Influence of Preaging Treatment on Bake-Hardening Response and Electrochemical Corrosion Behavior of High Strength Al-Zn-Mg-Cu-Zr Alloy
Abstract
:1. Introduction
2. Materials and Experimental Methods
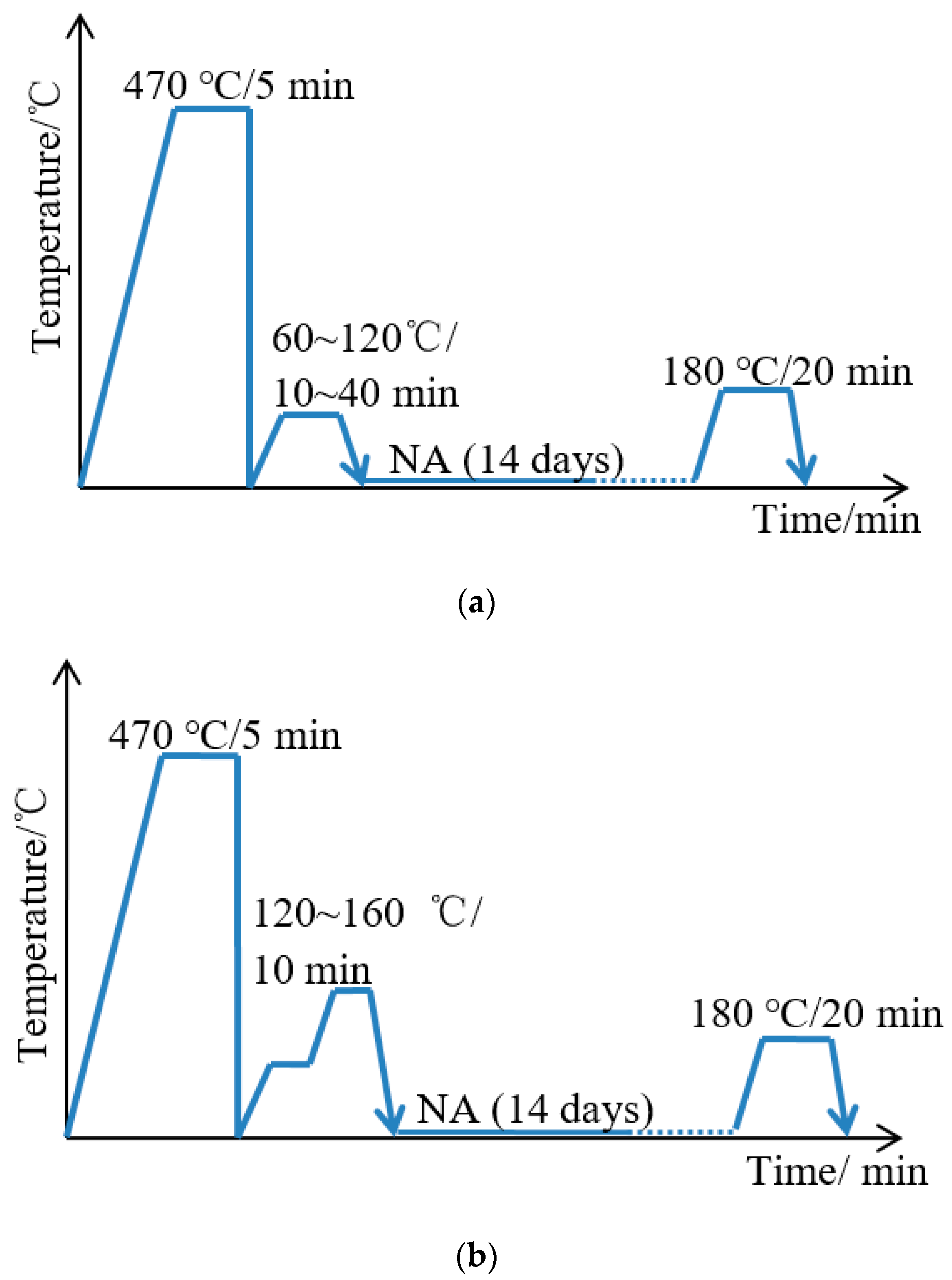
2.1. Materials and Heat Treatment
2.2. Mechanical Testing
2.3. Electrochemical Measurement
