Crystallization and Structure of AlSi10Mg0.5Mn0.5 Alloy with Dispersion Strengthening with Al–FexAly–SiC Phases
Abstract
:1. Introduction
2. Aim and Scope of the Paper
- -
- Investigation of the technological and material concepts needed to produce the casted alloy composite modified with powders FeAl, Al–FexAly, and Al–FexAly–SiC,
- -
- Determination of the method by which to produce the powders for modification of the aluminum matrix,
- -
- Preparation of the technological process for producing composites with varied contents of structural ingredients,
- -
- Determination of chemical and phase compositions and the structure of the alloy AlSi10Mg0.5Mn0.5.
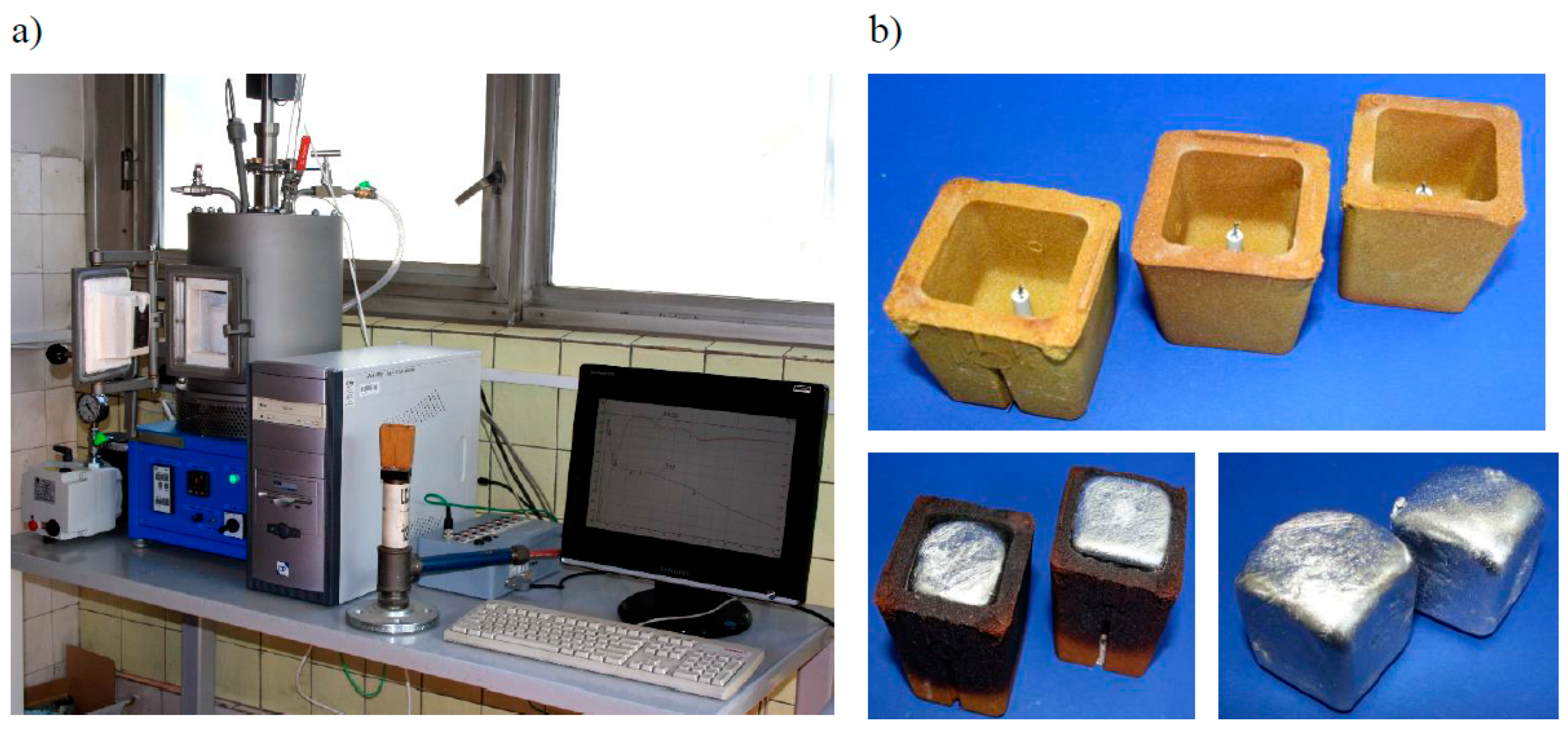
3. Material and Methodology of Tests
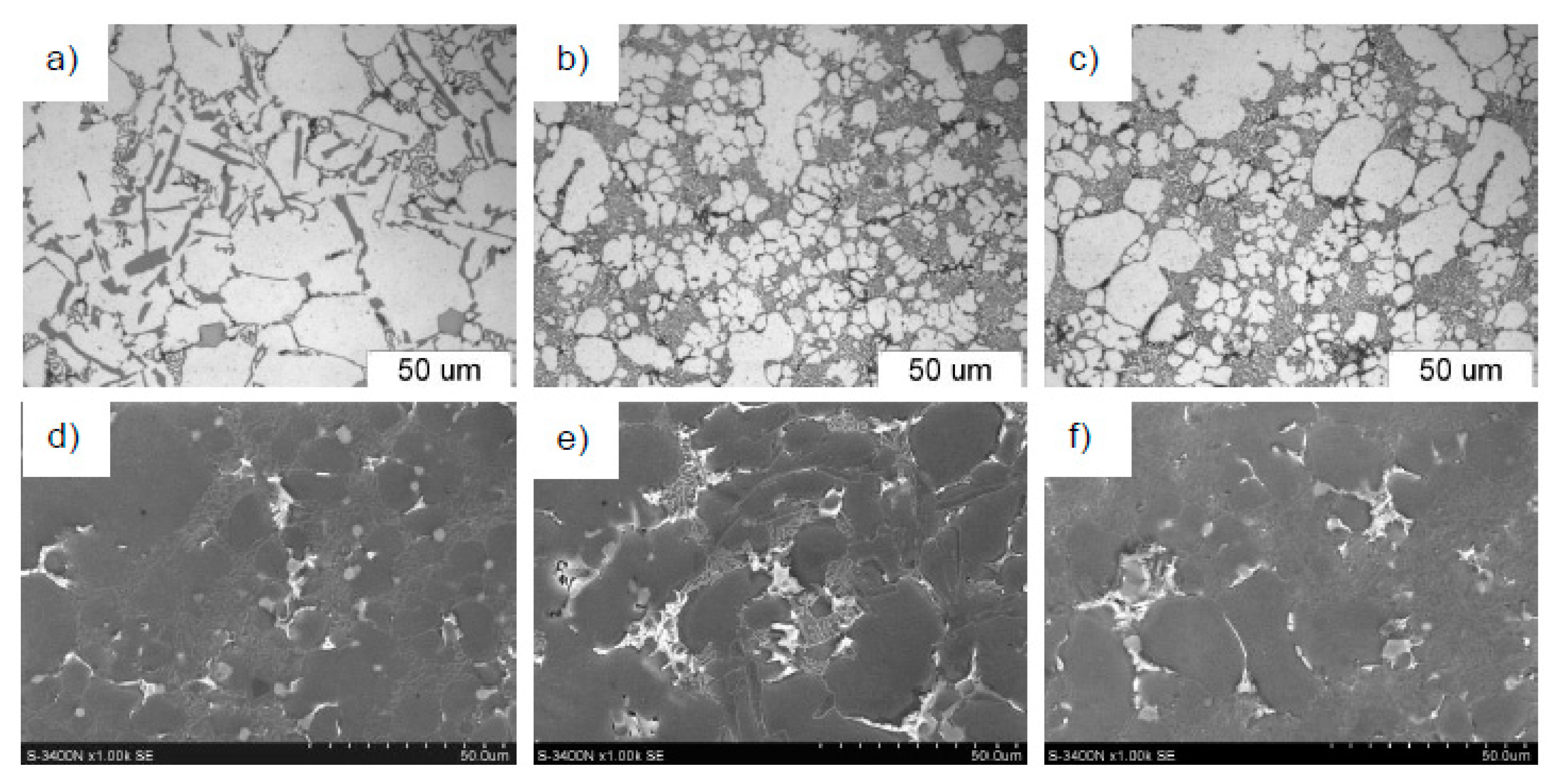
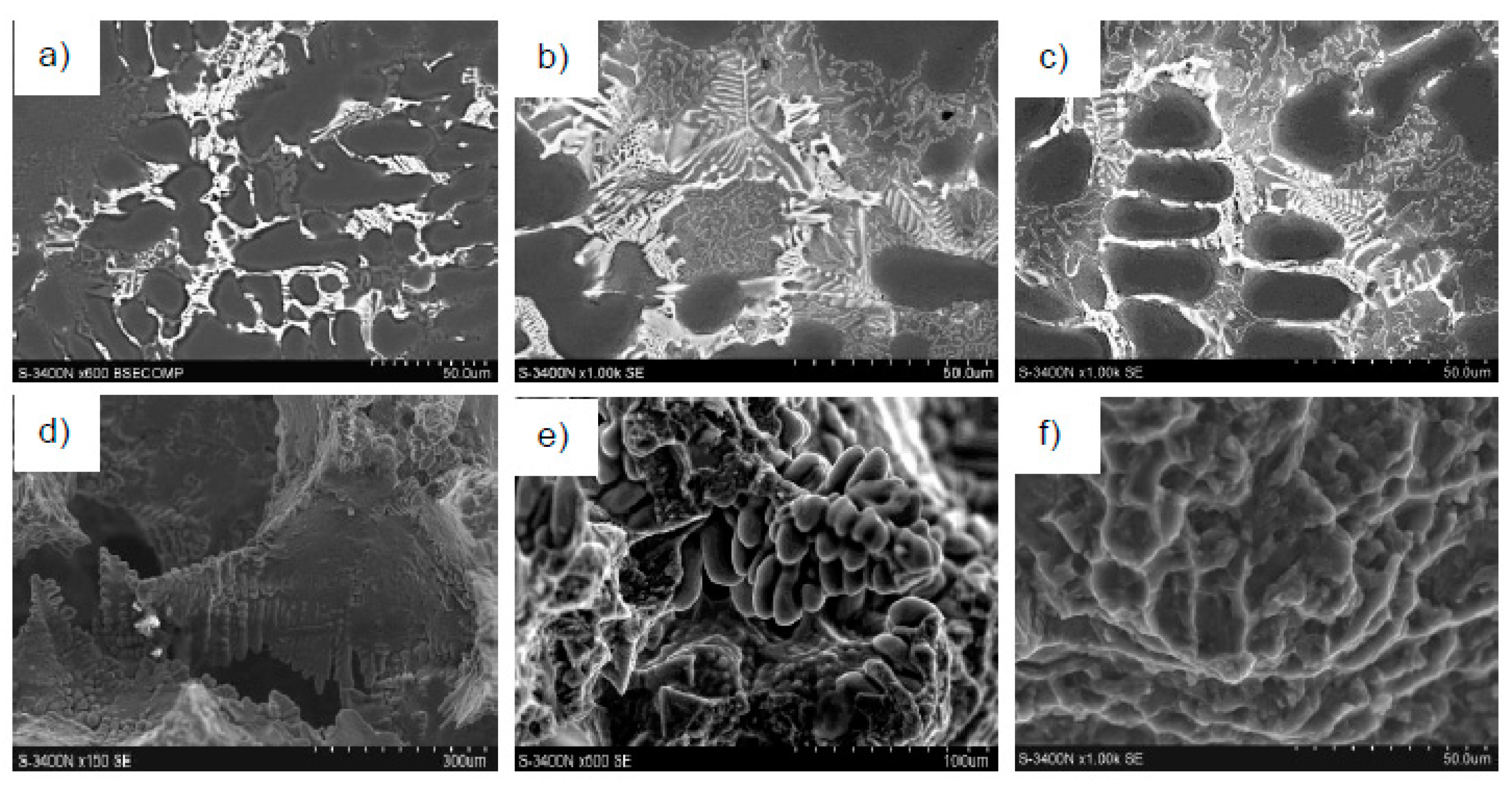
4. Results of Tests and Their Analysis
5. Discussion
6. Conclusion
