Calculation of Oxygen Diffusion Coefficients in Oxide Films Formed on Low-Temperature Annealed Zr Alloys and Their Related Corrosion Behavior
Abstract
1. Introduction
2. Materials and Methods
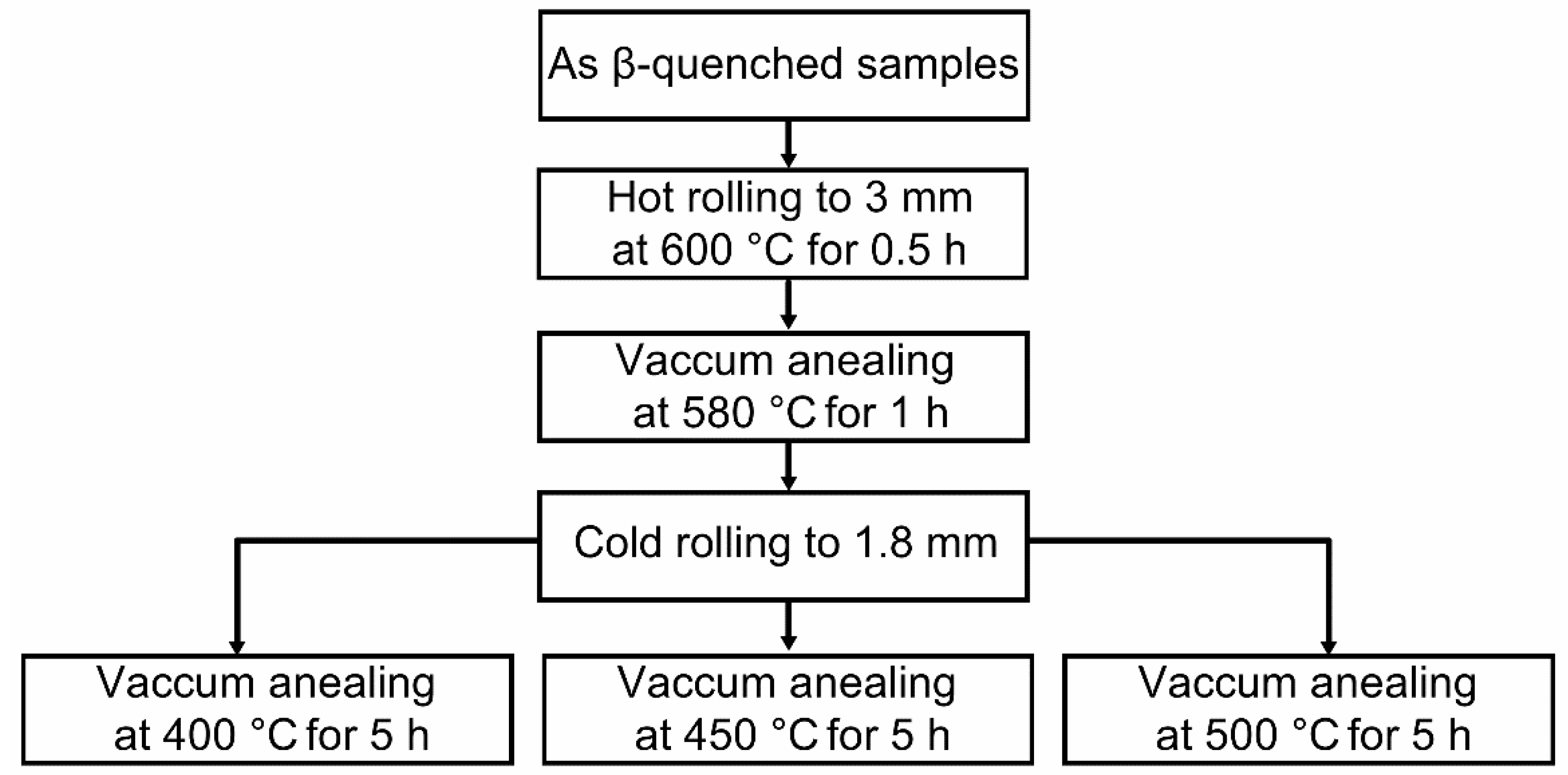
2.1. Sample Preparation and Corrosion Test
2.2. Characterizations
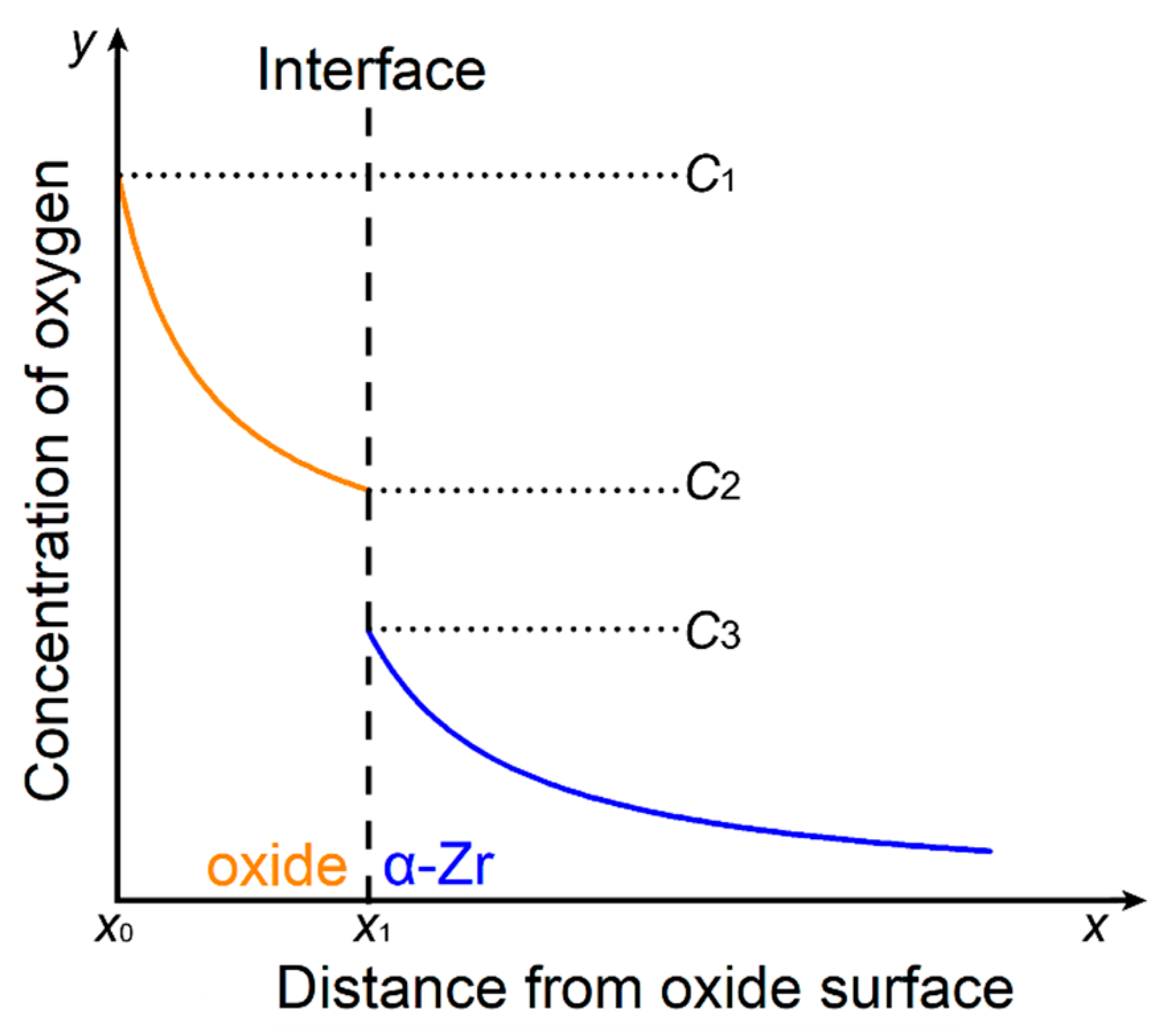
2.3. Model and Calculation Method
- (i)
- The diffusion of oxygen in the oxide film occurs via the vacancy mechanism;
- (ii)
- The diffusion coefficients of oxygen in the oxide and substrate are constant;
- (iii)
- The increase in the weight gain of the sample only results from the absorption of oxygen;
- (iv)
- No electric field or local space charge exists in the oxide film;
- (v)
- No sub-oxide is present between the oxide film and the substrate;
- (vi)
- No dissolution of the oxide film is considered.
3. Results and Discussion
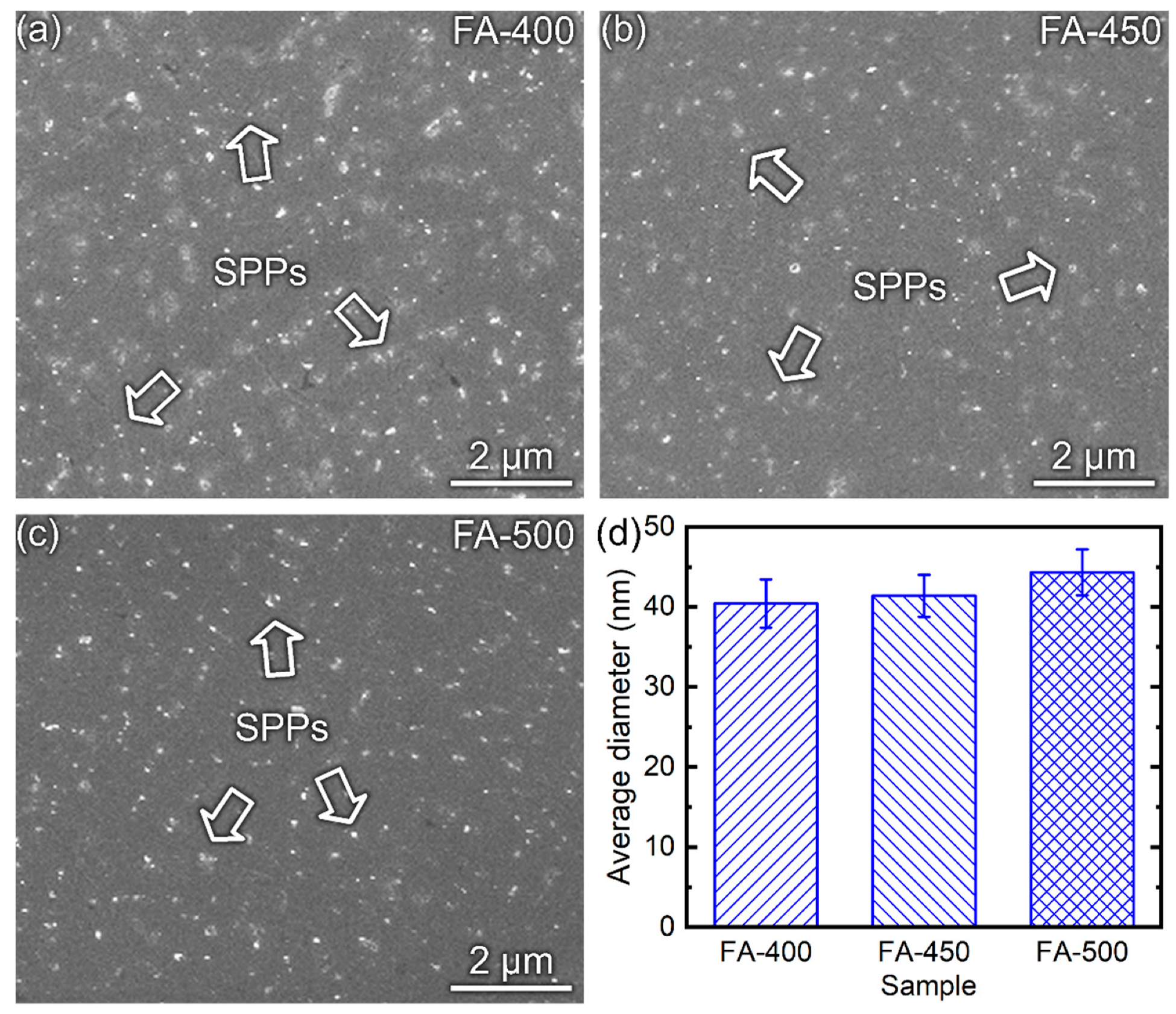
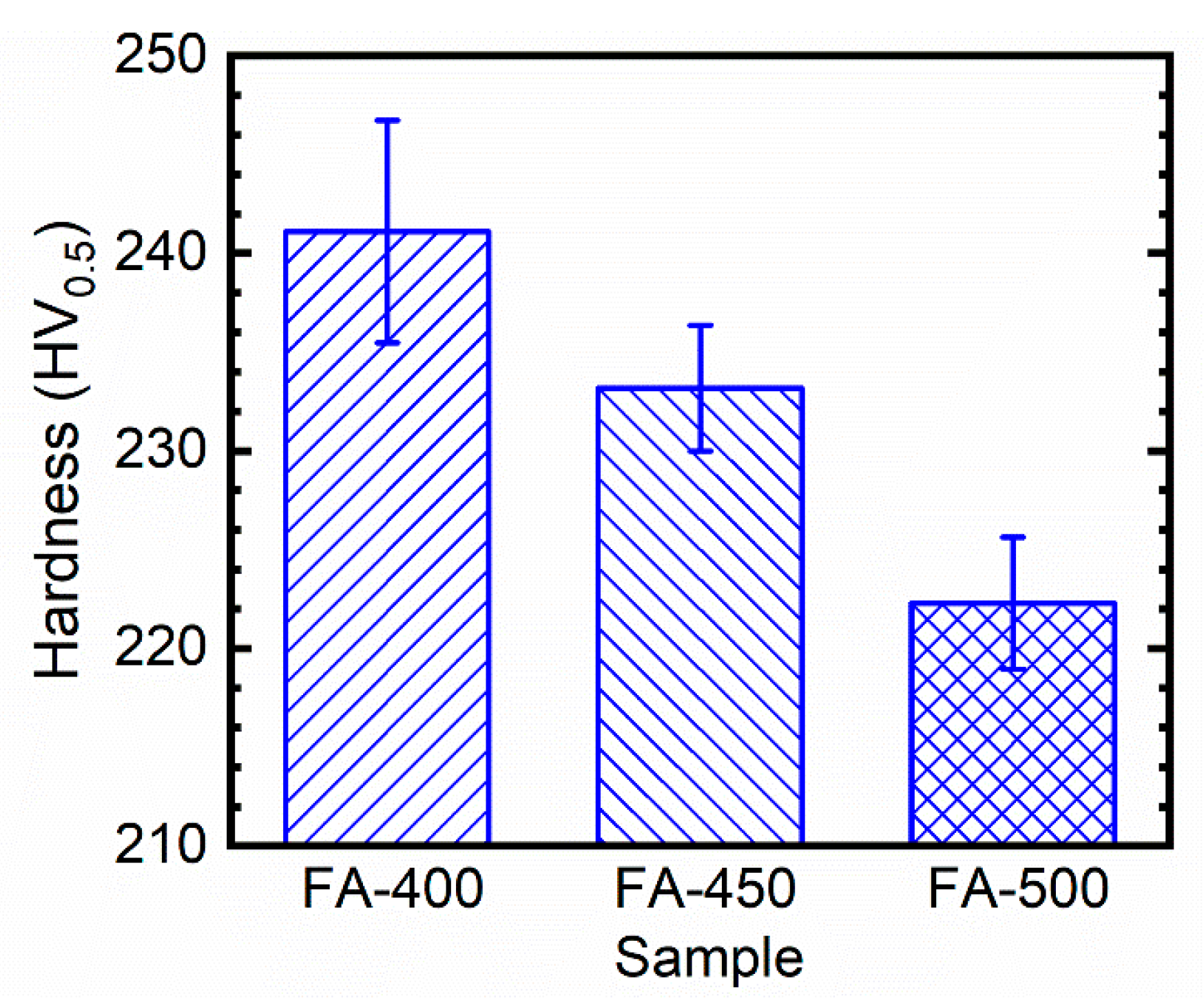
3.1. Characterizations of the Substrates
3.2. Corrosion Kinetics and the Phase Constituents of the Corroded Samples
3.3. Diffusion Coefficient of Oxygen in Oxide Film
3.4. Diffusion Coefficient of Oxygen in a Substrate
3.5. Oxide Growth of the Low-Temperature Annealed Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, H.-Y.; Wang, Z.; Shu, S.-L.; Lu, J.-B. Effect of Ta addition on the microstructures and mechanical properties of in situ bi-phase (TiB2-TiCxNy)/(Ni-Ta) cermets. Ceram. Int. 2019, 45, 4408–4417. [Google Scholar] [CrossRef]
- Kudiiarov, N.V.; Larionov, V.V.; Tyurin, I.Y. Mechanical Property Testing of Hydrogenated Zirconium Irradiated with Electrons. Metals 2018, 8, 207. [Google Scholar] [CrossRef]
- Yang, H.L.; Kano, S.; McGrady, J.; Shen, J.J.; Matsukawa, Y.; Chen, D.Y.; Murakami, K.; Abe, H. Surface orientation dependence of irradiation-induced hardening in a polycrystalline zirconium alloy. Scr. Mater. 2019, 162, 209–213. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Sang, P.; Zhang, L.; Song, D.; Chu, Y.-Q.; Chai, L.; Zhang, L.-C. Homogenization and growth behavior of second-phase particles in a deformed Zr–Sn-Nb-Fe-Cu-Si-O alloy. Metals 2018, 8, 759. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Zhang, L.C.; Lu, W.; Zhang, L.; Wang, L.; Zhang, D. Effects of alloyed Si on the autoclave corrosion performance and periodic corrosion kinetics in Zr-Sn-Nb-Fe-O alloys. Corros. Sci. 2015, 100, 651–662. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Q.; Li, J.; Lu, J.; Zhang, Y.; Zhang, L.-C.; Qin, X.; Lu, W.; Zhang, L.; Wang, L.; et al. Effect of microstructure on corrosion behavior of a Zr-Sn-Nb-Fe-Cu-O alloy. Mater. Des. 2016, 92, 888–896. [Google Scholar] [CrossRef]
