Influence of Nickel Powders on Corrosion Resistance of Cold Sprayed Coatings on Al7075 Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corrosion Test
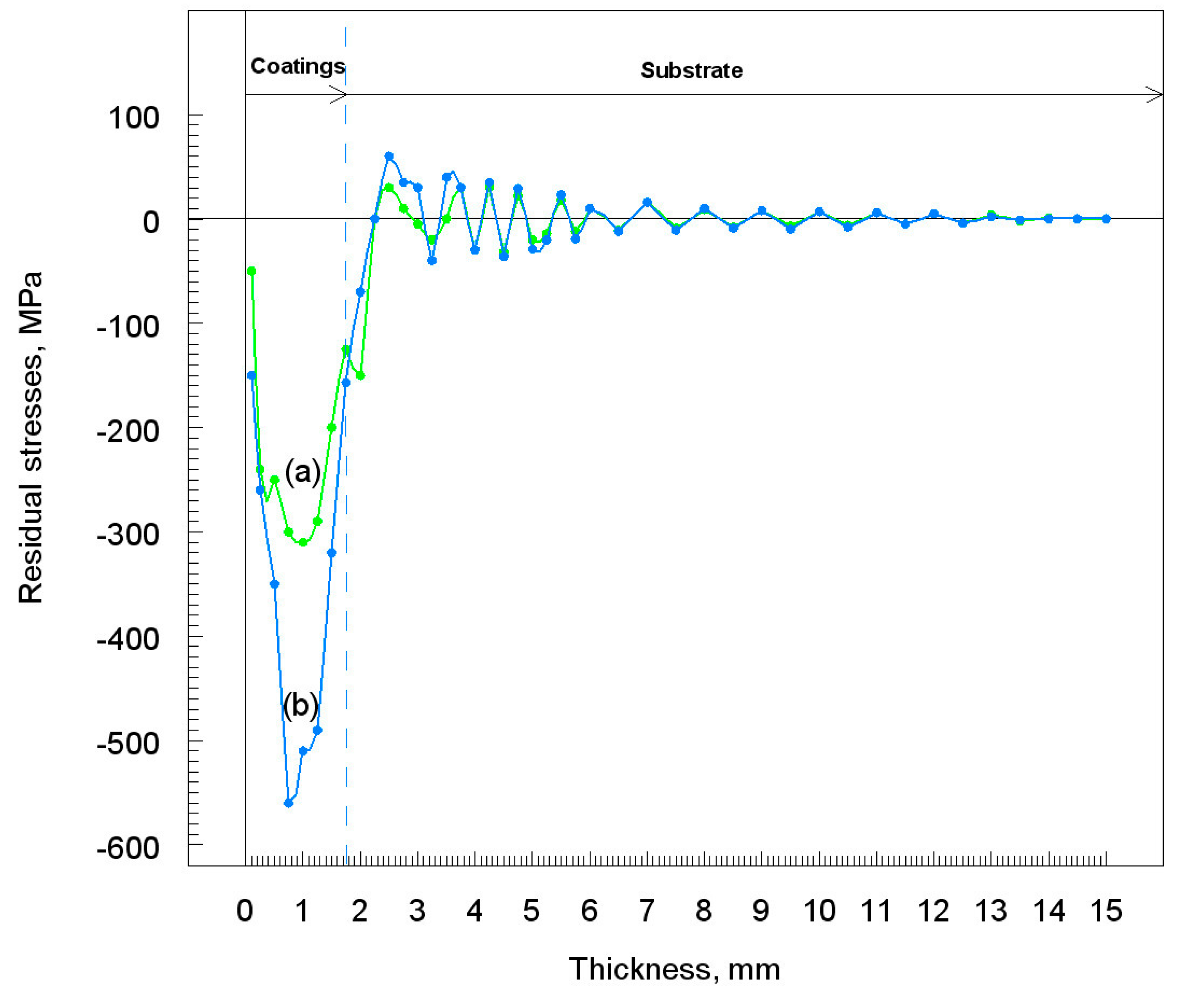
2.2. Residual Stress
3. Results and Discussion
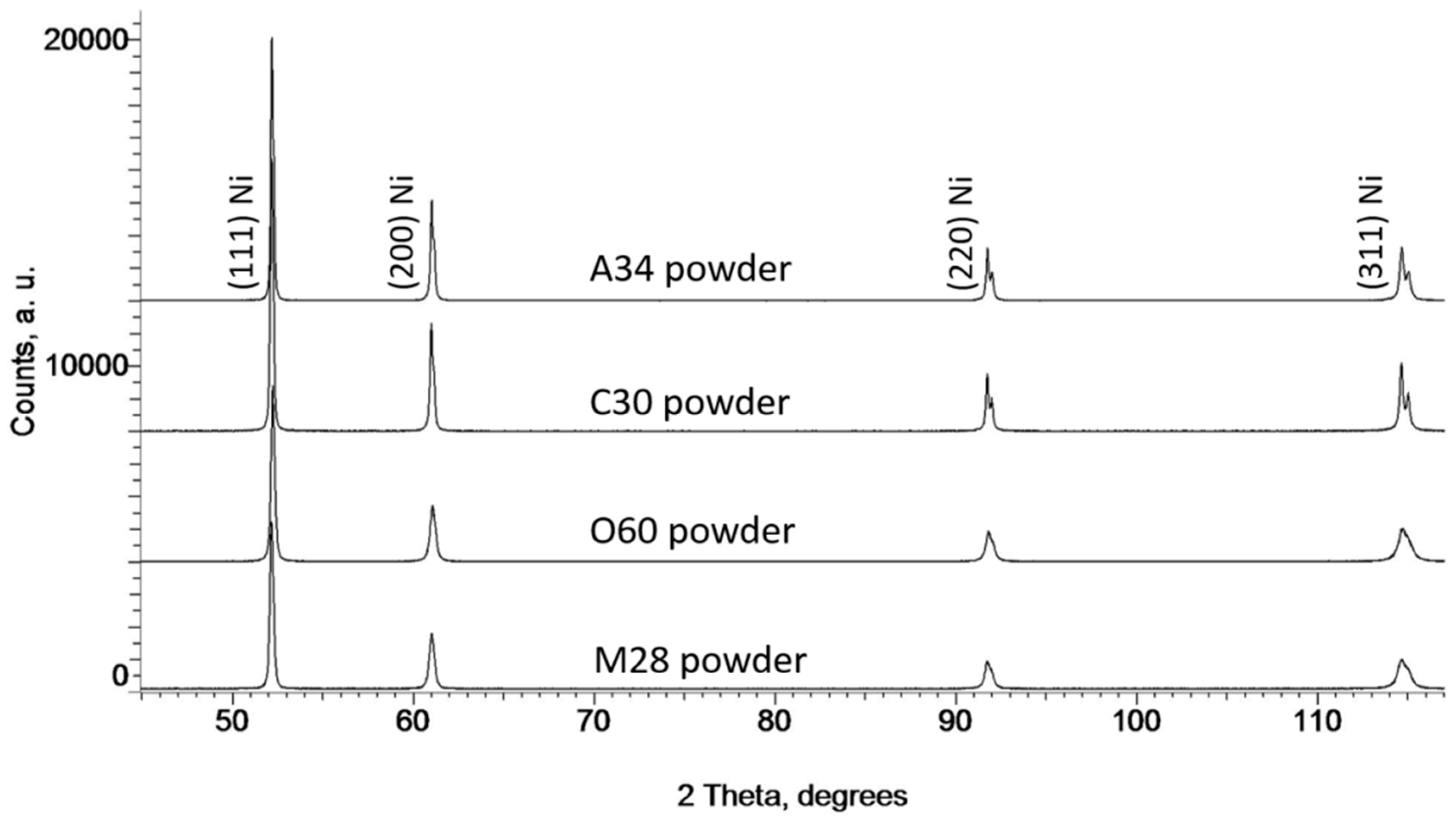
3.1. Microstructural Characteristics of Ni Powders
