Enhanced Stress Corrosion Cracking Resistance of Ultrafine-Grained Cu-Cr-Zr Alloy Fabricated via Equal-Channel Angular Pressing
Abstract
1. Introduction
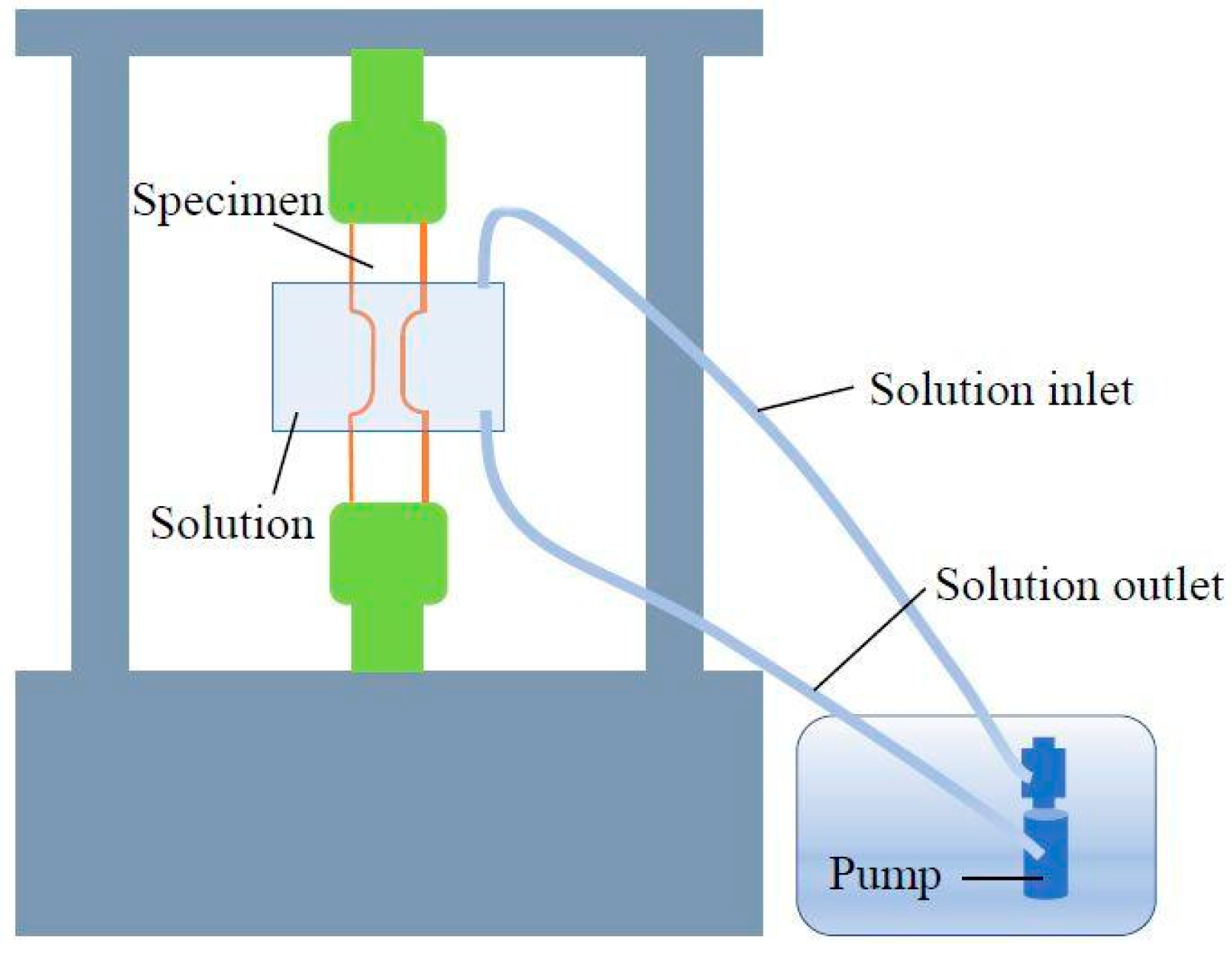
2. Materials and Methods
3. Results and Discussion
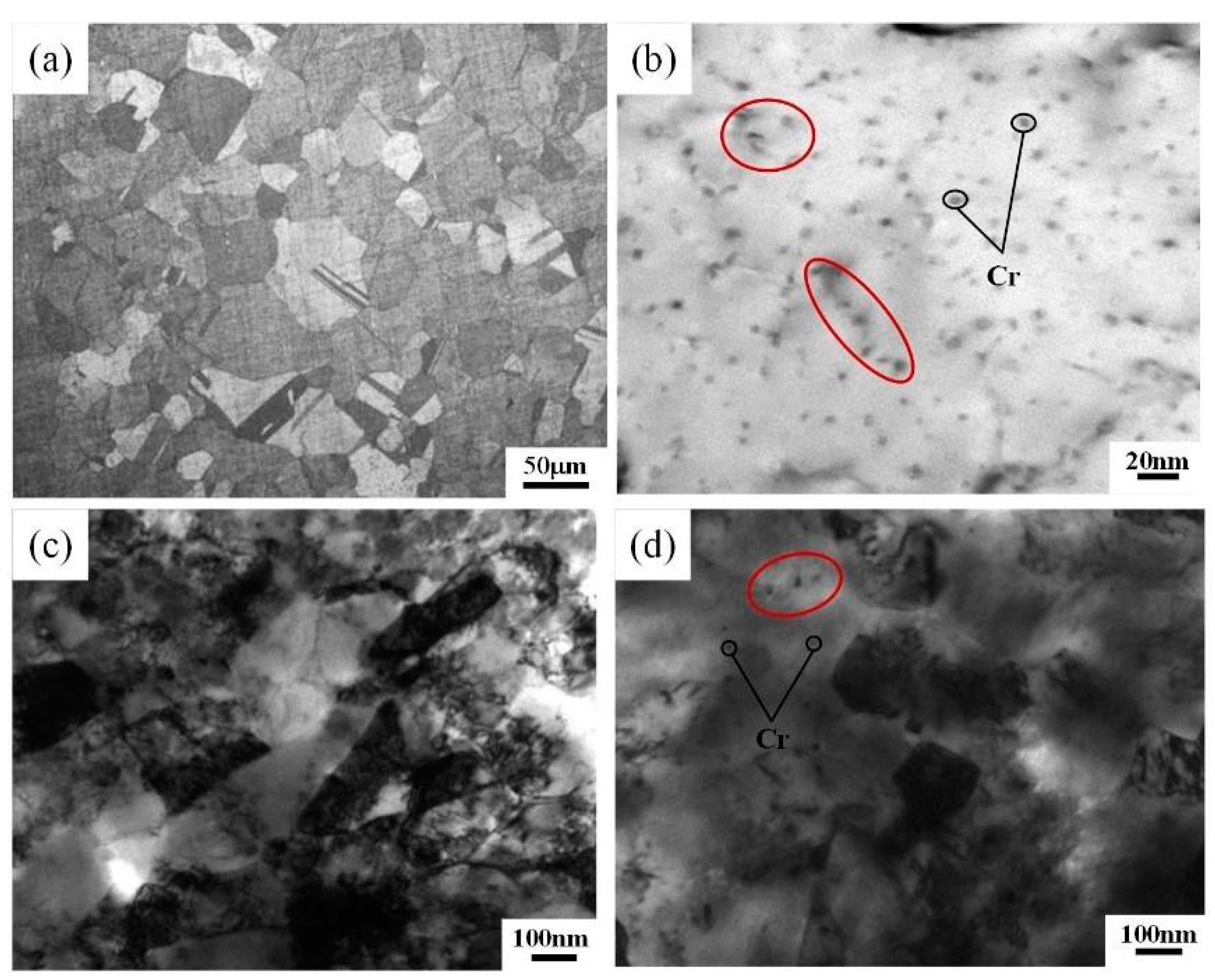
3.1. Microstructure
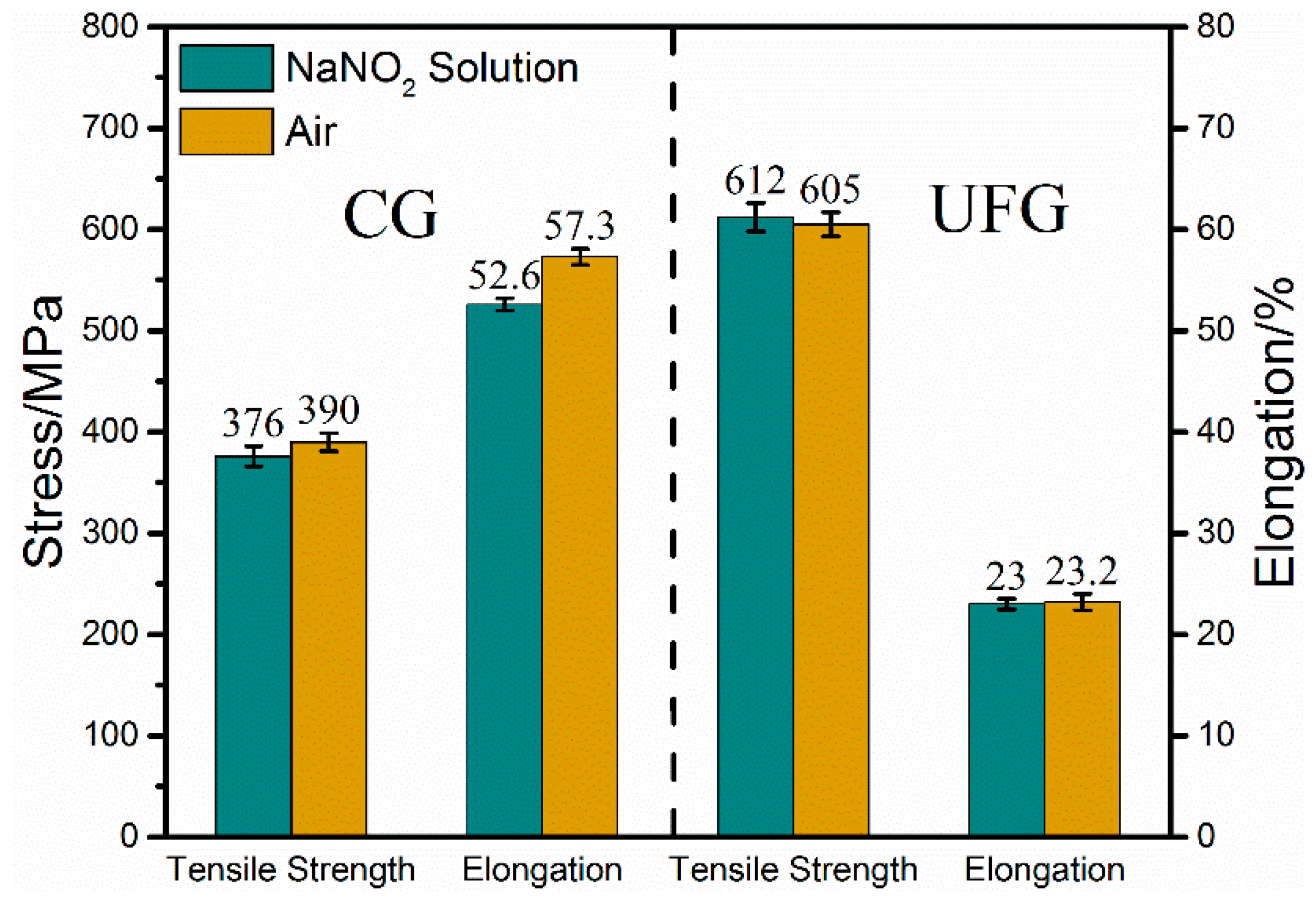
3.2. Stress Corrosion Behavior
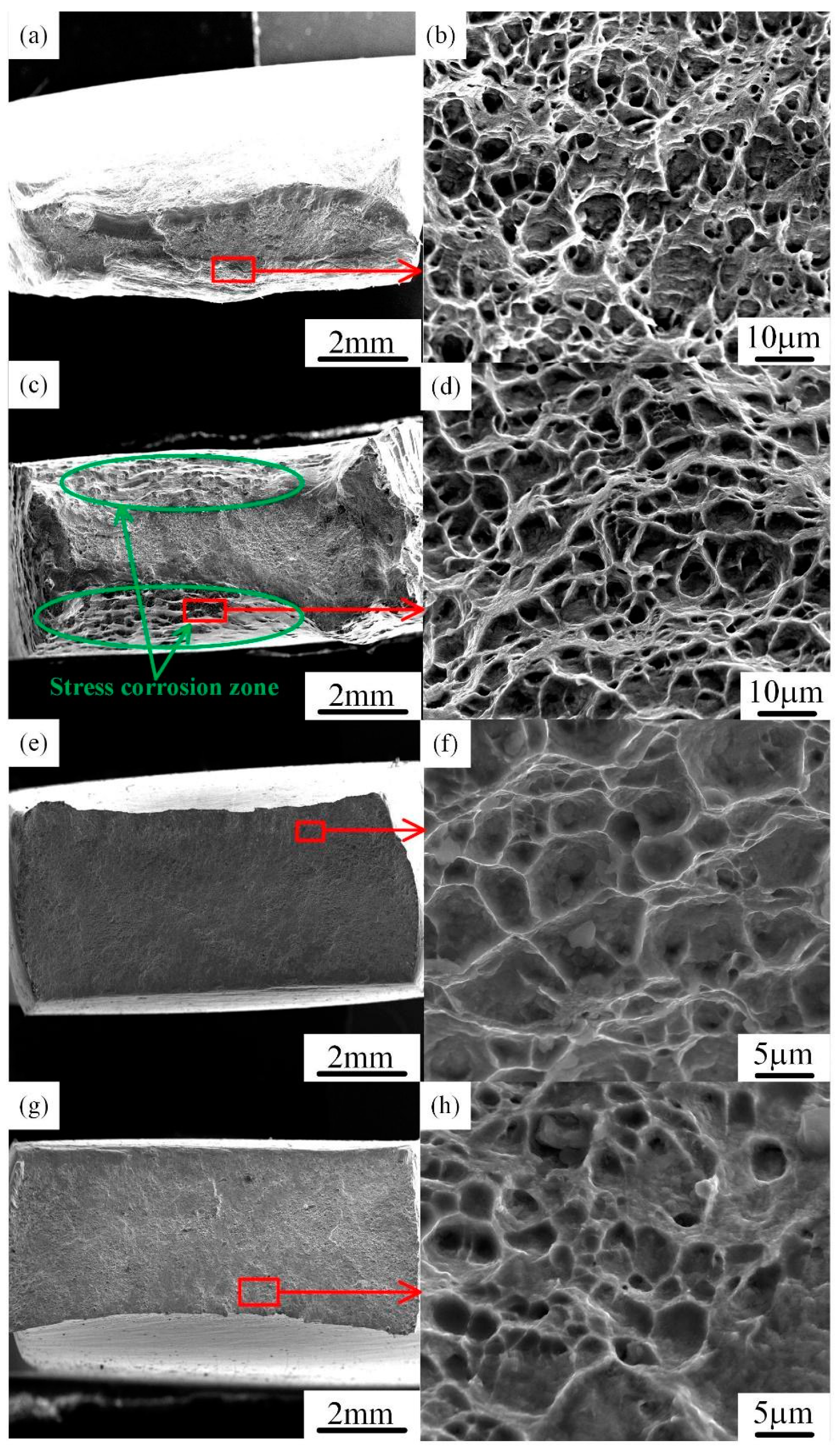
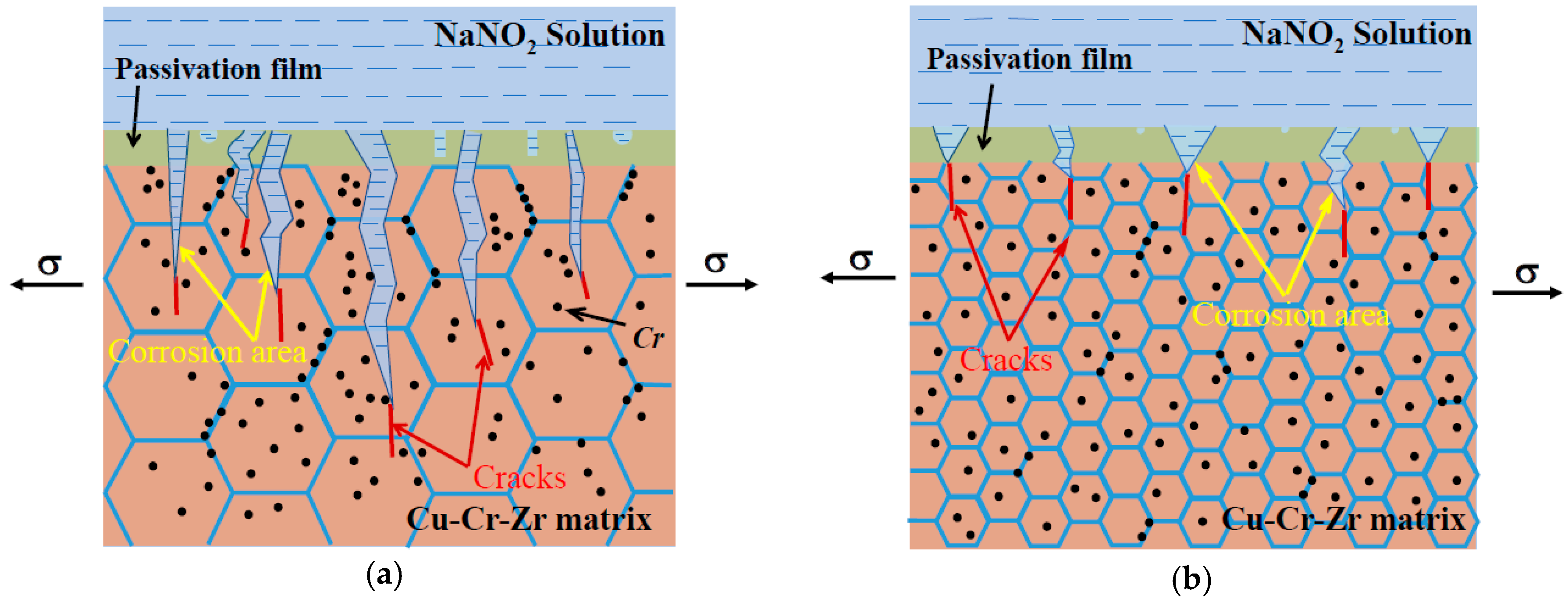
3.3. SCC Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishnev, R.; Shakhova, I.; Belyakov, A.; Kaibyshev, R. Deformation microstructures, strengthening mechanisms, and electrical conductivity in a Cu–Cr–Zr alloy. Mater. Sci. Eng. A 2015, 629, 29–40. [Google Scholar] [CrossRef]
- Peng, L.J.; Xie, H.F.; Huang, G.J.; Xu, G.L.; Yin, X.Q.; Feng, X.; Mi, X.J.; Yang, Z. The phase transformation and strengthening of a Cu-0.71wt% Cr alloy. J. Alloys Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Xiao, B.C.; Feng, J.; Jing, Y.J.; Pian, X.; Meng, M.T.; Zhong, Q.T. Precipitation, Recrystallization, and Evolution of Annealing Twins in a Cu-Cr-Zr Alloy. Metals 2018, 8, 227. [Google Scholar]
