Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview
Abstract
1. Introduction
2. Ball Milling Modification of Mg-Based Hydrogen Storage Alloys
2.1. Modification by Element Substitution of Mg2Ni
2.1.1. Modification by Substituting Mg Element
2.1.2. Modification by Substituting Ni Element
2.1.3. Modification by Substituting Mg and Ni Element
2.1.4. Mg-Ni Series Alloys
2.2. Modification Using Additives
2.2.1. Transition Metal Additives
2.2.2. Metal Oxide Additives
2.2.3. Transition Metal Halide Additives
2.2.4. Carbon Additives
2.2.5. Intermetallic Compounds
3. Summary
Funding
Conflicts of Interest
References
- Murashkina, T.L.; Syrtanov, M.S.; Laptev, R.S.; Stepanova, E.N.; Lider, A.M. Structure and defects evolution at temperature and activation treatments of the intermetallic compound of Laves phase C36-type. Int. J. Hydrogen Energy 2019, 44, 10732–10743. [Google Scholar] [CrossRef]
- Murashkina, T.L.; Syrtanov, M.S.; Laptev, R.S.; Lider, A.M. Cyclic stability of the C36-type Laves phase synthesized in the abnormal glow discharge plasma under hydrogenation. Int. J. Hydrogen Energy 2019, 44, 6709–6719. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Syrtanov, M.S.; Bordulev, Y.S.; Babikhina, M.N.; Lider, A.M.; Gubin, V.E.; Murashkina, T.L. The hydrogen sorption and desorption behavior in spherical powder of pure titanium used for additive manufacturing. Int. J. Hydrogen Energy 2017, 42, 15283–15289. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Kashkarov, E.B.; Syrtanov, M.S.; Lider, A.M. Hydrogen sorption by Ni-coated titanium alloy VT1-0. Int. J. Hydrogen Energy 2017, 42, 10604–10610. [Google Scholar] [CrossRef]
- Nikitina, L.; Laptev, R.; Abzaev, Y.; Lider, A.; Ivashutenko, A. Positron spectroscopy of nanodiamonds after hydrogen sorption. Nanomaterials 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Berdonosova, E.; Gammer, C.; Eckert, J.; Zadorozhnyy, M.; Bazlov, A.; Zheleznyi, M.; Kaloshkin, S.; Klyamkin, S. Mechanochemical synthesis and hydrogenation behavior of alloys. J. Alloy. Compd. 2019, 796, 42–46. [Google Scholar] [CrossRef]
- Hua, T.; Ahluwalia, R.; Eudy, L.; Singer, G.; Jermer, B.; Asselin-Miller, N.; Wessel, S.; Patterson, T.; Marcinkoski, J. Status of hydrogen fuel cell electric buses worldwide. J. Power Sour. 2014, 269, 975–993. [Google Scholar] [CrossRef]
- Huang, L.J.; Wang, H.; Liu, J.W.; Zhang, C.; Ouyang, L.Z.; Zhu, M. Low temperature de/hydrogenation in the partially crystallized metallic glasses induced by milling with process control agents. J. Alloy. Compd. 2019, 792, 835–843. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Kral, L.; Cermak, J. Improvement of hydrogen storage properties of Mg by catalytic effect of Al-containing phases in Mg-Al-Ti-Zr-C powders. Int. J. Hydrogen Energy 2019, 44, 13561–13568. [Google Scholar] [CrossRef]
- Wang, L.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Direct and reversible hydrogen storage of lithium hydride (LiH) nanoconfined in high surface area graphite. Int. J. Hydrogen Energy 2016, 41, 18088–18094. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. Chemsuschem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Pukazhselvan, D.; Kumar, V.; Singh, S.K. High capacity hydrogen storage: Basic aspects, new developments and milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ding, W.; Ying, Y.; Zou, J. Research progress of Mg-based energy materials. Mater. Chin. 2011, 30, 35–43. (In Chinese) [Google Scholar]
- Cortez, J.J.; Castro, F.J.; Troiani, H.E.; Pighin, S.A.; Urretavizcaya, G. Kinetic improvement of absorption and desorption properties in Mg/MgH2 by using niobium ethoxide as additive. Int. J. Hydrogen Energy 2019, 44, 11961–11969. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials:Development and optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.h.; Sun, C.h. Developments in the researches of Mg-based hydrogen storage materials. J. Shijiazhuang Vocat. Technol. Inst. 2016, 28, 50–53. (In Chinese) [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares-Fernández, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, J.; Jing, X.; Hu, B. The research status and development of hydrogen storage materials. Mater. Rev. 2007, 21, 118–120, 135. (In Chinese) [Google Scholar]
- Zhang, J.; Li, Z. Research Progress of preparation methods of Mg-based hydrogen storage Alloy. Hot Work Technol. 2014, 43, 7–9, 13. (In Chinese) [Google Scholar] [CrossRef]
- Ma, X.; Yue, L.; He, G.; He, D.; Zhang, J. Research development of mechanical alloying used to synthesize Mg-based hydrogen storage alloys (in Chinse). Mater. Rev. 2010, 24, 89–92, 98. [Google Scholar]
- Chen, J.; Shi, Y.; Zhang, F.; Han, Y. The mechanism of MA in high energy ball-milling technology. Machinery 2004, 31, 52–54. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, M.; Qin, X. Research and development of mechanical attrition method in nanostructural materials. Mater. Rev. 2003, 17, 20–22, 29. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhang, Y.H. Research progress in Mg-based hydrogen storage alloys. Rare Metals 2014, 33, 499–510. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Zhao, H.; Yu, X.; Shao, H. Mg-based metastable nano alloys for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 6007–6018. [Google Scholar] [CrossRef]
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Activation characteristics of graphite modified hydrogen absorbing materials. J. Alloy. Compd. 2001, 325, 245–251. [Google Scholar] [CrossRef]
- Xu, W.; Tao, Zh.; Chen, J. Progress of research on hydrogen storage. Prog. Chem. 2006, 18, 200–210. (In Chinese) [Google Scholar] [CrossRef]
- Lin, J.; Zhao, D.; Wang, L. Hydrogen storage materials and research progress. J. Suihua Univ. 2017, 37, 141–145. (In Chinese) [Google Scholar]
