Stress Corrosion Behavior of AM50Gd Magnesium Alloy in Different Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Preparation
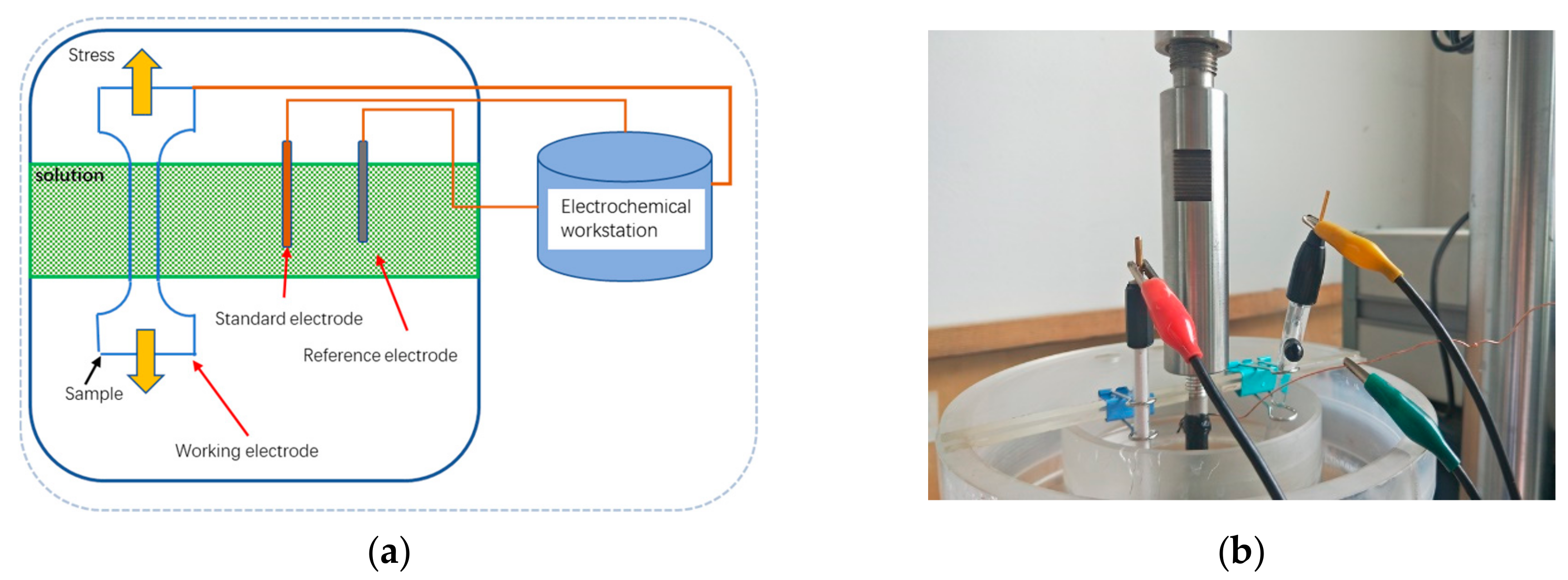
2.2. Slow Strain Rate Tensile (SSRT)
2.3. Electrochemical Polarization Test