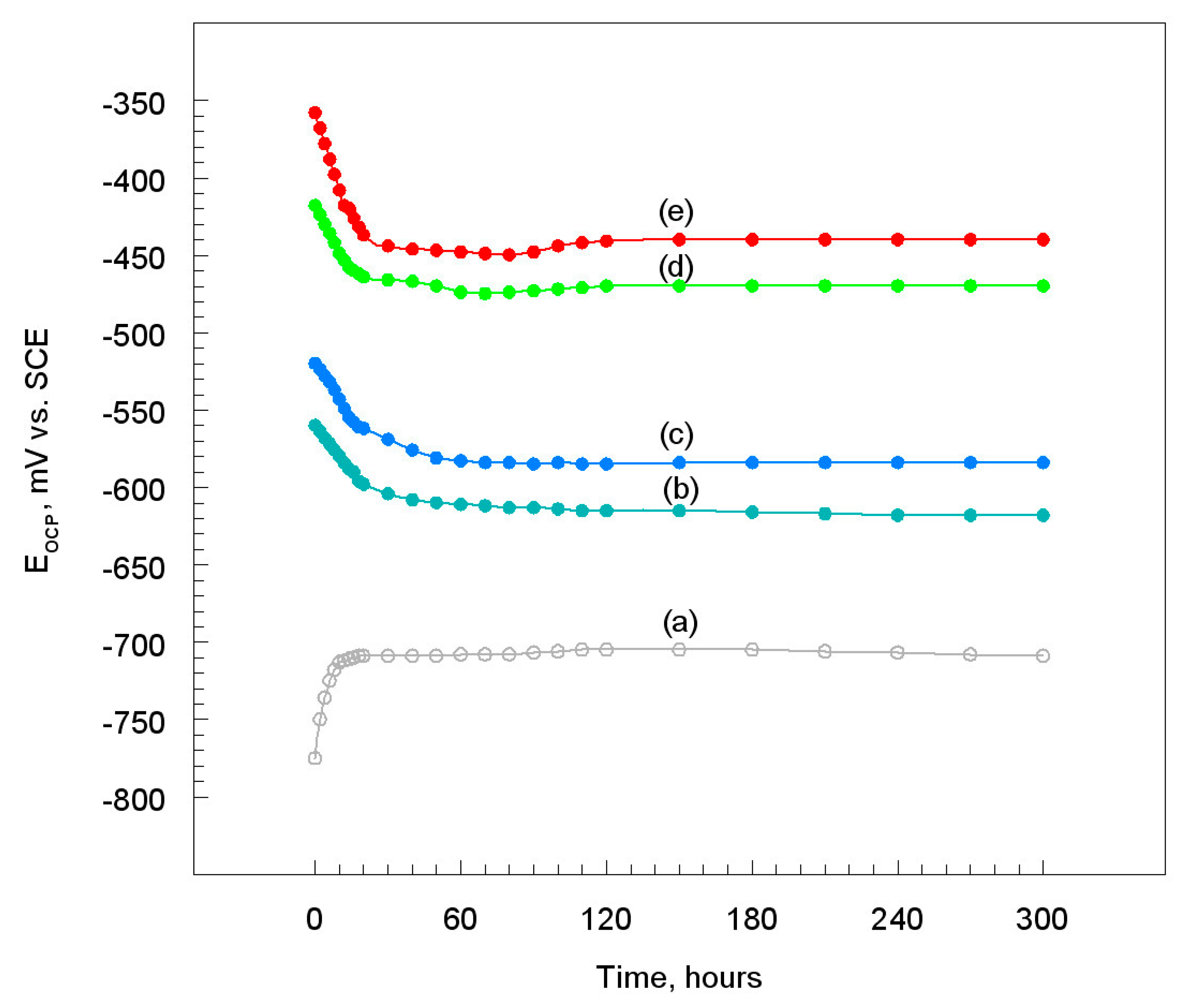
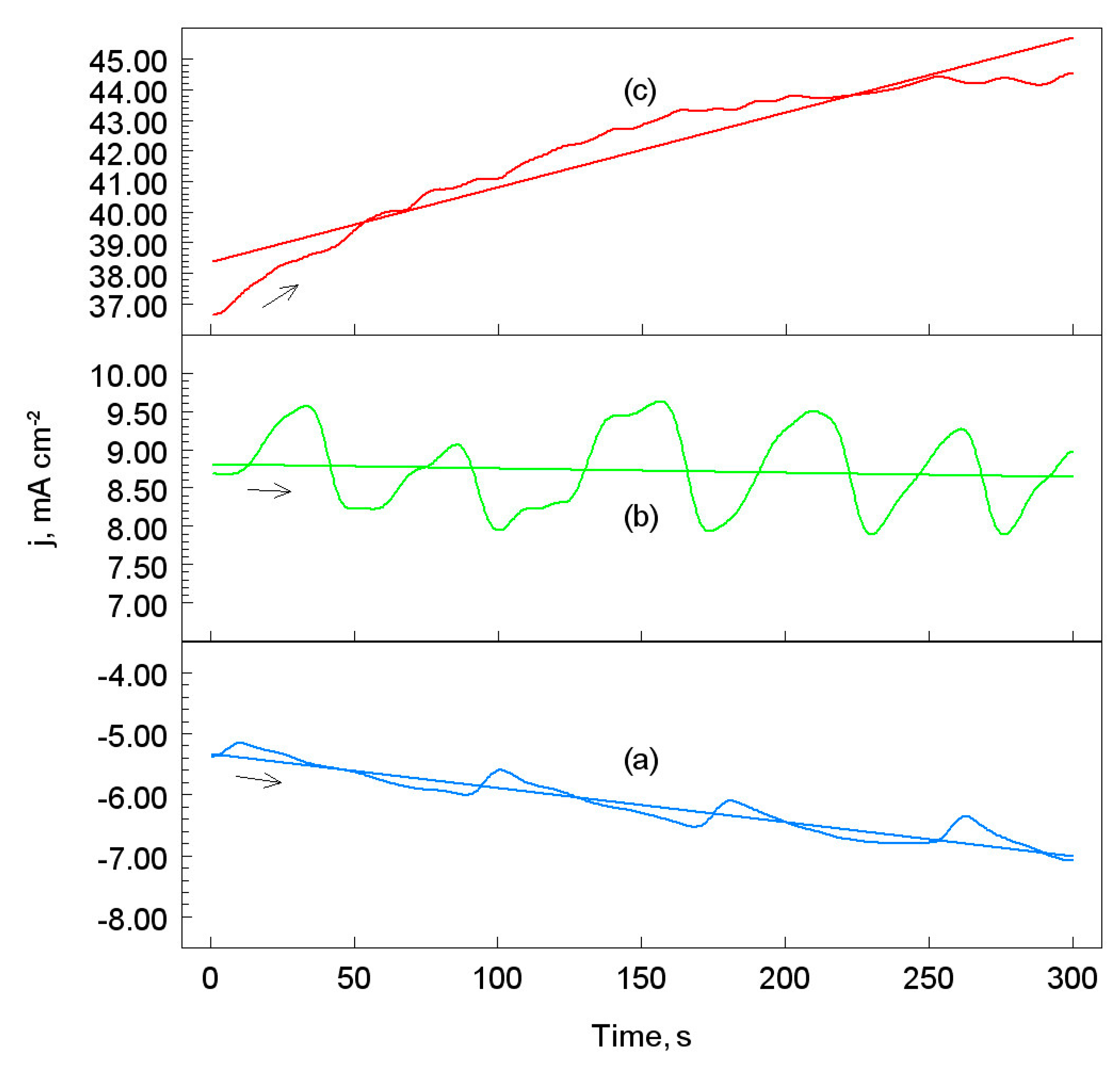
3.2. Chronopotentiometric Measurements
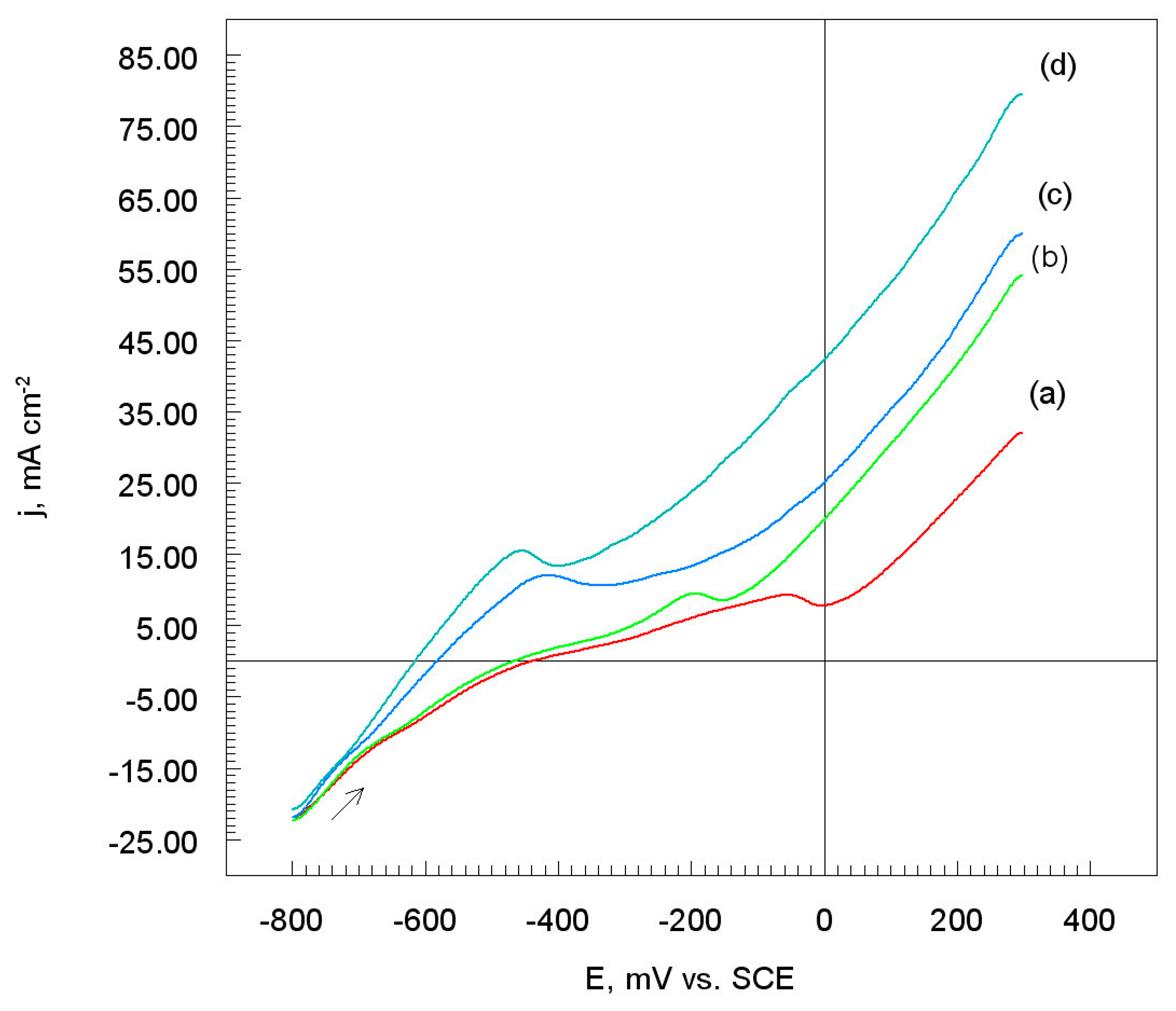
3.3. Potentiodynamic Polarization Measurements
3.3.1. Corrosion Electrochemical Parameters
3.3.2. Polarization Resistance and Corrosion Rate