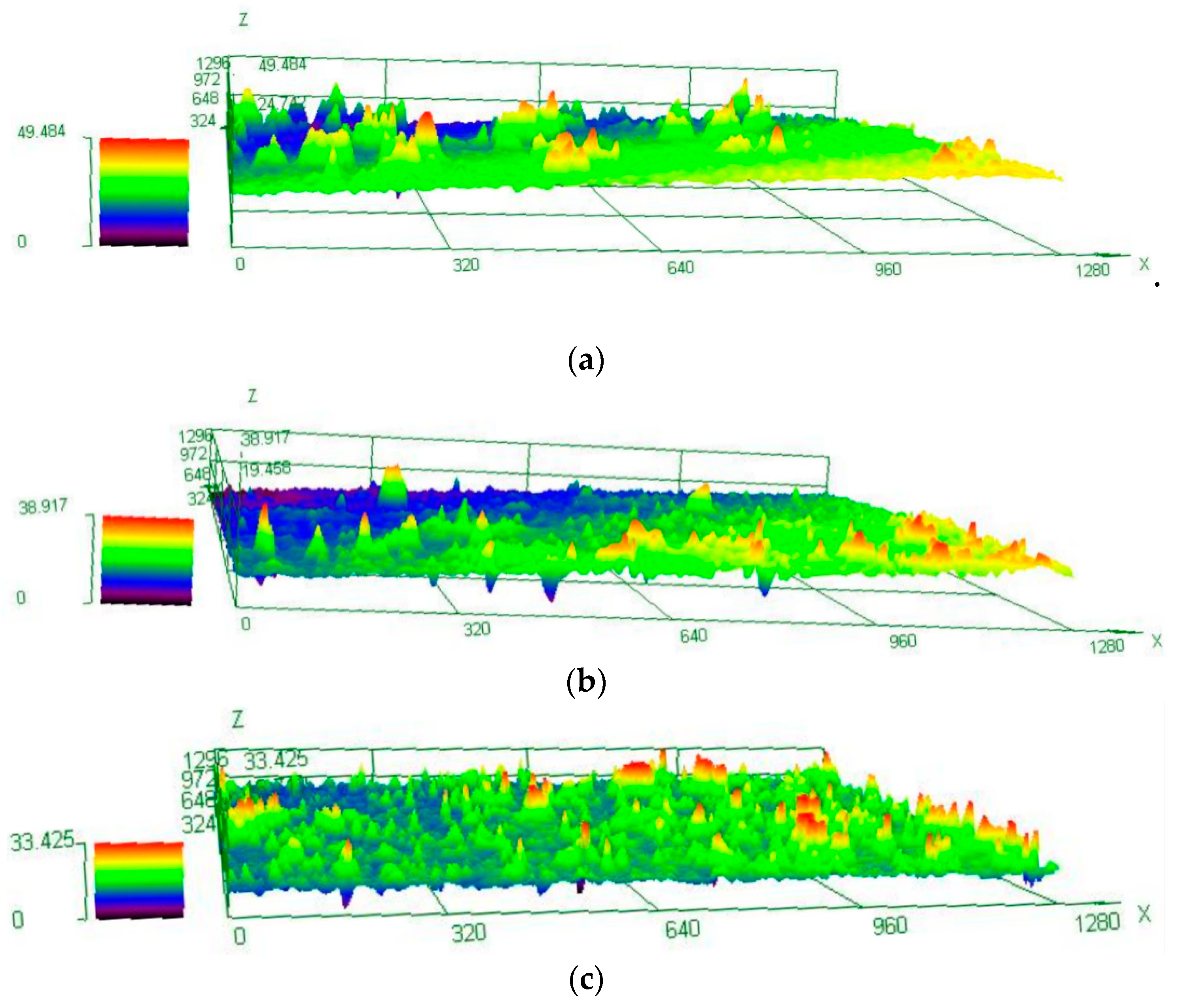
2.4. Microstructure Investigation
3. Results and Discussions
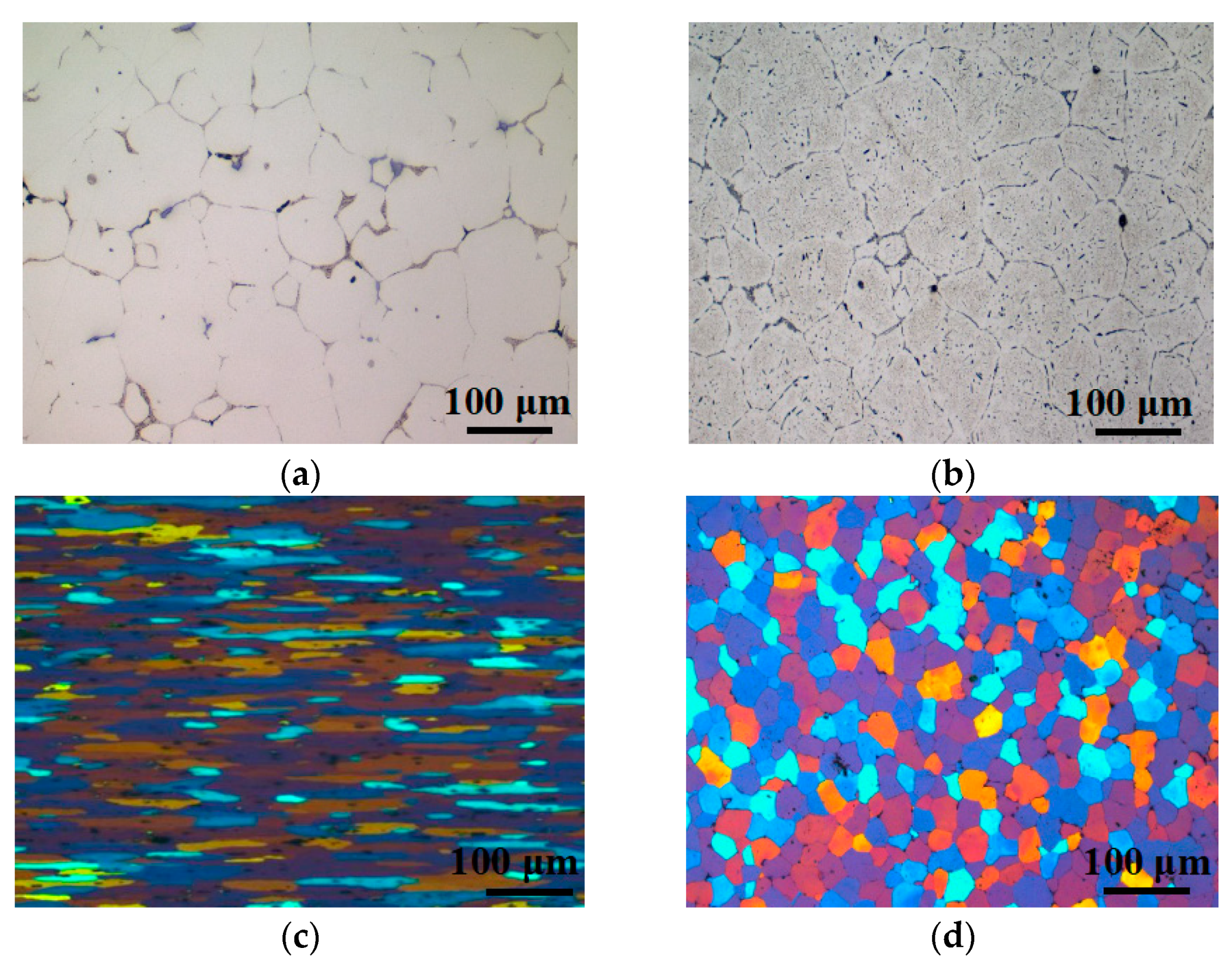
3.1. Metallurgical Microstructure
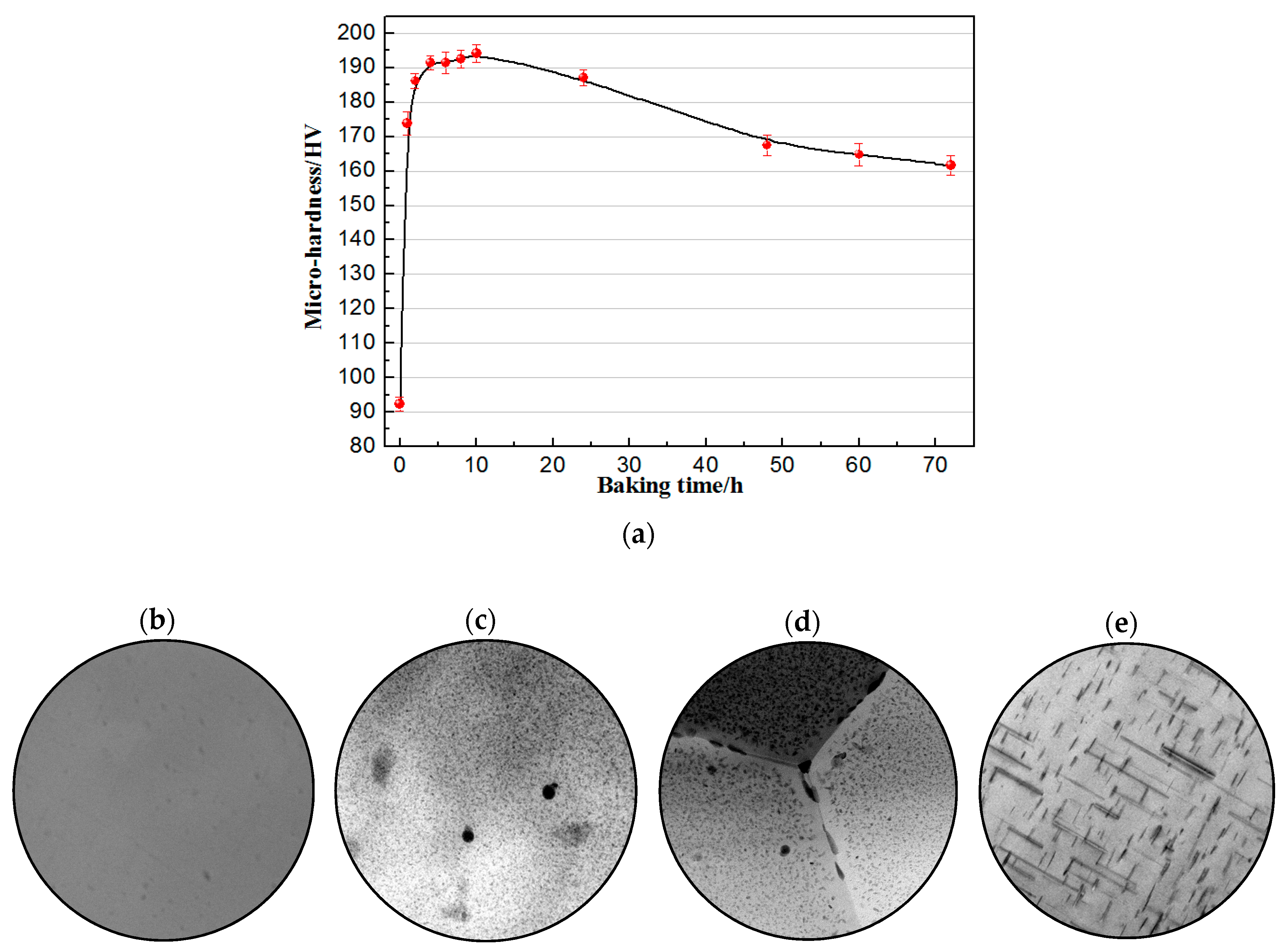
3.2. BH Response at 180 °C after Solution Treatment
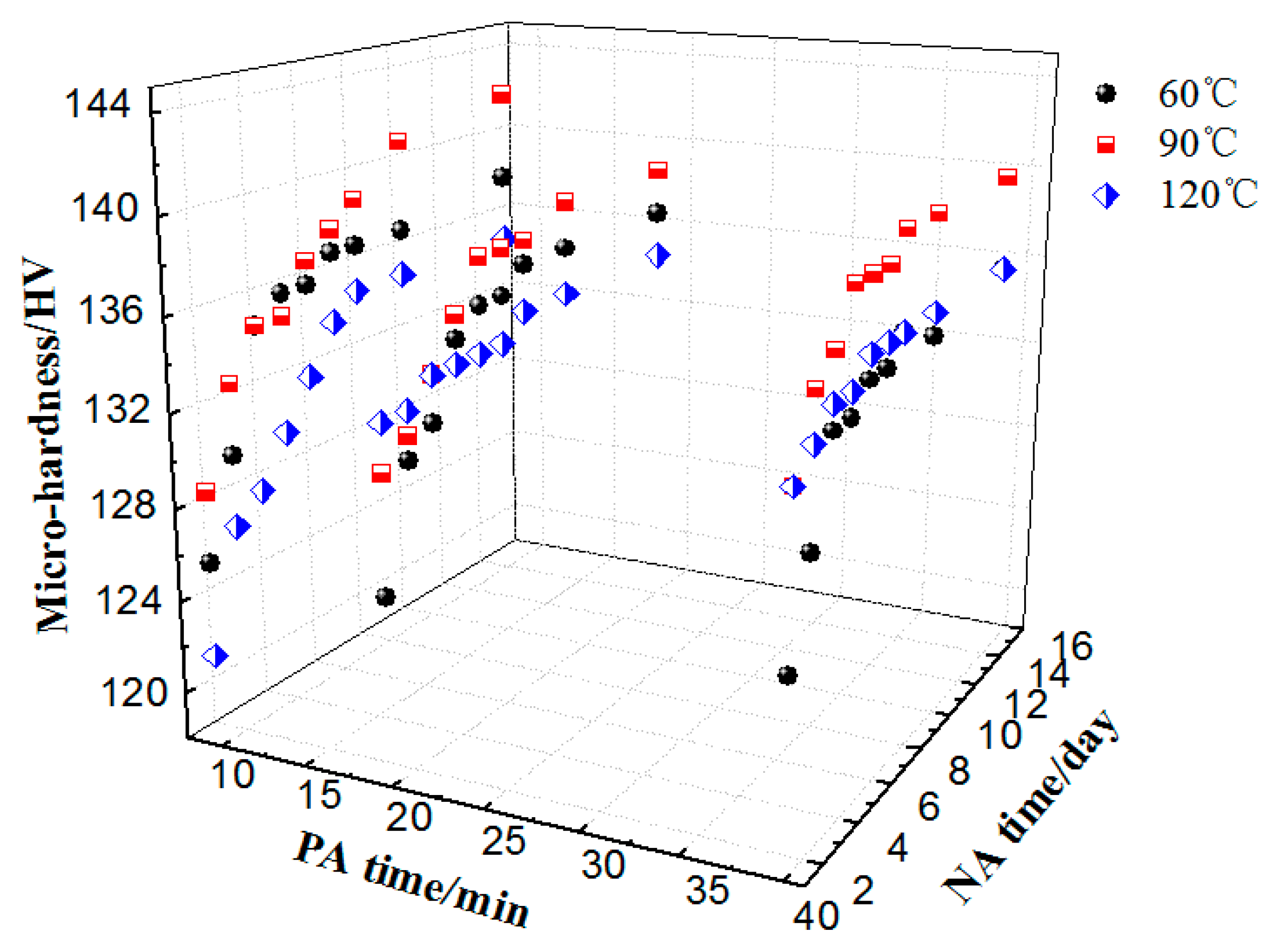
3.3. Influence of PA on Hardness and Microstructures
3.4. Novel Two-Step PA Treatment and Influence on Properties
4. Conclusions
- (1)
- While baking at 180 °C immediately after solution treatment, the micro-hardness increased sharply first and reached the peak value (194 HV) after 10 h holding, which was a percentage improvement of 110.87% compared to the hardness under the solution condition, then had a decreasing trend and tended to become steady after 60 h.
- (2)
- The PA treatments decreased NA adverse effect. The main strengthening phases were GP zones before BH treatment, whereas η’ were the dominant hardening phases after BH treatment. The specimen after PA under 120 °C/20 min had the lowest NA adverse effect and optimal BH response.