- Abib, K.; Balanos, J.A.M.; Alili, B.; Bradai, D. On the microstructure and texture of Cu-Cr-Zr alloy after severe plastic deformation by ECAP. Mater. Charact. 2016, 112, 252–258. [Google Scholar] [CrossRef]
- Xia, K.W.; Wei, W.; Wang, F.; Du, Q.B.; Alexandrov, I.V.; Hu, J. Microstructure, mechanical properties and electrical conductivity of industrial Cu–0.5%Cr alloy processed by severe plastic deformation. Mater. Sci. Eng. A 2011, 528, 1478–1484. [Google Scholar]
- Wu, S.H.; Zhang, P.; Shao, D.; Cheng, P.M.; Kuang, J.; Wu, K.; Zhang, J.Y.; Liu, G.; Sun, J. Grain size-dependent Sc microalloying effect on the yield strength-pitting corrosion correlation in Al-Cu alloys. Mater. Sci. Eng. A 2018, 721, 200–214. [Google Scholar] [CrossRef]
- Guo, M.X.; Du, J.Q.; Zheng, C.H.; Zhang, J.S.; Zhuang, L.Z. Influence of Zn contents on precipitation and corrosion of Al-Mg-Si-Cu-Zn alloys for automotive applications. Mater. Sci. Eng. A 2019, 778, 256–270. [Google Scholar] [CrossRef]