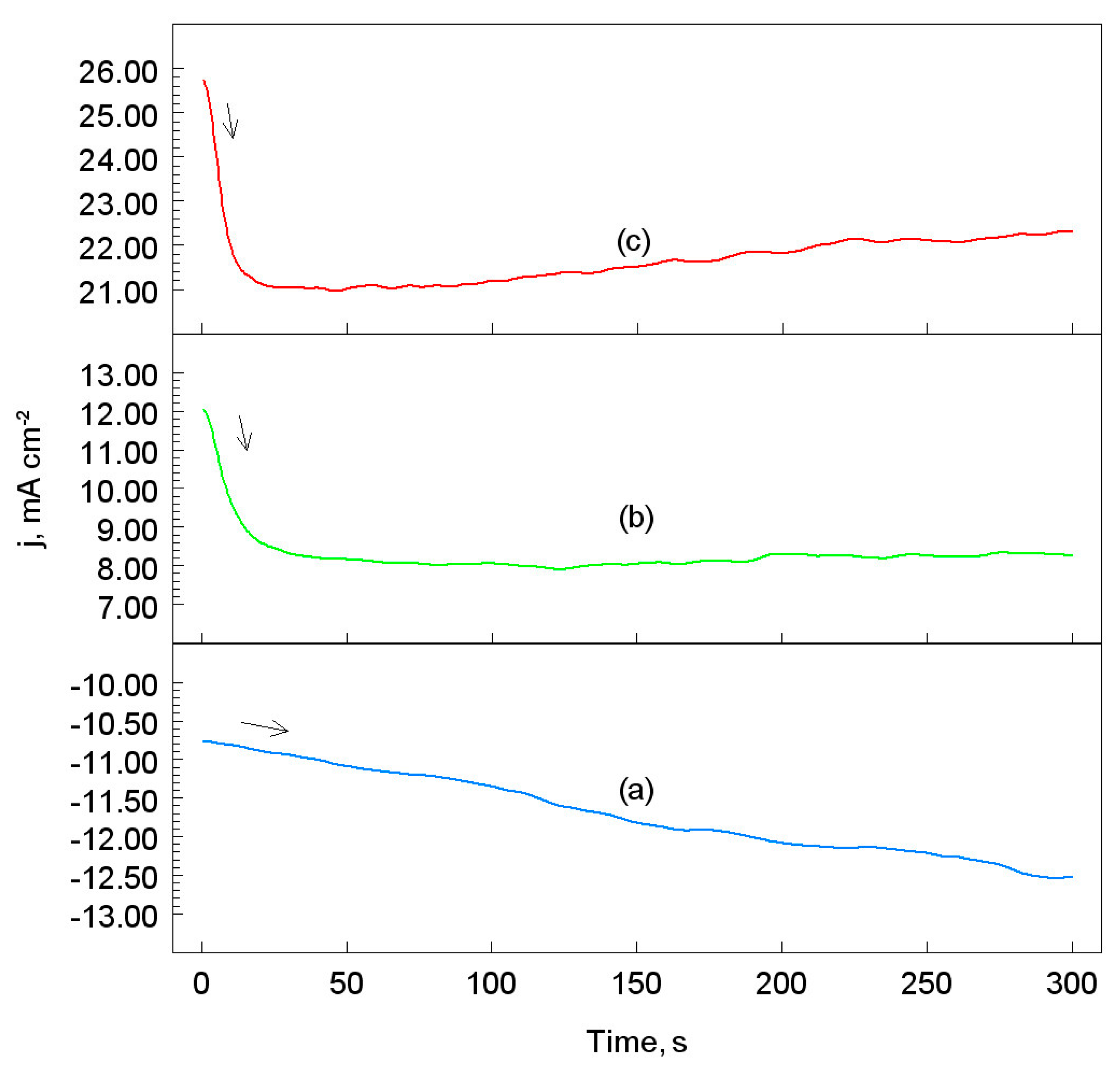
3.4. Chronoamperometric Measurements
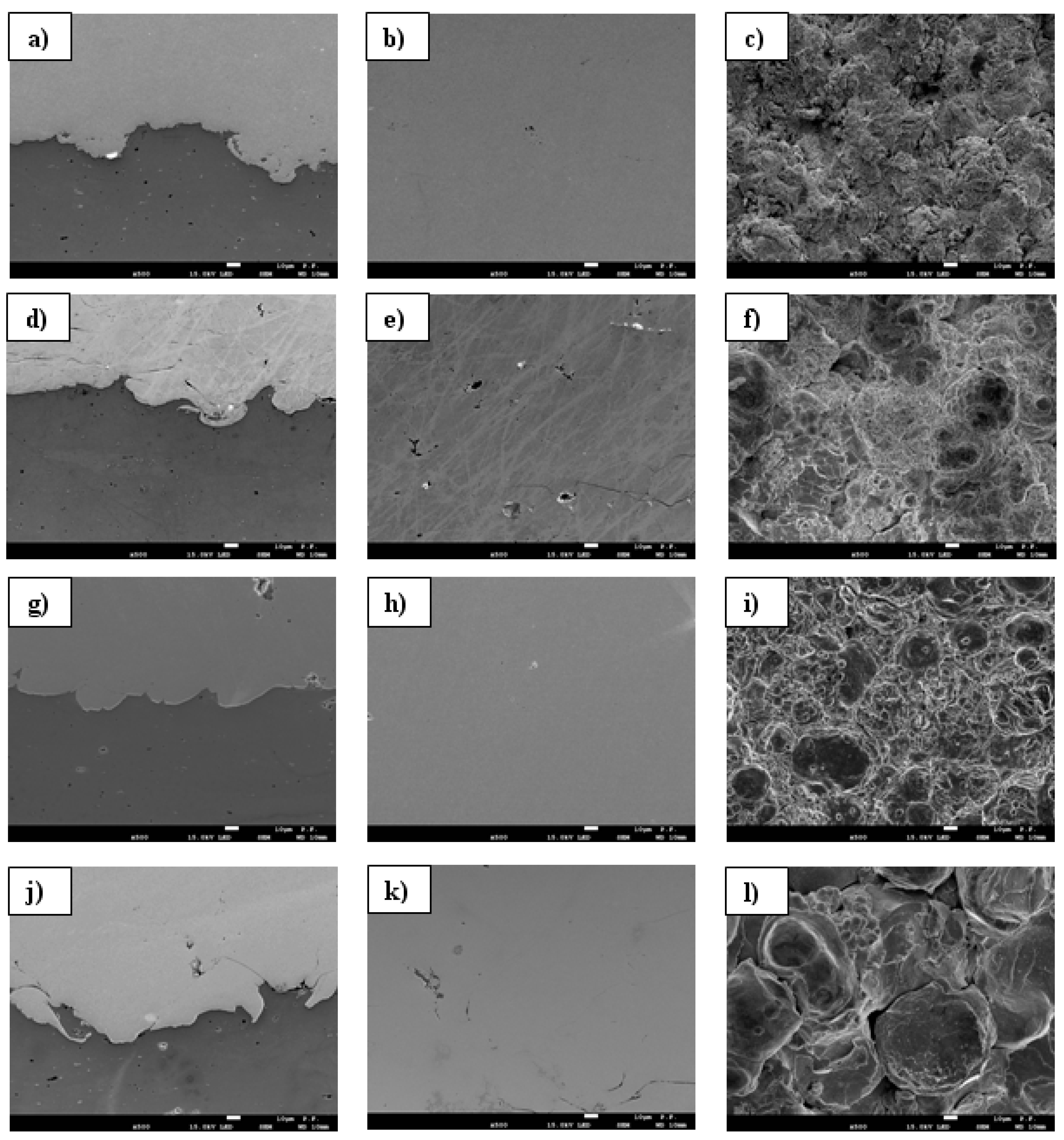
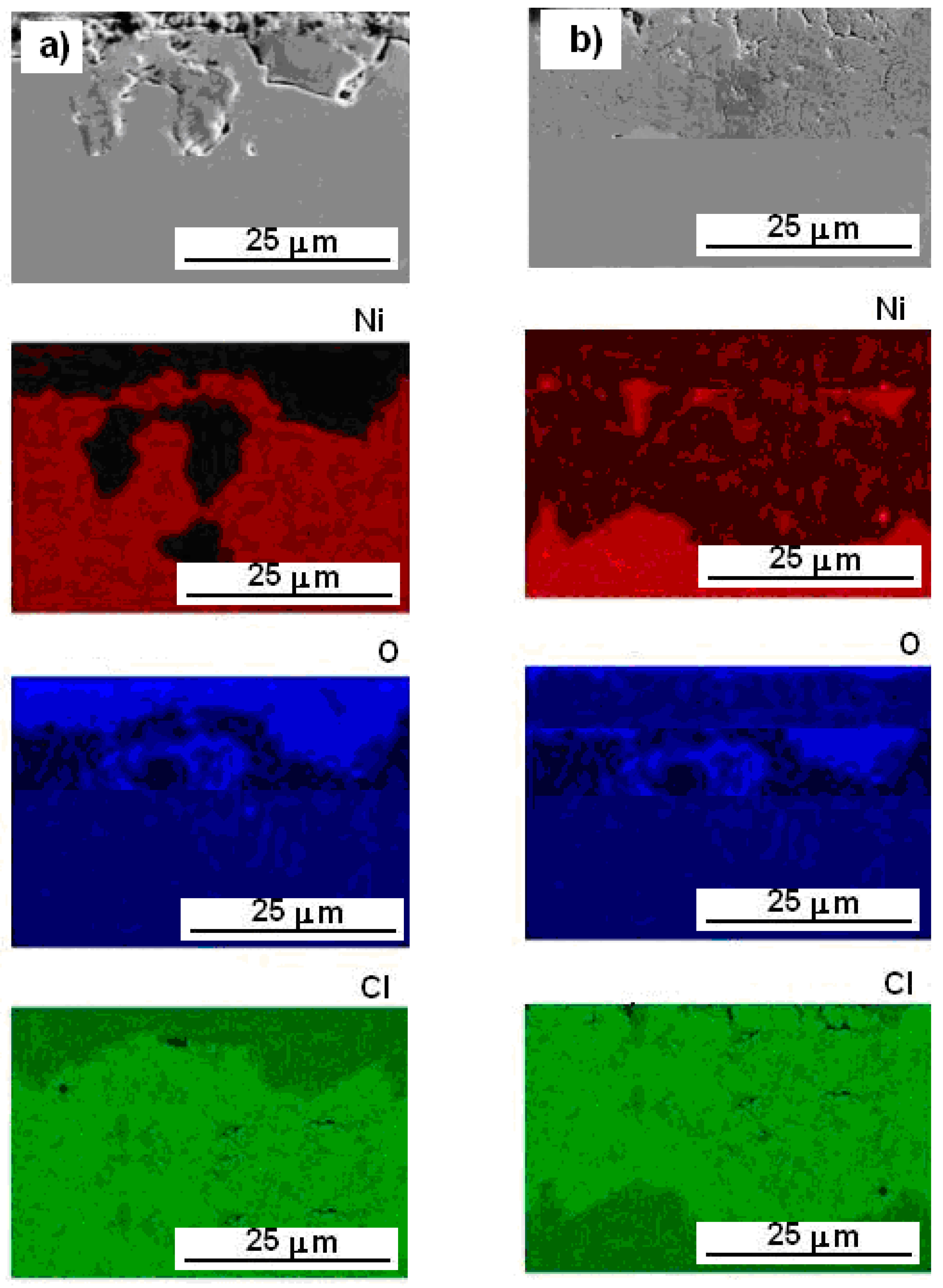
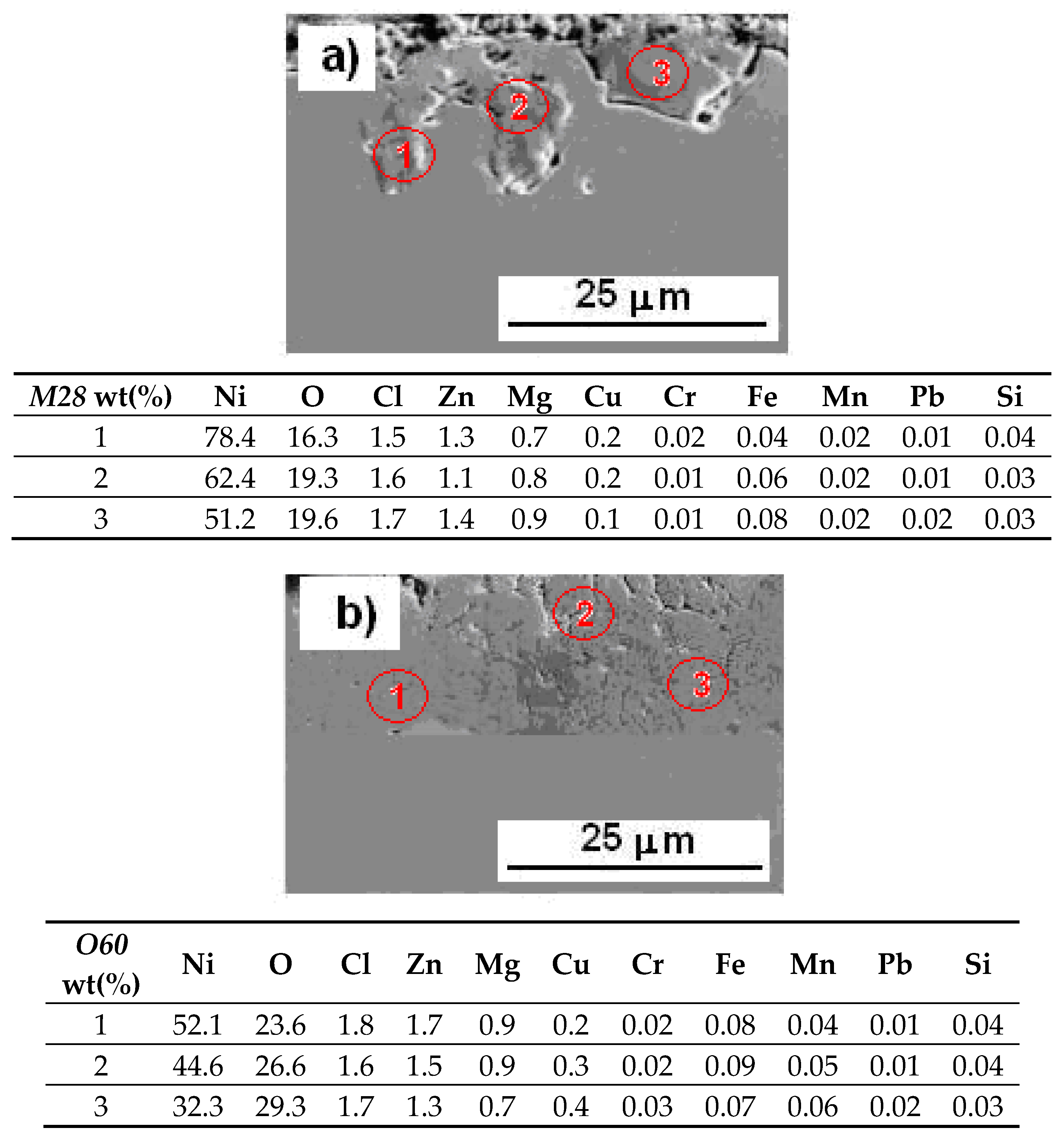
3.5. SEM/EDS Elemental Mapping
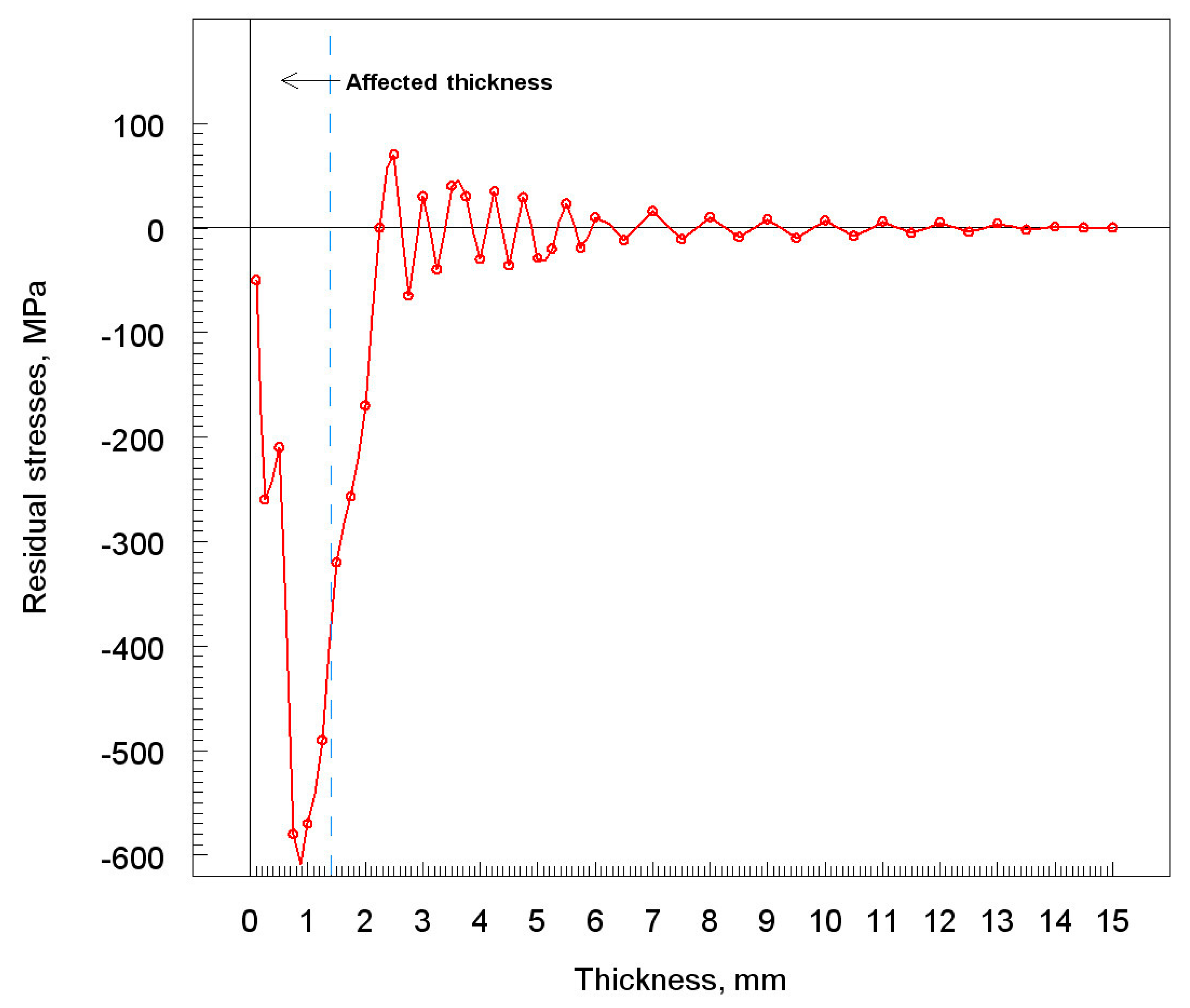
3.6. Residual Stress
4. Conclusions
- Nickel coatings adhere well on the Al7075 alloy, and the most smooth surface structure was obtained for Ni powders of irregular spherical and dendritic morphology.
- The mechanism of electrochemical corrosion of nickel coatings in acidic chloride solutions is a multi-stage process, and the main product of corrosion is NiOads. The oxide layer is porous and does not protect the Al7075 substrate from the penetration of an aggressive chloride solution.
- The polarization resistance of Ni coatings decreases with an increase in the size of nickel powders, which means that it is easy to replace the electrical charge and mass between the electrode and the electrolyte solution.