- (3)
- A novel two-step PA treatment, which further decreases the NA effect and increases the BH response compared to the optimal PA treatment, was designed. The BH value after two-step PA + NA treatment reached 168 MPa and was 21.7% higher than that of optimal PA + NA treatment. The characterizations of potentiodynamic polarization curves, EIS Nyquist plots, and 3D–MLM images showed its optimal corrosion resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tucker, R. Trends in automotive lightweighting. Met. Finish. 2013, 111, 23–25. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Vieregge, A. Recent development in aluminum alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Hirsch, J. Recent development in aluminum for automotive applications. Trans. Nonferr. Metal. Soc. 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Henriksson, F.; Johansen, K. On material substitution in automotive BIWs-from steel to aluminum body sides. Procedia CIRP 2016, 50, 683–688. [Google Scholar] [CrossRef]
- Hall, J.N.; Fekete, J.R. Steels for auto bodies: A general overview. In Automotive Steels; Woodhead Publishing: Cambridge, UK, 2007; pp. 19–45. [Google Scholar]
- Santos, J.; Gouveia, R.M.; Silva, F.J.G. Designing a new sustainable approach to the change for lightweight materials in structural components used in truck industry. J. Clean. Prod. 2017, 164, 115–123. [Google Scholar] [CrossRef]
- Ding, L.P.; He, Y.; Wen, Z.; Zhao, P.Z.; Jia, Z.H.; Liu, Q. Optimization of the pre-aging treatment for an AA6022 alloy at various temperatures and holding times. J. Alloy. Comp. 2015, 647, 238–244. [Google Scholar] [CrossRef]