- Li, X.; Xu, H.; Cheng, P. The Review of hydrogen storage materials. Hebei Chem. Ind. 2012, 35, 51–53. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Han, X.; Li, B.; Dong, X.; Wang, X. Electrochemical characteristics of mechanical alloyed Ni (x = 0–0.1) electrode alloys. Int. J. Hydrogen Energy 2007, 32, 2830–2835. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Q.; Song, H.; Wang, Y.; Liu, J. Electrochemical characteristics of -type alloys prepared by mechanical alloying. J. Alloy. Compd. 2003, 353, 322–326. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Li, B.; Ren, H.; Dong, X.; Wang, X. Electrochemical characteristics of Ni (x=0–0.6) electrode alloys prepared by mechanical alloying. J. Alloy. Compd. 2008, 450, 208–214. [Google Scholar] [CrossRef]
- Woo, J.H.; Jung, C.B.; Lee, J.H.; Lee, K.S. Electrochemical characteristics of nanocrystalline and type metal hydrides prepared by mechanical alloying. J. Alloy. Compd. 1999, 293–295, 556–563. [Google Scholar] [CrossRef]
- Anik, M.; Akay, I.; Özdemir, G.; Baksan, B. Electrochemical hydrogen storage performance of Mg–Ti–Zr–Ni alloys. Int. J. Hydrogen Energy 2009, 34, 9765–9772. [Google Scholar] [CrossRef]
- Kohno, T.; Yamamoto, M.; Kanda, M. Electrochemical properties of mechanically ground alloy. J. Alloy. Compd. 1999, 293–295, 643–647. [Google Scholar] [CrossRef]
- Hou, X.J.; Kou, H.C.; Zhang, T.B.; Hu, R.; Li, J.S.; Xue, X.Y. First-principles studies on the structures and properties of Ti- and Zn-substituted hydrogen storage alloys and their hydrides. Mater. Sci. Forum 2013, 743–744, 44–52. [Google Scholar] [CrossRef]
- Anik, M. Improvement of the electrochemical hydrogen storage performance of magnesium based alloys by various additive elements. Int. J. Hydrogen Energy 2012, 37, 1905–1911. [Google Scholar] [CrossRef]
- Huang, L.W.; Elkedim, O.; Nowak, M.; Chassagnon, R.; Jurczyk, M. Ni (x=0, 0.5) alloys prepared by mechanical alloying for electrochemical hydrogen storage: Experiments and first-principles calculations. Int. J. Hydrogen Energy 2012, 37, 14248–14256. [Google Scholar] [CrossRef]
- Huang, L.W.; Elkedim, O.; Nowak, M.; Jurczyk, M.; Chassagnon, R.; Meng, D.W. Synergistic effects of multiwalled carbon nanotubes and Al on the electrochemical hydrogen storage properties of -type alloy prepared by mechanical alloying. Int. J. Hydrogen Energy 2012, 37, 1538–1545. [Google Scholar] [CrossRef]
- Zeng, Y.; Fan, K.; Li, X.; Xu, B.; Gao, X.; Meng, L. First-principles studies of the structures and properties of Al- and Ag-substituted alloys and their hydrides. Int. J. Hydrogen Energy 2010, 35, 10349–10358. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Wang, Y.; Li, Q.; Song, H.; Yang, H. The hydrogenation properties of alloy. J. Alloy. Compd. 2002, 336, 297–300. [Google Scholar] [CrossRef]
- Xie, Zh.; Fu, A.; Chen, Y.; Pan, F.; Ding, P. Effect of Al addition on microstructure and hydrogen diffusion capability of alloy. J. Funct. Mater. 2006, 37, 601–603. (In Chinese) [Google Scholar] [CrossRef]
- Xie, Zh.; Pan, F.; Xiang, Y.; Chen, Y. Synergistic effects of Al and Cr addition on microstructure of hydrogen storage alloy. Mater. Sci. Forum 2009, 610–613, 960–963. [Google Scholar] [CrossRef]
- Kohno, T.; Kanda, M. Effect of partial substitution on hydrogen storage properties of alloy. J. Electrochem. Soc. 1997, 144, 2384–2388. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, T.; Fu, J.; Pan, F.; Deng, Sh.; Li, J.; Zhao, G. The effect of Al addition on hydrogenation properties of alloy. Mater. Rev. 2007, 21, 194–195, 207. (In Chinese) [Google Scholar]
- Shi, H.; Meng, L.; Li, X.; Zheng, S.H. Overviews of element partial substitution study of Mg–Ni-based hydrogen storage material. J. Handan Coll. 2010, 20, 51–57. (In Chinese) [Google Scholar] [CrossRef]
- Xue, J.; Li, G.; Hu, Y. Study on Electrochemical performances of system hydrogen storage alloys. Chin. J. Rare Met. 2000, 24, 128–130. (In Chinese) [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of nanocrystalline made by mechanical alloying. J. Alloy. Compd. 1999, 282, 286–290. [Google Scholar] [CrossRef]
- Iwakura, C.; Shin-ya, R.; Miyanohara, K.; Nohara, S.; Inoue, H. Effects of Ti–V substitution on electrochemical and structural characteristics of MgNi alloy prepared by mechanical alloying. Electrochim. Acta 2001, 46, 2781–2786. [Google Scholar] [CrossRef]
- Orimo, S.; Fujii, H. Materials science of Mg–Ni-based new hydrides. Appl. Phys. A 2001, 72, 167–186. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, H.; Ding, P. Recent Development of element partial substitution to improve the characteristics of Mg–Ni-based hydrogen storage alloys. Mater. Rev. 2004, 18, 29–33. (In Chinese) [Google Scholar] [CrossRef]
- Goo, N.; Lee, K. The electrochemical hydriding properties of Mg–Ni–Zr amorphous alloy. Int. J. Hydrogen Energy 2002, 27, 433–438. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, H.; Qiao, L.; Liu, Q. Preparation and electrochemical characteristics of (M = Cr, A1, Ti, Zr). Acta Sci. Nat. Univ. Nankaiensis (Nat. Sci. Ed.) 2005, 38, 74–79. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.T.; Wan, C.B.; Wang, R.L.; Meng, X.H.; Huang, M.F.; Ju, X. Effect of Cr substitution by Ni on the cycling stability of alloy using EXAFS. Int. J. Hydrogen Energy 2014, 39, 14858–14867. [Google Scholar] [CrossRef]
- Liang, G.X.; Boily, S.; Huot, J.; Van Neste, A.; Schulz, R. Hydrogen storage properties of nanocrystalline synthesized by mechanical alloying. Mater. Sci. Forum 1998, 269–272, 1049–1054. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ma, Z.; Guo, S.; Qi, Y.; Wang, X. Improved hydrogen storage behaviours of nanocrystalline and amorphous -type alloy by Mn substitution for Ni. Int. J. Hydrogen Energy 2010, 35, 11966–11974. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ding, X.; Liu, D.; Zhang, Q.; Si, T. Microstructure characterization and hydrogen storage properties study of (M = Ti, V, Fe or Si) alloys. Prog. Nat. Sci. Mater. Int. 2018, 28, 464–469. [Google Scholar] [CrossRef]
- Huang, L.W.; Elkedim, O.; Jarzebski, M.; Hamzaoui, R.; Jurczyk, M. Structural characterization and electrochemical hydrogen storage properties of (x=0, 0.125, 0.25, 0.375) alloys prepared by mechanical alloying. Int. J. Hydrogen Energy 2010, 35, 6794–6803. [Google Scholar] [CrossRef]