- Obrosov, A.; Sutygina, N.A.; Manakhov, A.; Bolz, S.; Weiß, S.; Kashkarov, B.E. Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals 2018, 8, 27. [Google Scholar] [CrossRef]
- Kashkarov, B.E.; Ryabchikov, I.A.; Kurochkin, V.A.; Syrtanov, S.M.; Shevelev, E.A.; Obrosov, A.; Weiß, S. Hydrogen Interaction with Deep Surface Modified Zr-1Nb Alloy by High Intensity Ti Ion Implantation. Metals 2018, 8, 1081. [Google Scholar] [CrossRef]
- Lee, C.M.; Sohn, D.S. Enhanced high-temperature oxidation resistance of a zirconium alloy cladding by high-temperature preformed oxide on the cladding. Corros. Sci. 2018, 131, 116–125. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, B.; Liang, X.; Liu, W.; Li, H.; Li, Q.; Yao, M.; Zhang, J. A novel mechanism for nodular corrosion of Zircaloy-4 corroded in 773 K superheated steam. Corros. Sci. 2017, 126, 44–54. [Google Scholar] [CrossRef]
- Huang, J.; Yao, M.; Chen, B.; Mao, Y.; Liang, X.; Zhang, J.; Zhou, B.; Li, Q. Oxidation behavior of Zr9S2 precipitates in Zr-0.8Sn-1.0Nb-0.3Fe-0.1Cr-xS alloys. Corros. Sci. 2017, 120, 82–89. [Google Scholar] [CrossRef]
- Chen, L.Y.; Shen, P.; Zhang, L.; Lu, S.; Chai, L.; Yang, Z.; Zhang, L.C. Corrosion behavior of non-equilibrium Zr-Sn-Nb-Fe-Cu-O alloys in high-temperature 0.01 M LiOH aqueous solution and degradation of the surface oxide films. Corros. Sci. 2018, 136, 221–230. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, B.X.; Pan, R.J.; Cao, X.X.; Wu, L.; Zhu, W.; Wen, B.; Fang, Z.Q.; Ovcharenko, Y.M.; He, W. Stress-driven grain re-orientation and merging behaviour found in oxidation of zirconium alloy using in-situ method and MD simulation. Corros. Sci. 2019, 147, 350–356. [Google Scholar] [CrossRef]
- Qin, W. Improvement and Application of Zirconium Alloys. Metals 2018, 8, 794. [Google Scholar] [CrossRef]
- Bakradze, G.; Jeurgens, L.P.H.; Acartürk, T.; Starke, U.; Mittemeijer, E.J.; Acartu, T. Atomic transport mechanisms in thin oxide films grown on zirconium by thermal oxidation, as-derived from 18 O-tracer experiments. Acta Mater. 2011, 59, 7498–7507. [Google Scholar] [CrossRef]