- The corrosion rate of nickel coatings in the chloride solution increases with an increase in the grain size of nickel powders. Moreover, the lowest corrosion rate of the tested coatings was observed for Ni grains of irregular spherical or dendritic morphology.
- The constant bombardment of particles on the substrate causes the emergence of residual stress. The highest residual stress value was observed for large grains of nickel powder.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karthikeyan, J. Cold Spray Technology. Adv. Mater. Process. 2005, 163, 33–35. [Google Scholar]
- King, P.C.; Bae, G.; Zahiri, S.H.; Jahedi, M.; Lee, C. An experimental and finite element study of cold spray copper impact onto two aluminum substrates. J. Thermal. Spray Technol. 2010, 19, 620–634. [Google Scholar] [CrossRef]
- Lee, M.S.; Choi, H.J.; Choi, J.W.; Kim, H.J. Application of Cold Spray Coating Technique to an Underground Disposal Copper Canister and its Corrosion Properties. Nucl. Eng. Technol. 2011, 43, 557–566. [Google Scholar] [CrossRef]
- Wang, Q.; Birbilis, N.; Zahang, X. On the Formation of a diffusion bond from cold-spray coatings. Metall. Mater. Trans. A: Phys. Metall. Mater. Sci. 2012, 43, 1395–1399. [Google Scholar] [CrossRef]
- Marrocco, T.; McCartney, D.G.; Shipway, P.H.; Sturgcon, A.J. Production of titanium deposits by cold-gas dynamic spray: Numerical modeling and experimental characterization. J. Thermal. Spray Technol. 2006, 15, 263–272. [Google Scholar] [CrossRef]
- Price, T.S.; Shipway, P.H.; McCartney, G.D. Effect of Cold Spray Deposition of a Titanium Coating on Fatigue Behavior of a Titanium Alloy. J. Thermal. Spray Technol. 2006, 15, 507–512. [Google Scholar] [CrossRef]
- Wong, W.; Rezaein, A.; Irissou, E.; Legoux, J.G.; Yue, S. Cold spray characteristics of commercially pure Ti and Ti-Al4V. Adv. Mater. Res. 2010, 89-91, 639–644. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Nakki, J.; Vuoristo, P. Corrosion properties of could-sprayed tantalum coatings. J. Thermal. Spray Technol. 2008, 18, 75–82. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Bolelli, G.; Lusvarghi, L.; Casadei, F.; Vuoristo, P. Corrosion resistance of cold-sprayed Ta coatings in very aggressive conditions. Surf. Coat. Technol. 2010, 205, 1103–1107. [Google Scholar] [CrossRef]
- Bolelli, G.; Bonferroni, B.; Koivuluoto, H.; Lusvarghi, L.; Vuoristo, P. Depth-sensing indentation for assessing the mechanical properties of cold-sprayed Ta. Surf. Coat. Technol. 2010, 205, 2209–2217. [Google Scholar] [CrossRef]
- Xiong, Y.; Bae, G.; Xiong, X.; Lee, C. The effects of successive impacts and cold welds on the deposition onset of cold spray coatings. J. Thermal. Spray Technol. 2010, 19, 575–585. [Google Scholar] [CrossRef]
- Wong, W.; Irissou, V.P.; Sone, M.; Bernier, F.; Legoux, G.; Fukanuma, H.; Yue, S. Cold spray forming of Inconel 718. J. Thermal. Spray Technol. 2012, 21, 1–9. [Google Scholar] [CrossRef]
- Luo, X.T.; Li, Y.J.; Li, C.X.; Yang, G.J.; Li, C.J. Effect of spray conditions on deposition behavior and microstructure of cold sprayed Ni coatings sprayed with a porous electrolytic Ni powder. Surf. Coat. Technol. 2016, 289, 85–93. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Milanti, A.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. High-pressure cold-sprayed Ni and Ni-Cu coatings: Improved structures and corrosion properties. J. Thermal. Spray Technol. 2014, 23, 98–103. [Google Scholar] [CrossRef]
