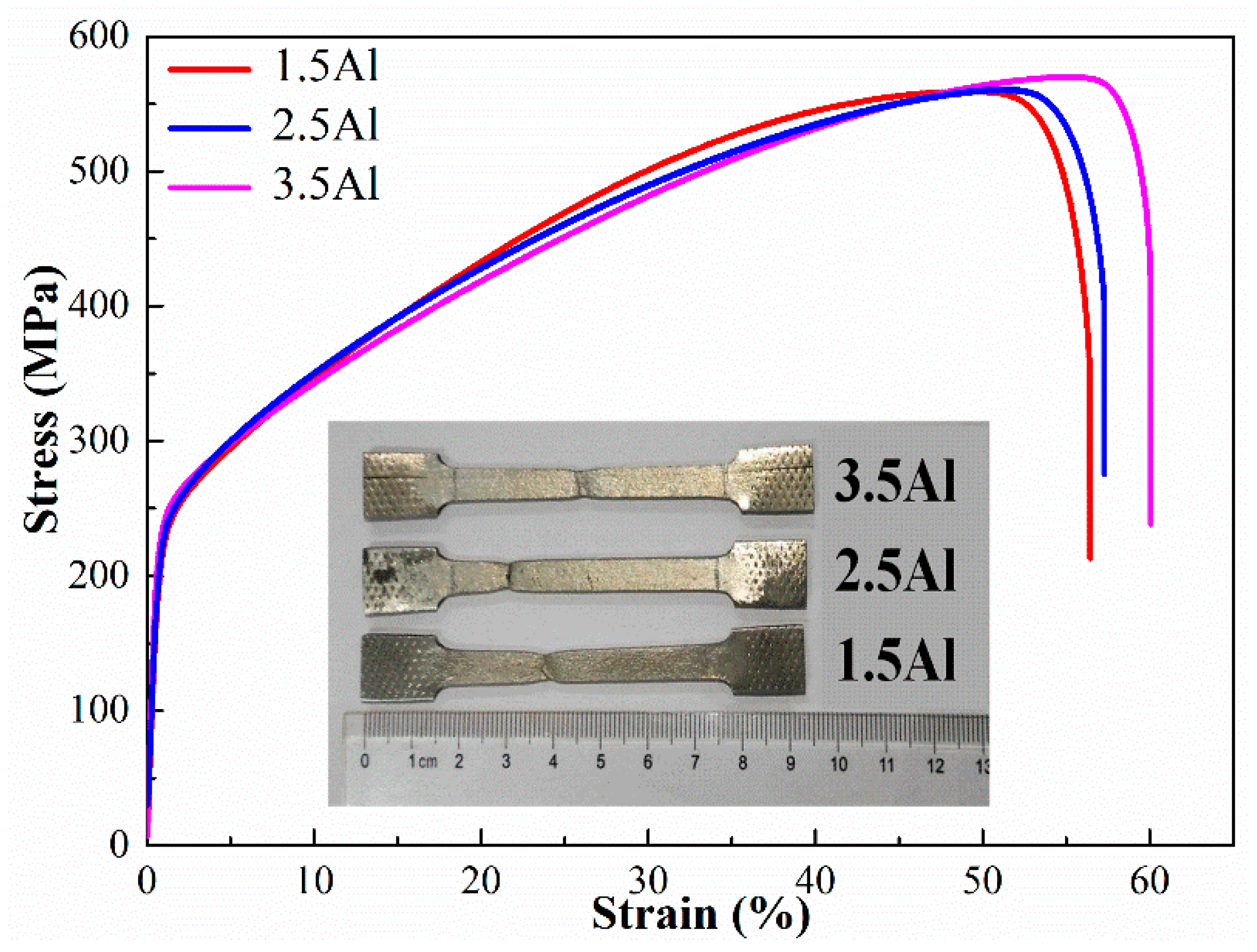
3.2. Mechanical Properties
3.3. Structure Stability and Strengthening and Toughening Mechanisms
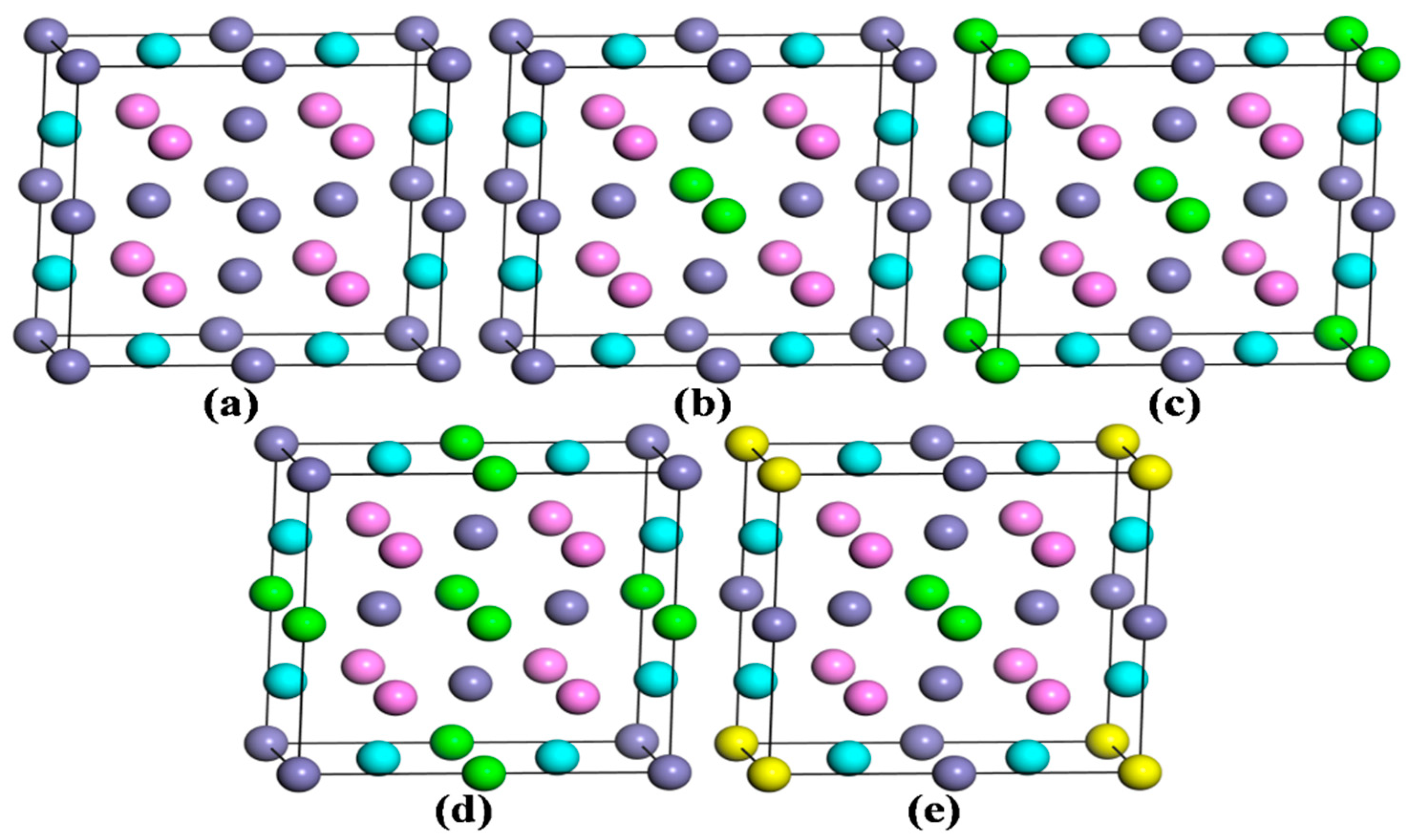
3.3.1. Calculation Models
3.3.2. Structural Stability
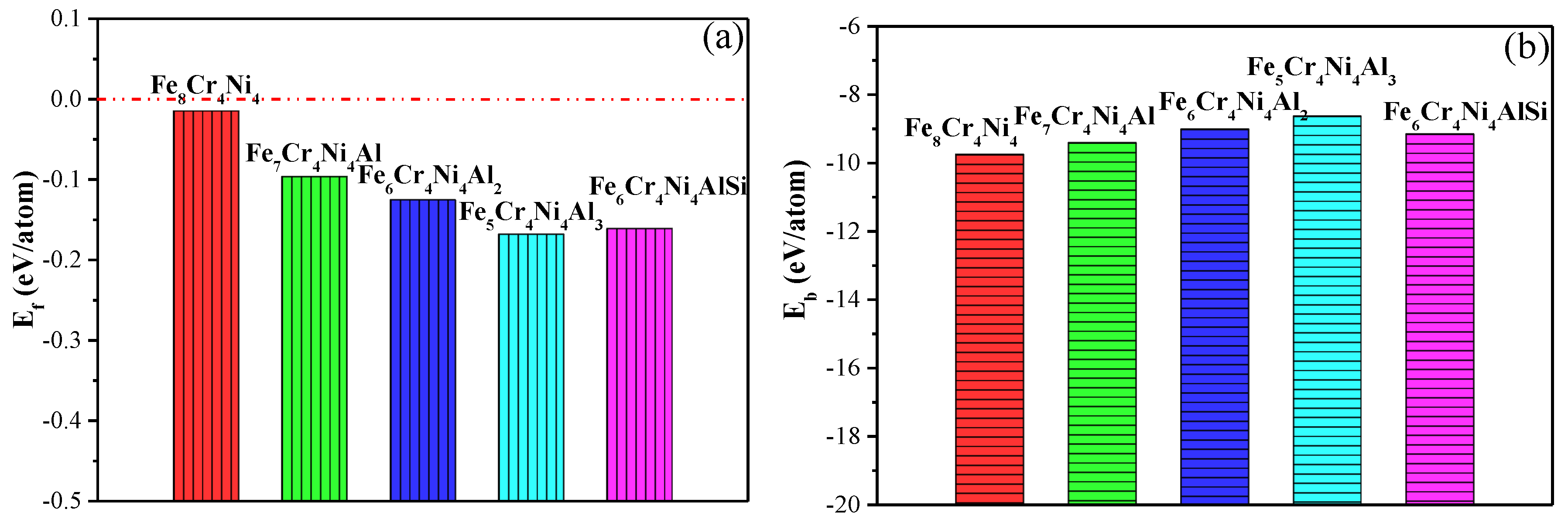
3.3.3. Thermodynamic Stability
3.3.4. Mechanical Properties
3.3.5. Electronic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussain, T.; Syed, A.U.; Simms, N.J. Fireside Corrosion of Superheater Materials in Coal/Biomass Co-fired Advanced Power Plants. Oxid. Met. 2013, 80, 529–540. [Google Scholar] [CrossRef]
- Maziasz, P.J. Development of creep-resistant and oxidation-resistant austenitic stainless steels for high temperature applications. JOM 2018, 70, 66–75. [Google Scholar] [CrossRef]
- Takahashi, T.; Sakakibara, M.; Kikuchi, M.; Ogawa, T.; Sakurai, H.; Nagao, K.; Yasuda, H. Development of High-Strength 20Cr-25Ni (NF709) Steel for USC Boiler Tubes; Nippon Steel Technical Report No. 38; Nippon Steel Corp: Tokyo, Japan, 1998. [Google Scholar]
- Viswanathan, R.; Sarver, J.; Tanzosh, J. Boiler Materials for Ultra-Supercritical Coal Power Plants—Steamside Oxidation. J. Mater. Eng. Perform. 2006, 15, 255–274. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Li, X.N. Microstructural evolution and change in hardness during creep of NF709 austenitic stainless steel. Acta Metall. Sin. 2011, 24, 220–224. [Google Scholar]
- Dudziak, T.; Łukaszewicz, M.; Simms, N.; Nicholls, J.R. Steam oxidation of TP347HFG, super 304H and HR3C-analysis of significance of steam flowrate and specimen surface finish. Corros. Eng. Sci. Technol. 2015, 50, 272–282. [Google Scholar] [CrossRef]