- Birol, Y. Pre-aging to improve bake hardening in a twin-roll cast Al-Mg-Si alloy. Mater. Sci. Eng. A 2005, 391, 175–180. [Google Scholar] [CrossRef]
- Hou, W.R.; Ji, W.B.; Zhang, Z.H.; Cheng, X. The effect of homogenization temperature on the corrosion resistance of extruded 7050 Al-alloy bars. J. Mater. Process. Technol. 2014, 214, 635–640. [Google Scholar] [CrossRef]
- Cong, F.G.; Zhao, G.; Jiang, F.; Tian, N.; Li, R. Effect of homogenization treatment on microstructure and mechanical properties of DC cast 7X50 aluminum alloy. Trans. Nonferr. Metal. Soc. 2015, 25, 1027–1034. [Google Scholar] [CrossRef]
- Jiang, F.L.; Zurob, H.S.; Purdy, G.R.; Zhang, H. Characterizing precipitate evolution of an Al-Zn-Mg-Cu based commercial alloy during artificial aging and non-isothermal heat treatments by in situ electrical resistivity monitoring. Mater. Charact. 2016, 117, 47–56. [Google Scholar] [CrossRef]
- Guo, W.; Guo, J.Y.; Wang, J.D.; Yang, M.; Li, H.; Wen, X.Y.; Zhang, J.W. Evolution of precipitate microstructure during stress aging of an Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2015, 634, 167–175. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, D.M.; Li, W.J. The effect of multistage ageing on microstructure and mechanical properties of 7050 alloy. J. Alloy. Compd. 2016, 671, 408–418. [Google Scholar] [CrossRef]
- Ozer, G.; Karaaslan, A. Properties of AA7075 aluminum alloy in aging and retrogression and reaging process. Trans. Nonferr. Metal. Soc. 2017, 27, 2357–2362. [Google Scholar] [CrossRef]
- Kumar, M.; Ross, N.G. Influence of temper on the performance of a high-strength Al-Zn-Mg alloy sheet in the warm forming processing chain. J. Mater. Process. Technol. 2016, 231, 189–198. [Google Scholar] [CrossRef]