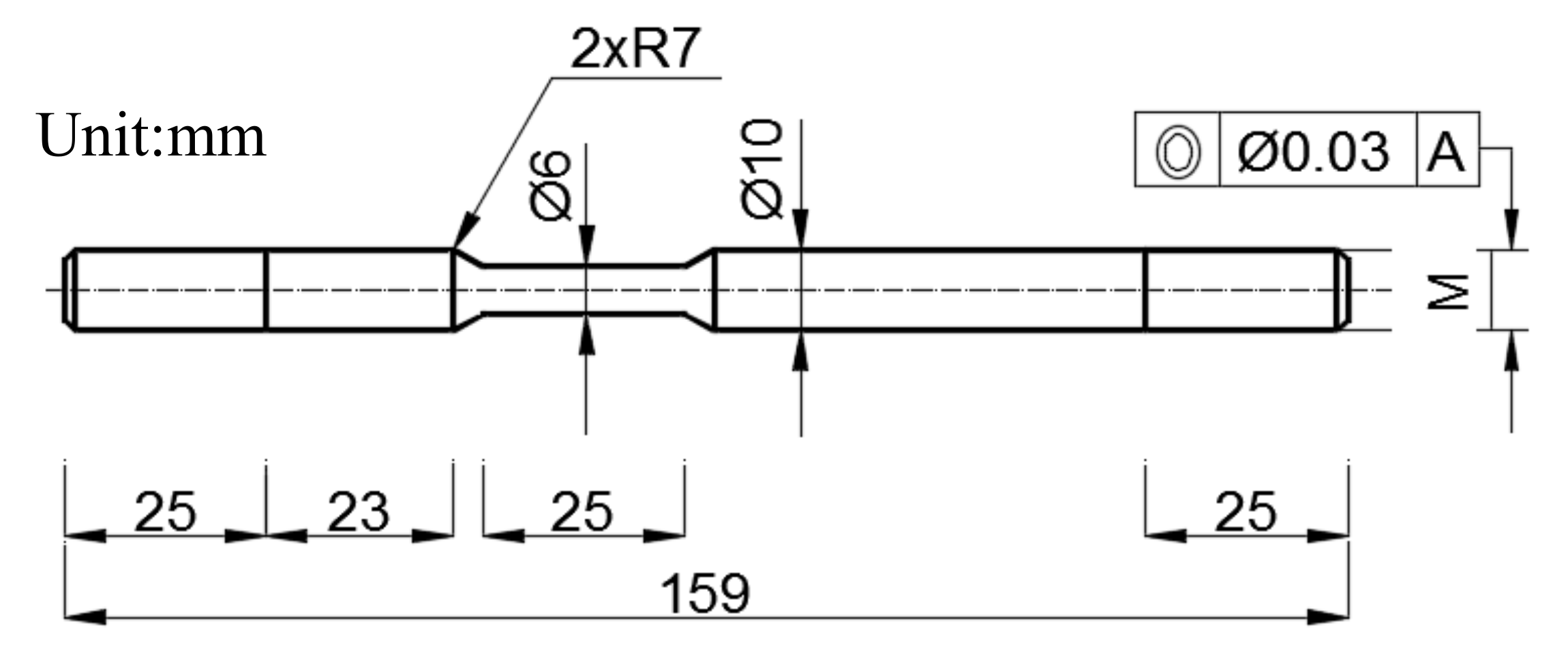
2.4. Sample Preparation
3. Results
3.1. Microstructure
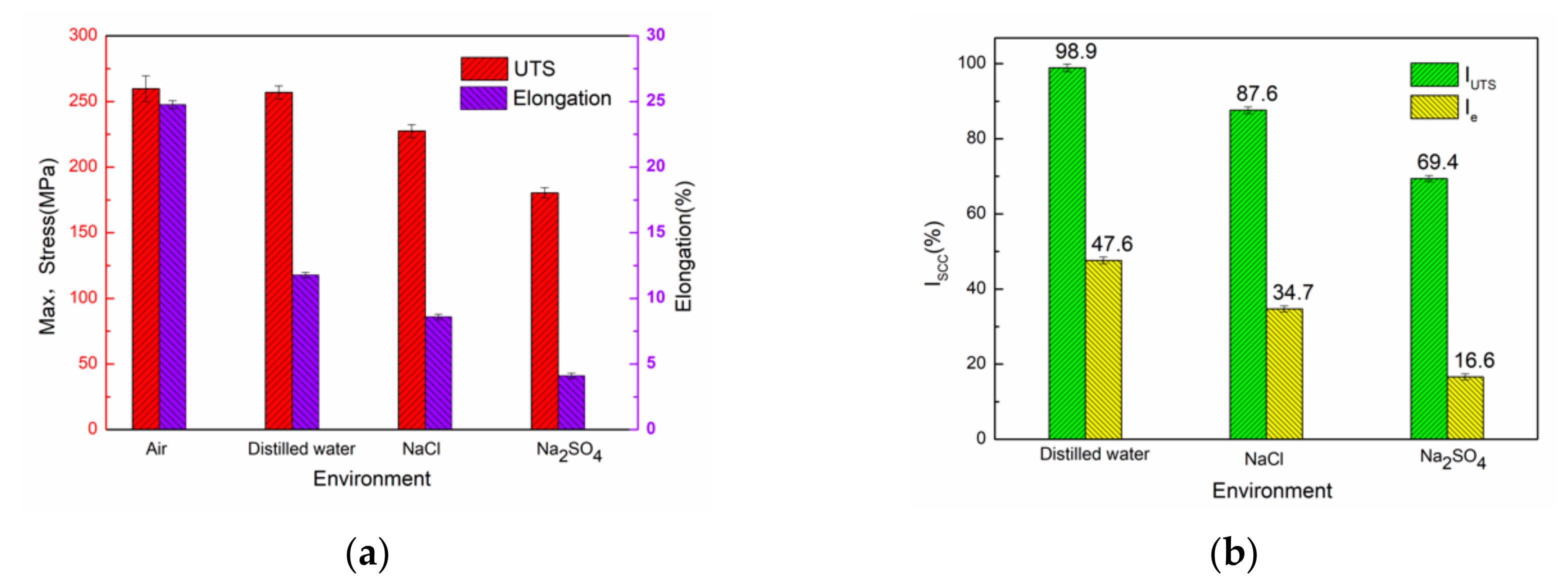
3.2. Slow Strain Rate Tensile (SSRT) Test
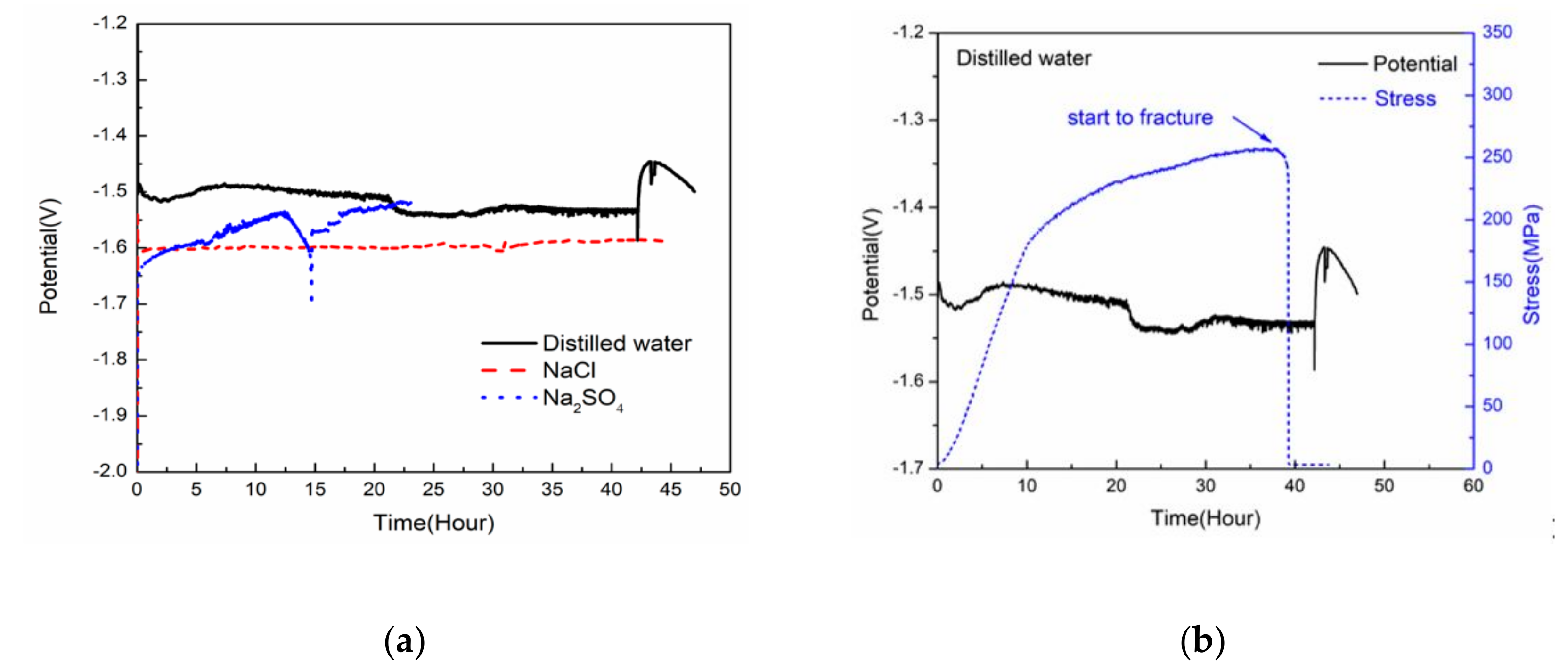