- Vujic, S.; Sandström, R.; Sommitsch, C. Precipitation evolution and creep strength modelling of 25Cr20NiNbN austenitic steel. Mater. High Temp. 2015, 32, 607–618. [Google Scholar] [CrossRef]
- Wright, I.G.; Dooley, R.B. A review of the oxidation behavior of structural alloys in steam. Int. Mater. Rev. 2010, 55, 129–167. [Google Scholar] [CrossRef]
- Saunders, S.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Jung, K.; Kim, C.S.; Pettit, F.S.; Meier, G.H. Interfacial failure via encapsulation of external particulates in an outward-growing thermal oxide. J. Power Sources 2011, 196, 4686–4694. [Google Scholar] [CrossRef]
- Chai, G.; Boström, M.; Olaison, M.; Forsberg, U. Creep and LCF Behaviors of Newly Developed Advanced Heat Resistant Austenitic Stainless Steel for A-USC. Procedia Eng. 2013, 55, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Iseda, A.; Okada, H.; Semba, H.; Igarashi, M. Long term creep properties and microstructure of SUPER304H, TP347HFG and HR3C for A-USC boilers. Energy Mater. 2007, 2, 199–206. [Google Scholar] [CrossRef]
- Liu, P.; Chu, Z.K.; Yuan, Y.; Wang, D.H.; Cui, C.Y.; Hou, G.C.; Zhou, Y.Z.; Sun, X.F. Microstructures and mechanical properties of a newly developed austenitic heat resistant steel. Acta Metall. Sin. 2019, 32, 517–525. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.; Yamamoto, Y.; Santella, M.; Pint, B.; Brady, M.; Pint, B. Effects of minor alloy additions and oxidation temperature on protective alumina scale formation in creep-resistant austenitic stainless steels. Scr. Mater. 2007, 57, 1117–1120. [Google Scholar] [CrossRef]