- Huang, L.W.; Elkedim, O.; Moutarlier, V. Synthesis and characterization of nanocrystalline prepared by mechanical alloying: Effects of substitution of Mn for Ni. J. Alloy. Compd. 2010, 504, S311–S314. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, H.; Ji, J.; Sun, H.; Zhou, Z.; Zhang, Y. Characteristics of (M=Ti, Cr, Mn, Fe, Co, Ni, Cu and Zn) alloys after surface treatment. J. Alloy. Compd. 2002, 330–332, 640–644. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Yuan, H.; Yang, E.; Zhou, Z.; Song, D. Dehydriding properties of ternary Mg2Ni(1 −x)Zrx hydrides synthesized by ball milling and annealing. J. Alloy. Compd. 1998, 269, 278–283. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Tessier, P.; Ström-Olsen, J.O.; Schulz, R. Catalytic effect of Pd on hydrogen absorption in mechanically alloyed , and FeTi. J. Alloy. Compd. 1995, 217, 295–300. [Google Scholar] [CrossRef]
- Janot, R.; Aymard, L.; Rougier, A.; Nazri, G.A.; Tarascon, J.M. Fast hydrogen sorption kinetics for ball-milled alloys☆. J. Phys. Chem. Solids 2004, 65, 529–534. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, T.; Fu, J.; Pan, F.; Deng, Sh.; Li, J.; Zhao, G. The effect of Cu addition on hydrogenation properties of alloy. J. Funct. Mater. 2007, 38, 952–954. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Fu, J.; Guo, Q.; Pan, F.; Deng, Sh.; Li, J.; Zhao, G. Hydrogen storage properties of (x=0, 0.2, 0.4) synthesized by induction melting followed by ball milling. Rare Met. Mat. Eng. 2010, 39, 149–152. (In Chinese) [Google Scholar]
- Wang, X.; Tu, J.; Zhang, X.; Rong, W.; Chen, Ch. Hydrogen storage properties of tetragonal nanocrystalline alloy. Chin. J. Nonferr. Met. 2004, 14, 1020–1024. (In Chinese) [Google Scholar] [CrossRef]
- Duan, R. Synthesis and hydrogen storage properties of Mg-based type Mg(2 − x)AlxNi(1 − y)Coy hydrogen storage alloys. Master’s Thesis, Inner Mongolia Normal University, Huhhot, China, May 2017. (In Chinese). [Google Scholar]
- Sun, H.; Feng, D.; Zhang, Y.; Ren, H. Gas hydrogen absorption and electrochemical properties of alloys improved by Y substitution, ball milling and Ni addition. Int. J. Hydrogen Energy 2018, 44, 5382–5388. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Kong, X.; Ding, Y.; Zhang, R.; Tang, J. Thermal stability of the -based hydrogen storage alloy doped Ti element. Int. J. Heat Technol. 2016, 34, 245–250. [Google Scholar] [CrossRef]
- Liu, Zh.; Zhu, Y.; Yang, Y.; Gu, H.; Li, L. Influence of vanadium on hydrogen storage properties of Mg-Based alloys. Chin. J. Rare Met. 2010, 34, 807–811. (In Chinese) [Google Scholar]
- Li, Y.; Hou, H.; Zhao, Y.; Yang, L.; Wang, N. Effects of Ti alloying on the hydrogen storage alloy. Special Casting & Nonferrous Alloy. 2016, 36, 341–344. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Zh.; Zhao, D.; Hou, Zh.; Yong, H.; Zhang, Y.; Wang, X. Hydrogen storage characteristics of ball-milling +xwt%Ti (x=0,1,3,5) alloys. Met. Funct. Mater. 2011, 18, 15–19. (In Chinese) [Google Scholar]
- Wang, H.; Nian, H.; Ren, X.; Li, X.; Zhou, Y. Hydrogen desorption properties of reactive ball milled Mg-Ni-M (M =Ni, Nb, Y, Ti) composite materials. Powder Metall. Technol. 2011, 29, 259–262. (In Chinese) [Google Scholar]
- Grigorova, E.; Khristov, M.; Khrussanova, M.; Bobet, J.L.; Peshev, P. Effect of additives on the hydrogen sorption properties of mechanically alloyed composites based on Mg and Ni. Int. J. Hydrogen Energy 2005, 30, 1099–1105. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Lin, G.; Zhou, G.; Zhang, J.; Lu, X. Hydriding reaction kinetics of Mg–Mg2Ni(1 − x)Mex compositions. Chin. J. Nonferr. Met. 2008, 18, 873–878. (In Chinese) [Google Scholar] [CrossRef]
- Sun, H.; Feng, D.; Ren, H.; Zhang, Y. Hydrogen storage properties of Mg22Y2Ni10Cu2+x% Ni composite prepared by ball milling. Chin. J. Rare Met. 2018, 42, 14–20. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Lei, Y.; Wang, Q. The reduction of cycling capacity degradation of Mg–Ni-based electrode alloys by Fe substitution. Int. J. Hydrogen Energy 2002, 27, 501–506. [Google Scholar] [CrossRef]
- Ye, H.; Chen, L.; Lei, Y. Effect of nickel on the structure and electrochemical properties of mechanically alloyed Mg–Ni based binary hydrogen storage alloys. Rare Met. Mat. Eng. 2000, 29, 193–196. (In Chinese) [Google Scholar] [CrossRef]
- Bhatnagar, A.; Johnson, J.K.; Shaz, M.A.; Srivastava, O.N. TiH2 as a dynamic additive for improving the de/rehydrogenation properties of MgH2: A combined experimental and theoretical mechanistic investigation. J. Phys. Chem. C 2018, 122, 21248–21261. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Li, Y.; Zhang, Q. Enhanced hydrogen storage kinetics of an Mg–Pr–Al composite by in situ formed Pr3Al11 nanoparticles. Dalton Trans. 2019, 48, 7735–7742. [Google Scholar] [CrossRef] [PubMed]
- Korablov, D.; Besenbacher, F.; Jensen, T.R. Kinetics and thermodynamics of hydrogenation-dehydrogenation for Mg-25%TM (TM=Ti, Nb or V) composites synthesized by reactive ball milling in hydrogen. Int. J. Hydrogen Energy 2018, 43, 16804–16814. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm=Ti, V, Mn, Fe and Ni) systems. J. Alloy. Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Polanski, M.; Nawra, D.; Zasada, D. Mg2FeH6 synthesized from plain steel and magnesium hydride. J. Alloy. Compd. 2019, 776, 1029–1040. [Google Scholar] [CrossRef]
- Khan, D.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of nanocrystalline Mg2Ni prepared from compressed 2MgH2–Ni powder. Int. J. Hydrogen Energy 2018, 43, 22391–22400. [Google Scholar] [CrossRef]
- Park, H.R.; Kwon, S.N.; Song, M.Y. Effects of milling time on the hydrogen storage properties of Mg-based transition metals-added alloys. Mater Sci. 2018, 24, 166–171. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH2–V nanocomposite. J. Alloy. Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]