- Huo, W.T.; Hou, L.G.; Zhang, Y.S.; Zhang, J.S. Warm formability and post-forming microstructure/property of high-strength AA7075-T6 Al alloy. Mater. Sci. Eng. A 2016, 675, 44–54. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Zhuang, L.Z.; Zhang, J.S. Improved age-hardening response and altered precipitation behavior of Al-5.2Mg-0.45Cu-2.0Zn alloy with pre-aging treatment. J. Alloy. Compd. 2017, 691, 40–43. [Google Scholar]
- Lee, Y.S.; Koh, D.H.; Kim, H.W.; Ahn, Y.S. Improved bake-hardening response of Al-Zn-Mg-Cu alloy through pre-aging treatment. Scr. Mater. 2018, 147, 45–49. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Evolution of precipitate microstructures during the retrogression and re-aging heat treatment of an Al-Zn-Mg-Cu alloy. Acta Mater. 2010, 58, 4814–4826. [Google Scholar] [CrossRef]
- Mondolfo, L.F.; Gjostein, N.A.; Levinson, D.W. Structural changes during the aging in an Al-Mg-Zn alloy. JOM 1956, 8, 1378–1385. [Google Scholar] [CrossRef]
- Engdahl, T.; Hansen, V.; Warren, P.J.; Stiller, K. Investigation of fine scale precipitates in Al-Zn-Mg alloys after various heat treatments. Mater. Sci. Eng. A 2002, 327, 59–64. [Google Scholar] [CrossRef]
- Graf, R. An X-Ray study of the precipitation phenomenon in an Al-Zn-Mg alloy containing 7% Zn and 3% Mg. Compt. Rend 1956, 242, 1311–1316. [Google Scholar]
- Ghiaasiaan, R.; Amirkhiz, B.S.; Shankar, S. Quantitative metallography of precipitating and secondary phases after strengthening treatment of net shaped casting of Al-Zn-Mg-Cu (7000) alloys. Mater. Sci. Eng. A 2017, 698, 206–217. [Google Scholar] [CrossRef]
- Swift, H.W. Plastic instability under plane stress. J. Mech. Phys. Solids 1952, 1, 1–18. [Google Scholar] [CrossRef]