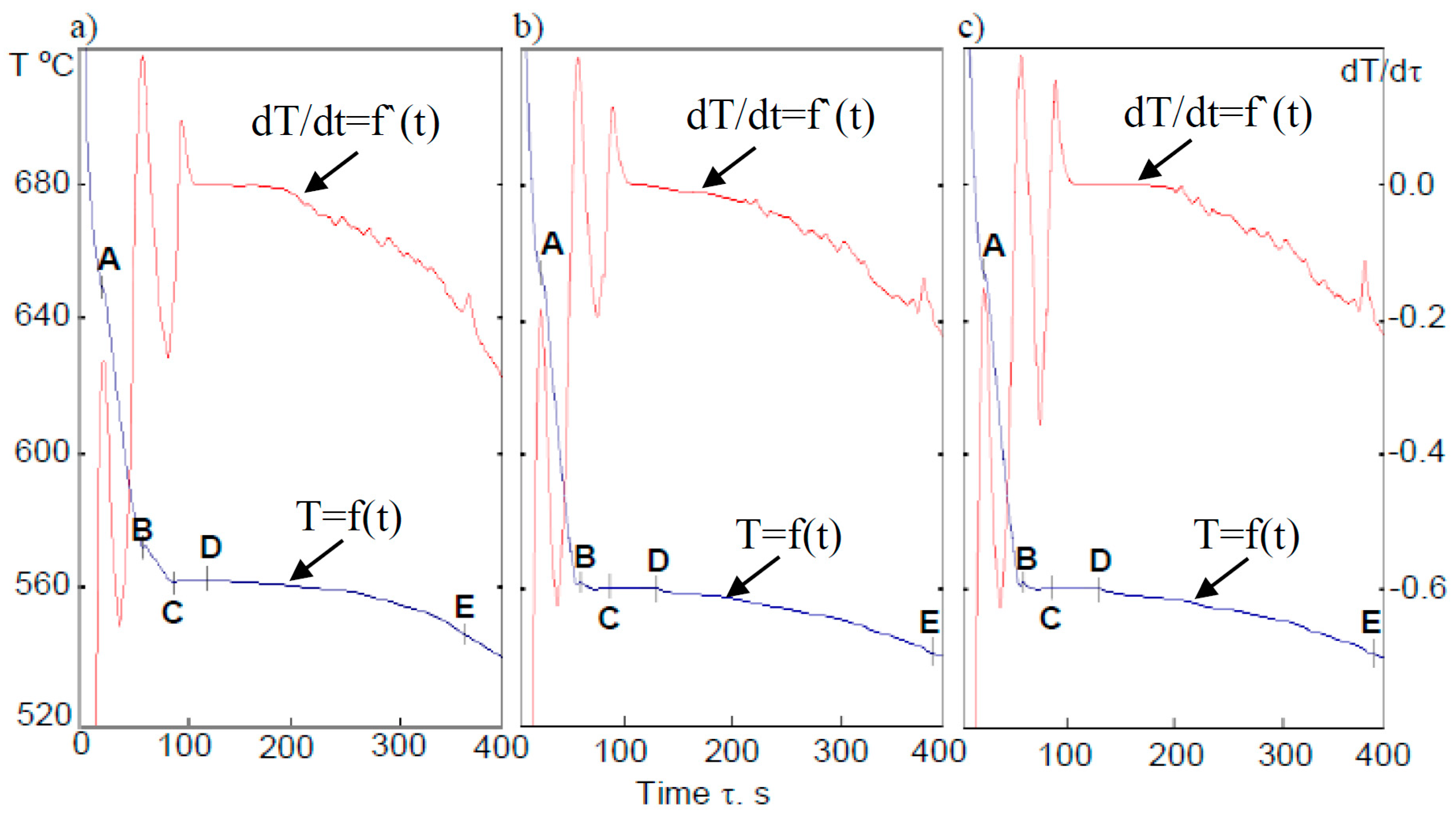
- Application of the assumed technological procedure of manufacturing the designed dispersion structure of the composite showed a significant decrease of crystallization temperature of the dendrites of the solution (Tliq). The decrease of temperature was of about 18 °C.