- Pelletier, J.F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A.R.; Lurio, L.B.; Mochrie, S.G.J. Hydrogen desorption mechanism in MgH2–Nb nanocomposites. Phys. Rev. B 2001, 63, 811–820. [Google Scholar] [CrossRef]
- Huot, J.; Pelletier, J.F.; Liang, G.; Sutton, M.; Schulz, R. Structure of nanocomposite metal hydrides. J. Alloy. Compd. 2002, 330–332, 727–731. [Google Scholar] [CrossRef]
- Huot, J.; Pelletier, J.F.; Lurio, L.B.; Sutton, M.; Schulz, R. Investigation of dehydrogenation mechanism of MgH2–Nb nanocomposites. J. Alloy. Compd. 2003, 348, 319–324. [Google Scholar] [CrossRef]
- De Castro, J.F.R.; Santos, S.F.; Costa, A.L.M.; Yavari, A.R.; Botta, W.J.; Ishikawa, T.T. Structural characterization and dehydrogenation behavior of Mg–5at%Nb nanocomposite processed by reactive milling. J. Alloy. Compd. 2004, 376, 251–256. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, G.; Zhu, Z.; Zheng, Q. Hydrogen Storage Properties and mechanism of Mg–In–Zn ternary solid solution by ball milling. J. Lujiang Univ. 2014, 22, 17–21. (In Chinese) [Google Scholar] [CrossRef]
- Janot, R.; Rougier, A.; Aymard, L.; Lenain, C.; Herrera-Urbina, R.; Nazri, G.A.; Tarascon, J.M. Enhancement of hydrogen storage in MgNi by Pd-coating. J. Alloy. Compd. 2003, 356–357, 438–441. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, I.; Bobet, J.L.; Song, M. Effect on the H2-sorption properties of Mg of Co (with various sizes) and CoO addition by reactive grinding. J. Alloy. Compd. 2004, 366, 279–288. [Google Scholar] [CrossRef]
- Luo, Y.; Meng, Ch.; Xu, Sh.; Feng, C.; Kang, L. Microstructure and hydrogen storage performance of alloys MgxTi(100 − x) prepared by high-energy ball milling. J. Lanzhou Univ. Technol. 2011, 37, 9–13. (In Chinese) [Google Scholar] [CrossRef]
- Ying, Y.; Zeng, X.; Chang, J.; Zou, J.; Ding, W. Research progress of catalysts for Mg-based hydrogen storage materials. Mater. Rev. 2011, 25, 134–138. (In Chinese) [Google Scholar]
- Liu, M.; Zhang, M.; Huo, Y.; Yan, Sh.; Zhang, H.; Sun, G. Study on modification of Mg-based hydrogen storage materials. Mater. Res. Appl. 2013, 7, 143–146. (In Chinese) [Google Scholar] [CrossRef]
- Chen, M.; Xiao, X.; Zhang, M.; Liu, M.; Huang, X.; Zheng, J.; Zhang, Y.; Jiang, L.; Chen, L. Excellent synergistic catalytic mechanism of in-situ formed nanosized Mg2Ni and multiple valence titanium for improved hydrogen desorption properties of magnesium hydride. Int. J. Hydrogen Energy 2019, 44, 1750–1759. [Google Scholar] [CrossRef]
- Liu, P.; Chen, H.; Yu, H.; Liu, X.; Jiang, R.; Li, X.; Zhou, S. Oxygen vacancy in magnesium/cerium composite from ball milling for hydrogen storage improvement. Int. J. Hydrogen Energy 2019, 44, 13606–13612. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Zhang, X.; Leng, Z.; Gao, M.; Hu, J.; Du, F.; Yao, J.; Pan, H.; Liu, Y. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2. J. Power Sour. 2018, 398, 183–192. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Nasrallah, E.; Banyan, M.; Al-Ajmi, F. Bulk nanocomposite MgH2/10wt%(8 Nb2O5/2 Ni) solid-hydrogen storage system for fuel cell applications. Int. J. Hydrogen Energy 2018, 43, 23382–23396. [Google Scholar] [CrossRef]
- Daryani, M.; Simchi, A.; Sadati, M.; Hosseini, H.M.; Targholizadeh, H.; Khakbiz, M. Effects of Ti-based catalysts on hydrogen desorption kinetics of nanostructured magnesium hydride. Int. J. Hydrogen Energy 2014, 39, 21007–21014. [Google Scholar] [CrossRef]
- AGUEYZINSOU, K.; ARESFERNANDEZ, J.; KLASSEN, T.; BORMANN, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrogen Energy 2007, 32, 2400–2407. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloy. Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.M.; Zhang, H.F.; Ding, B.Z.; Hu, Z.Q. Hydrogenation characteristics of Mg–TiO2 (rutile) composite. J. Alloy. Compd. 2000, 313, 218–223. [Google Scholar] [CrossRef]
- Kwon, I.H.; Bobet, J.L.; Bae, J.S.; Song, M.Y. Improvement of hydrogen-storage properties of Mg by reactive mechanical grinding with Fe2O3. J. Alloy. Compd. 2005, 396, 264–268. [Google Scholar] [CrossRef]
- Dehouche, Z.; Klassen, T.; Oelerich, W.; Goyette, J.; Bose, T.K.; Schulz, R. Cycling and thermal stability of nanostructured MgH2∓Cr2O3 composite for hydrogen storage. J. Alloy. Compd. 2002, 347, 319–323. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. J. Alloy. Compd. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Jung, K.S.; Lee, E.Y.; Lee, K.S. Catalytic effects of metal oxide on hydrogen absorption of magnesium metal hydride. J. Alloy. Compd. 2006, 421, 179–184. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, Zh. Cyclic hydrogen storage properties of Mg milled with nickel nano-powders and MnO2. J. Alloy. Compd. 2007, 443, 121–124. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Hydrogen sorption of nanocrystalline Mg at reduced temperatures by metal-oxide catalysts. Adv. Eng. Mater. 2001, 3, 487–490. [Google Scholar] [CrossRef]
- Castro, F.J.; Bobet, J.L. Hydrogen sorption properties of an Mg + WO3 mixture made by reactive mechanical alloying. J. Alloy. Compd. 2004, 366, 303–308. [Google Scholar] [CrossRef]
- Yu, Zh.; Wang, E.; Liu, Z. Hydorgen storage properties of nanocomposite Mg–Ni–V2O5. J. Funct. Mater. 2002, 33, 280–282. (In Chinese) [Google Scholar] [CrossRef]
- Yu, Zh.; Wang, E.; Liu, Z.; Xian, H. Properties of hydriding and dehydriding of nanocrystalline composite of Mg–Ni–Cr2O3. Chin. J. Nonferr. Met. 2002, 12, 743–748. (In Chinese) [Google Scholar] [CrossRef]
- Song, M.; Bobet, J.L.; Darriet, B. Improvement in hydrogen sorption properties of Mg by reactive mechanical grinding with Cr2O3, Al2O3 and CeO2. J. Alloy. Compd. 2002, 340, 256–262. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloy. Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Friedrichs, O.; Sanchez-Lopez, J.C.; López-Cartes, C.; Klassen, T.; Bormann, R.; Fernández, A. Nb2O5 “Pathway Effect” on Hydrogen Sorption in Mg. J. Phys. Chem. B 2006, 110, 7845–7850. [Google Scholar] [CrossRef] [PubMed]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Bhat, V.V.; Rougier, A.; Aymard, L.; Darok, X.; Nazri, G.; Tarascon, J.M. Catalytic activity of oxides and halides on hydrogen storage of MgH2. J. Power Sour. 2006, 159, 107–110. [Google Scholar] [CrossRef]