- Abriata, J.P.; Garces, J.; Versaci, R. The O−Zr (oxygen-zirconium) system. Bull. Alloy Phase Diagr. 1986, 7, 116–124. [Google Scholar] [CrossRef]
- Tapping, R.L. X-ray photoelectron and ultraviolet photoelectron studies of the oxidation and hydriding of zirconium. J. Nucl. Mater. 1982, 107, 151–158. [Google Scholar] [CrossRef]
- Lyapin, A.; Jeurgens, L.P.H.; Mittemeijer, E.J. Effect of temperature on the initial, thermal oxidation of zirconium. Acta Mater. 2005, 53, 2925–2935. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Huang, J.; Tu, L.; Yao, M.; Zhou, B. The corrosion resistance of Zr-0.7Sn-1Nb-0.2Fe-xCu-xGe alloys in 360 °C lithiated water. Corros. Sci. 2016, 111, 132–138. [Google Scholar] [CrossRef]
- Kim, H.; Kim, I.; Choi, B.; Park, J.; Jeong, Y.; Kim, K. Study of the corrosion and microstructure with annealing conditions of a β-quenched HANA-4 alloy. Corros. Sci. 2010, 52, 3162–3167. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, S.K. Effect of Mo addition on the corrosion resistance of Zr-based alloy in water containing LiOH. J. Nucl. Mater. 2003, 321, 238–248. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, S.J.; Choi, B.K.; Jeong, Y.H. Oxide microstructures of advanced Zr alloys corroded in 360 °C water loop. J. Alloys Compd. 2007, 437, 274–279. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Mechanically milling-induced amorphization in Sn-containing Ti-based multicomponent alloy systems. Mater. Sci. Eng. A 2005, 394, 204–209. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Wang, W.M.; Zhang, L.C. Rapid malachite green degradation using Fe73.5Si13.5B9Cu1Nb3 metallic glass for activation of persulfate under UV–Vis light. Mater. Des. 2017, 119, 244–253. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J.; Ma, E. Mechanically Alloyed Amorphous Ti50(Cu0.45Ni0.55)44–xAlxSi4B2 Alloys with Supercooled Liquid Region. J. Mater. Res. 2002, 17, 1743–1749. [Google Scholar] [CrossRef]
- Zhang, L.C.; Kim, K.B.; Yu, P.; Zhang, W.Y.; Kunz, U.; Eckert, J. Amorphization in mechanically alloyed (Ti, Zr, Nb)-(Cu, Ni)-Al equiatomic alloys. J. Alloys Compd. 2007, 428, 157–163. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y. A review on biomedical titanium alloys: recent progress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Luan, B.F.; Chai, L.J.; Chen, J.W.; Zhang, M.; Liu, Q. Growth behavior study of second phase particles in a Zr-Sn-Nb-Fe-Cr-Cu alloy. J. Nucl. Mater. 