Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
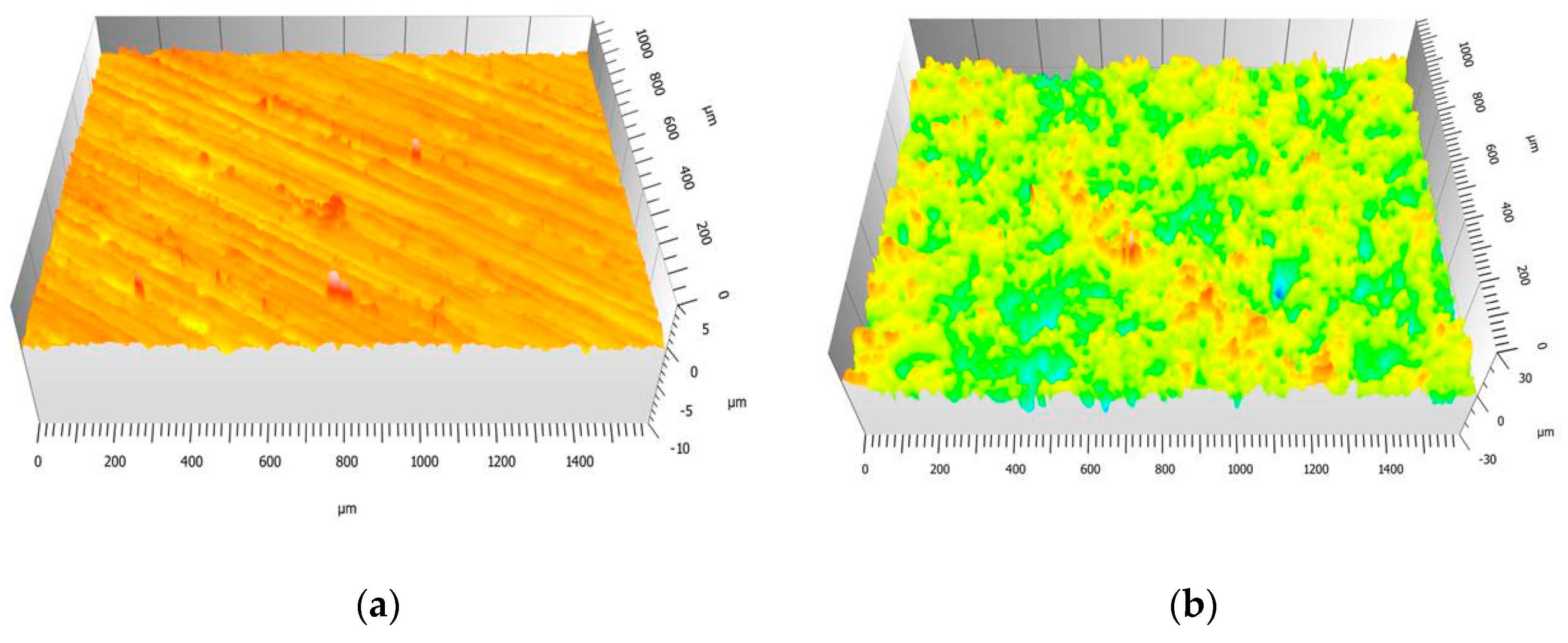
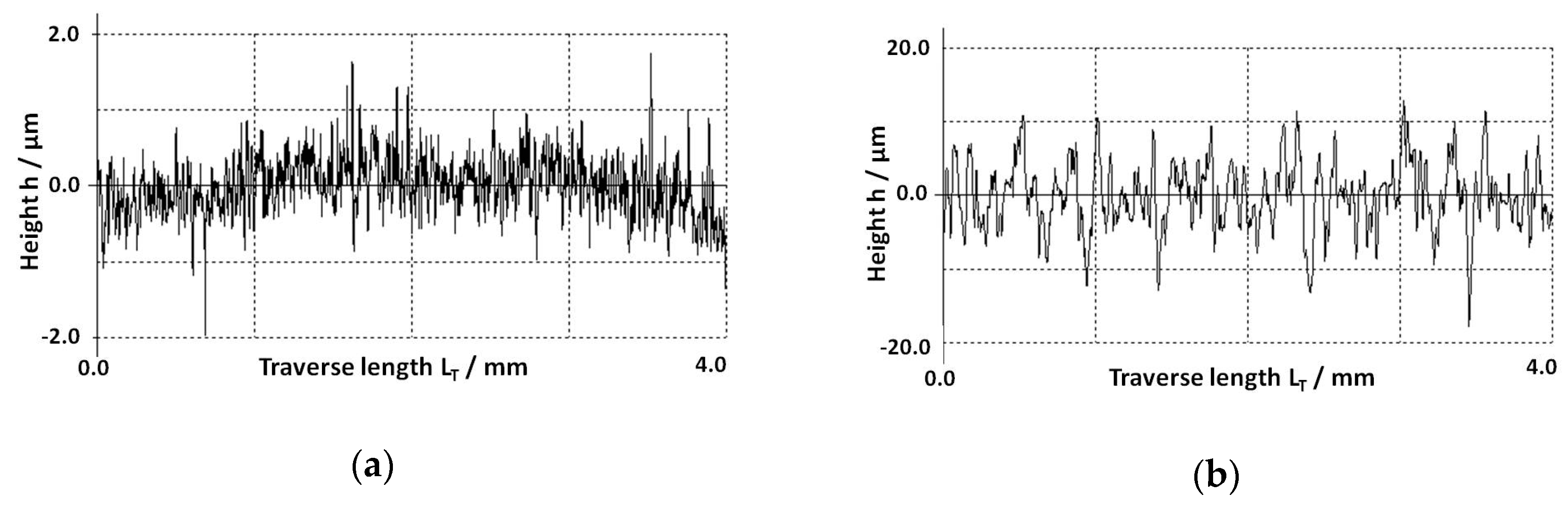
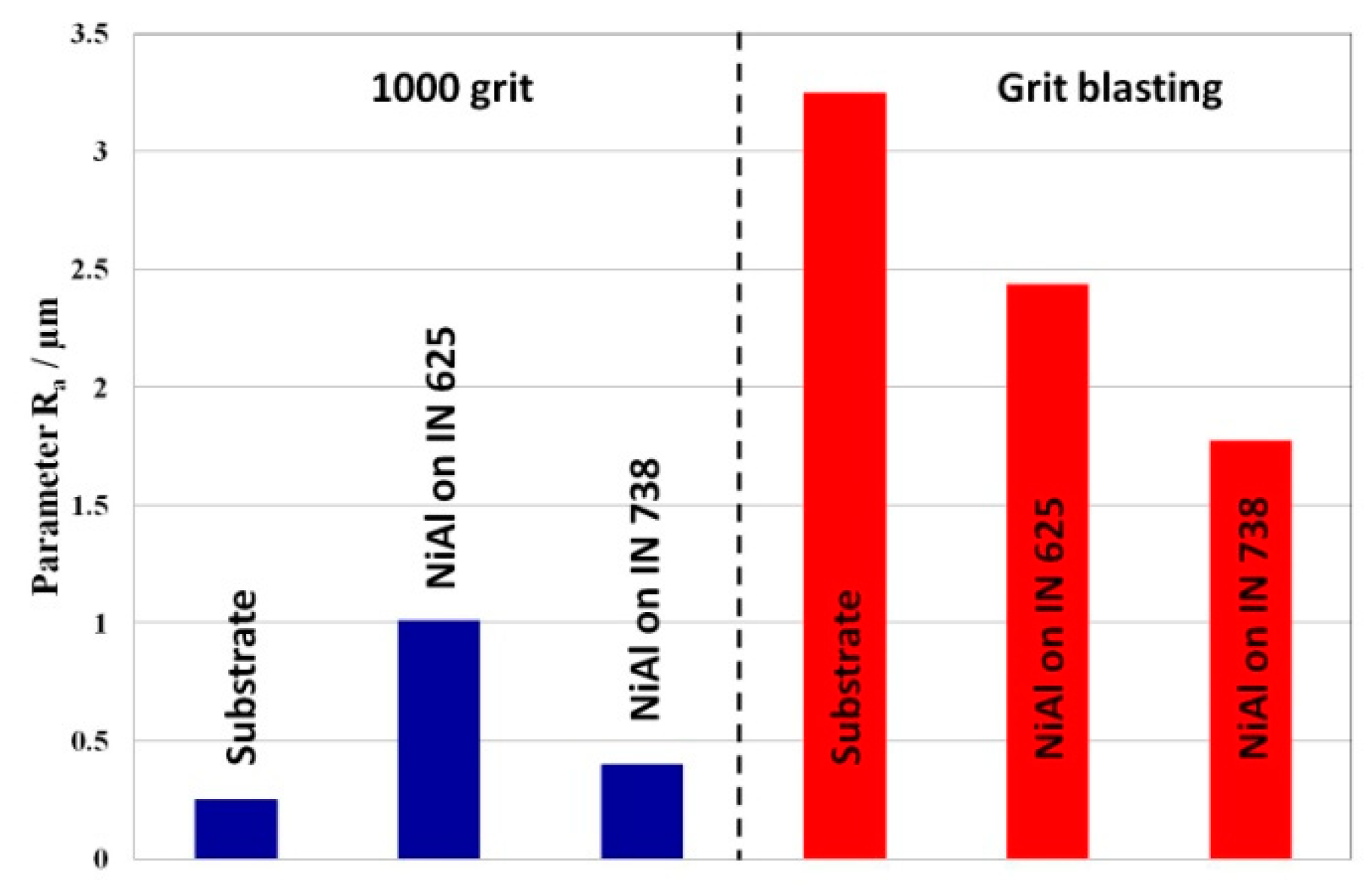
3.1. Roughness Evaluation of Ni-Base Substrates Surfaces
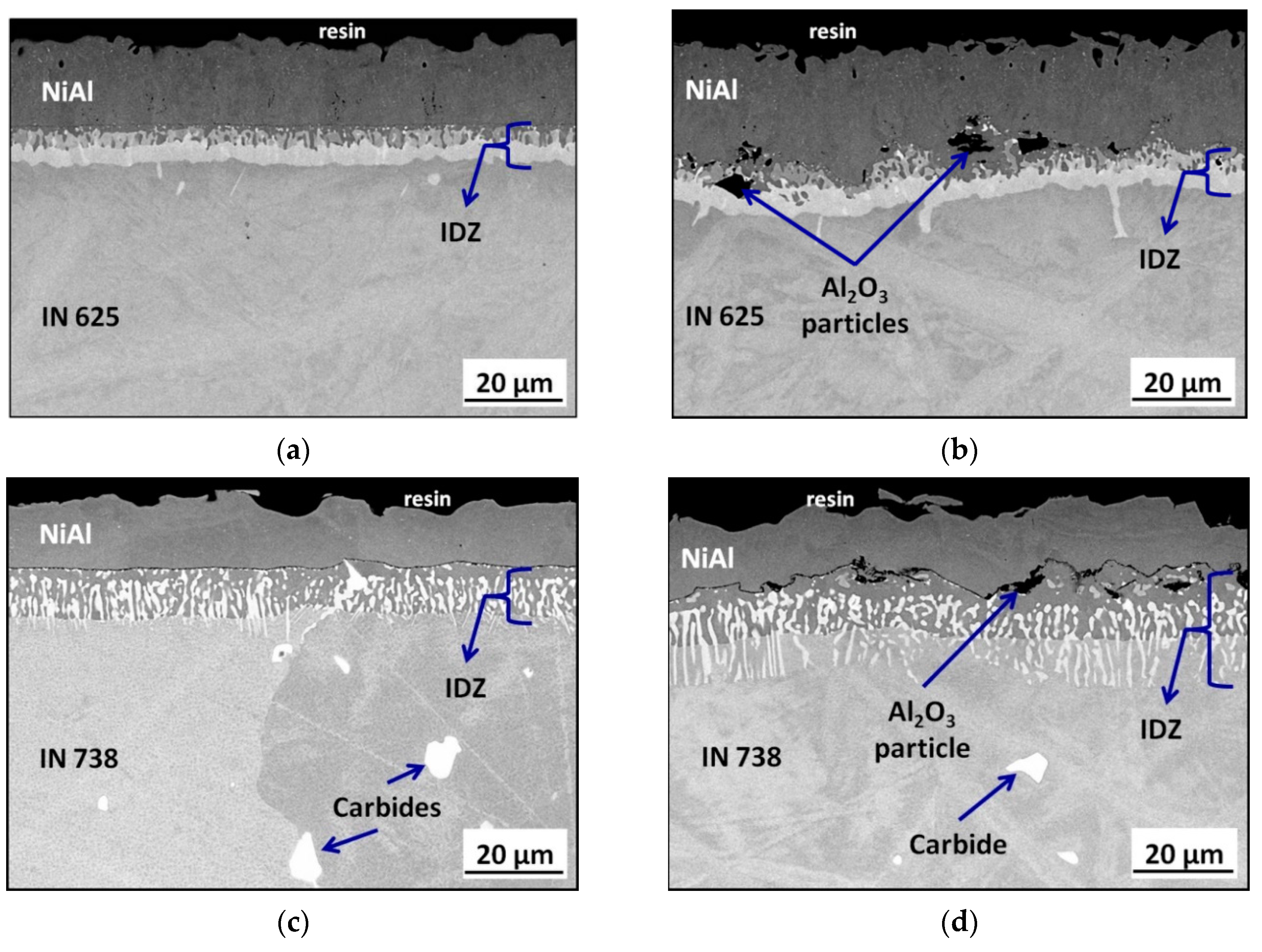
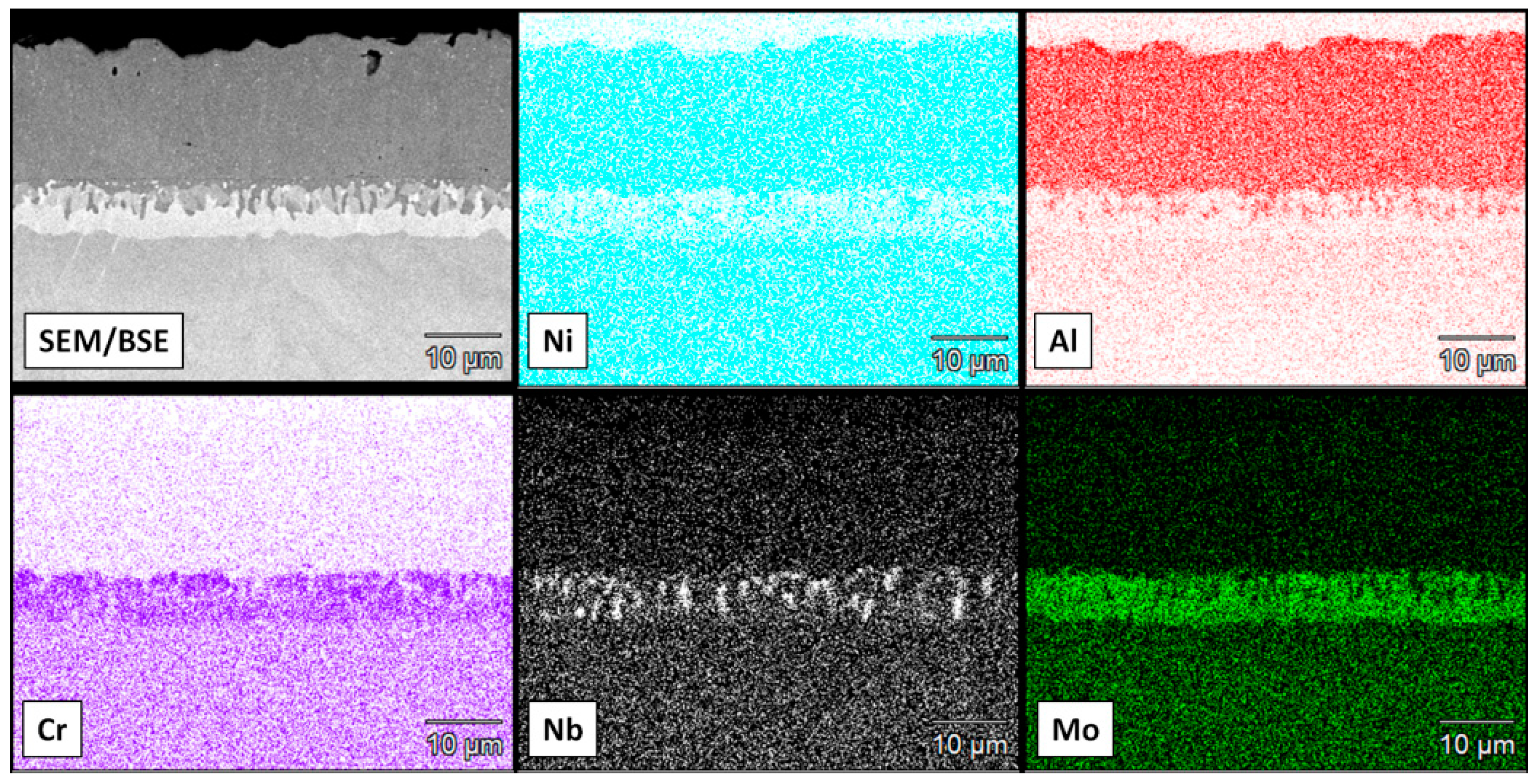
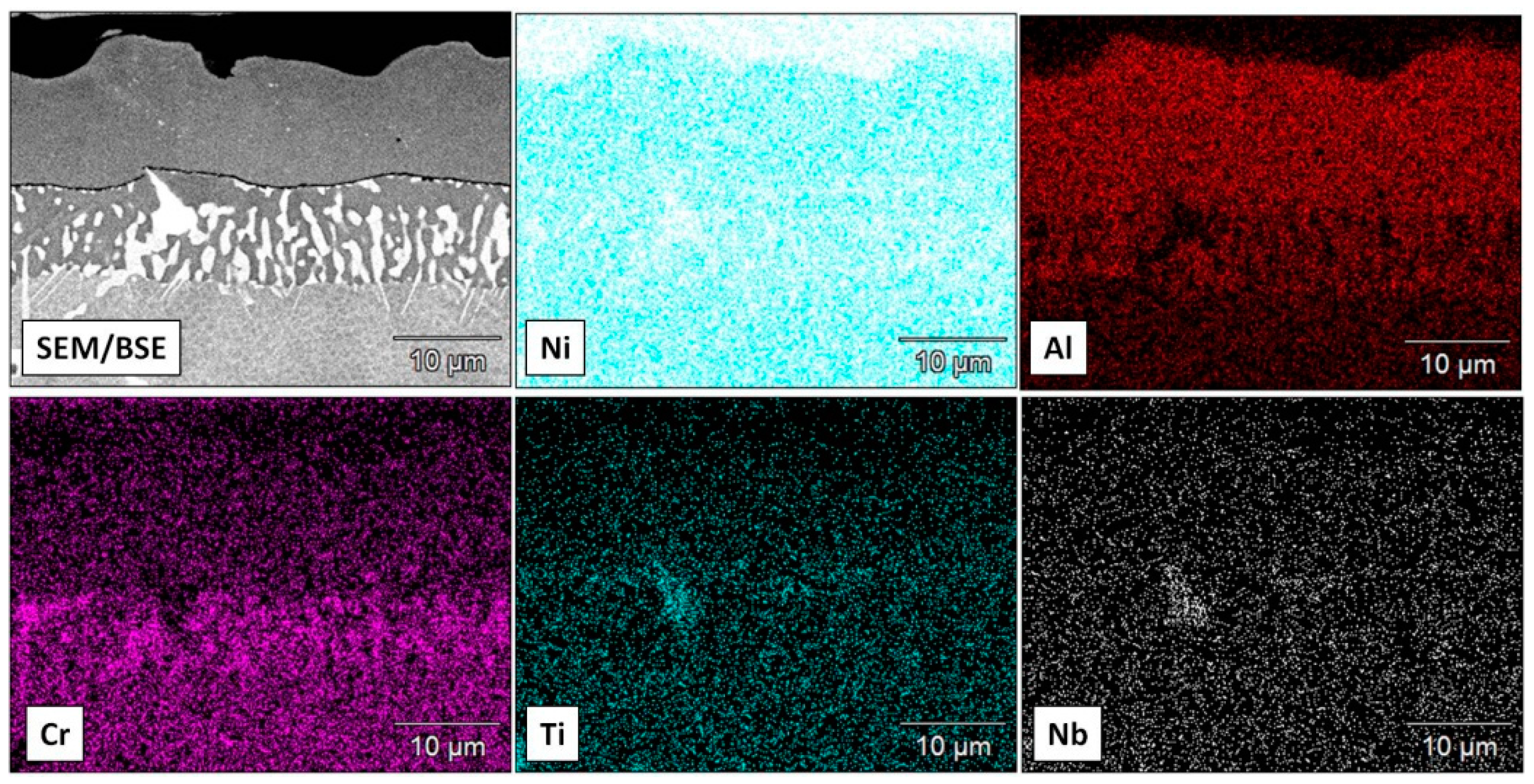
3.2. Aluminide Coatings in the As-Received Condition
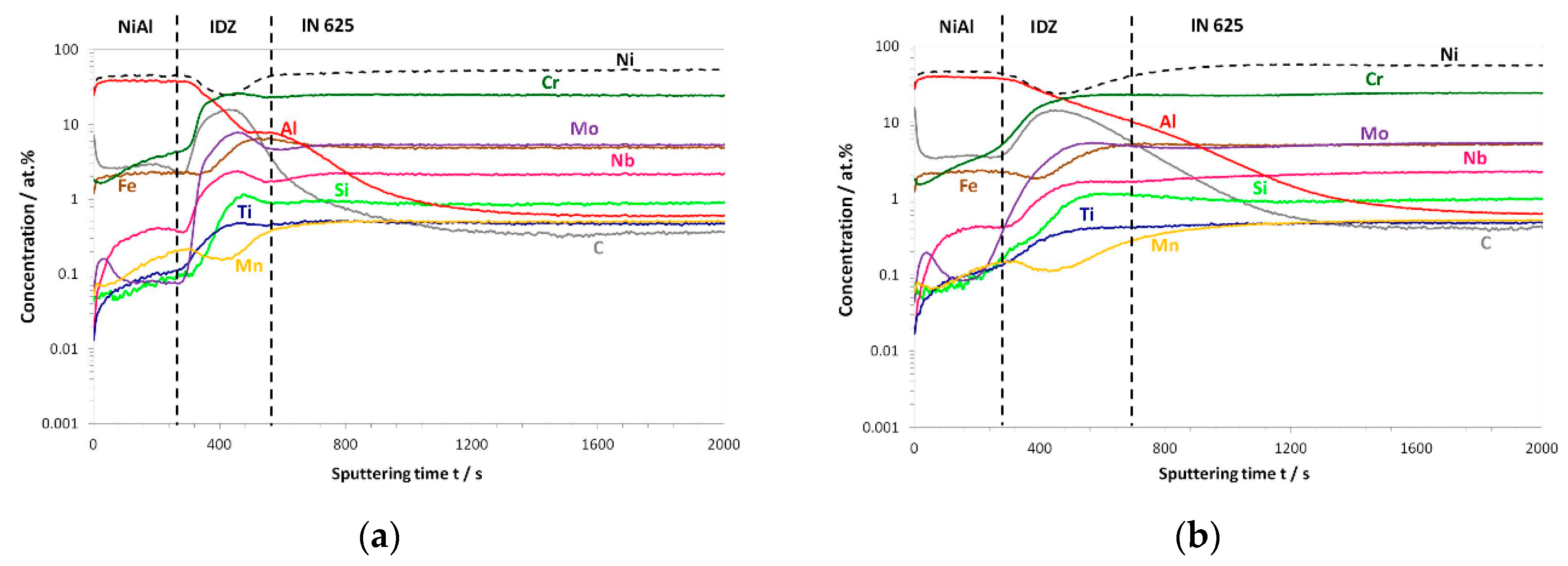
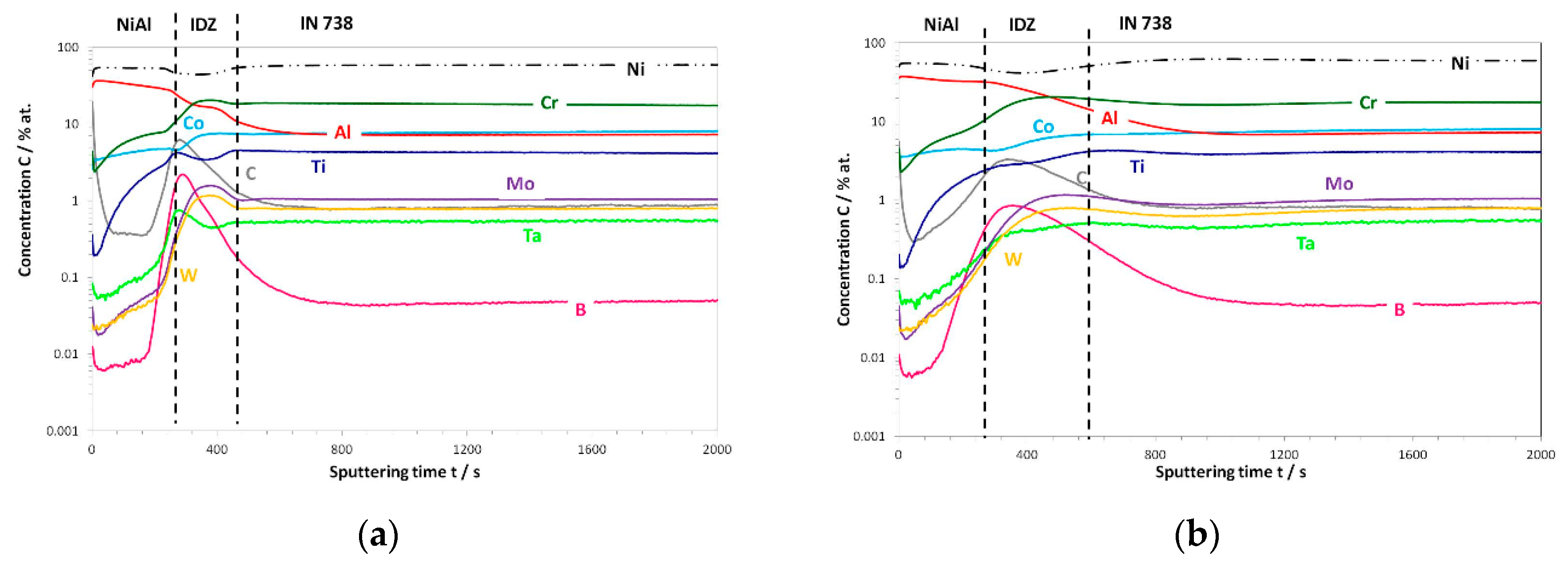
3.3. Post-Exposure Analyses
4. Conclusions
- Surface mechanical treatment influences the thickness and morphology of aluminized Ni-based superalloys: for grit-blasted IN 625, the thickening of NiAl coating was observed. Tor grit-blasted IN 738, a thicker IDZ was found.
- The presence of foreign particles, namely alumina, coming from grit-blasting process negatively affects diffusion processes at an elevated temperature. In addition, they can introduce additional stresses during the heating and cooling of the samples at the coating/substrate interface.