- Xu, X.Q.; Zhang, X.F.; Chen, G.L.; Lu, Z.P. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Brady, M.; Magee, J.; Yamamoto, Y.; Helmick, D.; Wang, L. Co-optimization of wrought alumina-forming austenitic stainless steel composition ranges for high-temperature creep and oxidation/corrosion resistance. Mater. Sci. Eng. A 2014, 590, 101–115. [Google Scholar] [CrossRef]
- Yanar, N.M.; Lutz, B.S.; Garcia-Fresnillo, L.; Brady, M.P.; Meier, G.H.; Brady, M. The Effects of Water Vapor on the Oxidation Behavior of Alumina Forming Austenitic Stainless Steels. Oxid. Met. 2015, 84, 541–565. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, W.; Mao, H.; Hu, Y.; Xu, X.; Sun, X.; Lu, Z.; Mao, H. Precipitate characteristics and their effects on the high-temperature creep resistance of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2015, 622, 91–100. [Google Scholar] [CrossRef]
- Gao, Q.; Qu, F.; Zhang, H.; Huo, Q. Austenite grain growth in alumina-forming austenitic steel. J. Mater. Res. 2016, 31, 1732–1740. [Google Scholar] [CrossRef]
- Yu, Z.D.; Chen, M.H.; Shen, C.B.; Zhu, S.L.; Wang, F.H. Oxidation of an austenitic stainless steel with or without alloyed aluminum in O2+10% H2O environment at 800 °C. Corro. Sci. 2017, 121, 105–115. [Google Scholar] [CrossRef]