2012, 423, 127–131. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Lu, W.; Zhang, L.C.; Wang, L.; Zhang, D. Effect of low-temperature pre-deformation on precipitation behavior and microstructure of a Zr-Sn-Nb-Fe-Cu-O alloy during fabrication. J. Nucl. Sci. Technol. 2016, 53, 496–507. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Zhang, L.; Lu, W.; Wang, L.; Zhang, L.-C.; Zhang, D. Zr-Sn-Nb-Fe-Si-O alloy for fuel cladding candidate: Processing, microstructure, corrosion resistance and tensile behavior. Corros. Sci. 2015, 100, 332–340. [Google Scholar] [CrossRef]
- Yang, Z.N.; Wang, X.B.; Liu, F.; Zhang, F.C.; Chai, L.J.; Qiu, R.S.; Chen, L.Y. Effect of intercritical annealing temperature on microstructure and mechanical properties of duplex Zr-2.5Nb alloy. J. Alloys Compd. 2019, 776, 242–249. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.N.; Zhang, F.C.; Wang, X.B.; Chen, L.Y.; Yang, Z.N. Effect of annealing treatment on the microstructure and mechanical properties of a duplex Zr-2.5 Nb alloy. Mater. Sci. Eng. A 2017, 706, 236–241. [Google Scholar] [CrossRef]
- Yang, H.; Shen, J.; Matsukawa, Y.; Satoh, Y.; Kano, S.; Zhao, Z.; Li, Y.; Li, F.; Abe, H. Effects of alloying elements (Sn, Nb, Cr, and Mo) on the microstructure and mechanical properties of zirconium alloys. J. Nucl. Sci. Technol. 2015, 52, 1162–1173. [Google Scholar] [CrossRef]
- Cao, G.; Yun, Y.; Xu, H.; Yuan, G.; Hu, J.; Shao, G. A mechanism assessment for the anti-corrosion of zirconia coating under the condition of subcritical water corrosion. Corros. Sci. 2019, 152, 54–59. [Google Scholar] [CrossRef]
- Almarshad, A.I.A.; Klein, A.C. A model for waterside oxidation of Zircaloy fuel cladding in pressurized water reactors. J. Nucl. Mater. 1991, 183, 186–194. [Google Scholar] [CrossRef]
- Ishchenko, N.I. Determination of oxygen diffusion coefficient in oxide on zirconium alloys and adjacent metal from weight gain and oxide thickness measurement data. Probl. At. Sci. Technol. 2014, 4, 88–93. [Google Scholar]
- Santamaria, M.; Di Franco, F.; Di Quarto, F.; Pisarek, M.; Zanna, S.; Marcus, P. Photoelectrochemical and XPS characterisation of oxide layers on 316L stainless steel grown in high-temperature water. J. Solid State Electrochem. 2015, 19, 3511–3519. [Google Scholar] [CrossRef]