- The grit-blasting of substrates results in a worse oxidation behavior of studied aluminized systems.
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, J.; Xiao, G.; Wang, Y.; Liu, Y. High temperature oxidation behavior of the wrought Ni-based superalloy GH4037 in the solid and semi-solid state. J. Alloy. Compd. 2019, 784, 394–404. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Simms, N.J. Shreir’s Corrosion; Richardson, T., Ed.; Elsevier: Amsterdam, NL, USA, 2010; Volume 1, pp. 518–540. [Google Scholar]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Zielińska, M.; Zagula-Yaworska, M.; Sieniawski, J.; Filip, R. Microstructure and oxidation resistance of an aluminide coating on the nickel based superalloy MAR M247 deposited by the CVD aluminizing process. Arch. Metall. Mater. 2013, 58, 697–701. [Google Scholar] [CrossRef]
- Gao, F.; Huang, X.; Liu, R.; Yang, Q. A Study of Pack Aluminizing Process for NiCrAlY Coatings Using Response Surface Methodology. J. Mater. Eng. Perform. 2014, 23, 83–91. [Google Scholar] [CrossRef]
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion mechanisms and microstructure development in pack aluminizing of Ni-based alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Romanowska, J.; Zagula-Yavorska, M.; Sieniawski, J.; Markowski, J. Zirconium Modified Aluminide Coatings Obtained by the CVD and PVD Methods. Open J. Met. 2013, 3, 92–99. [Google Scholar] [CrossRef][Green Version]
- Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative Isothermal Oxidation Behaviour of New Aluminide Coatings from Slurries Containing Al Particles and Conventional Out-of-Pack Aluminide Coatings. Oxid. Met. 2014, 81, 139–149. [Google Scholar] [CrossRef]
- Rannou, B.; Velasco, F.; Guzmán, C.; Kolarik, V.; Pedraza, F. Aging and thermal behavior of a PVA/Al microspheres slurry for aluminizing purposes. Mater. Chem. Phys. 2012, 134, 360–365. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating Techniques for Functional Enhancement of Metal Implants for Bone Replacement: A Review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef]