- Baker, I.; Afonina, N.; Wang, Z.; Wu, M. Preliminary creep testing of the alumina-forming austenitic stainless steel Fe-20Cr-30Ni-2Nb-5Al. Mater. Sci. Eng. A 2018, 718, 492–498. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Pint, B.A.; Liu, C.T.; Lu, Z.P.; Bei, H.; Brady, M.; Maziasz, P. The development of alumina-forming austenitic stainless steels for high-temperature structural use. JOM 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Bei, H.; Yamamoto, Y.; Brady, M.; Santella, M.; Brady, M. Aging effects on the mechanical properties of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2010, 527, 2079–2086. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.Y.; Phaniraj, M.P.; Han, H.N.; Jang, J.S.; Zhou, Z.J. Evolution of microstructure and tensile properties of Fe-18Ni-12Cr based AFA steel during aging at 700 °C. Mater. Sci. Eng. A 2016, 672, 23–31. [Google Scholar] [CrossRef]
- Hu, B.; Baker, I. The effect of thermo-mechanical treatment on the high temperature tensile behavior of an alumina-forming austenitic steel. Mater. Sci. Eng. A 2016, 651, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Peraldi, R.; Pint, B.A. Effect of Cr and Ni Contents on the Oxidation Behavior of Ferritic and Austenitic Model Alloys in Air with Water Vapor. Oxid. Met. 2004, 61, 463–483. [Google Scholar] [CrossRef]
- Sun, Y.F.; Lv, Y.Z.; Zhang, Y.; Zhao, J.Y.; Wu, Y. Microstructural and properties evolution of austenitic heat resistant steel after addition of aluminium. Mater. Sci. Technol. 2013, 29, 511–516. [Google Scholar] [CrossRef]