- Ma, X.; Toffolon-Masclet, C.; Guilbert, T.; Hamon, D.; Brachet, J.C. Oxidation kinetics and oxygen diffusion in low-tin Zircaloy-4 up to 1523 K. J. Nucl. Mater. 2008, 377, 359–369. [Google Scholar] [CrossRef]
- García, E.A.; Kovacs, J. Diffusion model for the oxidation of zirconium at 573 and 623 K. J. Nucl. Mater. 1994, 210, 78–83. [Google Scholar] [CrossRef]
- García, E.A. Dynamical diffusion model to simulate the oxide crystallization and grain growth during oxidation of zirconium at 573 and 623 K. J. Nucl. Mater. 1995, 224, 299–304. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.F.; Zhang, S.D.; Guo, J.H.; Jiang, S.S.; Li, D.Y. Diffusion behavior of carbon and its hardening effect on plasma carburized M50NiL steel: Influences of treatment temperature and duration. Surf. Coat. Technol. 2018, 333, 96–103. [Google Scholar] [CrossRef]
- Yang, Z.; Chu, C.; Jiang, F.; Qin, Y.; Long, X.; Wang, S.; Chen, D.; Zhang, F. Accelerating nano-bainite transformation based on a new constructed microstructural predicting model. Mater. Sci. Eng. A 2019, 748, 16–20. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Li, X.F.; Wang, W.M.; Lin, H.C.; Zhang, L.C. Ultrafast activation efficiency of three peroxides by Fe78Si9B13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Appl. Catal. B Environ. 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Chen, K.; Zeng, L.; Li, Z.; Chai, L.; Wang, Y.; Chen, L.-Y.; Yu, H. Effects of laser surface alloying with Cr on microstructure and hardness of commercial purity Zr. J. Alloys Compd. 2019, 784, 1106–1112. [Google Scholar] [CrossRef]
- Chai, L.; Xia, J.; Zhi, Y.; Gou, Y.; Chen, L.; Yang, Z.; Guo, N. Deformation mode-determined misorientation and microstructural characteristics in rolled pure Zr sheet. Sci. China Technol. Sci. 2018, 61, 1346–1352. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Lv, Y.; Zhang, L.-C.; Chen, L.; Meng, Q.; Qu, J.; Zhang, D.; Lu, W. Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing. Acta Mater. 2017, 131, 499–510. [Google Scholar] [CrossRef]
- Su, L.H.; Lu, C.; He, L.Z.; Zhang, L.C.; Guagliardo, P.; Tieu, A.K.; Samarin, S.N.; Williams, J.F.; Li, H.J. Study of vacancy-type defects by positron annihilation in ultrafine-grained aluminum severely deformed at room and cryogenic temperatures. Acta Mater. 2012, 60, 4218–4228. [Google Scholar] [CrossRef][Green Version]