- Gouda, V.; Selim, I.; Khedr, A.; Fathi, A. Pitting corrosion of Monel-400 alloy in chloride solutions. J. Mater. Sci. Technol. 1999, 15, 208–212. [Google Scholar]
- Sherif, E.; Almajid, A.; Bairamov, A.; Al-Zahrani, E. Corrosion of Monel-400 in aerated stagnant arabian gulf seawater after different exposure intervals. Int. J. Electrochem. Sci. 2011, 6, 5430–5444. [Google Scholar]
- Al-Abdallah, M. Impedance measurements on Inconel and Monel alloys in dead sea water and sulphate solution. Br. Corros. J. 1996, 31, 213–217. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Prakash, S. Performance of cold sprayed Ni based coatings in actual boiler environment. Surf. Coat. Technol. 2017, 318, 50–61. [Google Scholar] [CrossRef]
- Lima, R.S.; Karthikeyan, J.; Kay, C.M.; Lindemann, J.; Berndt, C.C. Microstructural characteristics of cold-sprayed microstructural WC-Co coatings. Thin Solid Films 2002, 416, 129–135. [Google Scholar] [CrossRef]
- Couto, M.; Dosta, S.; Fernández, J.; Guilemany, J.M. Comparison of the mechanical and electrochemical properties of WC-25Co coatings obtained by high velocity oxy-fuel and cold gas spraying. J. Therm. Spray. Technol. 2014, 28, 1251–1258. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, J.; Cui, X.; Wu, J.; Li, T.; Xiong, T. Microstructure characteristics and mechanical properties of Al-12Si coatings on AZ31 magnesium alloy produced by cold spray technique. J. Therm. Spray. Technol. 2016, 25, 1020–1028. [Google Scholar] [CrossRef]
- Li, C.-J.; Li, W.-Y. Deposition characteristics of titanium coating in cold spraying. Surf. Coat. Technol. 2003, 167, 278–283. [Google Scholar] [CrossRef]
- Goldhaum, D.; Shockley, J.M.; Chromik, R.R.; Rezaeian, A.; Yue, S.; Legoux, J.-G. The effect of deposition conditions on adhesion strength of Ti and Ti6Al4V cold spray splats. J. Therm. Spray. Technol. 2012, 21, 288–303. [Google Scholar] [CrossRef]
- Birt, A.M.; Champagne, V.K.; Sisson, R.D.; Apelian, D. Microstructural analysis of cold-sprayed Ti-6Al-4V at the micro- and nano-scale. J. Therm. Spray. Technol. 2015, 24, 1277–1288. [Google Scholar] [CrossRef]
- Khum, N.W.; Tan, A.W.Y.; Liu, E. Mechanical and tribological properties of cold-sprayed Ti coatings on Ti-6Al-4V substrates. J. Therm. Spray. Technol. 2016, 25, 715–724. [Google Scholar] [CrossRef]
- Ajdelsztajn, L.; Jodoin, B.; Schoenung, J.M. Synthesis and mechanical properties of nanocrystalline Ni coatings produced by cold gas dynamic spraying. Surf. Coat. Technol. 2006, 201, 1166–1172. [Google Scholar] [CrossRef]
- Góral, A.; Żórawski, W.; Czaja, P.; Lityńska-Dobrzyńska, L.; Makrenek, M.; Kowalski, S. Effect of powder morphology on the microstructure and properties of cold sprayed Ni coatings. Int. J. Mater. Res. 2019, 110, 49–59. [Google Scholar] [CrossRef]
- Faria, M.I.S.T.; Robin, A.; Prisco, L.P.; Puccini, M.C.; Goncalves, D.C.; Lourenc, J.C. Corrosion of Al 7075 Alloy during the production of aeronautic components: Influence of process parameters at the deburring stage. Mater. Corros. 2013, 64, 1114–1120. [Google Scholar] [CrossRef]
- Pruthviraj, R.D.; Rashmi, M. Electrochemical studies of aluminium 7075 alloy in different concentration of acid chloride medium. Mater. Sci. Eng. 2016, 5:2, 1–4. [Google Scholar]
- Scendo, M.; Radek, N.; Trela, J. Influence of laser treatment on the corrosive resistance of WC-Cu coating produced by electrospark deposition. Int. J. Electrochem. Sci. 2013, 8, 9264–9277. [Google Scholar]