- Guo, X.; Chen, K.; Gao, W.; Shen, Z.; Zhang, L. Corrosion behavior of alumina-forming and oxide dispersion strengthened austenitic 316 stainless steel in supercritical water. Corros. Sci. 2018, 138, 297–306. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.D.; Cheng, S.C.; Liu, C.M.; Wang, J.Z.; Liu, Z.C. Microstructure Evolution and Mechanical Properties of HR3C Steel during Long-term Aging at High Temperature. J. Iron Steel Res. Int. 2014, 21, 765–773. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Guo, G.Y.; Wang, H.H. Gradient-corrected density functional calculation of elastic constants of Fe, Co and Ni in bcc, fcc and hcp structures. Chin. J. Phys. 2000, 38, 949–961. [Google Scholar]
- Haglund, J.; Guillermet, A.F.; Grimvall, G.; Körling, M. Theory of bonding in transition-metal carbides and nitrides. Phys. Rev. B 1993, 48, 11685–11691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chang, K.; Fan, J.; Chen, Z.; Dong, X.; Zhang, L. Annealing effects on precipitation and high-temperature properties of a Cu-containing alumina-forming austenitic steel. Mater. Lett. 2016, 176, 83–86. [Google Scholar] [CrossRef]
- Zhao, B.; Fan, J.; Chen, Z.; Dong, X.; Sun, F.; Zhang, L. Evolution of precipitates in a Cu-containing alumina-forming austenitic steel after short-term mechanical tests. Mater. Charact. 2017, 125, 37–45. [Google Scholar] [CrossRef]
- Chi, C.Y.; Yu, H.Y.; Xie, X.S. Research and development of austenitic heat-resistant steels for 600 °C superheat/reheater tubes of USC power plant boilers. World Iron Steel 2012, 4, 50–65. [Google Scholar]
- Moon, J.; Lee, T.H.; Heo, Y.U.; Han, Y.S.; Kang, J.Y.; Ha, H.Y.; Suh, D.W. Precipitation sequence and its effect on age hardening of alumina-forming austenitic stainless steel. Mater. Sci. Eng. A 2015, 645, 72–81. [Google Scholar] [CrossRef]