- Yahya, M.S.; Sulaiman, N.N.; Mustafa, N.S.; Halim Yap, F.A.; Ismail, M. Improvement of hydrogen storage properties in MgH2 catalysed by K2NiF7. Int. J. Hydrogen Energy 2018, 43, 14532–14540. [Google Scholar] [CrossRef]
- Kim, J.W.; Ahn, J.P.; Jin, S.A.; Lee, S.H.; Chung, H.S.; Shim, J.H.; Cho, Y.W.; Oh, K.H. Microstructural evolution of NbF2-doped MgH2 exhibiting fast hydrogen sorption kinetics. J. Power Sour. 2008, 178, 373–378. [Google Scholar] [CrossRef]
- Peng, S.; Xiao, X.; Xu, R.; Li, L.; Wu, F.; Li, S.; Wang, Q.; Chen, L. Hydrogen storage behaviors and microstructure of MF3 (M=Ti, Fe)-doped magnesium hydride. Trans. Nonferr. Met. Soc. 2010, 20, 1879–1884. [Google Scholar] [CrossRef]
- Yin, Y.; Li, B.; Yuan, Z.; Qi, Y.; Zhang, Y. A comparison of TiF3 and NbF5 catalytic effects on hydrogen absorption and desorption kinetics of a ball-milled Mg85Zn5Ni10 alloy. RSC Adv. 2018, 8, 34525–34535. [Google Scholar] [CrossRef]
- Jin, S.A.; Shim, J.H.; Cho, Y.W.; Yi, K.W. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides. J. Power Sour. 2007, 172, 859–862. [Google Scholar] [CrossRef]
- Jin, S.A.; Shim, J.H.; Ahn, J.P.; Cho, Y.W.; Yi, K.W. Improvement in hydrogen sorption kinetics of MgH2 with Nb hydride catalyst. Acta Mater. 2007, 55, 5073–5079. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z. Particle size, grain size and γ-MgH2 effects on the desorption properties of nanocrystalline commercial magnesium hydride processed by controlled mechanical milling. Nanotechnology 2006, 17, 3856–3865. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Ma, L.; Cheng, H. Hydrogen sorption kinetics of MgH2 catalyzed with NbF5. J. Alloy. Compd. 2008, 453, 138–142. [Google Scholar] [CrossRef]
- Recham, N.; Bhat, V.V.; Kandavel, M.; Aymard, L.; Tarascon, J.M.; Rougier, A. Reduction of hydrogen desorption temperature of ball-milled MgH2 by NbF5 addition. J. Alloy. Compd. 2008, 464, 377–382. [Google Scholar] [CrossRef]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with magnesium hydride. Int. J. Hydrogen Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- De Castro, J.F.R.; Yavari, A.R.; LeMoulec, A.; Ishikawa, T.T.; Botta, F.W.J. Improving H-sorption in MgH2 powders by addition of nanoparticles of transition metal fluoride catalysts and mechanical alloying. J. Alloy. Compd. 2005, 389, 270–274. [Google Scholar] [CrossRef]
- Deledda, S.; Borissova, A.; Poinsignon, C.; Botta, W.J.; Dornheim, M.; Klassen, T. H-sorption in MgH2 nanocomposites containing Fe or Ni with fluorine. J. Alloy. Compd. 2005, 404–406, 409–412. [Google Scholar] [CrossRef]
- Malka, I.E.; Pisarek, M.; Czujko, T.; Bystrzycki, J. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH2 sorption properties. Int. J. Hydrogen Energy 2011, 36, 12909–12917. [Google Scholar] [CrossRef]
- Ma, L.P.; Kang, X.D.; Dai, H.B.; Liang, Y.; Fang, Z.Z.; Wang, P.J.; Wang, P.; Cheng, H.M. Superior catalytic effect of TiF3 over TiCl3 in improving the hydrogen sorption kinetics of MgH2: Catalytic role of fluorine anion. Acta Mater. 2009, 57, 2250–2258. [Google Scholar] [CrossRef]
- Ivanov, E.; Konstanchuk, I.; Bokhonov, B.; Boldyrev, V.V. Hydrogen interaction with mechanically alloyed magnesium-salt composite materials. J. Metastab. Nanocryst. Mater. 2003, 15–16, 579–584. [Google Scholar] [CrossRef]
- Tian, M.; Shang, C. Mg-based composites for enhanced hydrogen storage performance. Int. J. Hydrogen Energy 2019, 44, 338–344. [Google Scholar] [CrossRef]
- Thongtan, P.; Dansirima, P.; Thiangviriya, S.; Thaweelap, N.; Suthummapiwat, A.; Plerdsranoy, P.; Utke, R. Reversible hydrogen sorption and kinetics of hydrogen storage tank based on MgH2 modified by TiF4 and activated carbon. Int. J. Hydrogen Energy 2018, 43, 12260–12270. [Google Scholar] [CrossRef]
- Alsabawi, K.; Webb, T.A.; Gray, E.M.; Webb, C.J. The effect of C60 additive on magnesium hydride for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 10508–10515. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Improvement of hydrogen storage properties of MgH2 catalyzed by K2NiF7 and multiwall carbon nanotube. J. Phys. Chem. C 2018, 122, 11222–11233. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.M.; Beregovsky, M.; Sezen, M.; Amouyal, Y.; Rabkin, E. Hydrogen storage and thermal transport properties of pelletized porous Mg-2 wt.% multiwall carbon nanotubes and Mg-2 wt.% graphite composites. Int. J. Hydrogen Energy 2016, 41, 14461–14474. [Google Scholar] [CrossRef]
- Rud, A.D.; Lakhnik, A.M. Effect of carbon allotropes on the structure and hydrogen sorption during reactive ball-milling of Mg–C powder mixtures. Int. J. Hydrogen Energy 2012, 37, 4179–4187. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.M.; Amouyal, Y.; Rabkin, E. Tuning the thermal conductivity of hydrogenated porous magnesium hydride composites with the aid of carbonaceous additives. Int. J. Hydrogen Energy 2017, 42, 22395–22405. [Google Scholar] [CrossRef]
- Spassov, T.; Zlatanova, Z.; Spassova, M.; Todorova, S. Hydrogen sorption properties of ball-milled Mg–C nanocomposites. Int. J. Hydrogen Energy 2010, 35, 10396–10403. [Google Scholar] [CrossRef]
- Alsabawi, K.; Gray, E.M.; Webb, C.J. The effect of ball-milling gas environment on the sorption kinetics of MgH2 with/without additives for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 2976–2980. [Google Scholar] [CrossRef]
- Yu, Zh.; Sun, H.; Wang, E.; Liang, J.; Fang, W. Hydrogen storage properties of Mg-based materials with CNTs. Chin. J. Nonferr. Met. 2005, 15, 876–881. (In Chinese) [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, S.; Yan, Y. Ball milling process and its effect on hydrogen adsorption storage of MWNTS. Chin. J. Process. Eng. 2006, 6, 837–840. (In Chinese) [Google Scholar] [CrossRef]