- Haynes, J.A.; Pint, B.A.; More, K.L.; Zhang, Y.; Wright, I.G. Influence of Sulfur, Platinum, and Hafnium on the Oxidation Behavior of CVD NiAl Bond Coatings. Oxid. Met. 2002, 58, 513–554. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Kubiak, K.; Sieniawski, J. Oxidation behaviour of palladium modified aluminide coatings deposited by CVD method on nickel-based superalloys under air atmosphere. J. Achiev. Mater. Manuf. Eng. 2012, 55, 848–854. [Google Scholar]
- Monceau, D.; Bouhanek, K.; Peraldi, R.; Malie, A.; Pieraggi, B. Transition in high-temperature oxidation kinetics of Pd-modified aluminide coatings: Role of oxygen partial pressure, heating rate, and surface treatment. J. Mater. Res. 2000, 15, 665–675. [Google Scholar] [CrossRef]
- Chen, M.; Shen, M.; Zhu, S.; Wang, F.; Wang, X. Effect of sand blasting and glass matrix composite coating on oxidation resistance of a nickel-based superalloy at 1000 °C. Corros. Sci. 2013, 73, 331–341. [Google Scholar] [CrossRef]
- Dong, J.; Sun, Y.; He, F.; Huang, H.; Zhen, J. Effects of substrate surface roughness and aluminizing agent composition on the aluminide coatings by low-temperature pack cementation. Mater. Res. Express 2018, 6. [Google Scholar] [CrossRef]
- Nowak, W.J.; Serafin, D.; Wierzba, B. Effect of surface mechanical treatment on the oxidation behavior of FeAl-model alloy. J. Mater. Sci. 2019, 54, 9185–9196. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B. Effect of Surface Treatment on High Temperature Oxidation Behavior of IN 713C. J. Mater. Eng. Perform. 2018, 27, 5280–5290. [Google Scholar] [CrossRef]
- Serafin, D.; Nowak, W.J.; Wierzba, B. The effect of surface preparation on high temperature oxidation of Ni, Cu and Ni-Cu alloy. Appl. Surf. Sci. 2019, 476, 442–451. [Google Scholar] [CrossRef]