- Huang, A.H.; Wang, Y.F.; Wang, M.S.; Song, L.Y.; Li, Y.S.; Gao, L.; Huang, C.X.; Zhu, Y.T. Optimizing the strength, ductility and electrical conductivity of a Cu-Cr-Zr alloy by rotary swaging and aging treatment. Mater. Sci. Eng. A 2019, 746, 211–216. [Google Scholar] [CrossRef]
- Qiu, Y.X.; Ai, B.M.; Jing, H.J.; Zhao, J.C.; Dan, S.; Yu, C.Y.; Huan, L. Stress Corrosion Cracking Behavior of Fine-Grained AZ61 Magnesium Alloys Processed by Equal-Channel Angular Pressing. Metals 2017, 9, 343. [Google Scholar]
- Purcek, G.; Yanar, H.; Demirtas, M.; Alemdag, Y.; Shangina, D.V.; Dobatkin, S.V. Optimization of strength, ductility and electrical conductivity of Cu–Cr–Zr alloy by combining multi-route ECAP and aging. Mater. Sci. Eng. A 2016, 649, 114–122. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Chen, J.B.; Li, J.F. Microstructure and properties of rare earth-containing Cu-Cr-Zr alloy. Trans. Nonferr. Met. Soc. China 2015, 25, 1206–1214. [Google Scholar] [CrossRef]