- Chen, D.; Chen, L.; Liu, S.; Ma, C.X.; Chen, D.M.; Wang, L.B. Microstructure and hydrogen storage property of Mg/MWNTs composites. J. Alloy. Compd. 2004, 372, 231–237. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Study of the activation process of Mg-based hydrogen storage materials modified by graphite and other carbonaceous compounds. J. Mater. Res. 2001, 16, 2893–2905. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride. J. Alloy. Compd. 2006, 414, 259–264. [Google Scholar] [CrossRef]
- Wu, C.; Wang, P.; Yao, X.; Liu, C.; Chen, D.; Lu, G.Q.; Cheng, H. Effects of SWNT and metallic catalyst on hydrogen absorption/desorption performance of MgH2. J. Phys. Chem. B 2005, 109, 22217–22221. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Hydrogen storage properties of MgH2/SWNT composite prepared by ball milling. J. Alloy. Compd. 2006, 420, 278–282. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, M.; Ma, H.; Zhang, T.; Zhang, G. The effect of microlitic carbon in Mg-based composite hydrogen-stored materials. J. Shandong Univ. Sci. Technol. 2009, 28, 54–57. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Li, T.; Wang, N.; Chen, H.; Zhang, T.; Yu, H.; Niu, H.; Liu, D. Nano-confined magnesium for hydrogen storage from reactive milling with anthracite carbon as milling aid. Int. J. Hydrogen Energy 2014, 39, 13628–13633. [Google Scholar] [CrossRef]
- Lu, G.; Tang, D. Preparation of Mg–C nanocomposite study on phase transformation rate of hydrogen desorption. Mater. Rev. 2009, 23, 28–30. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, P.; Mao, C.; Zhou, D. Influence and micro-mechanism of carbon materials doping on dehydrogenation properties of magnesium based hydride. Chin. J. Nonferr. Met. 2015, 25, 2464–2470. (In Chinese) [Google Scholar]
- Yao, X.; Wu, C.Z.; Wang, H.; Cheng, H.M.; Lu, G.Q. Effects of carbon nanotubes and metal catalysts on hydrogen storage in magnesium nanocomposites. J. Nanosci. Nanotechnol. 2006, 6, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wu, C.; Du, A.; Zou, J.; Zhu, Z.; Wang, P.; Cheng, H.; Smith, S.; Lu, G. Metallic and carbon nanotube-catalyzed coupling of hydrogenation in magnesium. J. Am. Chem. Soc. 2007, 129, 15650–15654. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cheng, H.M. Effects of carbon on hydrogen storage performances of hydrides. J. Mater. Chem. 2010, 20, 5390–5400. [Google Scholar] [CrossRef]
- Yao, X.; Wu, C.; Du, A.; Liu, G.Q.; Cheng, H.; Smith, S.C.; Zou, J.; He, Y. Mg-based nanocomposites with high capacity and fast kinetics for hydrogen storage. J. Phys. Chem. B 2006, 110, 11697–11703. [Google Scholar] [CrossRef]
- Li, W.; Hu, S.; Hao, Y.; Dai, J.; Wang, Q. Hydrogen storage property of Mg–Ni–CNTs composites. J. Gansu Sci. 2009, 21, 54–56. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Ritter, J.A. Kinetic behavior of Ti-doped NaAlH4 when cocatalyzed with carbon nanostructures. J. Phys. Chem. B 2006, 110, 17353–17358. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yuan, T.; Yang, J.; Gao, M.; Pan, H.; Liu, Y.; Zheng, S. In situ formation of Al3Ti, MgF2 and Al and their superior synergetic effects on reversible hydrogen storage of MgH2. Catal. Today 2017, 318, 107–112. [Google Scholar] [CrossRef]
- Cermak, J.; Kral, L.; Roupcova, P. Improved hydrogen sorption kinetics in Mg modified by chosen catalysts. Int. J. Hydrogen Energy 2019, 44, 8315–8324. [Google Scholar] [CrossRef]
- Lototskyy, M.; Goh, J.; Davids, M.W.; Linkov, V.; Khotseng, L.; Ntsendwana, B.; Denys, R.; Yartys, V.A. Nanostructured hydrogen storage materials prepared by high-energy reactive ball milling of magnesium and ferrovanadium. Int. J. Hydrogen Energy 2019, 44, 6687–6701. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Z.; Jian, N.; Hu, J.; Du, F.; Yao, J.; Gao, M.; Liu, Y.; Pan, H. A novel complex oxide TiVO3.5 as a highly active catalytic precursor for improving the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2018, 43, 23327–23335. [Google Scholar] [CrossRef]
- Pighin, S.A.; Capurso, G.; Lo Russo, S.; Peretti, H.A. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloy. Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- Zaranski, Z.; Czujko, T. The influence of ball milling process on hydrogenation properties of MgH2–FeTiHx composites. J. Alloy. Compd. 2011, 509, S608–S611. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Ajmi, F.; Banyan, M. Mechanically-induced catalyzation of MgH2 powders with Zr2Ni-Ball Milling Media. Catalysts 2019, 9, 382. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Yao, Z.; Ji, L.; Ze, S.; Yan, N.; Zhang, B.; Xiao, B.; Du, J.; Zhu, X.; et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- Santos, S.F.; Ishikawa, T.T.; Botta, W.J.; Huot, J. MgH2+FeNb nanocomposites for hydrogen storage. Mater. Chem. Phys. 2014, 147, 557–562. [Google Scholar] [CrossRef]
- Zhou, C.S.; Fang, Z.G.Z.; Sun, P.; Xu, L.; Liu, Y. Capturing low-pressure hydrogen using Ve-Ti-Cr catalyzed magnesium hydride. J. Power Sour. 2019, 423, 139–147. [Google Scholar] [CrossRef]
- Li, F.; Jiang, L.; Du, J.; Wang, Sh.; Liu, X.; Zhan, F. Synthesis and hydrogenation properties of Mg–La–Ni–H system by reactive mechanical alloying. Int. J. Hydrogen Energy 2006, 31, 581–585. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage in mechanically milled Mg–LaNi5 and MgH2∓LaNi5 composite. J. Alloy. Compd. 2000, 297, 261–265. [Google Scholar] [CrossRef]
- Fu, Y.; Groll, M.; Mertz, R.; Kulenovic, R. Effect of LaNi5 and additional catalysts on hydrogen storage properties of Mg. J. Alloy. Compd. 2008, 460, 607–613. [Google Scholar] [CrossRef]
- Zhou, Ch.; Fang, Z.Z.; Ren, Ch.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Ren, Ch.; Fang, Z.Z.; Zhou, Ch.; Lu, J.; Ren, Y.; Zhang, X. Hydrogen storage properties of magnesium hydride with V-based additives. J. Phys. Chem. C 2014, 118, 21778–21784. [Google Scholar] [CrossRef]