- Calin, M.; Zhang, L.C.; Eckert, J. Tailoring of microstructure and mechanical properties of a Ti-based bulk metallic glass-forming alloy. Scr. Mater. 2007, 57, 1101–1104. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Chaubet, D.; Bacroix, B.; Brisset, F. A study of recovery and primary recrystallization mechanisms in a Zr-2Hf alloy. Acta Mater. 2005, 53, 5131–5140. [Google Scholar] [CrossRef]
- Dong, B.-X.; Yang, H.-Y.; Qiu, F.; Li, Q.; Shu, S.-L.; Zhang, B.-Q.; Jiang, Q.-C. Design of TiC nanoparticles and their morphology manipulating mechanisms by stoichiometric ratios: Experiment and first-principle calculation. Mater. Des. 2019, 181, 107951. [Google Scholar] [CrossRef]
- Yang, H.L.; Kano, S.; Matsukawa, Y.; Li, Y.F.; Shen, J.J.; Zhao, Z.S.; Li, F.; Satoh, Y.; Abe, H. Study on recrystallization and correlated mechanical properties in Mo-modified Zr-Nb alloys. Mater. Sci. Eng. A 2016, 661, 9–18. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, H.; Qiao, Y.; Tian, H.; Ni, X.; Li, Z.; Wang, X. Grain morphology and crystal structure of pre-transition oxides formed on Zircaloy-4. Corros. Sci. 2013, 74, 323–331. [Google Scholar] [CrossRef]
- Garner, A.; Preuss, M.; Frankel, P. A method for accurate texture determination of thin oxide films by glancing-angle laboratory X-ray diffraction. J. Appl. Crystallogr. 2014, 47, 575–583. [Google Scholar] [CrossRef]
- Wei, J.; Frankel, P.; Polatidis, E.; Blat, M.; Ambard, A.; Comstock, R.J.; Hallstadius, L.; Hudson, D.; Smith, G.D.W.; Grovenor, C.R.M.; et al. The effect of Sn on autoclave corrosion performance and corrosion mechanisms in Zr-Sn-Nb alloys. Acta Mater. 2013, 61, 4200–4214. [Google Scholar] [CrossRef]
- Vermaak, N.; Parry, G.; Estevez, R.; Bréchet, Y. New insight into crack formation during corrosion of zirconium-based metal-oxide systems. Acta Mater. 2013, 61, 4374–4383. [Google Scholar] [CrossRef]
- Parise, M.; Sicardy, O.; Cailletaud, G. Modelling of the mechanical behavior of the metal–oxide system during Zr alloy oxidation. J. Nucl. Mater. 1998, 256, 35–46. [Google Scholar] [CrossRef]
- Chai, L.; Wang, T.; Ren, Y.; Song, B.; Guo, N.; Chen, L. Microstructural and Textural Differences Induced by Water and Furnace Cooling in Commercially Pure Zr Annealed in the α + β Region. Met. Mater. Int. 2018, 24, 673–680. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, H.; Zhao, C.; Lu, S.; Wang, Z.-X.; Sha, J.; Chen, S.; Zhang, L.-C. Automatic remelting and enhanced mechanical performance of a plasma sprayed NiCrBSi coating. Surf. Coat. Technol. 2019, 369, 31–43. [Google Scholar] [CrossRef]