- Fast, L.; Wills, J.M.; Johansson, B.; Eriksson, O. Elastic constants of hexagonal transition metals: Theory. Phys. Rev. B 1995, 51, 17431–17438. [Google Scholar] [CrossRef]
- Meradji, H.; Drablia, S.; Ghemid, S.; Belkhir, H.; Bouhafs, B.; Tadjer, A. First-principles elastic constants and electronic structure of BP, BAs, and BSb. Phys. Status Solidi (b) 2004, 241, 2881–2885. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, J.; Xu, S.; Peng, P. Thermal stability and elastic properties of Mg3Sb2 and Mg3Bi2 phases from first-principles calculations. Phys. B Condens. Matter 2010, 405, 2863–2868. [Google Scholar] [CrossRef]
- Huang, Z.W.; Zhao, Y.H.; Hou, H.; Han, P.D. Electronic structural, elastic properties and thermodynamics of Mg17Al12, Mg2Si and Al2Y phases from first-principles calculations. Phys. B 2002, 407, 1075–1081. [Google Scholar] [CrossRef]
- Du, J.; Wen, B.; Melnik, R.; Kawazoe, Y. Phase stability, elastic and electronic properties of Cu–Zr binary system intermetallic compounds: A first-principles study. J. Alloy. Compd. 2014, 588, 96–102. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Crystal structures and elastic properties of superhardIrN2andIrN3from first principles. Phys. Rev. B 2007, 76, 1–15. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, M.; Zhang, Y.; Zhang, C. First-principles investigation of structural, mechanical and electronic properties for Cu–Ti intermetallics. Comput. Mater. Sci. 2016, 123, 70–78. [Google Scholar] [CrossRef]