- Shangina, D.V.; Bochvar, N.R.; Morozova, A.I.; Belyakov, A.N.; Kaibyshev, R.O.; Dobatkin, S.V. Effect of chromium and zirconium content on structure, strength and electrical conductivity of Cu-Cr-Zr alloys after high pressure torsion. Mater. Lett. 2017, 199, 46–49. [Google Scholar] [CrossRef]
- Rebak, R.B.; Carranza, R.M.; Galvele, J.R. Stress Corrosion Cracking Mechanism of α-Brass in NaNO2 Solutions. Corros. Sci. 1988, 28, 1089–1106. [Google Scholar] [CrossRef]
- Yamasaki, T.; Miyamoto, H.; Mimaki, T.; Vinogradov, A.; Hashimoto, S. Stress corrosion cracking susceptibility of ultra-fine grain copper produced by equal-channel angular pressing. Mater. Sci. Eng. A 2001, 318, 122–128. [Google Scholar] [CrossRef]
- Wang, L.C.; Li, D.Y. Mechanical, electrochemical and tribological properties of nanocrystalline surface of brass produced by sandblasting and annealing. Surf. Coat. Technol. 2003, 167, 188–196. [Google Scholar] [CrossRef]
- Sadawy, M.M.; Ghanem, M. Grain refinement of bronze alloy by equal-channel angular pressing (ECAP) and its effect on corrosion behavior. Def. Technol. 2016, 12, 316–323. [Google Scholar] [CrossRef]
- Vinogradov, A.; Mimaki, T.; Hashimoto, S.; Valiev, R. On the corrosion behaviour of ultra-fine grain copper. Scr. Mater. 1999, 41, 319–326. [Google Scholar] [CrossRef]
- Miyamoto, H.; Harada, K.; Mimaki, T.; Vinogradov, A.; Hashimoto, S. Corrosion of ultra-fine grained copper fabricated by equal-channel angular pressing. Corros. Sci. 2008, 50, 1215–1220. [Google Scholar] [CrossRef]
- Jang, Y.H.; Kim, S.S.; Han, S.Z.; Lim, C.Y.; Kim, C.J. Corrosion and stress corrosion cracking behavior of equal channel angular pressed oxygen-free copper in 3.5% NaCl solution. J. Mater. Sci. 2006, 41, 4293–4297. [Google Scholar] [CrossRef]
- Balusamy, T.; Kumar, S.; Sankara Narayanan, T.S.N. Effect of surface nanocrystallization on the corrosion behaviour of AISI 409 stainless steel. Corros. Sci. 2010, 52, 3826–3834. [Google Scholar] [CrossRef]