- Liu, L.H.; Yang, C.; Wang, F.; Qu, S.G.; Li, X.Q.; Zhang, W.W.; Li, Y.Y.; Zhang, L.C. Ultrafine grained Ti-based composites with ultrahigh strength and ductility achieved by equiaxing microstructure. Mater. Des. 2015, 79, 1–5. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, T.; Lu, S.; Wang, Z.X.; Chen, S.; Zhang, L.C. Improved hardness and wear resistance of plasma sprayed nanostructured NiCrBSi coating via short-time heat treatment. Surf. Coat. Technol. 2018, 350, 436–444. [Google Scholar] [CrossRef]
- Li, C.-L.; Qiu, F.; Chang, F.; Zhao, X.-M.; Geng, R.; Yang, H.-Y.; Zhao, Q.-L.; Jiang, Q.-C. Simultaneously Enhanced Strength, Toughness and Ductility of Cast 40Cr Steels Strengthened by Trace Biphase TiCx-TiB2 Nanoparticles. Metals 2018, 8, 707. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Liang, S.-X. Fe-based Metallic Glasses in Functional Catalytic Applications. Chem. Asian J. 2018, 13, 3575–3592. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, F.; Gao, Y.-Y.; Dong, B.-X.; Shu, S.-L.; Lv, M.-M.; Yang, H.-Y.; Zhao, Q.-L.; Jiang, Q.-C. Microstructure refinement and strengthening mechanisms of bimodal-sized and dual-phased (TiCn-Al3Tim)/Al hybrid composites assisted ultrasonic vibration. J. Alloys Compd. 2019, 788, 1309–1321. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Liu, Y.J.; Zhang, W.; Wang, W.; Lu, J.; Zhang, L.C. Compelling Rejuvenated Catalytic Performance in Metallic Glasses. Adv. Mater. 2018, 30, 1802764. [Google Scholar] [CrossRef]
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Panicaud, B.; Retraint, D.; Grosseau-Poussard, J.-L.; Li, L.; Guérain, M.; Goudeau, P.; Tamura, N.; Kunz, M. Experimental and numerical study of the effects of a nanocrystallisation treatment on high-temperature oxidation of a zirconium alloy. Corros. Sci. 2012, 60, 224–230. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, B.X.; Chen, B.; Zhu, W.; Wen, B.; Wu, L.; Tang, H.K.; Fang, Z.Q.; Li, Q.; Yao, M. In-situ oxidation and short-time corrosion investigation on strain and dislocation during the generation and growth of ZrO2. Corros. Sci. 2017, 122, 26–31. [Google Scholar] [CrossRef]
- Smeltzer, W.W.; Haering, R.R.; Kirkaldy, J.S. Oxidation of metals by short circuit and lattice diffusion of oxygen. Acta Metall. 1961, 9, 880–885. [Google Scholar] [CrossRef]