- Ramsay, J.D.; Evans, H.E.; Child, D.J.; Taylor, M.P.; Hardy, M.C. The influence of stress on the oxidation of a Ni-based superalloy. Corr. Sci. 2019, 154, 277–285. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, J.; Zhao, P.; Dou, B. Formation and phase transformation of aluminide coating prepared by low temperature aluminizing process. Surf. Coat. Technol. 2017, 234–240. [Google Scholar] [CrossRef]
- Pfeifer, J.P.; Holzbrecher, H.; Quadakkers, W.J.; Speier, J. Quantitative analysis of oxide films on ODS-alloys using MCs+-SIMS and e-beam SNMS. J. Anal. Chem. 1993, 346, 186–191. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Elschner, A.; Speier, W.; Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. Appl. Surf. Sci. 1991, 52, 271–287. [Google Scholar] [CrossRef]
- Nowak, W.J. Characterization of oxidized Ni-based superalloys by GD-OES. J. Anal. At. Spectrom. 2017, 32, 1730–1738. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Steven, R.A.; Flewitt, P.E.J. Microstructural changes which occur during isochronal heat treatment of the nickel-base superalloy IN-738. J. Mater. Sci. 1978, 13, 367–376. [Google Scholar] [CrossRef]
- Evans, H.E.; Taylor, M.P. Diffusion Cells and Chemical Failure of MCrAlY Bond Coats in Thermal-Barrier Coating Systems. Oxid. Met. 2001, 55, 17–34. [Google Scholar] [CrossRef]
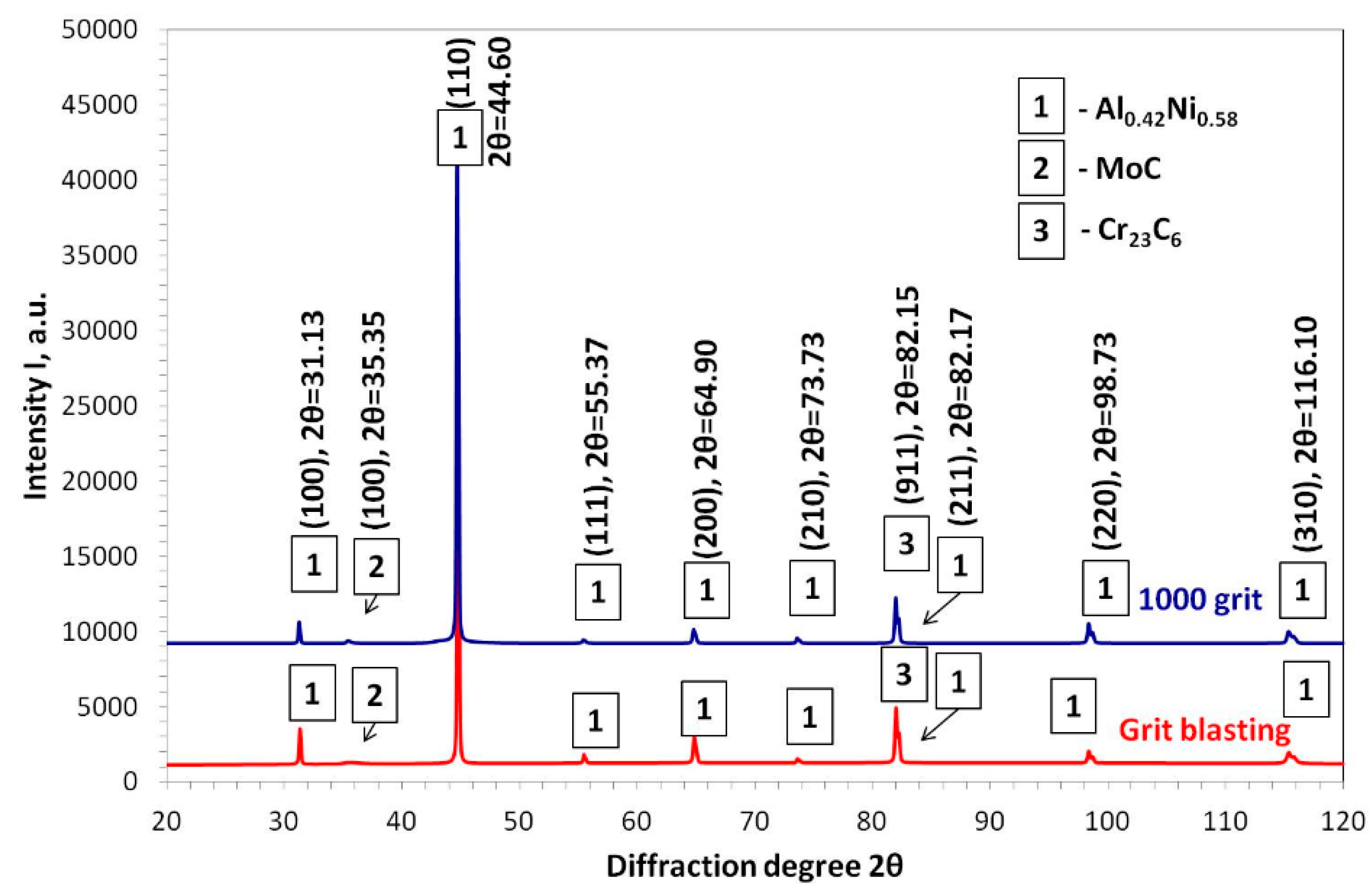
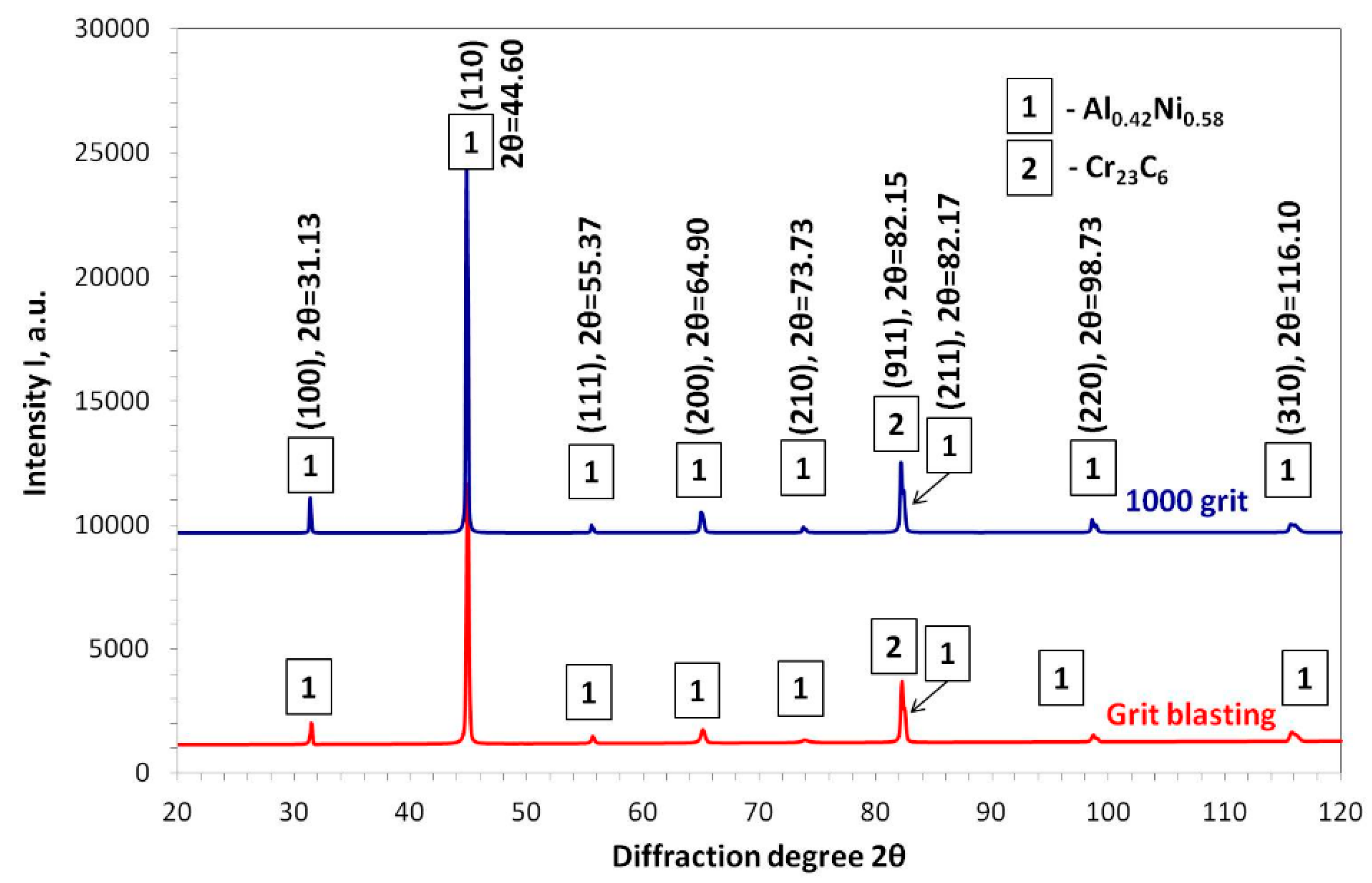
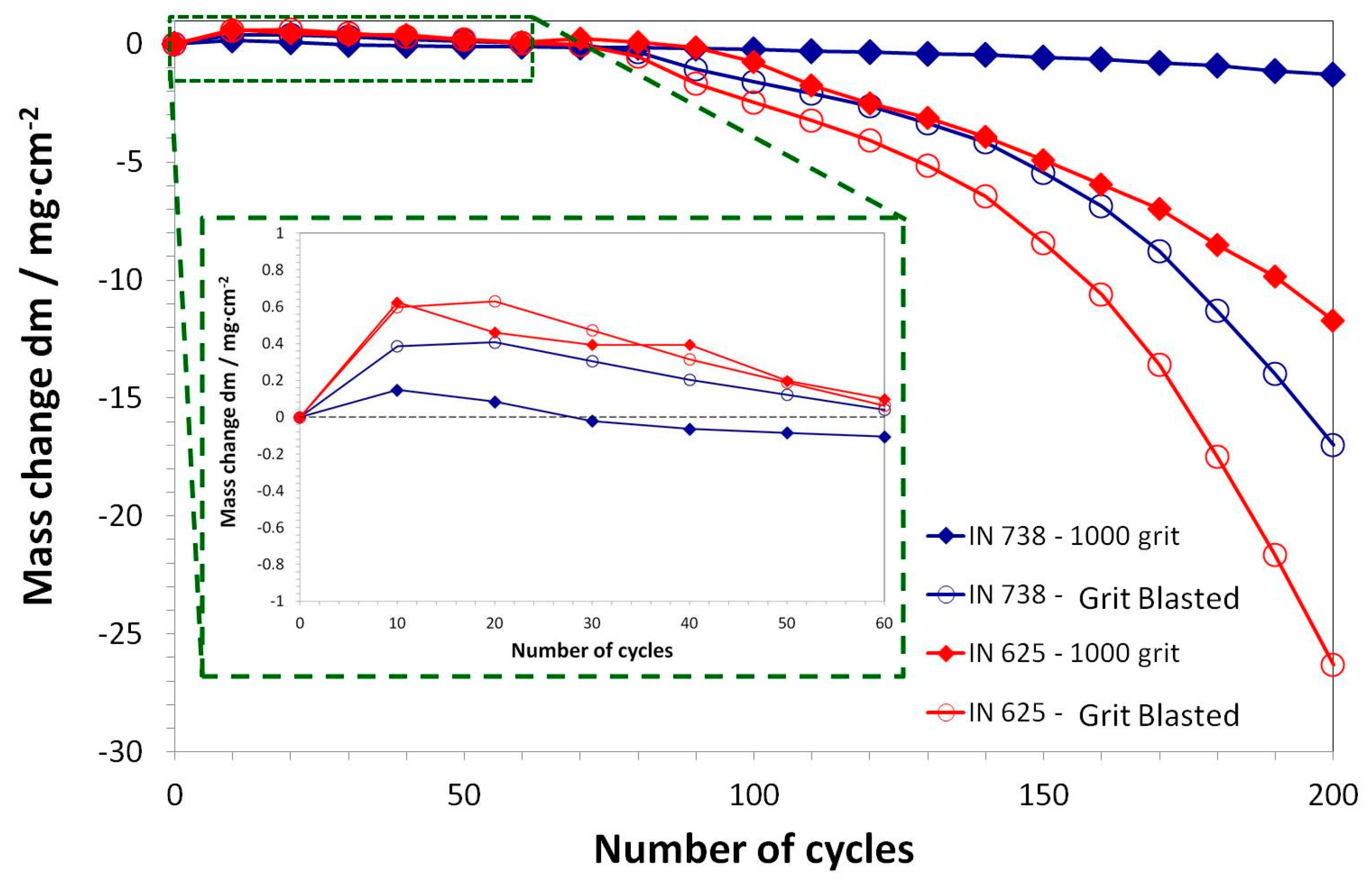
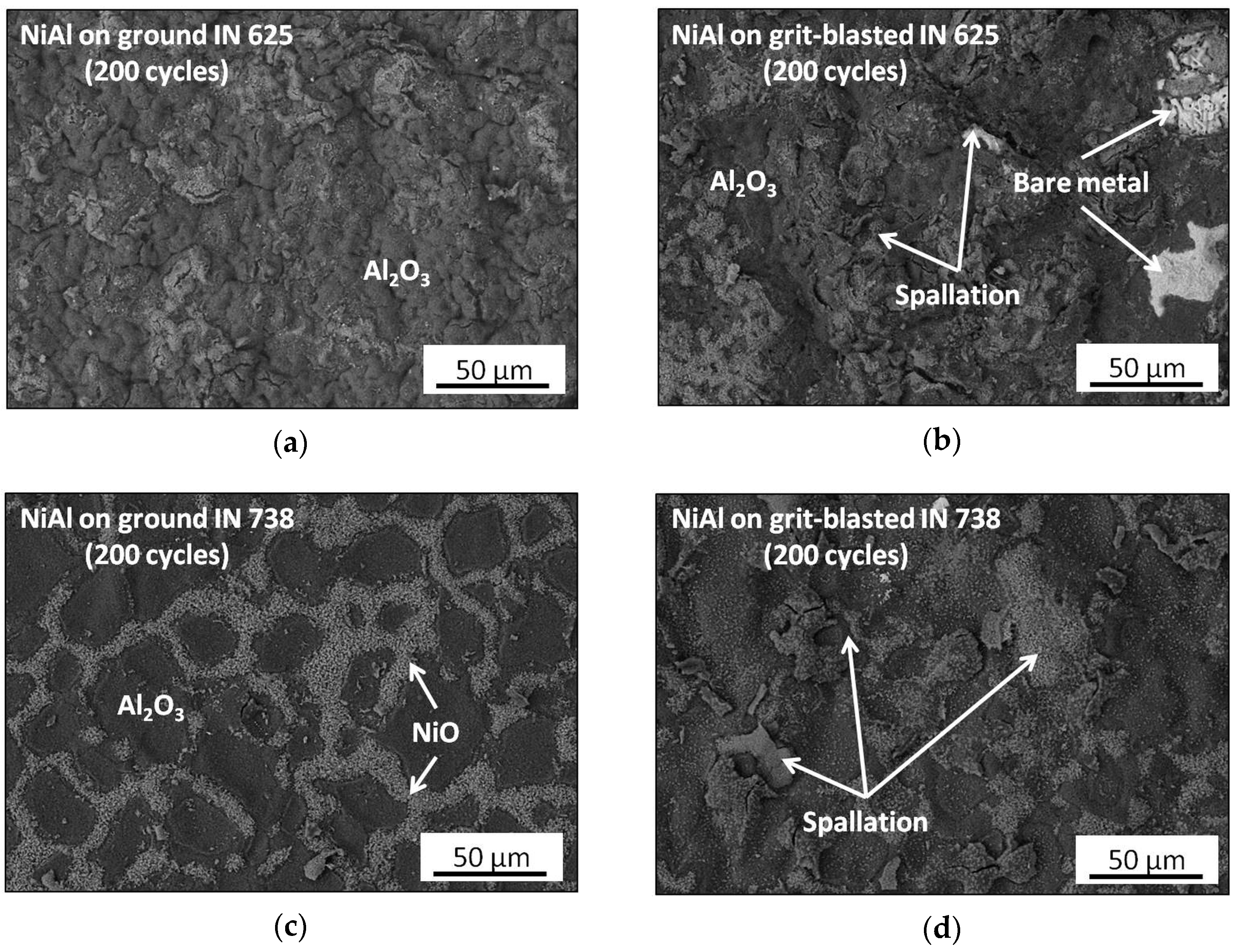
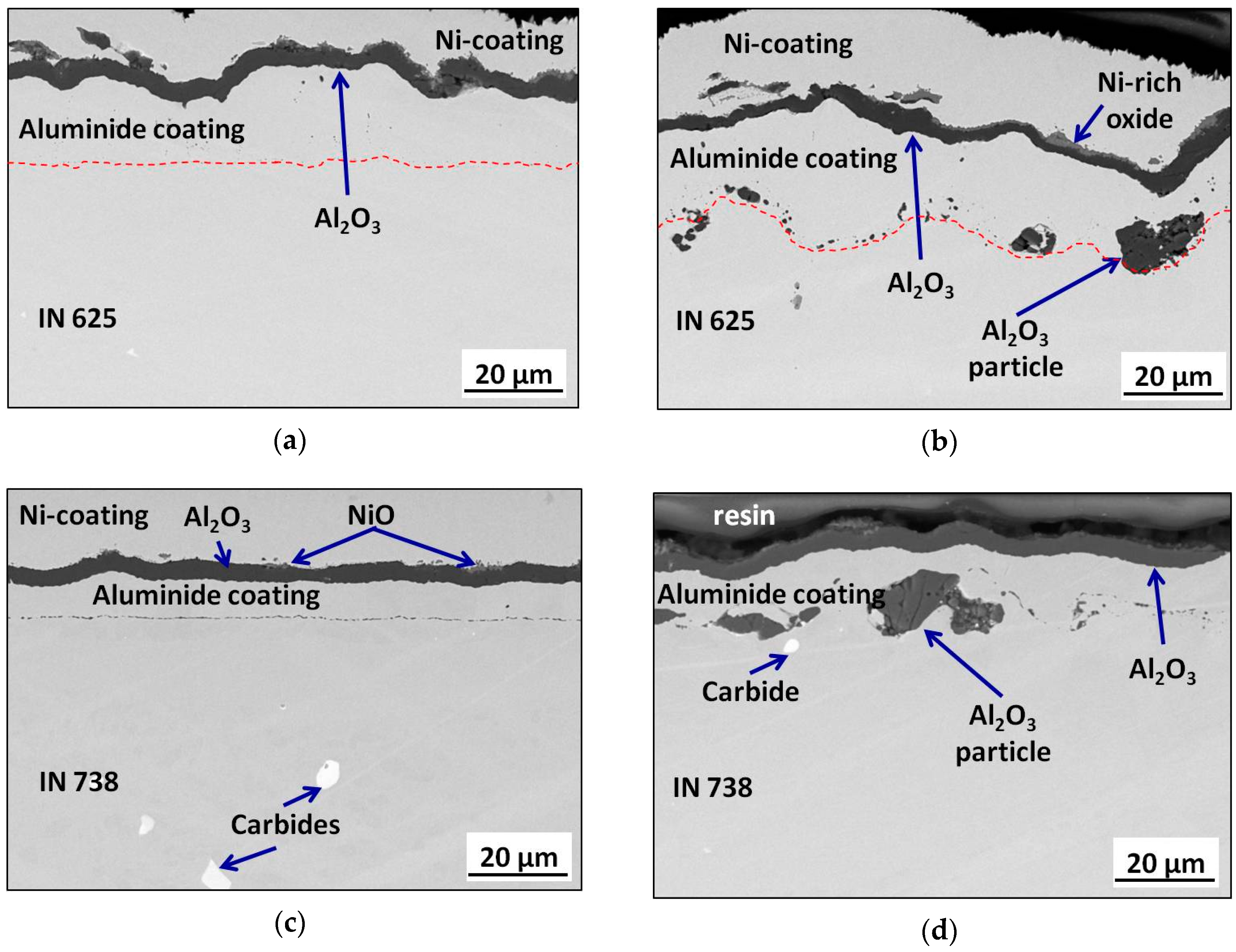
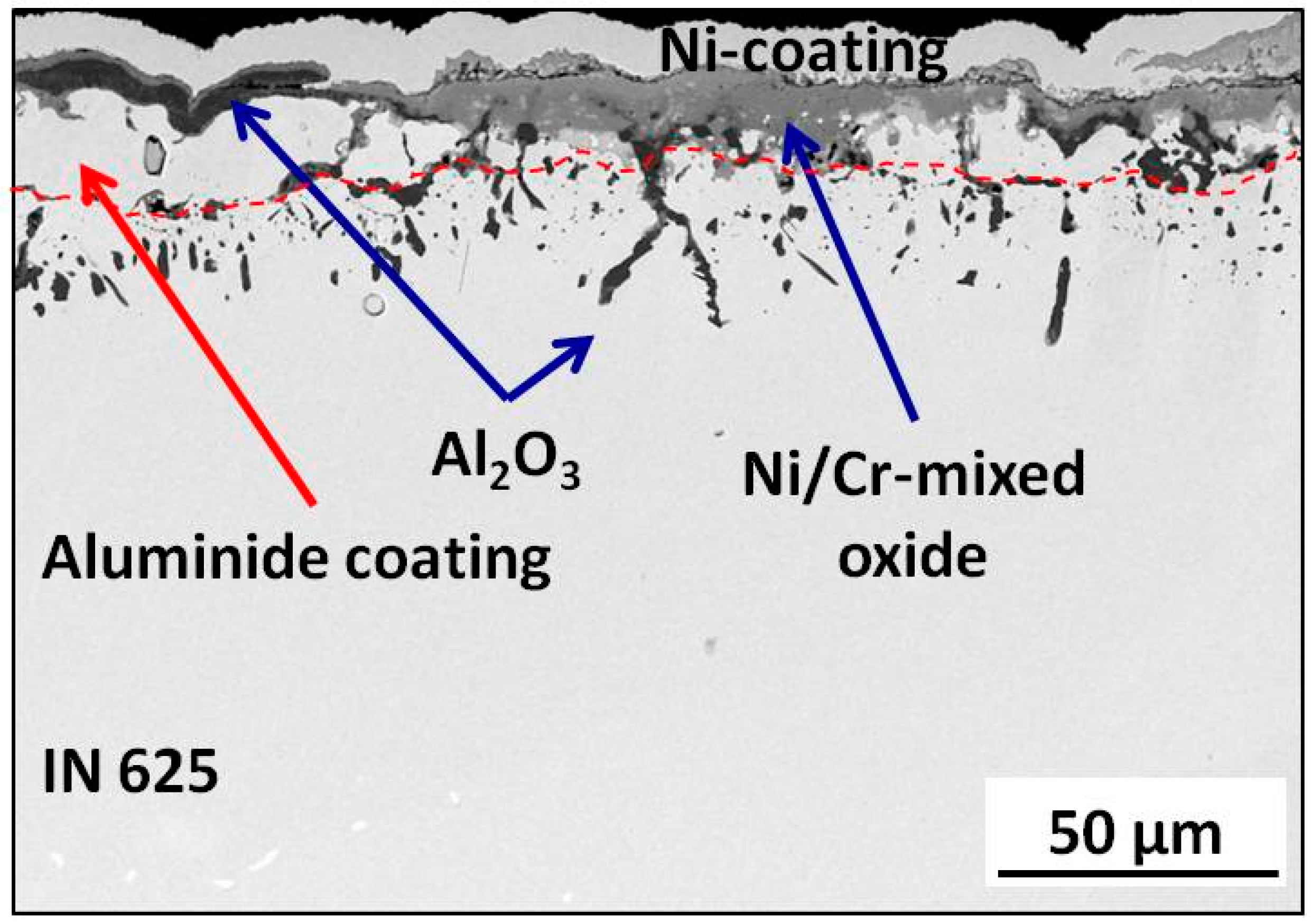

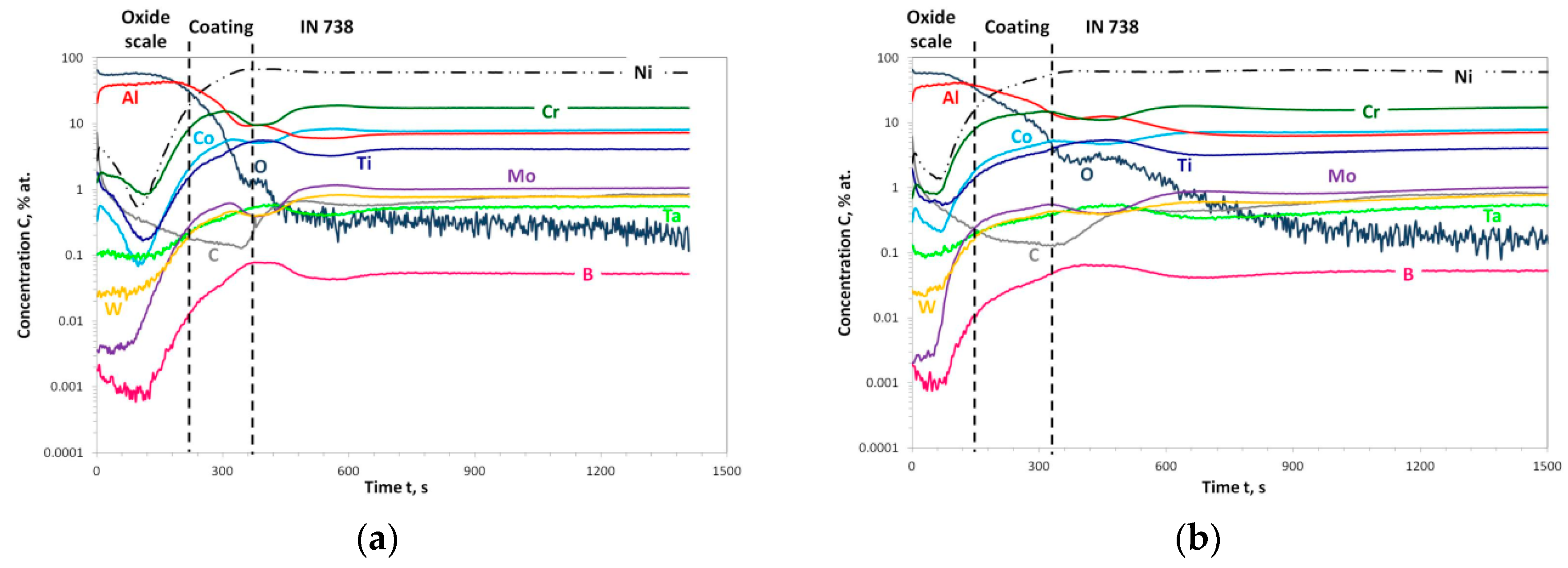
| Alloy | Elements Content (wt %) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Ta | Co | Mo | W | Nb | Al | Ti | Fe | Mn | Si | B | C | |
| IN 625 | BASE | 21.50 | 3.65 * | 1.00 | 9.00 | - | 3.65 * | 0.40 | 0.40 | 5.00 | 0.5 | 0.5 | - | 0.10 |
| IN 738 | BASE | 16.00 | 1.8 | 8.5 | 1.8 | 2.6 | 0.8 | 3.5 | 3.5 | - | - | - | 0.01 | 0.18 |
| Alloy | IN 625 | IN 738 | ||
|---|---|---|---|---|
| Surface Preparation | 1000 Grit | Grit-Blasting | 1000 Grit | Grit-Blasting |
| Outer NiAl thickness [µm] | 16.93 | 20.90 | 12.97 | 13.50 |
| Standard deviation | 1.36 | 1.77 | 1.77 | 2.06 |
| IDZ thickness [µm] | 8.13 | 11.70 | 10.90 | 18.93 |
| Standard deviation | 0.90 | 3.40 | 1.12 | 3.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, W.J.; Ochał, K.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals 2019, 9, 782. https://doi.org/10.3390/met9070782
Nowak WJ, Ochał K, Wierzba P, Gancarczyk K, Wierzba B. Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals. 2019; 9(7):782. https://doi.org/10.3390/met9070782
Chicago/Turabian StyleNowak, Wojciech J., Kamil Ochał, Patrycja Wierzba, Kamil Gancarczyk, and Bartek Wierzba. 2019. "Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy" Metals 9, no. 7: 782. https://doi.org/10.3390/met9070782
APA StyleNowak, W. J., Ochał, K., Wierzba, P., Gancarczyk, K., & Wierzba, B. (2019). Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals, 9(7), 782. https://doi.org/10.3390/met9070782