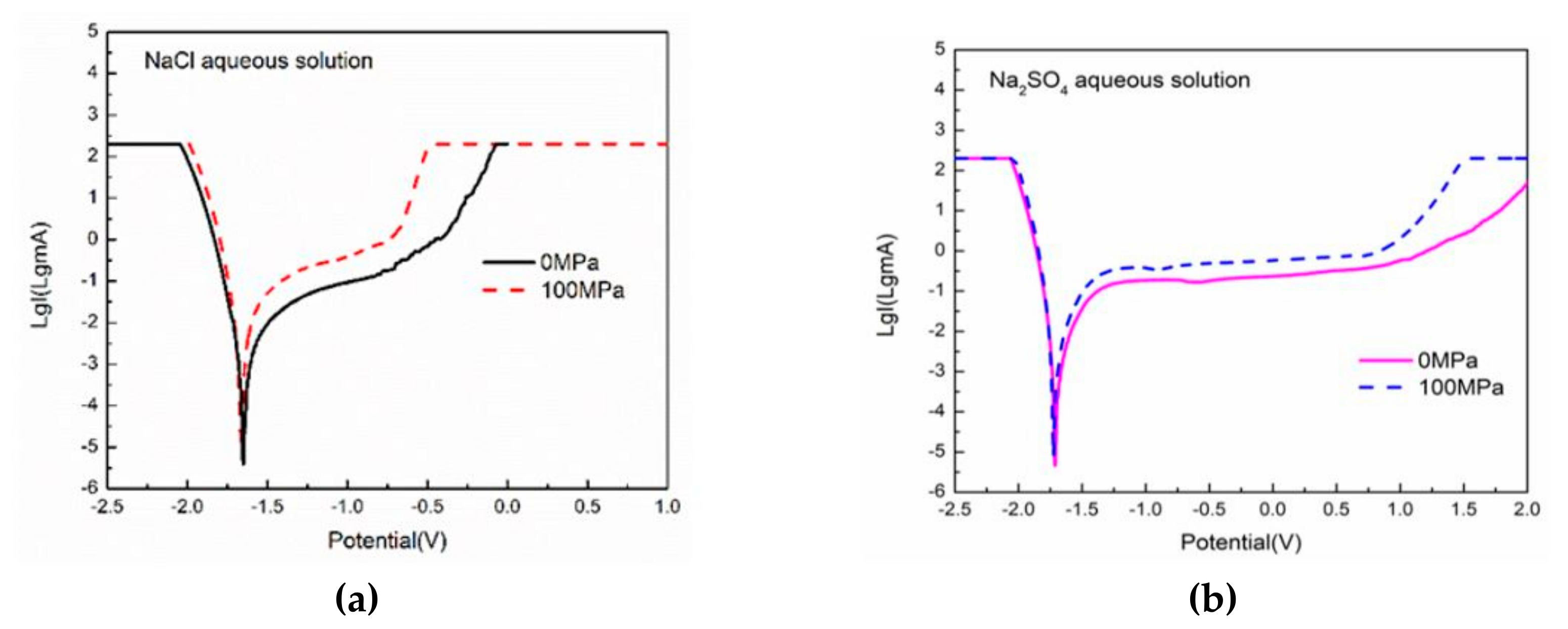
3.3. Electrochemical Tests
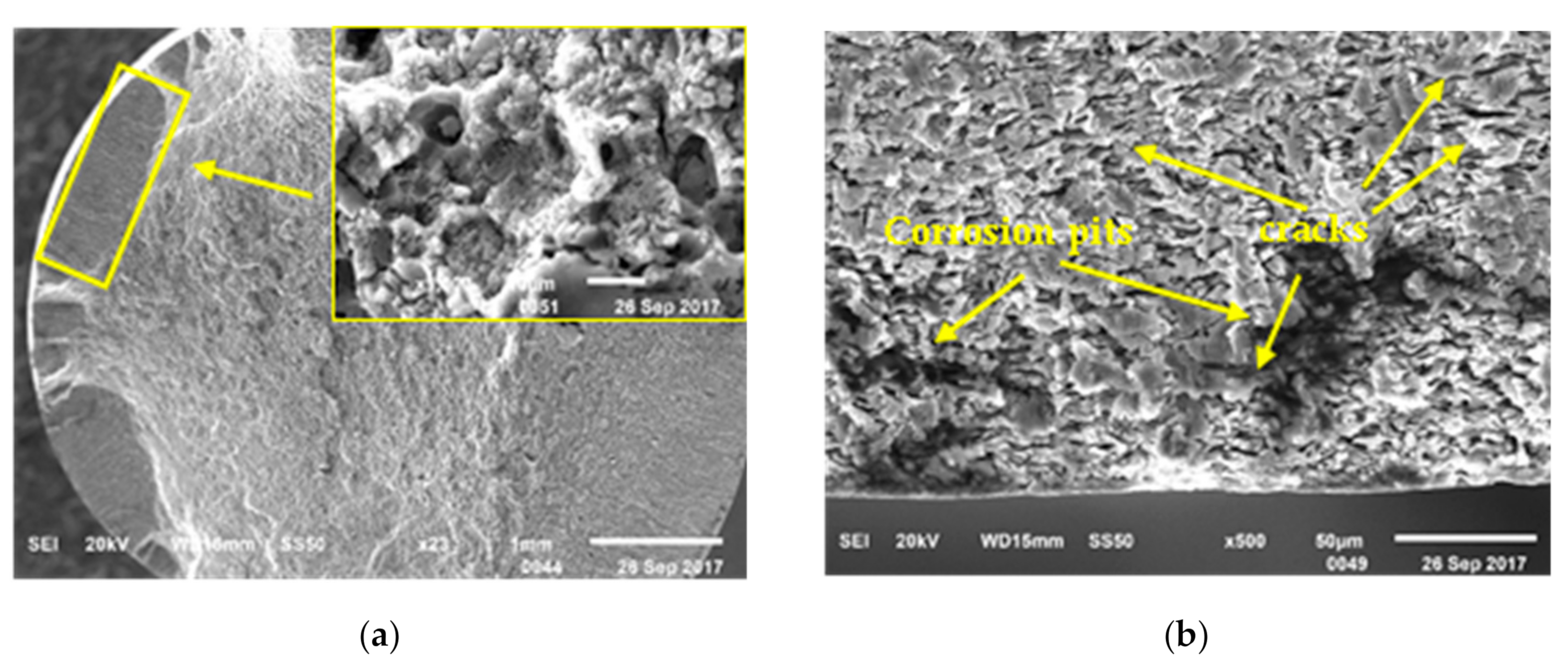
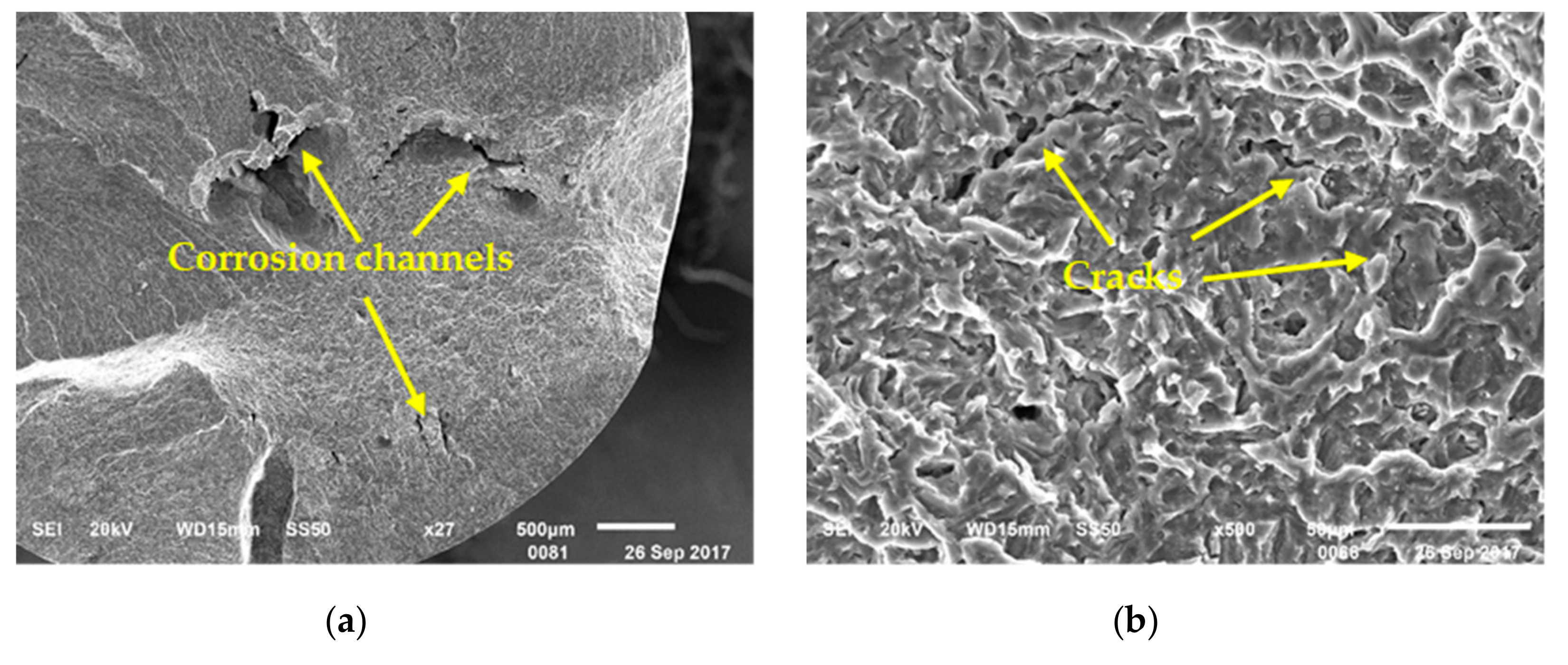
3.4. Surface and Fracture Morphology
4. Conclusions
- 1)
- The stress corrosion sensitivity of the material in different environments was Na2SO4 > NaCl > distilled water > air, respectively.
- 2)
- According to the Tafel curves measured at 0 and 100 MPa, the corrosion voltage decreased little and the corrosion current density increased rapidly under 100 Pa. This was because the film of the corrosion product ruptured to form a large cathode and a small anode, which resulted in a large instantaneous corrosion current.
- 3)
- The mechanism of hydrogen embrittlement and anodic dissolution together affected the stress corrosion behavior of the alloy. In distilled water, hydrogen embrittlement played a major role, while in NaCl and Na2SO4 solution hydrogen embrittlement and anodic dissolution were both affected.