- Gu, Y.X.; Ma, A.B.; Jiang, J.H.; Li, H.Y.; Song, D.; Wu, H.R.; Yuan, Y.C. Simultaneously improving mechanical properties and corrosion resistance of pure Ti by continuous ECAP plus short-duration annealing. Mater. Charact. 2018, 138, 38–47. [Google Scholar] [CrossRef]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.A.; Stolyarov, V.V.; Valiev, R.Z.; Liao, X.Z.; Zhao, Y.H.; Jiang, Y.B.; Xu, H.F.; Lowe, T.C.; et al. Corrosion resistance of ultra fine-grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Brunner, J.G.; May, J.; Höppel, H.W.; Göken, M.; Virtanen, S. Localized corrosion of ultrafine-grained Al–Mg model alloys. Electrochim. Acta 2010, 55, 1966–1970. [Google Scholar] [CrossRef]
- Zhou, P.; Ogle, K. The corrosion of copper and copper alloys. In Encyclopedia of Interfacial Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 478–489. [Google Scholar]
- Mimaki, T.; Nakazawa, Y.; Hashimoto, S.; Miura, S. Stress corrosion cracking of copper bicrystals with 〈110〉 -Tilt ∑3, ∑9, and ∑11 coincident site lattice boundaries. Metall. Trans. A 1990, 21, 2355–2361. [Google Scholar] [CrossRef]