- Pugh, S. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Benyelloul, K.; Aourag, H. Elastic constants of austenitic stainless steel: Investigation by the first-principles calculations and the artificial neural network approach. Comput. Mater. Sci. 2013, 67, 353–358. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, J.; Li, X. Precipitates in hr3c steel aged at high temperature. Acta Met. Sin. 2010, 46, 844–849. [Google Scholar] [CrossRef]
- Li, K.; Xie, H.; Liu, J.; Ma, Z.; Zhou, Y.; Xue, D. From chemistry to mechanics: Bulk modulus evolution of Li–Si and Li–Sn alloys via the metallic electronegativity scale. Phys. Chem. Chem. Phys. 2013, 15, 17658–17663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Z.; Wang, Y.; Zou, Y.; Lei, W.; Pan, Y.; Lu, C. A first principles study of the mechanical properties of Li–Sn alloys. RSC Adv. 2015, 5, 36022–36029. [Google Scholar] [CrossRef]
- Yu, R.; Chong, X.; Jiang, Y.; Zhou, R.; Yuan, W.; Feng, J. The stability, electronic structure, elastic and metallic properties of manganese nitrides. RSC Adv. 2015, 5, 1620–1627. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, L.; Wan, B.; Zhang, J.; Li, Z.; Gou, H. Structural evolution, mechanical properties, and electronic structure of Al–Mg–Si compounds from first principles. J. Mater. Sci. 2015, 50, 6498–6509. [Google Scholar] [CrossRef]
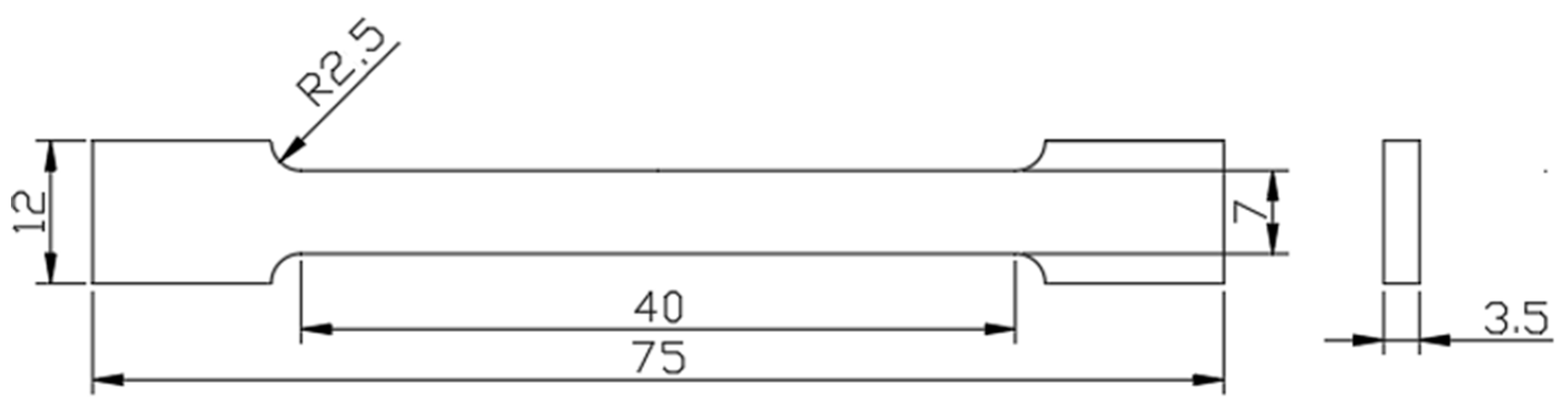


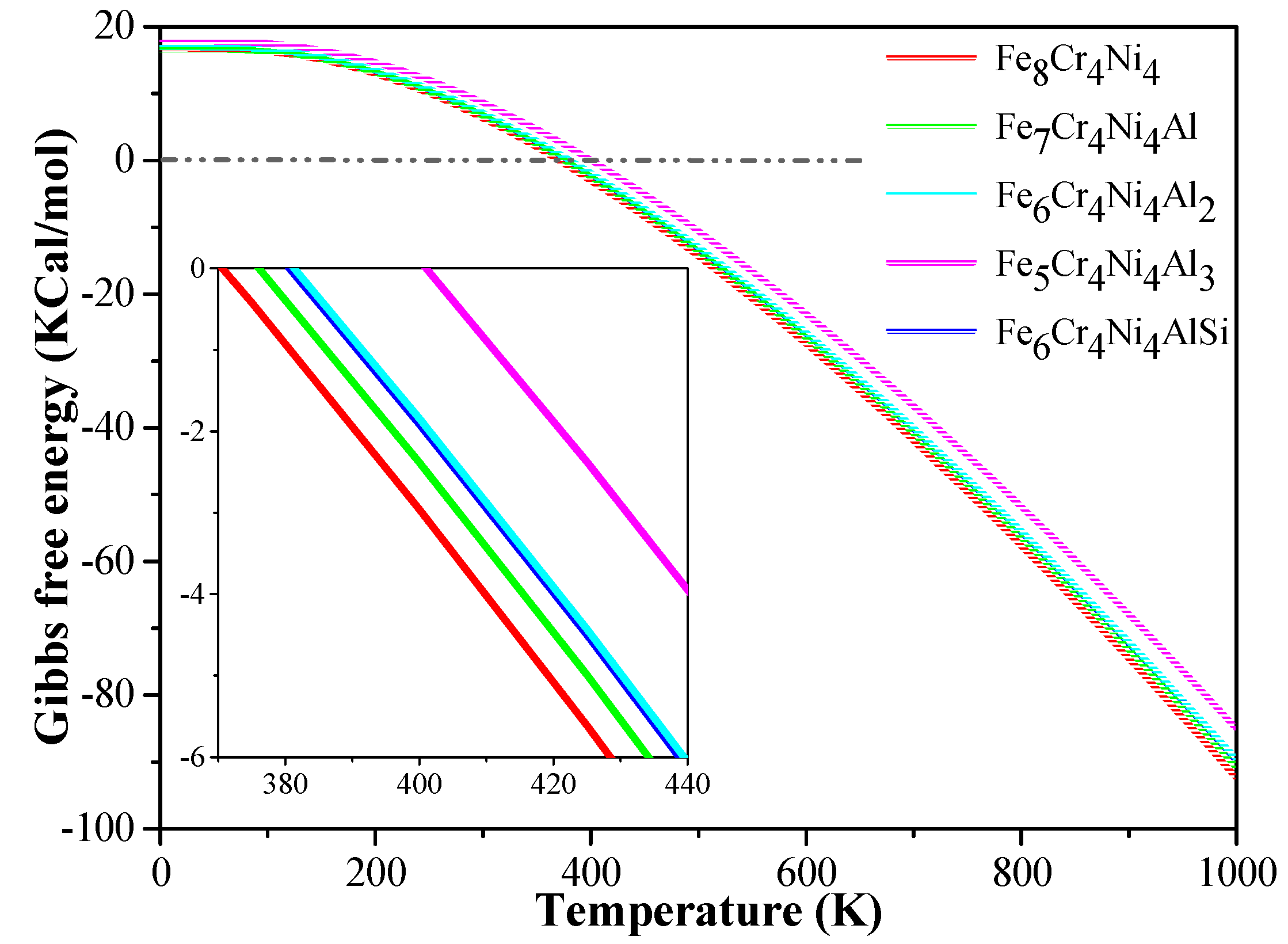

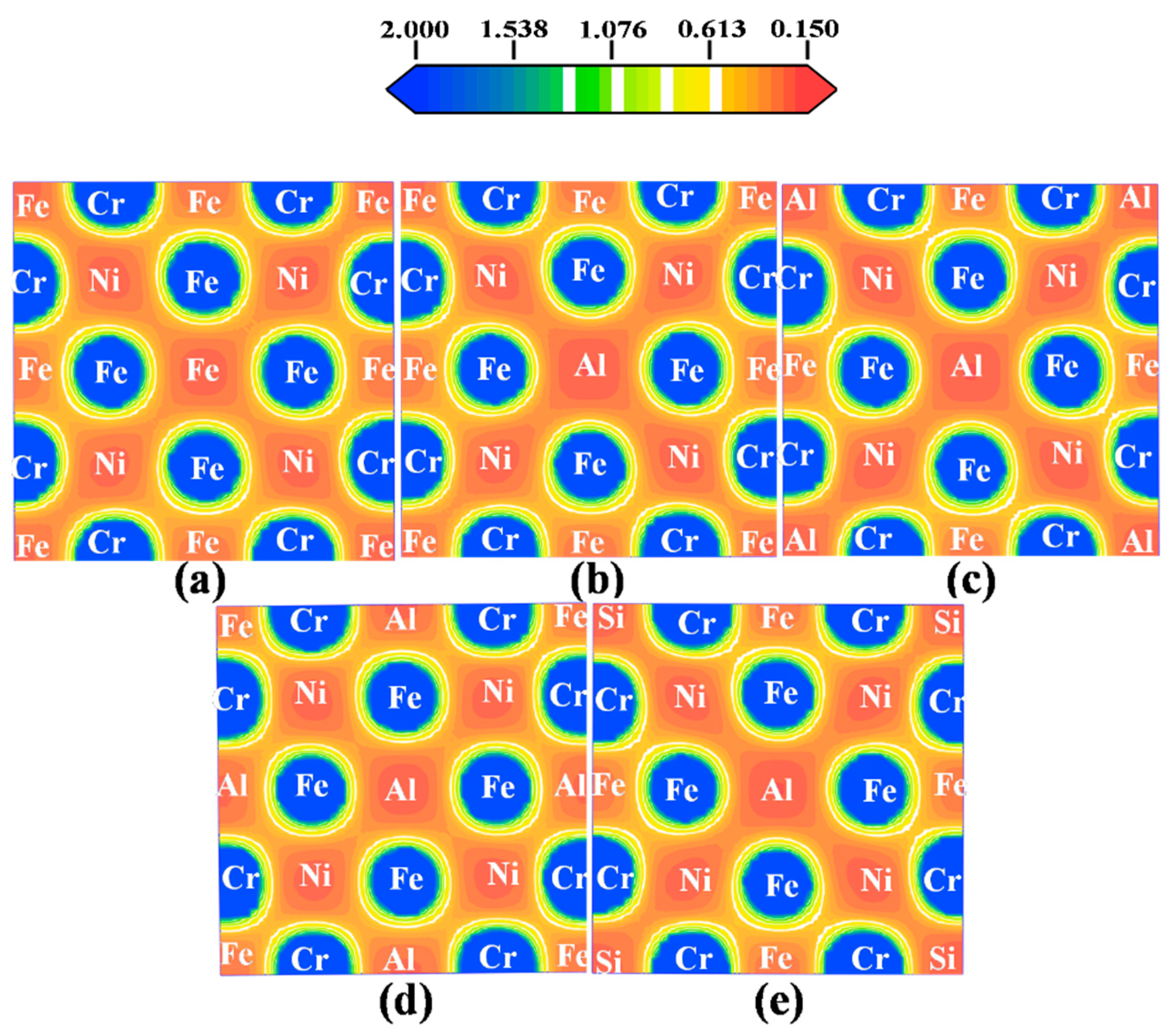
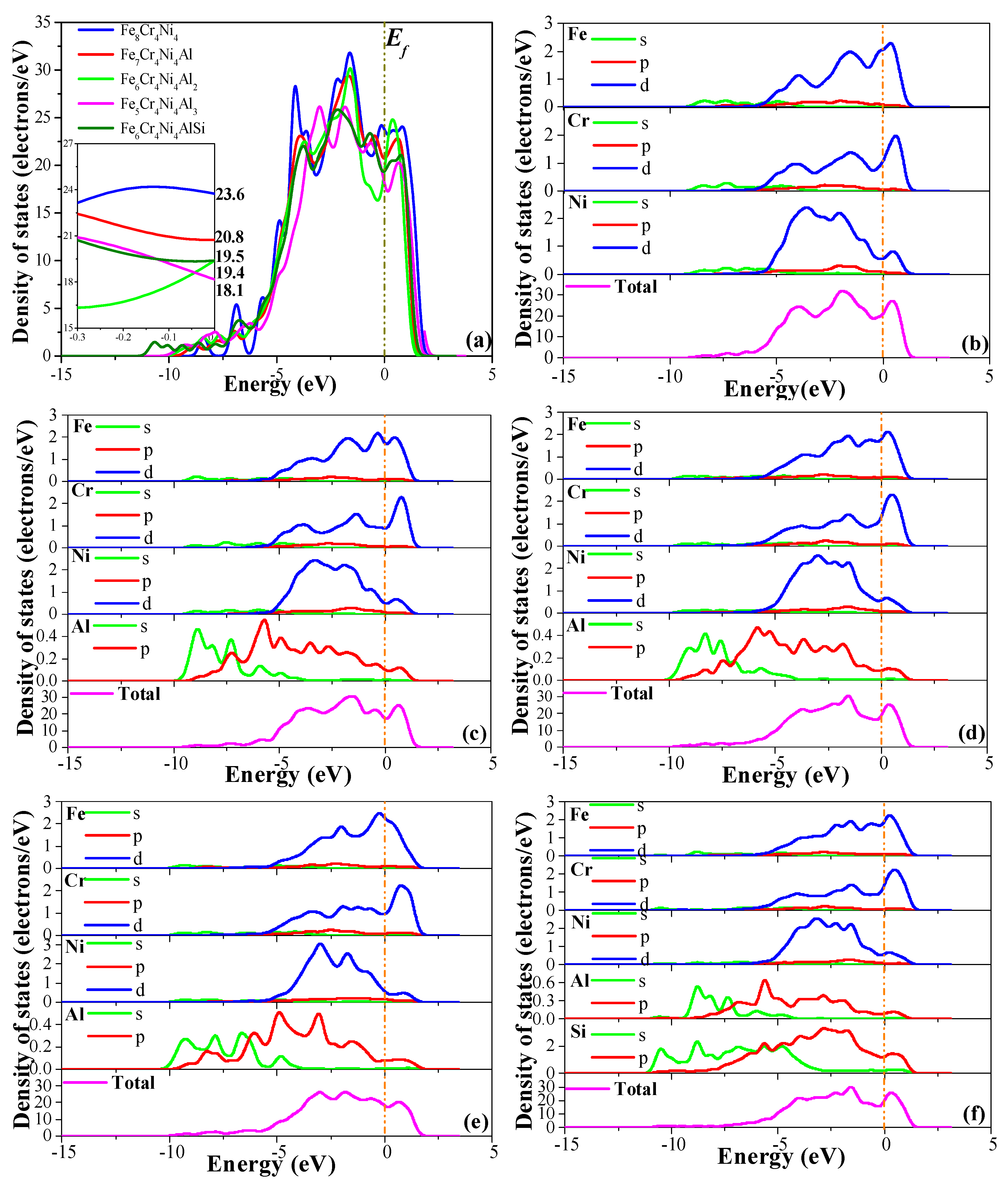