- Molinas, B.; Ghilarducci, A.A.; Melnichuk, M.; Corso, H.L.; Peretti, H.A.; Agresti, F.; Bianchin, A.; Lo Russo, S.; Maddalena, A.; Principi, G. Scaled-up production of a promising Mg-based hydride for hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 4597–4601. [Google Scholar] [CrossRef]
- Makihara, Y.; Umeda, K.; Shoji, F.; Kato, K.; Miyairi, Y. Cooperative dehydriding mechanism in a mechanically milled Mg–50mass% ZrMn2 composite. J. Alloy. Compd. 2008, 455, 385–391. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.; Zhang, H.; Ding, B.; Hu, Z. Hydriding properties of a mechanically milled Mg–50wt.% ZrFe1.4Cr0.6 composite. J. Alloy. Compd. 2000, 297, 240–245. [Google Scholar] [CrossRef]
- Wang, Zh.; Gu, Zh.; Cheng, G.; Cheng, J. Influences of the factors on electrochemical characteristics of Mg2Ni alloys. Electr. Eng. Mater. 2003, 3, 40–46. (In Chinese) [Google Scholar] [CrossRef]
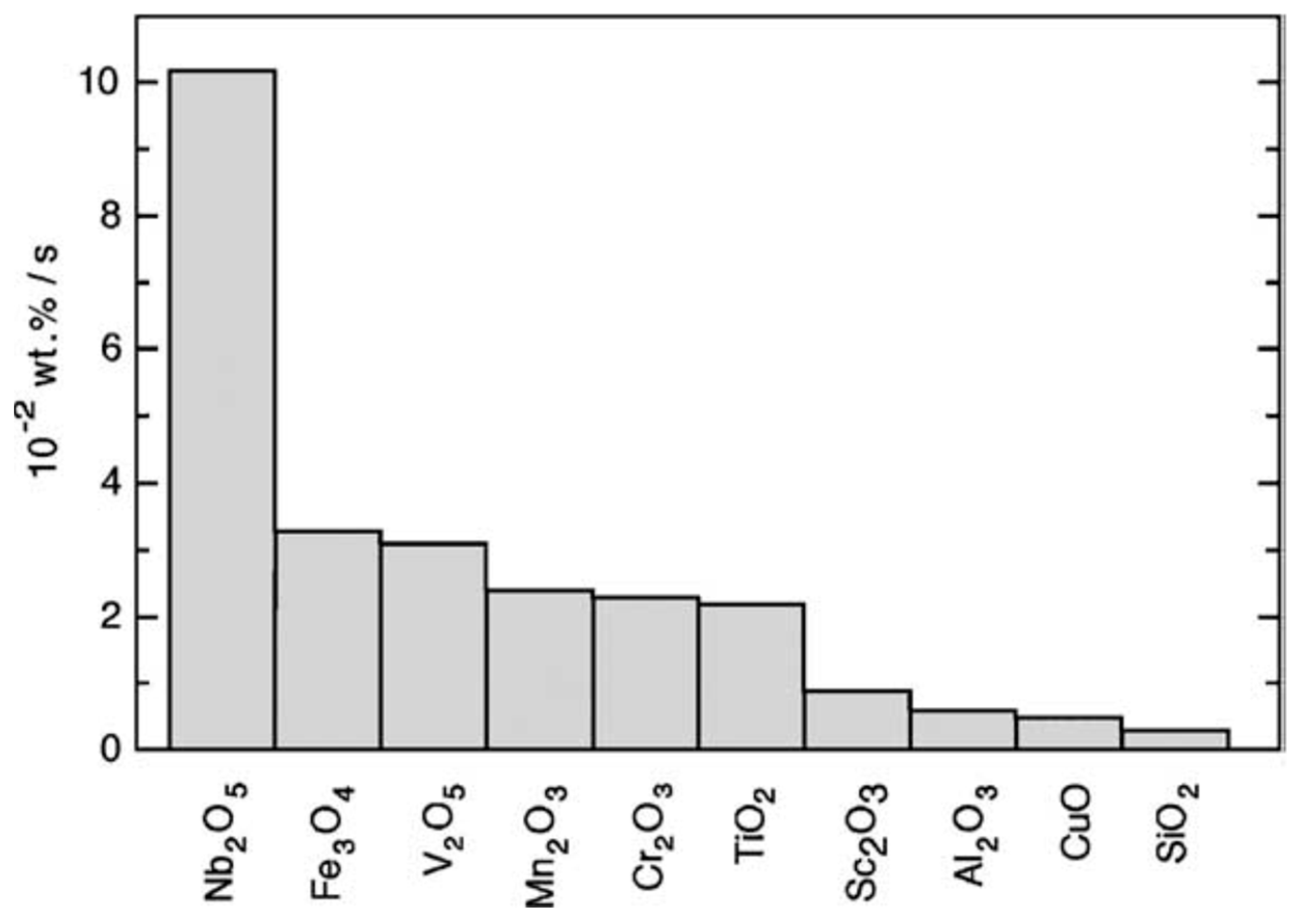

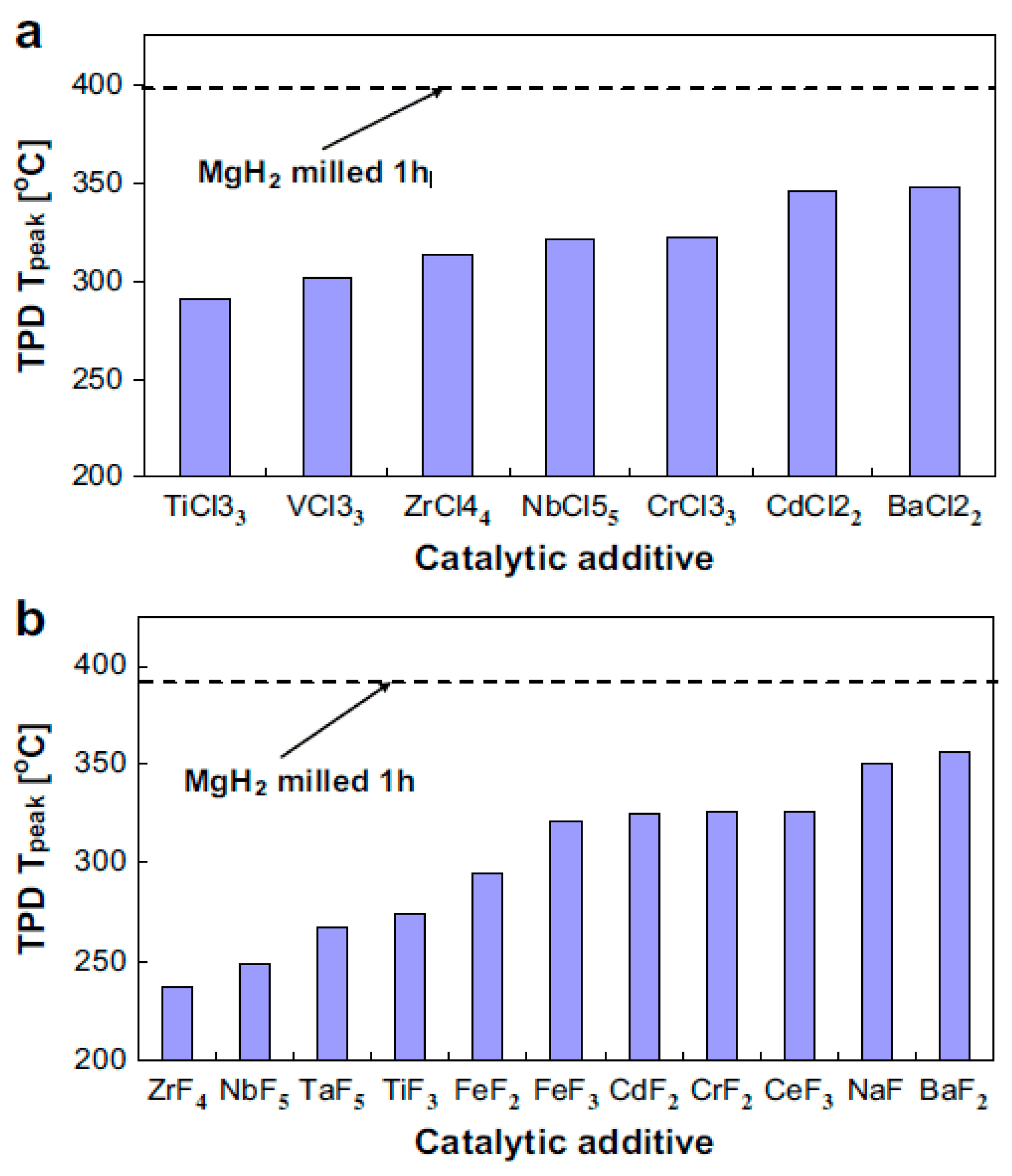

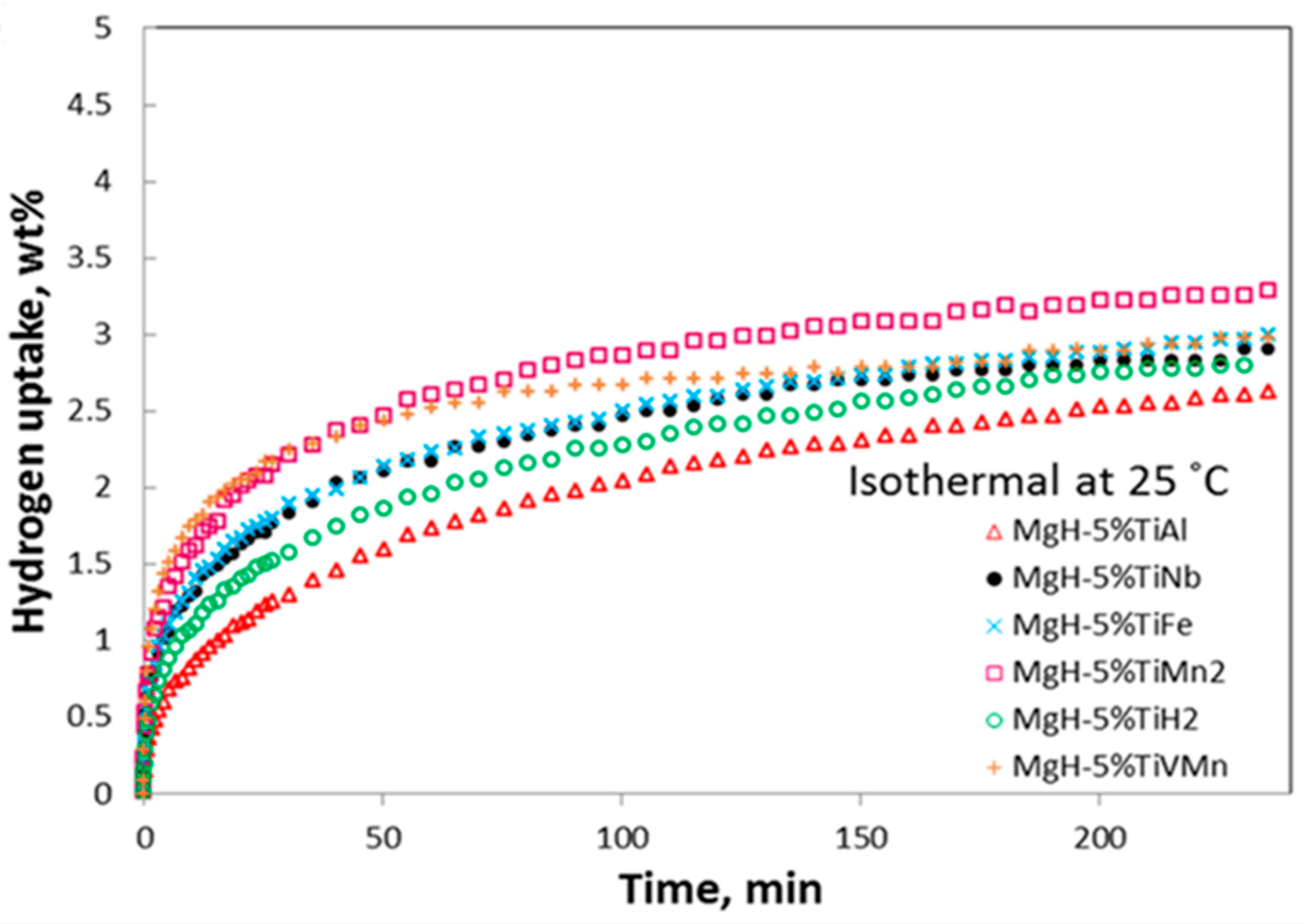
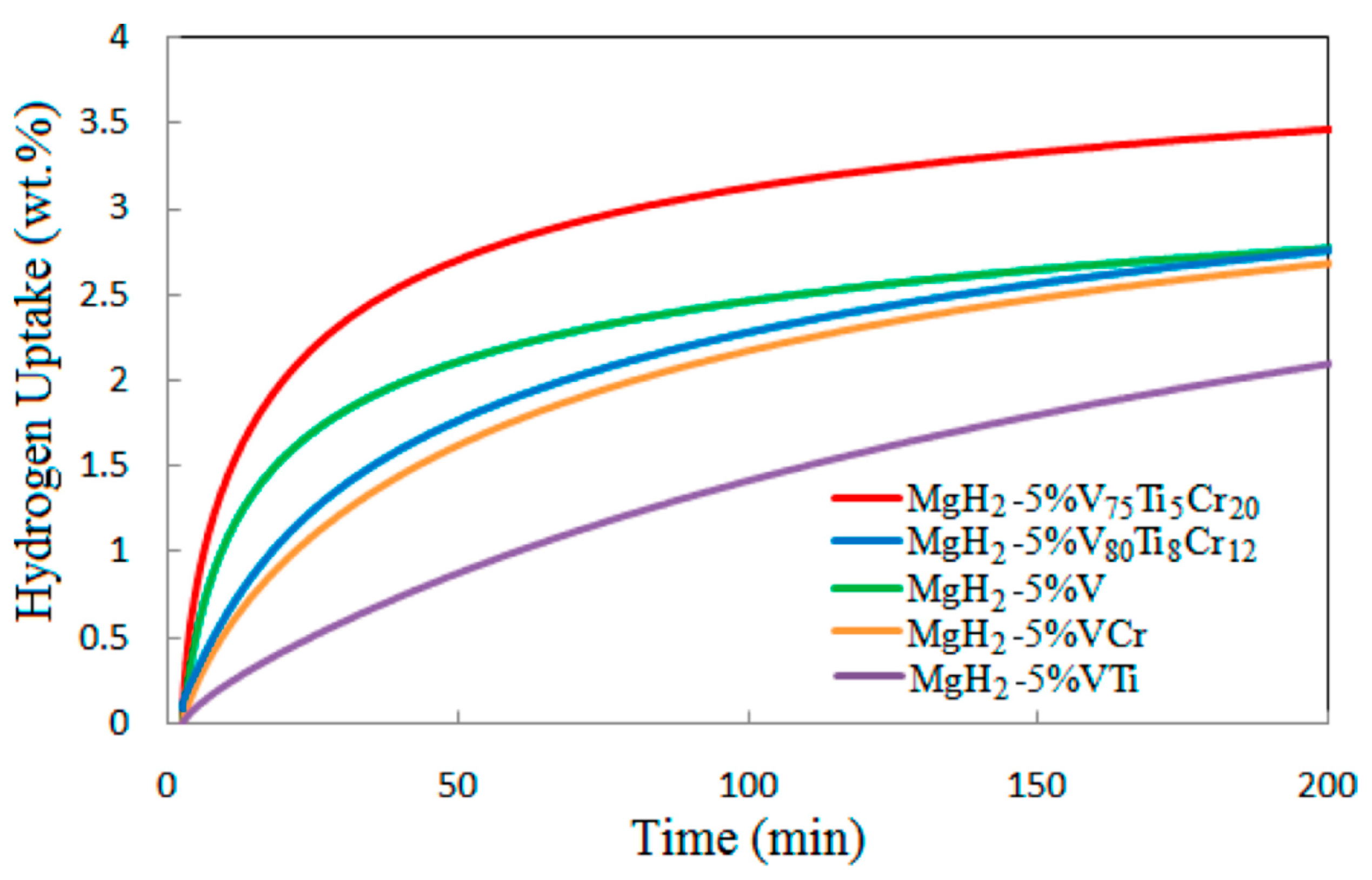
| Material | Rotation Speed, rpm | Time, h | Atmosphere | Ball-to-Powder Weight Ratio | Hydrogen Storage Properties | Ref. |
|---|---|---|---|---|---|---|
| Mg1.8Ag0.2Ni | 400 | 2 | 0.2 MPa Ar | - | Abs: 2.5wt%/0.2 atm/200 °C ΔHabs = −51.96 kJ·mol−1·H2 ΔSabs = −2.24 J mol−1·K−1 H2 | [45] |
| Mg2Ni0.9Zr0.1 | 300 | 2 | Ar | 20:1 | Des: 3.25wt%/15 min/1 atm /340 °C Desorption equilibrium pressure (340 °C): 9.0 atm Des temperature: 251 °C ΔHabs = −62.7 kJ·mol−1·H2 | [65] |
| Mg2Ni0.7Zr0.3 | 300 | 2 | Ar | 20:1 | Des: 3.25wt%/60 min/1 atm /340 °C Desorption equilibrium pressure (340 °C): 10.1 atm Des temperature: 248 °C ΔHabs = −59.8 kJ·mol−1·H2 | [65] |
| Mg2Ni | - | 72 | Ar | 8:1 | Abs: 3.6wt%/10 h/150 °C Des: 3.0wt%/10 h/150 °C Absorption equilibrium pressure (150 °C): 2 bar Desorption equilibrium pressure (150 °C): 0.5 bar | [67] |
| Mg2Ni0.9Cu0.1 | 600 | 60 | Ar | 20:1 | Abs: 2.5wt%/100 s/400 °C | [68] |
| Mg2Ni0.7Cu0.3 | 600 | 60 | Ar | 20:1 | Abs: 2.3wt%/100 s/400 °C Desorption equilibrium pressure (400 °C): 0.13 MPa | [68] |
| Mg1.95Ti0.05Ni0.8Cr0.2 | 280 | 80 | Ar | 10:1 | Abs: 3.12wt%/2 min/4.0 MPa/393 K | [70] |
| Mg–10wt%(1/4Y–3/4Ni) | 225 | Ar 2 h H2 30 h | 40:1 | Des: 3wt%/300 °C | [77] | |
| Material | Rotation Speed, rpm | Time, h | Atmosphere | Ball-to-Powder Weight Ratio | Hydrogen Storage Properties | Ref. |
|---|---|---|---|---|---|---|
| Mg–10.7wt%Ti–10.9wt%Al–0.7wt%Zr–0.1wt%C | 400 | 12 | Ar | 60:1 | Abs: 4.17wt%/10 min/1.5 MPa/573 K Des: 4.7wt%/20 min/0.0005 MPa/573 K ΔHdes = 74.9 kJ·mol−1·H2 ΔSdes = 136.6 J mol−1·K−1 H2 ΔHdes = 75.9 kJ·mol−1·H2 ΔSdes = 137.5 J mol−1·K−1 H2 | [11] |
| MgH2–5wt%TiH2 | 320 | 30 | - | 50:1 | Abs: 5.8wt%/2.5 min /15 atm/300 °C Des: 5.9wt%/20 min/1 atm/300 °C Full Des: 6.0wt%/374 °C Des temperature: 260 °C | [83] |
| MgH2 | 320 | 30 | - | 50:1 | Abs: 1.7wt%/2.5 min/15 atm/300 °C Des: 0.6wt%/20 min/1 atm/300 °C Full Des: 6.70wt%/410 °C Des temperature: 354 °C | [83] |
| MgH2–Fe | 650 | 2 | Ar | - | Des temperature: 256.4 °C | [87] |
| Mg–5wt%Ni–2.5wt%Fe–2.5wt%Ti | 250 | 4 | H2 (12 bar) | 45:1 | Abs: 4.0wt%/10 min/12 bar/300 °C Des: 4.2wt%/60 min/1.0 bar/300 °C | [89] |
| Mg–5wt%Ni–2.5wt%Fe–2.5wt%Ti | 250 | 8 | H2 (12 bar) | 45:1 | Abs: 4.7wt%/10 min/12 bar/300 °C Des: 1.5wt%/60 min/1.0 bar/300 °C | [89] |
| Mg92In5Zn3 | 300 | 80 | Ar | 30:1 | Abs: 3.5wt%/1800 s/1.0 MPa/573 K ΔHdes = −68.6 kJ·mol−1·H2 ΔSdes = 134.5 J mol−1·K−1 H2 Ea (abs) = −62.5 kJ·mol−1·H2 | [95] |
| Mg–10wt%Co(0.5–1.5 μm) | 200 | 2 | H2 (11 bar) | 9:1 | Abs: 1.3wt%/60 min/11.2 bar/598 K | [97] |
| Mg–10wt%Co(3–7 μm) | 200 | 2 | H2 (11 bar) | 9:1 | Abs: 0.3wt%/60 min/11.2 bar/598 K | [97] |
| Mg–10wt%Co(44 μm) | 200 | 2 | H2 (11 bar) | 9:1 | Abs: 0.7wt%/60 min/11.2 bar/598 K | [97] |
| Material | Rotation Speed, rpm | Time, h | Atmosphere | Ball-to-Powder Weight Ratio | Hydrogen Storage Properties | Ref. |
|---|---|---|---|---|---|---|
| MgH2–4wt%Ni | 400 | 10 | H2 (10 bar) | 40:1 | Des: 7.0wt%/300 °C Des temperature: 264 °C | [101] |
| MgH2–6wt%TiO2 | 400 | 10 | H2 (10 bar) | 40:1 | Des: 5.6wt%/350 °C Des temperature: 308 °C | [101] |
| MgH2–4wt%Ni–6wt%TiO2 | 400 | 10 | H2 (10 bar) | 40:1 | Des: 6.0wt%/300 °C Des temperature: 259 °C | [101] |
| MgH2–10wt%Ni/TiO2 (Ni/TiO2 is nanocomposite with a mass ratio of 4:6) | 400 | 10 | H2 (10 bar) | 40:1 | Des: 6.5wt%/300 °C Des temperature: 231 °C | [101] |
| MgH2–8wt%Nb2O5–2wt%Ni | 200 | 50 | H2 (10 bar) | 34:1 | Des: 5.5wt%/7 min/0.2 bar/225 °C ΔHabs = 72.74 kJ·mol−1·H2 | [105] |
| (MgH2)84(TiH2)10(TiO2)6 | - | 4 | Ar | 10:1 | Abs: 5wt%/60 s/20 atm/300 °C Des: 4.8wt%/1250 s/0.2 atm/300 °C (des) = 118 kJ·mol−1·H2 | [106] |
| MgH2–17wt%Nb2O5 | - | 20 | Ar | - | Abs: 6wt%/400 s/1 MPa/300 °C Des: 6wt%/1000 s/0.1 kPa/300 °C Des temperature: 300 °C | [107] |
| MgH2–17wt%Nb2O5 | - | 200 | Ar | - | Abs: 6wt%/100 s/1 MPa/300 °C Des: 6wt%/250s/0.1 kPa/300 °C Des temperature: 264 °C | [107] |
| MgH2–5mol%CuO | - | 100 | Ar | 10:1 | Abs: 5.5wt%/90 s/8.4 bar/300 °C | [108] |
| MgH2–5mol%Mn2O3 | - | 100 | Ar | 10:1 | Abs: 4.8wt%/90 s/8.4 bar/300 °C | [108] |
| MgH2–5mol%Cr2O3 | - | 100 | Ar | 10:1 | Abs: 4.5wt%/90 s/8.4 bar/300 °C | [108] |
| –5mol%Fe3O4 | - | 100 | Ar | 10:1 | Abs: 4.2wt%/60 s/8.4 bar/300 °C | [108] |
| MgH2–5mol%V2O5 | - | 100 | Ar | 10:1 | Abs: 4wt%/60 s/8.4 bar/300 °C | [108] |
| Mg–20wt%TiO2 | - | - | H2 (0.8 MPa) | 20:1 | Abs: 3.7wt%/10 min/2.0 MPa/200 °C | [109] |
| Mg–10wt%Fe2O3 | 250 | 24 | H2 (10 bar) | 9:1 | Abs: 5wt%/60 min/12 bar/593 K | [110] |
| Mg–10wt%Fe2O3 | 250 | 8 | H2 (10 bar) | 9:1 | Abs: 3.3wt%/60 min/12 bar/593 K | [110] |
| Mg–10wt%Fe2O3 | 200 | 1 | H2 (10 bar) | 9:1 | not absorb hydrogen | [110] |
| Mg–10wt%Al2O3 | - | 2 | H2 | 9:1 | Abs: 5.4wt%/60 min/11 bar/573 K | [120] |
| Mg–10wt%CeO2 | - | 2 | H2 | 9:1 | Abs: 3.2wt%/60 min/11 bar/573 K | [120] |
| Material | Rotation Speed, rpm | Time, h | Atmosphere | Ball-to-Powder Weight Ratio | Hydrogen Storage Properties | Ref. |
|---|---|---|---|---|---|---|
| Mg–15wt%TiF4 | 400 | 2 | Ar (0.1 MPa) | 20:1 | Des temperature: 150 °C Ea (des) = 77 kJ·mol−1·H2 | [123] |