- Scendo, M.; Trela, J.; Radek, N. Influence of laser power on the corrosive resistance of WC-Cu coating. Surf. Coat. Technol. 2014, 259, 401–407. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Staszewska, K.; Makrenek, M.; Goral, A. Influence of surface pretreatment on the corrosion resistance of cold spray nickel coatings in acidic chloride solution. J. Mater. Eng. Perform. 2018, 27, 1725–1737. [Google Scholar] [CrossRef]
- Luo, X.-T.; Li, Y.-J.; Li, C.-J. A comparison of cold spray deposition behavior between gas atomized and dendritic porous electrolytic Ni powders under the same spray conditions. Mater. Lett. 2016, 163, 58–60. [Google Scholar] [CrossRef]
- Bae, G.; Jang, J.; Lee, C. Correlation of particle impact conditions with bonding, nanocrystal formation and mechanical properties in kinetic sprayed nickel. Acta Mater. 2012, 60, 3524–3535. [Google Scholar] [CrossRef]
- Grujicic, M.; Zhao, C.L.; DeRosset, W.S.; Helfritch, D. Adiabatic shear instability based mechanism for particles/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Luo, X.-T.; Li, C.-X.; Shang, F.-L.; Yang, G.-J.; Wang, Y.-Y.; Li, C.-J. High velocity impact induced microstructure evolution during deposition of cold spray coatings: A review. Surf. Coat. Technol. 2014, 254, 11–20. [Google Scholar] [CrossRef]
- Hawang, I.J.; Hawang, D.Y.; Kim, Y.M.; Yoo, B.; Shin, D.H. Formation of uniform passive oxide layers on high Si content Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2010, 504, 527–530. [Google Scholar] [CrossRef]
- Chin, R.J.; Nobe, K. Electrodissolution kinetics of iron in chloride solutions. J. Electrochem. Soc. 1972, 119, 1457–1461. [Google Scholar] [CrossRef]
- Larib, M.; Mesrati, N.; Vannes, A.B.; Treheux, D. Metallurgical characterization and determination of residual stresses of coating formed by thermal spraying. J. Therm. Spray Technol. 2003, 12, 234–239. [Google Scholar] [CrossRef]

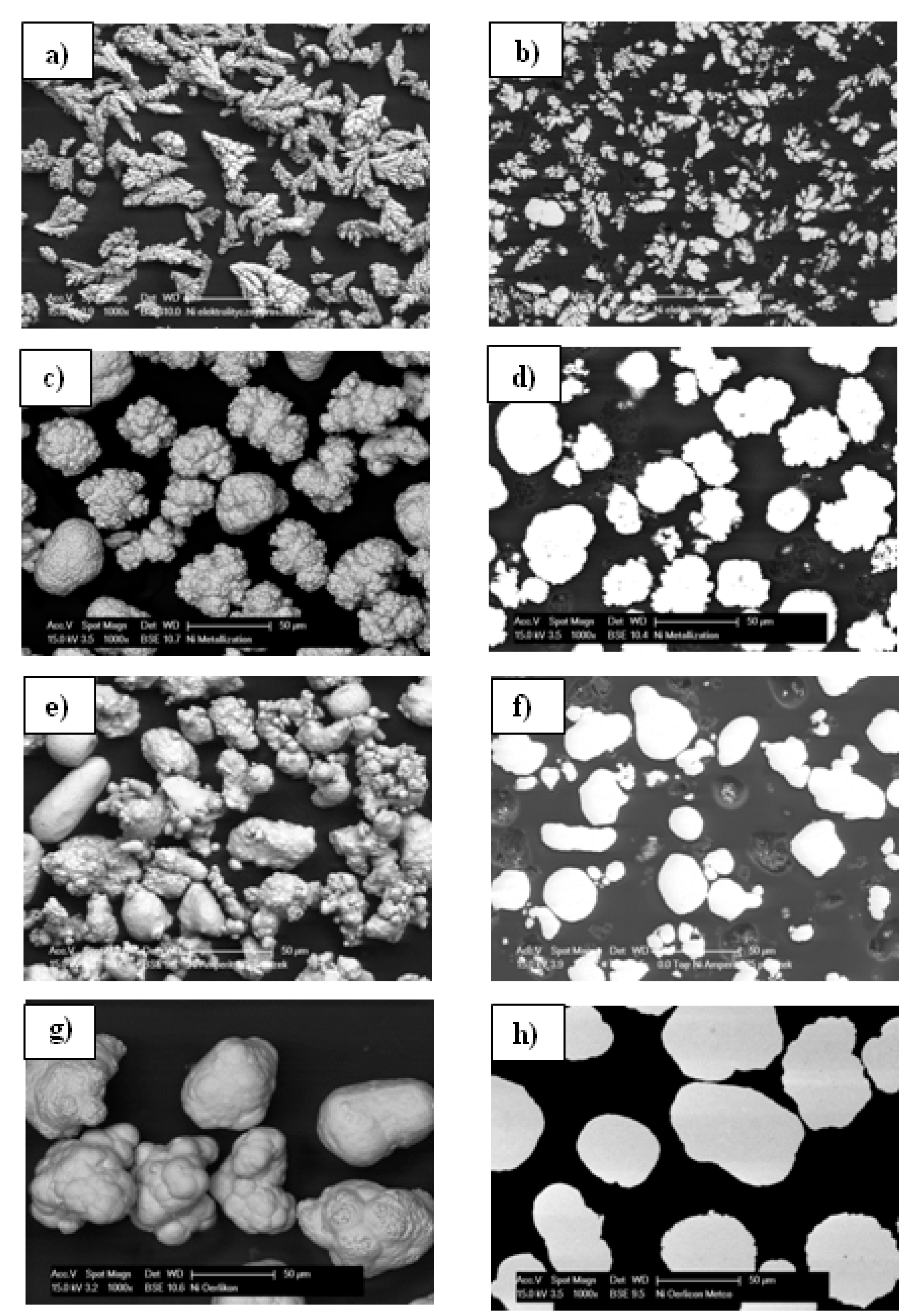
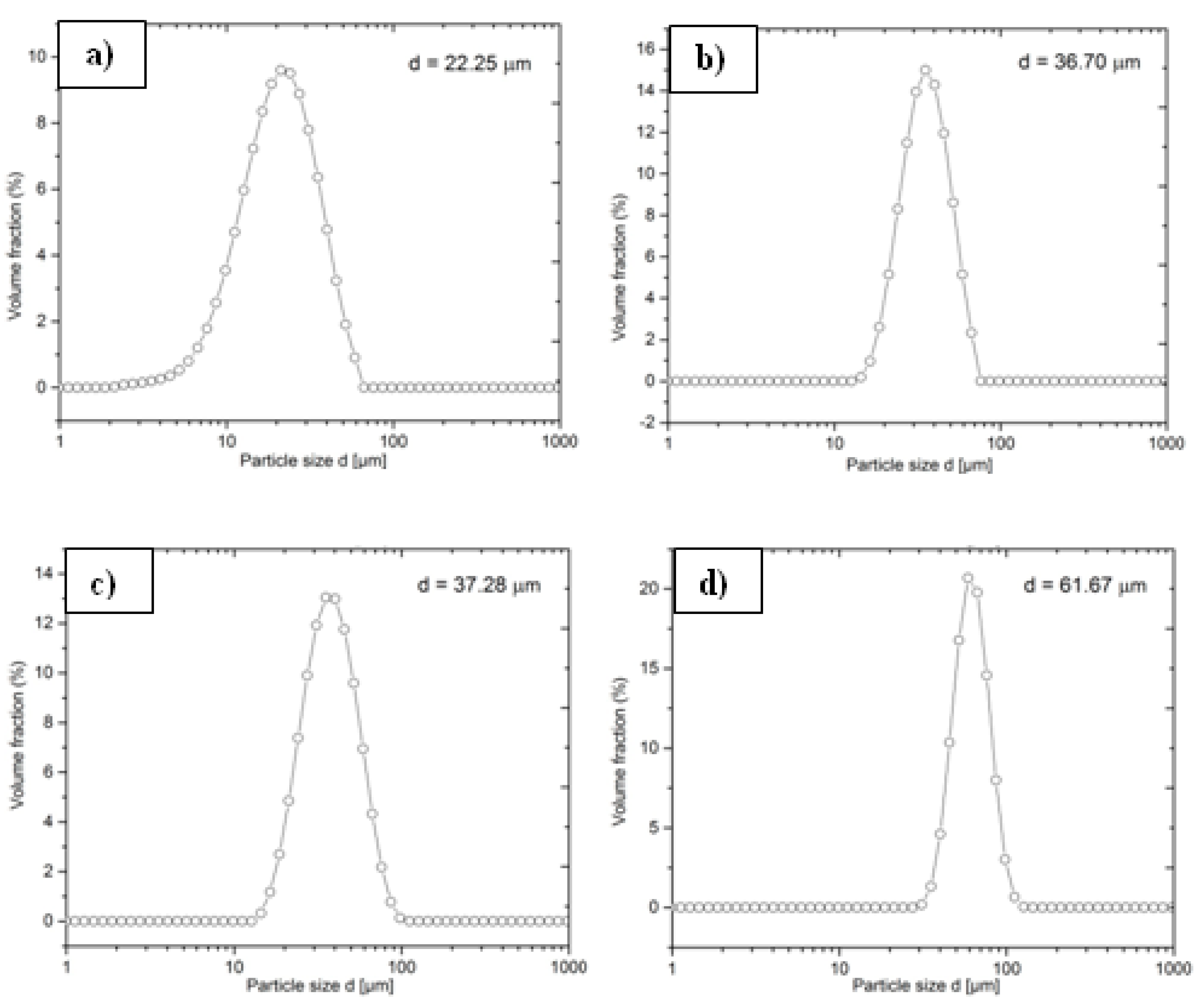
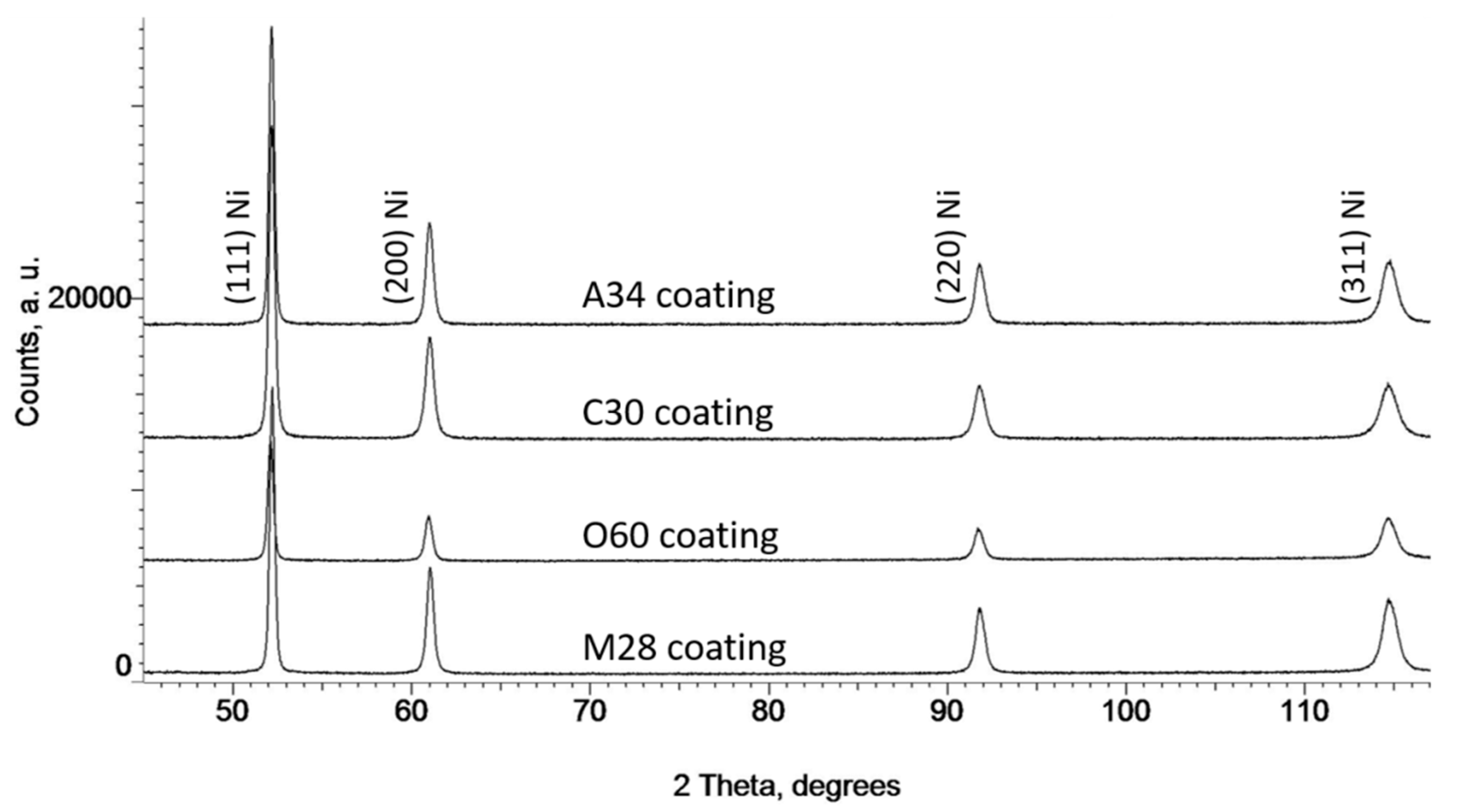
| Name powder/coating | C30 | M28 | A34 | O60 |
| Grain size, μm | 15–30 | 20–40 | 22–45 | 50–75 |
| Name Powder/Coating | Ecorr mV vs. SCE | jcorr mA cm−2 | −βc | βa |
|---|---|---|---|---|
| mV dec−1 | ||||
| M28 | −440 | 3.1 | 400 | 720 |
| C30 | −470 | 3.4 | 390 | 640 |
| A34 | −584 | 5.5 | 360 | 440 |
| O60 | −618 | 6.9 | 350 | 430 |
| Name Powder/Coating | Rp kΩ cm2 | υcorr mm year−1 |
|---|---|---|
| M28 | 36 | 3.3 |
| C30 | 31 | 3.7 |
| A34 | 16 | 5.9 |
| O60 | 12 | 7.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scendo, M.; Zorawski, W.; Goral, A. Influence of Nickel Powders on Corrosion Resistance of Cold Sprayed Coatings on Al7075 Substrate. Metals 2019, 9, 890. https://doi.org/10.3390/met9080890
Scendo M, Zorawski W, Goral A. Influence of Nickel Powders on Corrosion Resistance of Cold Sprayed Coatings on Al7075 Substrate. Metals. 2019; 9(8):890. https://doi.org/10.3390/met9080890
Chicago/Turabian StyleScendo, Mieczyslaw, Wojciech Zorawski, and Anna Goral. 2019. "Influence of Nickel Powders on Corrosion Resistance of Cold Sprayed Coatings on Al7075 Substrate" Metals 9, no. 8: 890. https://doi.org/10.3390/met9080890
APA StyleScendo, M., Zorawski, W., & Goral, A. (2019). Influence of Nickel Powders on Corrosion Resistance of Cold Sprayed Coatings on Al7075 Substrate. Metals, 9(8), 890. https://doi.org/10.3390/met9080890