- Anna, M.; Elijah, B.; Vladimir, B.; Sergey, Z.; Andrey, B.; Rustam, K. Grain refinement kinetics in a low alloyed Cu–Cr–Zr alloy subjected to large strain deformation. Materials 2017, 10, 1394. [Google Scholar]
- Edwards, D.J.; Singh, B.N.; Tähtinen, S. Effect of heat treatments on precipitate microstructure and mechanical properties of a CuCrZr alloy. J. Nucl. Mater. 2007, 367, 904–909. [Google Scholar] [CrossRef]
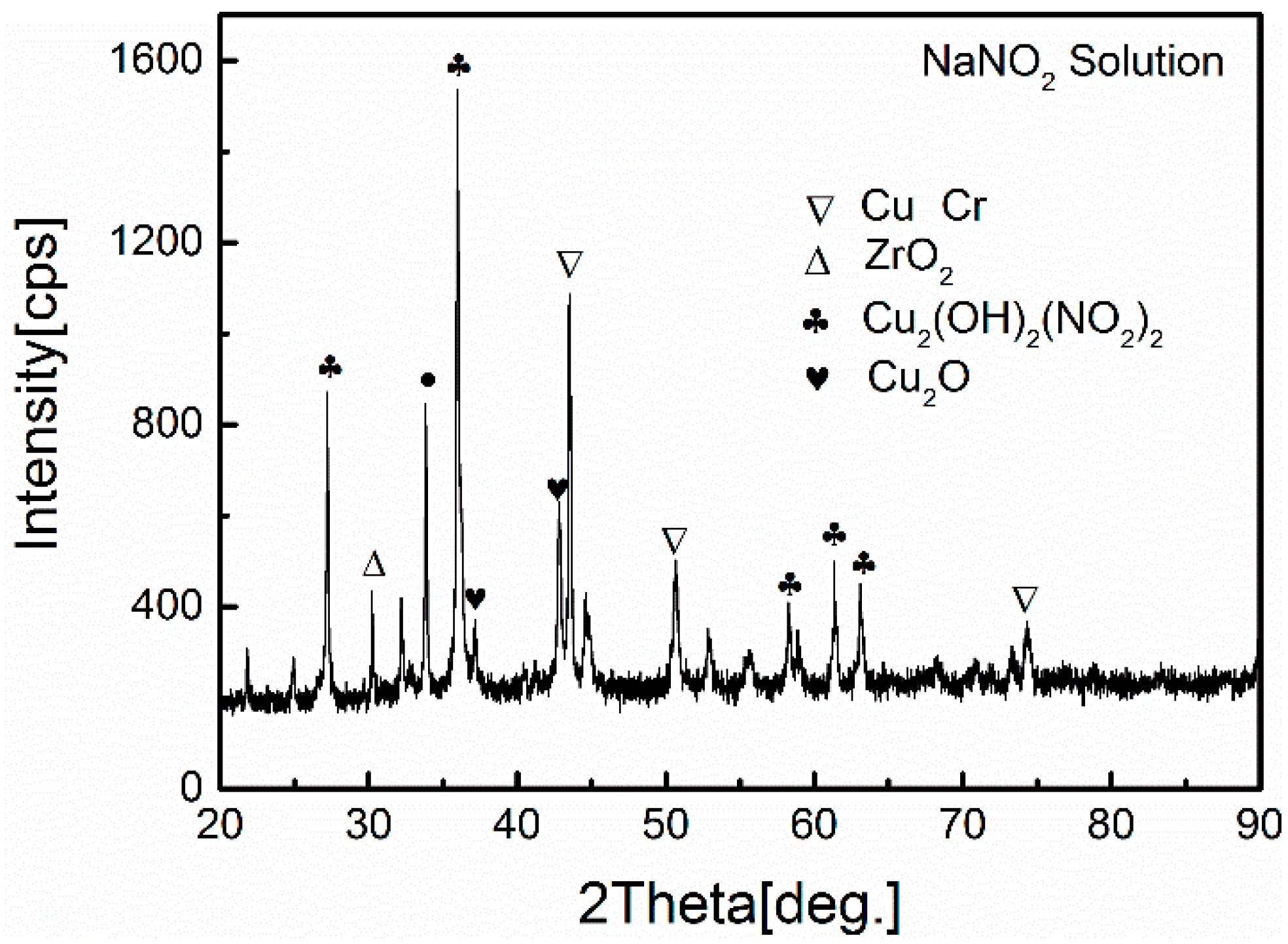
| Alloy | Chemical Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Cr | Zr | Fe | Ni | Pb | Zn | Cu | |
| Cu-Cr-Zr | 0.83–0.84 | 0.15–0.20 | ≤0.03 | ≤0.02 | ≤0.002 | ≤0.003 | Bal. |
| Sample | CG | UFG |
|---|---|---|
| Average residual stress (MPa) | 35 | 42 |
| Parameter | Solution | ||||||
|---|---|---|---|---|---|---|---|
| CG Cu-Cr-Zr | NaNO2 | 376 | 3.6 | 52.6 | 8.4 | 14 | 1.14 |
| In air | 390 | 57.3 | 16 | ||||
| UFG Cu-Cr-Zr | NaNO2 | 612 | –1.2 | 23 | 0.9 | 6.38 | 1.01 |