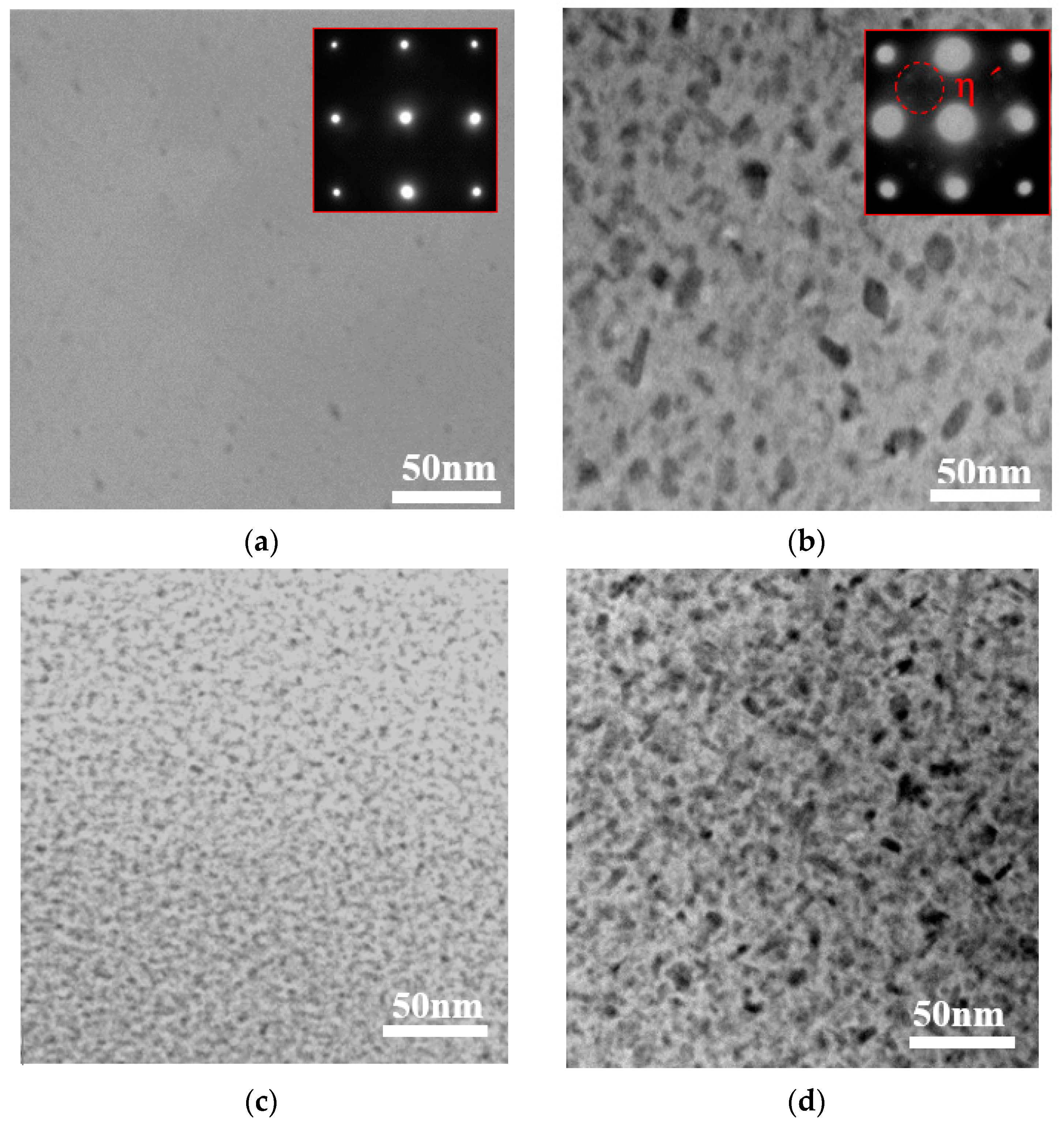
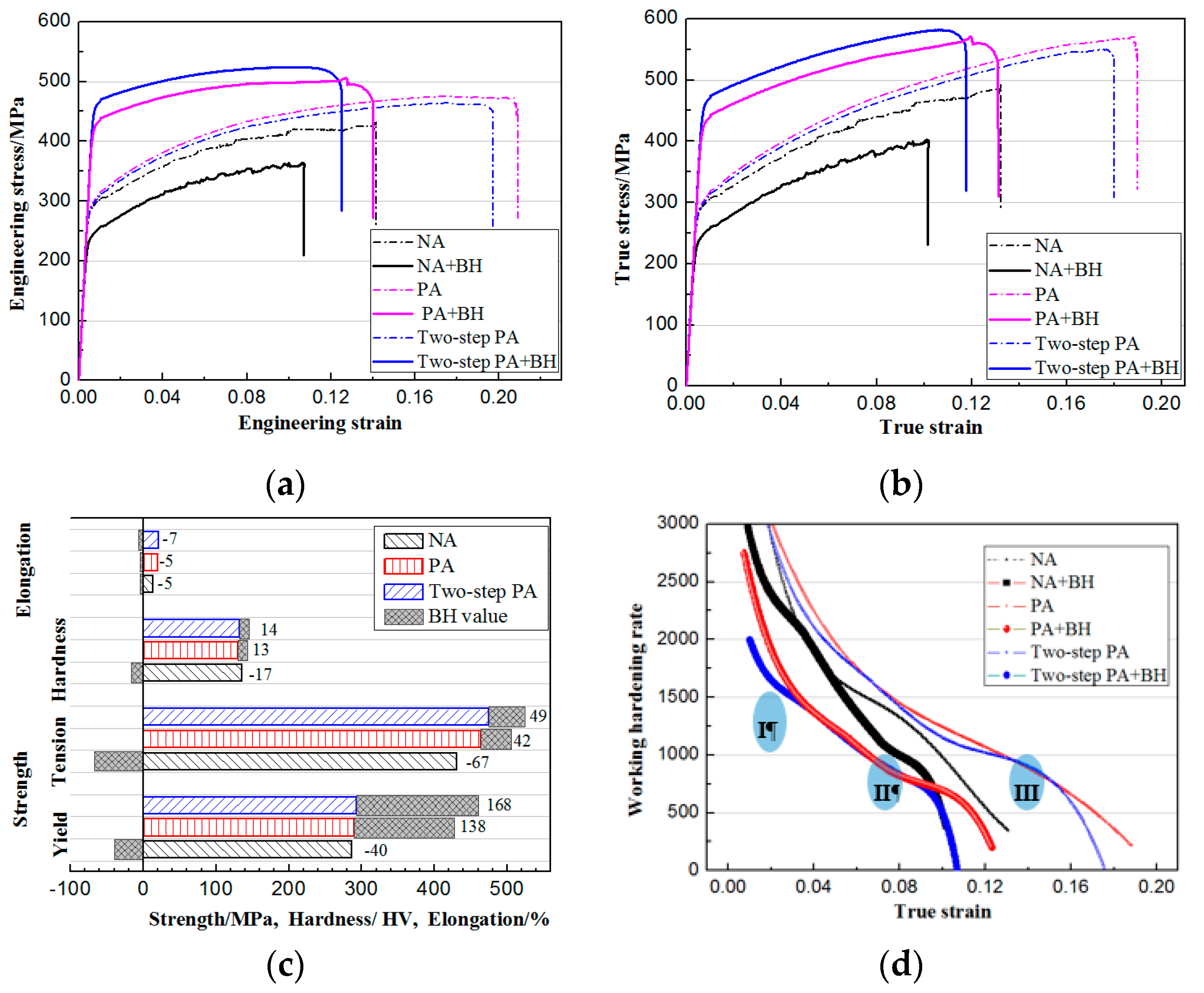
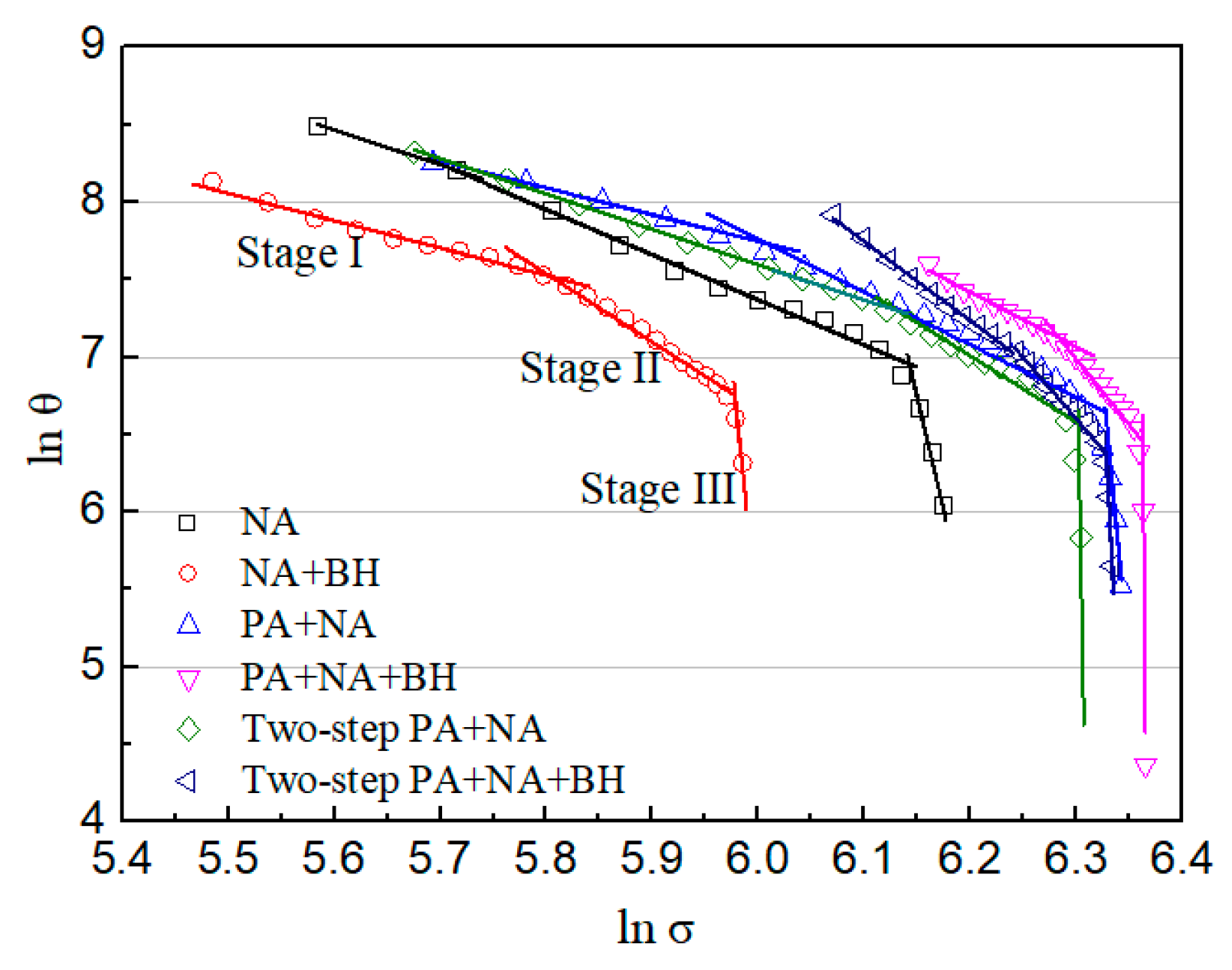
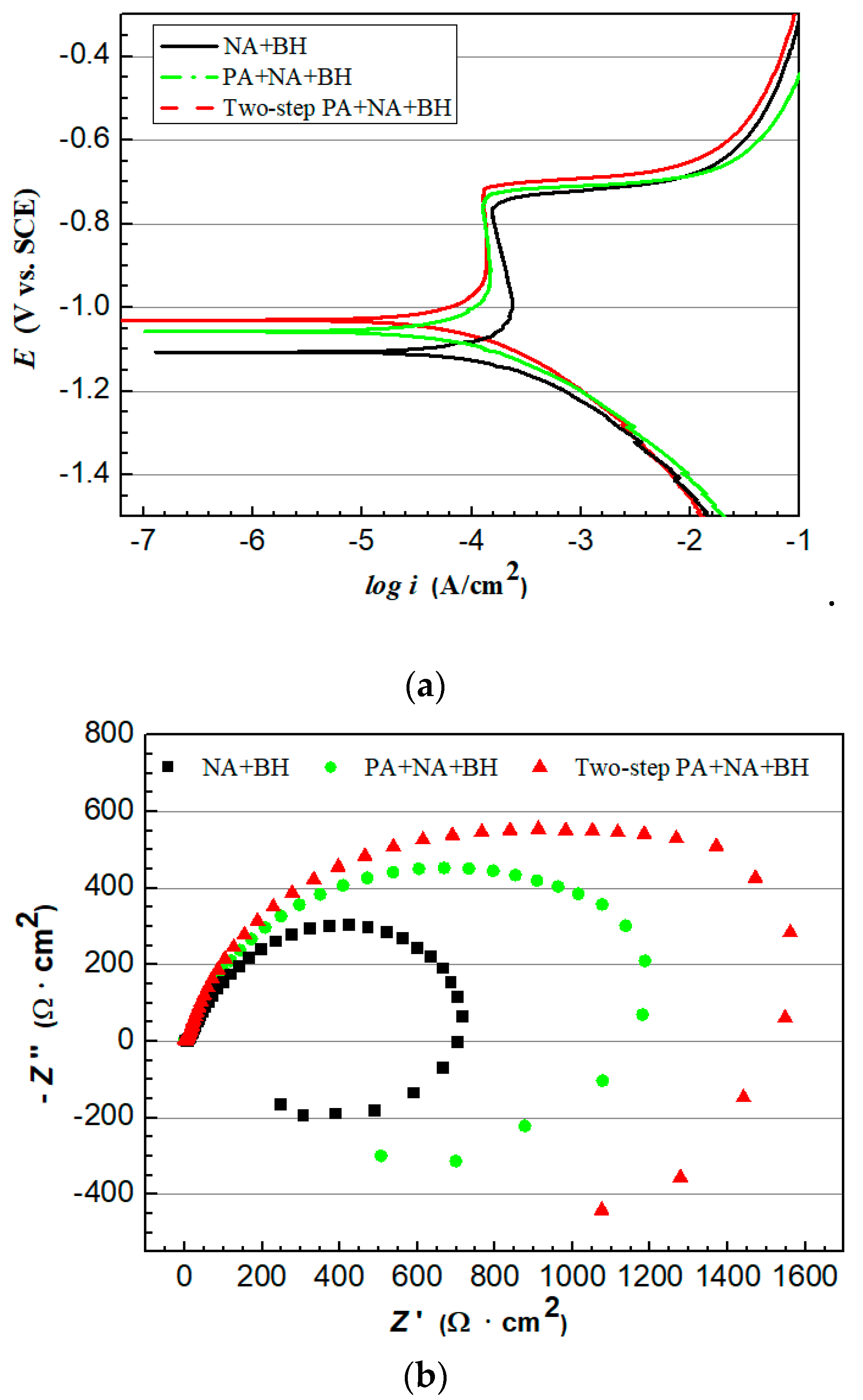
| Stage | Stage I | Stage II | Stage III | |||
|---|---|---|---|---|---|---|
| Heat Treatment | nSwift1 | εtr1 | nSwift2 | εtr2 | nSwift3 | |
| NA | 3.239 | 0.01189 | 3.912 | 0.09691 | 29.303 | |
| NA + BH | 2.756 | 0.04187 | 5.412 | 0.09049 | 62.158 | |
| PA | 2.744 | 0.04038 | 4.409 | 0.15059 | 63.115 | |
| PA + BH | 6.108 | 0.06277 | 8.877 | 0.10948 | 150.413 | |
| Two-step PA | 3.285 | 0.07753 | 5.276 | 0.15436 | 370.775 | |
| Two-step PA + BH | 4.566 | 0.04899 | 9.464 | 0.0958 | 777.251 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, X.-T.; Wang, J.-Y. Influence of Preaging Treatment on Bake-Hardening Response and Electrochemical Corrosion Behavior of High Strength Al-Zn-Mg-Cu-Zr Alloy. Metals 2019, 9, 895. https://doi.org/10.3390/met9080895
Li H, Liu X-T, Wang J-Y. Influence of Preaging Treatment on Bake-Hardening Response and Electrochemical Corrosion Behavior of High Strength Al-Zn-Mg-Cu-Zr Alloy. Metals. 2019; 9(8):895. https://doi.org/10.3390/met9080895
Chicago/Turabian StyleLi, Hui, Xiao-Teng Liu, and Jia-Yi Wang. 2019. "Influence of Preaging Treatment on Bake-Hardening Response and Electrochemical Corrosion Behavior of High Strength Al-Zn-Mg-Cu-Zr Alloy" Metals 9, no. 8: 895. https://doi.org/10.3390/met9080895
APA StyleLi, H., Liu, X.-T., & Wang, J.-Y. (2019). Influence of Preaging Treatment on Bake-Hardening Response and Electrochemical Corrosion Behavior of High Strength Al-Zn-Mg-Cu-Zr Alloy. Metals, 9(8), 895. https://doi.org/10.3390/met9080895