| Alloy | Cr | Ni | Al | Nb | Mn | Cu | Si | C | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 1.5Al | 21.19 | 25.12 | 1.50 | 0.44 | 0.80 | 2.60 | 0.30 | 0.07 | Bal |
| 2.5Al | 21.36 | 24.59 | 2.48 | 0.42 | 0.77 | 2.65 | 0.28 | 0.07 | Bal |
| 3.5Al | 22.14 | 25.29 | 3.51 | 0.40 | 0.78 | 2.63 | 0.29 | 0.07 | Bal |
| Elastic Constants | C11 | C12 | C13 | C33 | C44 | C66 |
|---|---|---|---|---|---|---|
| Fe8Cr4Ni4 | 371.484 | 204.667 | 170.828 | 417.936 | 195.492 | 209.082 |
| Fe7Cr4Ni4Al | 323.440 | 186.422 | 157.412 | 354.115 | 184.691 | 174.932 |
| Fe6Cr4Ni4Al2 | 271.592 | 169.982 | 149.656 | 298.950 | 170.620 | 147.576 |
| Fe5Cr4Ni4Al3 | 225.141 | 199.483 | 126.016 | 297.716 | 134.792 | 103.835 |
| Fe6Cr4Ni4AlSi | 298.478 | 211.721 | 178.404 | 364.793 | 168.795 | 133.751 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, N.; Jia, R.; Wang, J.; Fan, G.; Fang, X.; Han, P. Composition Optimum Design and Strengthening and Toughening Mechanisms of New Alumina-Forming Austenitic Heat-Resistant Steels. Metals 2019, 9, 921. https://doi.org/10.3390/met9090921
Dong N, Jia R, Wang J, Fan G, Fang X, Han P. Composition Optimum Design and Strengthening and Toughening Mechanisms of New Alumina-Forming Austenitic Heat-Resistant Steels. Metals. 2019; 9(9):921. https://doi.org/10.3390/met9090921
Chicago/Turabian StyleDong, Nan, Ruirui Jia, Jian Wang, Guangwei Fan, Xudong Fang, and Peide Han. 2019. "Composition Optimum Design and Strengthening and Toughening Mechanisms of New Alumina-Forming Austenitic Heat-Resistant Steels" Metals 9, no. 9: 921. https://doi.org/10.3390/met9090921