| In air | 605 | 23.2 | 6.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, D.; Tong, L.; Zhou, Y.; Wang, W.; Zhou, H.; Fan, R. Enhanced Stress Corrosion Cracking Resistance of Ultrafine-Grained Cu-Cr-Zr Alloy Fabricated via Equal-Channel Angular Pressing. Metals 2019, 9, 824. https://doi.org/10.3390/met9080824
Wang Q, Liu D, Tong L, Zhou Y, Wang W, Zhou H, Fan R. Enhanced Stress Corrosion Cracking Resistance of Ultrafine-Grained Cu-Cr-Zr Alloy Fabricated via Equal-Channel Angular Pressing. Metals. 2019; 9(8):824. https://doi.org/10.3390/met9080824
Chicago/Turabian StyleWang, Qingjuan, Dan Liu, Libo Tong, Ying Zhou, Wei Wang, Haixiong Zhou, and Ruixue Fan. 2019. "Enhanced Stress Corrosion Cracking Resistance of Ultrafine-Grained Cu-Cr-Zr Alloy Fabricated via Equal-Channel Angular Pressing" Metals 9, no. 8: 824. https://doi.org/10.3390/met9080824
APA StyleWang, Q., Liu, D., Tong, L., Zhou, Y., Wang, W., Zhou, H., & Fan, R. (2019). Enhanced Stress Corrosion Cracking Resistance of Ultrafine-Grained Cu-Cr-Zr Alloy Fabricated via Equal-Channel Angular Pressing. Metals, 9(8), 824. https://doi.org/10.3390/met9080824