- Introduction of the aluminum powders FexAly and FexAly–SiC achieved by ASHS process did not cause a change of crystallization temperature of the eutectic composite, which included a phase rich in Mn and the binary eutectic α(Al) + β(Si).
- As a result of the introduction of FexAly and FexAly–SiC powders, a decrease in the crystallization temperature of the complex eutectic composites was observed, which probably included the intermetallic phase Mg2Si (a decrease of about 10 °C). There was also a temperature Tsol. decrease observed (of about 12 °C), and therefore there was an extension of the crystallization process after the addition of FeAl powder.
- The suggested technological procedure of composite preparation on the basis of sub-eutectic silumin AlSi10Mg0.5Mn0.5 showed refinement of the dendrites of solution α(Al), and transition of plate eutectics α(Al) + β(Si) into modified eutectics. This was confirmed by microstructures.
- Due to insufficiently small exothermic effects from the iron content (around 0.5% mass) and introduced powders FexAly and FexAly–SiC, the ATD thermal tests should be completed with calorimetric analysis DSC.
- For a more complete vision of the influence of modification with FexAly and FexAly–SiC powders, there should be tests of mechanical properties conducted.
Author Contributions
Funding
Conflicts of Interest
References
- Makhlouf, M.M.; Guthy, H.V. The aluminium-silicon eutectic reaction: Mechanisms and crystallography. J. Light Met. 2001, 1, 199–218. [Google Scholar] [CrossRef]
- Ares, A.E.; Gueijman, S.F.; Caram, R.; Schvezov, C.E. Analysis of solidification parameters during solidification of lead and aluminium base alloys. J. Cryst. Growth 2005, 275, 319–327. [Google Scholar] [CrossRef]
- Piątkowski, J. Modification of silumin AK9 with complex additives. Ores Non-Ferr. Met. 2004, 49, 181–184. [Google Scholar]
- Piątkowski, J. Physical and Chemical Phenomena which Shape the Structure, Mechanical Properties and Technological Stability of Hyper-Eutectic Alloys Al-Si-Me Subject to Overheating; Publishing House of Silesian University of Technology: Gliwice, Poland, 2013; pp. 175–184. [Google Scholar]
- Selivorstov, V.; Dotsenko, Y.; Borodianskiy, K. Influence of Low-Frequency Vibration and Modification on Solidification and Mechanical Properties of Al-Si Casting Alloy. Materials 2017, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Sigworth, G.; Campbell, J.; Jorstad, J. The Modification of Al-Si Casting Alloys: Important Practical and Theoretical Aspects. Int. J. Met. 2009, 3, 65–78. [Google Scholar] [CrossRef]
- Bondhus, E.; Sagstad, T. Strobloy—The New Combined Grain Refiner and Modifier for Hypoeutectic AlSi Foundry Alloys. In Essential Readings in Light Metals; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Panuskova, M.; Tillova, E.; Chalupova, M. Relation between mechanical properties and microstructure of cast aluminium alloy. Strength Mater. 2008, 40, 98–101. [Google Scholar] [CrossRef]