- Ly, A.; Ambard, A.; Blat-Yrieix, M.; Legras, L.; Frankel, P.; Preuss, M.; Curfs, C.; Parry, G.; Brechet, Y.; Barberis, P.; et al. Understanding Crack Formation at the Metal/Oxide Interface During Corrosion of Zircaloy-4 Using a Simple Mechanical Model. J. ASTM Int. 2011, 8, 103550. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Park, J.Y.; Kim, H.G.; Jeong, Y.H. Oxide structure and corrosion mechanism of ZrSnNbFeCrCu alloy studied with transmission electron microscopy and nano-indentation: Relation to corrosion kinetics. Mater. Chem. Phys. 2010, 122, 408–416. [Google Scholar] [CrossRef]
- Kim, H.G.; Choi, B.K.; Park, J.Y.; Jeong, Y.H. Influence of the manufacturing processes on the corrosion of Zr-1.1Nb-0.05Cu alloy. Corros. Sci. 2009, 51, 2400–2405. [Google Scholar] [CrossRef]
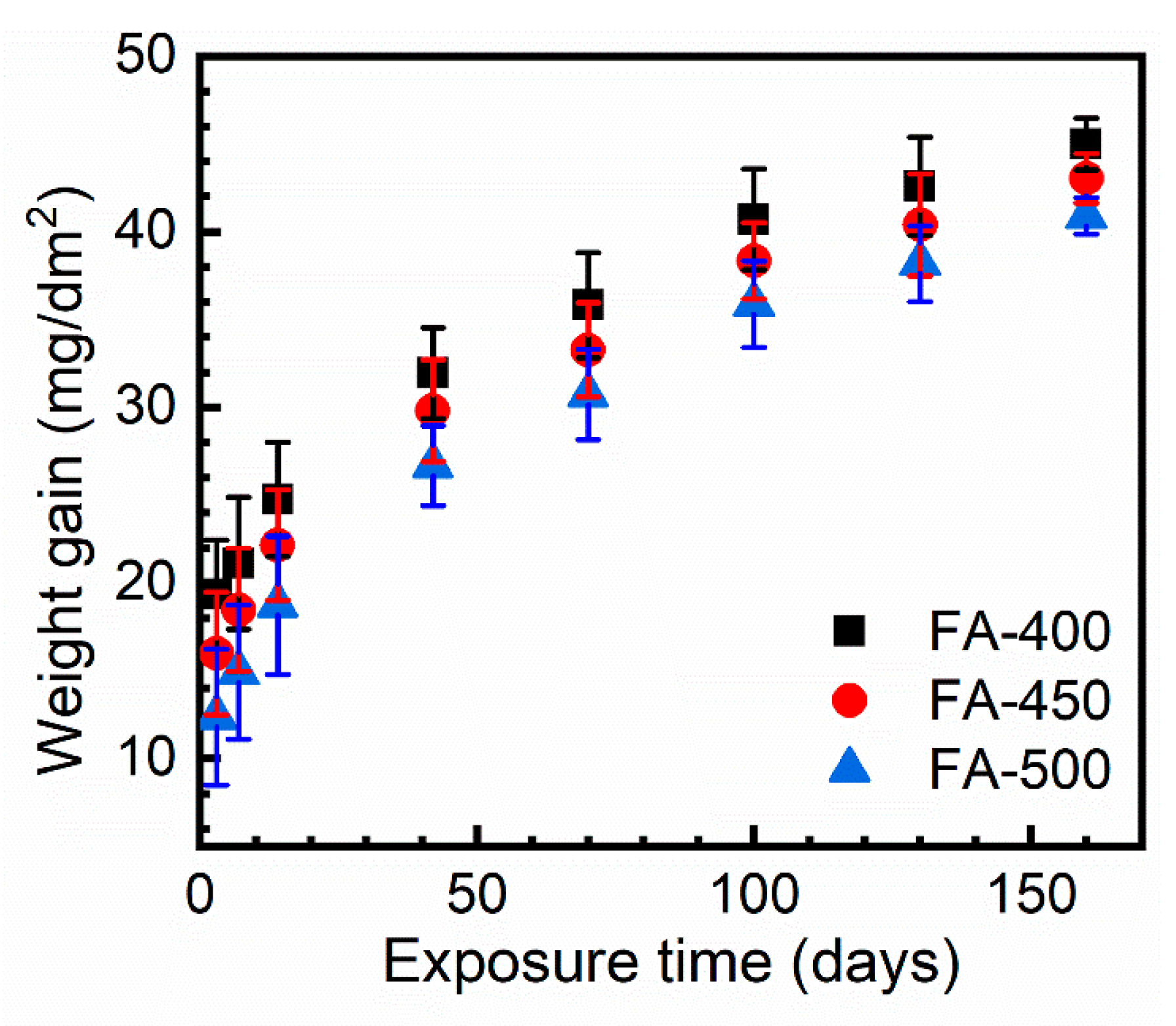
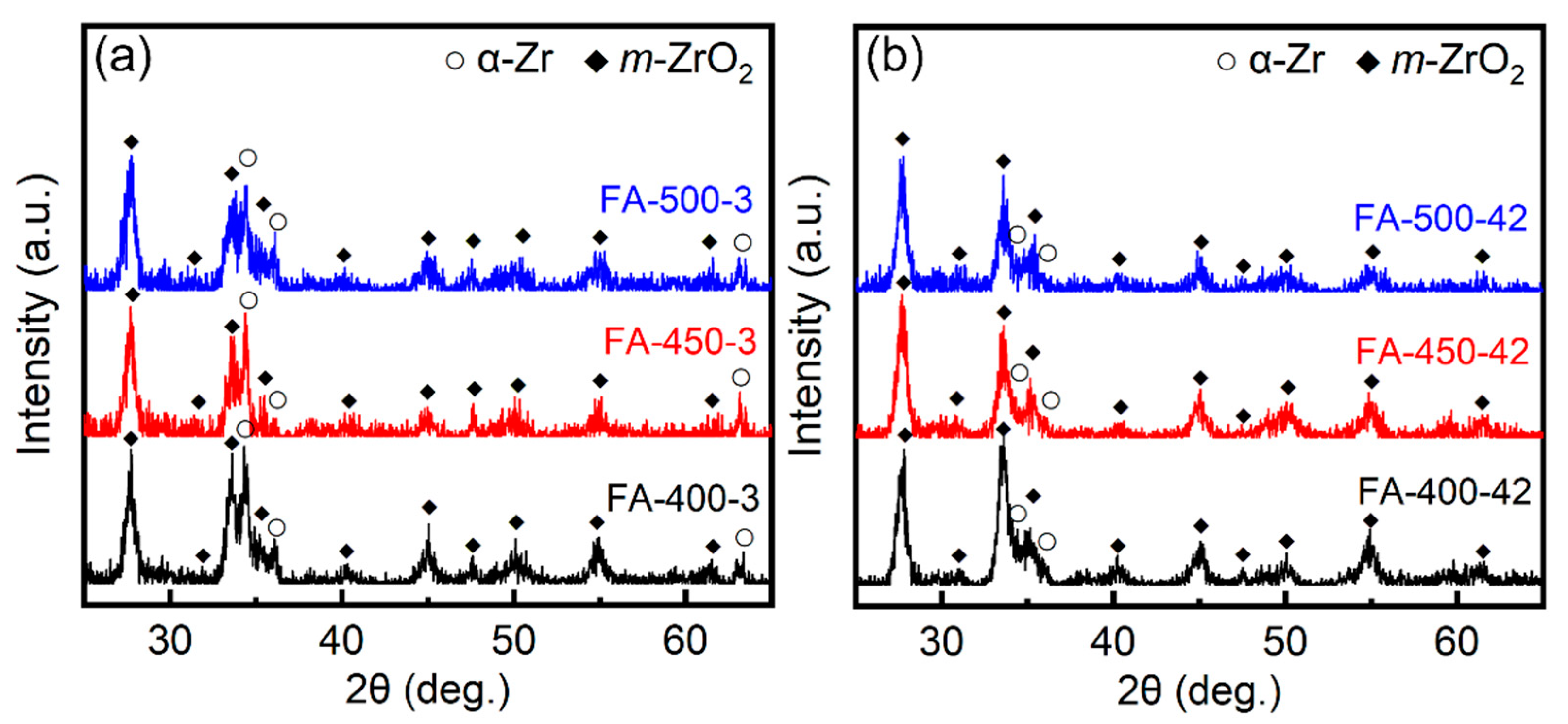
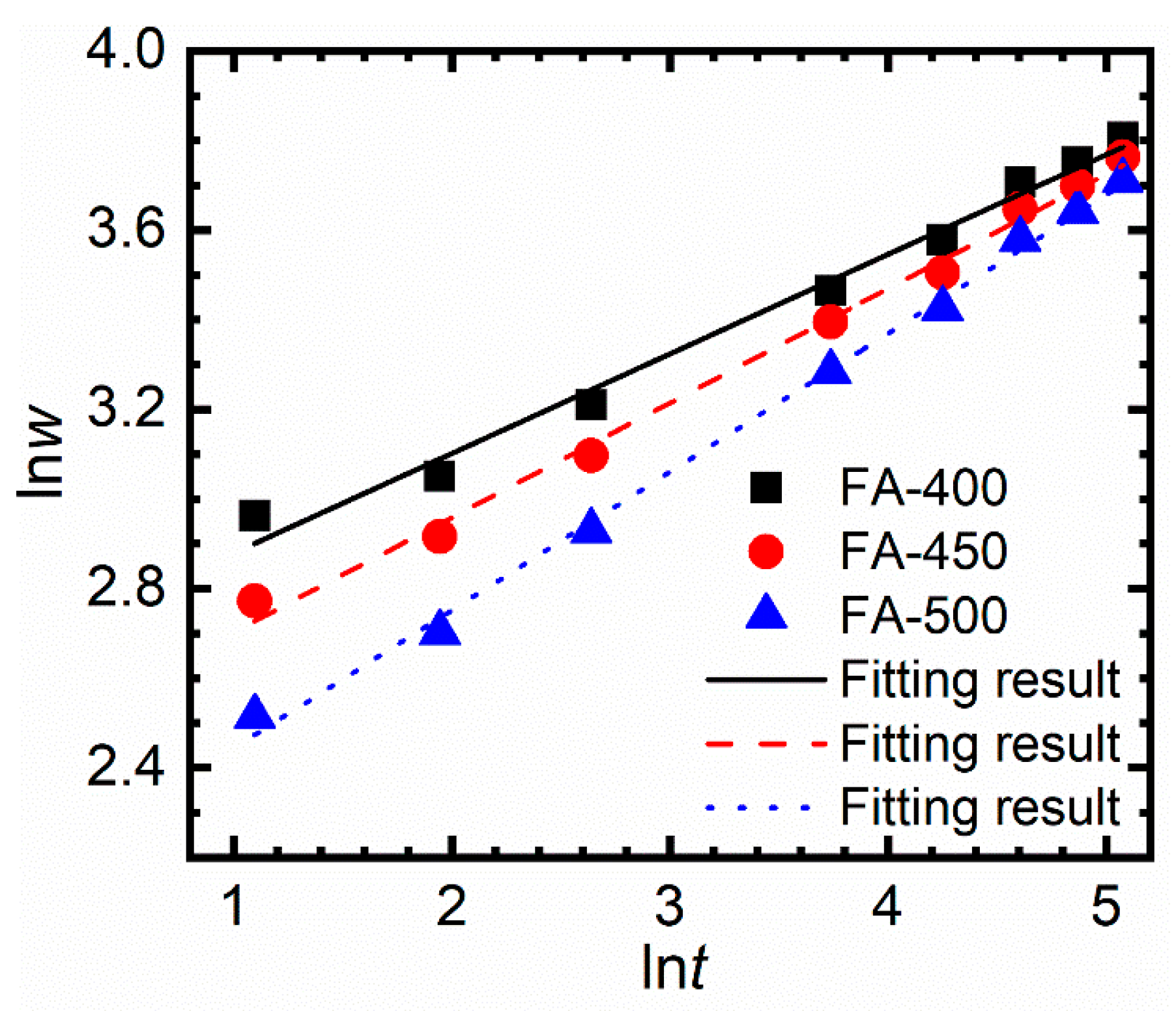
| Constant | FA-400 | FA-450 | FA-500 |
|---|---|---|---|
| 0.222 | 0.256 | 0.308 | |
| 2.656 | 2.447 | 2.134 | |
| (mg/dm2)/dayn | 14.240 | 11.554 | 8.449 |
| Diffusion Coefficient | FA-400 | FA-450 | FA-500 |
| (cm2/s) | 3.252 × 10−11 | 3.464 × 10−11 | 3.740 × 10−11 |
| Diffusion Coefficient | FA-400 | FA-450 | FA-500 |
| (cm2/s) | 5.472 × 10−11 | 6.862 × 10−11 | 8.993 × 10−11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, L.-Y.; Zhao, C.; Liu, Y.; Zhang, L.-C. Calculation of Oxygen Diffusion Coefficients in Oxide Films Formed on Low-Temperature Annealed Zr Alloys and Their Related Corrosion Behavior. Metals 2019, 9, 850. https://doi.org/10.3390/met9080850
Zhang L, Chen L-Y, Zhao C, Liu Y, Zhang L-C. Calculation of Oxygen Diffusion Coefficients in Oxide Films Formed on Low-Temperature Annealed Zr Alloys and Their Related Corrosion Behavior. Metals. 2019; 9(8):850. https://doi.org/10.3390/met9080850
Chicago/Turabian StyleZhang, Lina, Liang-Yu Chen, Cuihua Zhao, Yujing Liu, and Lai-Chang Zhang. 2019. "Calculation of Oxygen Diffusion Coefficients in Oxide Films Formed on Low-Temperature Annealed Zr Alloys and Their Related Corrosion Behavior" Metals 9, no. 8: 850. https://doi.org/10.3390/met9080850
APA StyleZhang, L., Chen, L.-Y., Zhao, C., Liu, Y., & Zhang, L.-C. (2019). Calculation of Oxygen Diffusion Coefficients in Oxide Films Formed on Low-Temperature Annealed Zr Alloys and Their Related Corrosion Behavior. Metals, 9(8), 850. https://doi.org/10.3390/met9080850