| MgH2–0.4mol%NbCl5 | - | 2 | Ar | - | Des:5.2wt%/50 min/primary vacuum/300 °C | [124] |
| MgH2–0.4mol%CaF2 | - | 2 | Ar | - | Des:5.8wt%/50 min/primary vacuum/300 °C | [124] |
| MgH2–5wt%K2NbF7 | 400 | 3 | Ar | 80:1 | Abs: 5.7wt%/2.3 min/27 atm/320 °C Des: 5.7wt%/4.6 min/1 atm/320 °C Full Des: 6.5wt%/350 °C Des temperature: 255 °C | [125] |
| MgH2 as milled | 400 | 3 | Ar | 80:1 | Abs: 7.0wt%/6.5 min/27 atm/320 °C Des: 0.2wt%/4.6 min/1 atm/320 °C Full Des: 6.8wt%/450 °C Des temperature: 330 °C | [125] |
| Pristine MgH2 | - | - | - | - | Des: Full Des: 7.0wt%/425 °C Des temperature: 414 °C | [125] |
| MgH2–10wt%FeF3 | 400 | 15 | H2 (1 MPa) | 40:1 | Abs: 4.03wt%/5 min/3.5 MPa/473 K Des: 5.92wt%/8 min/0.01 MPa/573 K Ea (des) = 77.6 kJ·mol−1·H2 | [127] |
| MgH2–10wt%TiF3 | - | - | - | - | Abs: 4.2wt%/5 min/3.5 MPa/473 K Des: 6.04wt%/8 min/0.01 MPa/573 K Ea (des) = 74.1 kJ·mol−1·H2 | [127] |
| MgH2–0.2mol% NbF5 | - | 25 | Ar | 16:1 | Des: 5.6wt%/25 min/10−1 mbar/300 °C | [133] |
| MgH2–2mol% NbF5 | - | 25 | Ar | 16:1 | Des: 5.8wt%/25 min/10−1 mbar/300 °C Ea (des) = 88 kJ·mol−1·H2 | [133] |
| MgH2–2mol%NbF5 | - | 1 | Ar | 16:1 | Des: 4.4wt%/40 min/10−1 mbar/250 °C Ea (des) = 72 kJ·mol−1·H2 | [133] |
| MgH2–7wt%TiCl3 | 650 | 1 | Ar | 25:1 | Abs: 6.4wt%/1.9 min/10 bar/325 °C Des: 6.1wt%/4 min/1 bar/325 °C | [137] |
| MgH2–7wt%ZrF4 | 650 | 1 | Ar | 25:1 | Abs: 6.4wt%/2.0 min/10 bar/325 °C Des: 6.3wt%/1.6 min/1 bar/325 °C | [137] |
| Material | Rotation Speed, rpm | Time, h | Atmosphere | Ball-to-Powder Weight Ratio | Hydrogen Storage Properties | Ref. |
|---|---|---|---|---|---|---|
| MgH2 | - | 20 | H2 (10 bar) | 21:1 | Abs: 6.3wt%/35 min/50 bar/503 K | [142] |
| MgH2–10wt%C60 | 600 | 10 | H2 (10 bar) | 21:1 | Abs: 5.3wt%/35 min/50 bar/503 K | [142] |
| MgH2–10wt%C60 | 600 | 20 | H2 (10 bar) | 21:1 | Abs: 5.0wt%/35 min/50 bar/503 K | [142] |
| MgH2–10wt%C60 | 600 | 0.5 | H2 (10 bar) | 21:1 | Abs: 5.0wt%/35 min/50 bar/503 K | [142] |
| Mg–2wt%graphite | 800 | 0.5 | - | 20:1 | Abs: 6.0wt%/12.5 min Des:5.0wt%/200 min | [144] |
| Mg–2wt%graphite | 800 | 4 | - | 20:1 | Abs: 6.0wt%/12.5 min Des: 4.5wt%/200 min | [144] |
| Mg–2wt%graphite | 800 | 38 | - | 20:1 | Abs: 5.0wt%/12.5 min Des: 5.0wt%/200 min | [144] |
| Mg–2wt%MWCNTs | 800 | 0.5 | - | 20:1 | Abs: 6.5wt%/10 min Des: 6.5wt%/200 min | [144] |
| Mg–2wt%MWCNTs | 800 | 4 | - | 20:1 | Abs: 6.0wt%/12.5 min Des: 6.0wt%/200 min | [144] |
| Mg–2wt%MWCNTs | 800 | 38 | - | 20:1 | Abs: 5.0wt%/12.5 min Des: 2.8wt%/200 min | [144] |
| MgH2–25at%CB (carbon black) | - | 10 | Ar | 12:1 | Abs: 7.0wt%/90 min/25 bar/300 °C | [147] |
| MgH2–1mol%C60 | 600 | 2 | H2 (10 bar) | 21:1 | Abs: 3.1wt%/20 min/400 °C Des temperature: 325 °C | [148] |
| MgH2–1mol%C60 | 600 | 2 | Ar (1 bar) | 21:1 | Abs: 5.1wt%/20 min/400 °C Des temperature: 325 °C | [148] |
| Mg–5wt%MWNTs | - | 3 | H2 (0.1 MPa) | 20:1 | Abs: 4.3wt%/15 min/2.0 MPa/373 K Abs: 4.9wt%/1 min/2.0 MPa/553 K | [151] |
| MgH2–5wt%SWNTs | - | 10 | Ar | 40:1 | Abs: 5.3wt%/180 min/2.0 MPa/423 K Abs: 6.7wt%/2 min/2.0 MPa/573 K | [153] |
| MgH2–5wt%BNNT (boron nitride nanotubes) | - | 10 | Ar | 40:1 | Abs: 1.3wt%/180 min/2.0 MPa/423 K Abs: 5.4wt%/10 min/2.0 MPa/573 K | [153] |
| MgH2–5wt%AC (activated carbon) | - | 10 | Ar | 40:1 | Abs: 3.7wt%/180 min/2.0 MPa/423 K Abs: 5.8wt%/2 min/2.0 MPa/573 K | [153] |
| Material | Rotation Speed, rpm | Time, h | Atmosphere | Ball-to-Powder Weight Ratio | Hydrogen Storage Properties | Ref. |
|---|---|---|---|---|---|---|
| Mg–11.5wt%LiCl–12.49wt%CB | - | 15 | H2 (6N) | 60:1 | Abs: 5.2wt%/5 h/2.0 MPa/2.5 MPa 623K, ΔHdes = 74.2 kJ·mol−1·H2 | [167] |
| Mg–9.09wt%V0.81Fe0.19 | 500 | 5 | H2 (30 bar) | 36.4:1 | Abs: 4.8wt%/10 min/10 bar/250 °C, Ea (des) = 60 kJ·mol−1·H2 | [168] |
| Mg–16.67wt%V0.81Fe0.19 | 500 | 5 | H2 (30 bar) | 33.3:1 | Abs: 3.8wt%/40 min/10 bar/25 °C, Ea (des) = 33 kJ·mol−1·H2 | [168] |
| MgH2–10wt%TiVO3.5 | 500 | 24 | H2 (50 bar) | 120:1 | Des: 6.6wt%/60 min/0.001 Torr/300 °C | [169] |
| MgH2–5wt%Zr8Ni21 | 20 | Ar | 10:1 | Abs: 5.3wt%/16 s/10 bar/250 °C Des: 6.5wt%/300 s/0.1 bar/300 °C | [170] | |
| MgH2–10wt%FeNiHx | 175 | 10 | H2 (0.7 MPa) | 50:1 | Abs: 5.6wt%/25 bar/300 °C Des: 6.4wt%/1 atm/325 °C | [171] |
| Mg–15wt%Mg2Ni0.9Co0.1 | 200 | 0.5 | Ar | 10:1 | Abs: 5.5wt%/40 min/1 MPa/473 K Des: 5.3wt%/90 min/0.15 MPa/573 K | [172] |
| Mg–15wt%Mg2Ni0.9Fe0.1 | 200 | 0.5 | Ar | 10:1 | Abs: 5.3wt%/10 min/1 MPa/573 K Des: 5.8wt%/60 min/0.15 MPa/573 K | [173] |
| Mg–7wt%FeNb granule | - | 10 | Ar | 10:1 | Abs: 5.5wt%/100 s/2.0 MPa/350 °C Des: 6.4wt%/450 s/0.01 MPa/350 °C | [174] |
| Mg–30wt%LaNi2.28 | - | 80 | H2 (3.0 MPa) | 10:1 | Abs: 5.35wt%/50 s/3.0 MPa/553 K | [176] |
| Mg–30wt%LaNi2.28 | - | 40 | H2 (3.0 MPa) | 10:1 | Abs: 4.5wt%/420 s/3.0 MPa/553 K | [176] |
| MgH2–30wt%LaNi5 | - | 20 | Ar | 10:1 | Abs: 4.5wt%/400 s/1.0 MPa/423 K Des: 4.6wt%/200 s/0.015 MPa/573 K | [177] |
| Mg–30wt%LaNi5 | - | 20 | Ar | 10:1 | Abs: 4.1wt%/1000 s/1.0 MPa/423 K Des: 5.0wt%/150 s/0.015 MPa/573 K | [177] |
| MgH2–5wt%LaNi5 | 300 | 40 | H2 (1 bar) | 15:1 | Abs: 5.5wt%/180 s/20 bar/245 °C Des: 3.6wt%/2 h/245 °C | [178] |
| MgH2–5wt%LaNi5 | 300 | 20 | H2 (1 bar) | 15:1 | Abs: 5.5wt%/1 h/20 bar/245 °C Des: 3.0wt%/2 h/245 °C | [178] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, J.; Lider, A.; Kudiiarov, V. Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview. Metals 2019, 9, 768. https://doi.org/10.3390/met9070768
Lyu J, Lider A, Kudiiarov V. Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview. Metals. 2019; 9(7):768. https://doi.org/10.3390/met9070768
Chicago/Turabian StyleLyu, Jinzhe, Andrey Lider, and Viktor Kudiiarov. 2019. "Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview" Metals 9, no. 7: 768. https://doi.org/10.3390/met9070768
APA StyleLyu, J., Lider, A., & Kudiiarov, V. (2019). Using Ball Milling for Modification of the Hydrogenation/Dehydrogenation Process in Magnesium-Based Hydrogen Storage Materials: An Overview. Metals, 9(7), 768. https://doi.org/10.3390/met9070768