- Dercz, G.; Piątkowski, J. Rietveld quantitative and structural analysis of the Al-W master alloy for silumina modification. Solid State Phenom. 2010, 63, 161–164. [Google Scholar] [CrossRef]
- Tebib, M.; Samuel, A.M.; Ajersch, F.; Chen, X.-G. Effect of P and Sr additions on the microstructure of hypereutectic Al-15Si-14 Mg-4Cu alloy. Mater. Charact. 2014, 89, 112–123. [Google Scholar] [CrossRef]
- Górny, Z.; Sobczak, J. Modern Casting Materials on the Basis of Non-Ferrous Metals; Publishing House ZA-PIS: Kraków, Poland, 2005; pp. 98–107. [Google Scholar]
- Formanek, B.; Piątkowski, J.; Szymszal, J. Aluminium composite casting dispersion reinforced with iron-aluminium and silicon carbide phases. Arch. Foundry Eng. 2010, 10, 35–39. [Google Scholar]
- Hu, X.P.; Zhao, G.Q.; Wang, W.M. Solidifying pressure and microstructure of AlSi10Cu3 in die sleeve in high pressure die casting. J. Cast Met. Res. 2010, 23, 289–295. [Google Scholar] [CrossRef]
- Piątkowski, J.; Formanek, B. Cast AlSi9Cu4 alloy with hybrid strengthened by FexAly-Al2O3 composite powder. Mater. Sci. Eng. 2011, 22, 12–20. [Google Scholar]
- Formanek, B.; Piątkowski, J. Patent Application—Method of Modification and Strengthening of Casting Alloys Al-Si; Silesian University of Technology: Katowice, Poland, 2010. [Google Scholar]
| Tested Alloy | Si | Cu | Fe | Mn | Mg | Ni | Al |
|---|---|---|---|---|---|---|---|
| alloy EN AC-43400 | 9.82 | 0.08 | 0.47 | 0.48 | 0.47 | 0.13 | the rest |
| alloy + powder-1 | 9.88 | 0.09 | 0.51 | 0.44 | 0.43 | 0.11 | the rest |
| alloy + powder-2 | 10.03 | 0.06 | 0.53 | 0.49 | 0.48 | 0.12 | the rest |
| Point | A | B | C | D | E |
|---|---|---|---|---|---|
| temperature | TE(Mn) | T(Al) | TEmin. | TE | TE(Mg) |
| alloy EN AC-43400 | 648 | 578 | 565 | 568 | 550 |
| alloy + powder-1 | 652 | 559 | 562 | 566 | 540 |
| alloy + powder-2 | 650 | 560 | 568 | 570 | 539 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątkowski, J.; Wieszała, R. Crystallization and Structure of AlSi10Mg0.5Mn0.5 Alloy with Dispersion Strengthening with Al–FexAly–SiC Phases. Metals 2019, 9, 865. https://doi.org/10.3390/met9080865
Piątkowski J, Wieszała R. Crystallization and Structure of AlSi10Mg0.5Mn0.5 Alloy with Dispersion Strengthening with Al–FexAly–SiC Phases. Metals. 2019; 9(8):865. https://doi.org/10.3390/met9080865
Chicago/Turabian StylePiątkowski, Jarosław, and Robert Wieszała. 2019. "Crystallization and Structure of AlSi10Mg0.5Mn0.5 Alloy with Dispersion Strengthening with Al–FexAly–SiC Phases" Metals 9, no. 8: 865. https://doi.org/10.3390/met9080865
APA StylePiątkowski, J., & Wieszała, R. (2019). Crystallization and Structure of AlSi10Mg0.5Mn0.5 Alloy with Dispersion Strengthening with Al–FexAly–SiC Phases. Metals, 9(8), 865. https://doi.org/10.3390/met9080865