- 4)
- The direct reason of the SCC sample failure was the cracks expanding rapidly at the bottom of pit, which was caused by corrosion.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Liu, Y.; Fang, S.; Song, Y.; Zhang, D.; Zhang, L.; Li, C. Evaluating the improvement of corrosion residual strength by adding 1.0 wt.% yttrium into an AZ91D magnesium alloy. Mater. Charact. 2010, 61, 674–682. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, G.Z.; Shao, Y.W.; Cui, Z.Y.; Wang, F.H. Corrosion of hot extrusion AZ91 magnesium alloy. Part II: Effect of rare earth element neodymium (Nd) on the corrosion behavior of extruded alloy. Corros. Sci. 2011, 53, 2934–2942. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.H.; Liu, J.A.; Song, Y.L. Effect of T6 heat treatment on corrosion resistance and mechanical properties of AM50 magnesium alloy. Mater. Res. Innova. 2016, 19, 259–264. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, F.; Song, D.; He, S.; Lin, J.; Yu, F. Stress corrosion cracking of a forged Mg-Al-Zn alloy with different surface conditions. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Klein, M.; Frieling, G.; Walther, F. Corrosion fatigue assessment of creep-resistant magnesium alloys DieMag422 and AE42. Eng. Fract. Mech. 2017, 185, 33–45. [Google Scholar]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E.H. Pitting corrosion of a rare earth Mg alloy GW93. J. Mater. Sci. Technol. 2017, 33, 954–960. [Google Scholar] [CrossRef]
- Song, G.; Bowles, A.L.; Stjohn, D.H. Corrosion resistance of aged die cast magnesium alloy AZ91D. Mat. Sci. Eng. A 2004, 366, 74–86. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.S.; Davies, C.H.; Hofstetter, J.; Uggowitzer, P.J.; Löffler, J.F. Stress corrosion cracking and corrosion fatigue characterisation of MgZn1Ca0. 3 (ZX10) in a simulated physiological environment. J. Mech. Behav Biomed. 2017, 65, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Song, R.G.; Blawert, C.; Dietzel, W.; Atrens, A. A study on stress corrosion cracking and hydrogen embrittlement of AZ31 magnesium alloy. Mater. Sci. Eng. A 2005, 399, 308–317. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Raja, V.; Song, G.; Kainer, K. Characterisation of stress corrosion cracking (SCC) of Mg–Al alloys. Mater. Sci. Eng. A 2008, 488, 339–351. [Google Scholar] [CrossRef]
- Padekar, B.S.; Raja, V.S.; Raman, R.K.S.; Lyon, P. Stress corrosion cracking behavior of magnesium alloys EV31A and AZ91E. Mater. Sci. Eng. A 2013, 583, 169–176. [Google Scholar] [CrossRef]
- Padekar, B.S.; Raja, V.S.; Raman, R.K.; Paul, L. Stress corrosion cracking of a new rare- earth containing magnesium alloy, Elektron21 compared with AZ91E. Mater. Sci. Forum 2011, 690, 361–364. [Google Scholar] [CrossRef]
- Mirzadeh, H. Quantification of the strengthening effect of rare earth elements during hot deformation of Mg-Gd-Y-Zr magnesium alloy. J. Mater. Res. Technol. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Huang, Y.; Gan, W.; Kainer, K.U.; Hort, N. Role of multi-microalloying by rare earth elements in ductilization of magnesium alloys. J. Magnesium Alloys 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; De Negri, S.; Saccone, A.; Delfino, S. Effect of erbium addition on the corrosion behaviour of Mg–Al alloys. Intermetallics 2005, 13, 55–60. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Liu, Y.; Han, X. Corrosion and mechanical properties of AM50 magnesium alloy after modified by different amounts of rare earth element Gadolinium. Open Phy. 2016, 14, 444–451. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.L.; Liu, M.; Dargusch, M.S.; Atrens, A. Stress corrosion cracking of several hot-rolled binary Mg–X alloys. Corro. Sci. 2015, 98, 6–19. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Liu, Y.; Yang, M.; Qu, Q. Influence of Erbium, Cerium on the stress corrosion cracking behavior of AZ91 alloy in humid atmosphere. Adv. Eng. Mater. 2017, 19, 1700021. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Liu, J.; Song, Y. Corrosion and mechanical properties of AM50 magnesium alloy after being modified by 1 wt.% rare earth element gadolinium. J. Rare Earths 2014, 32, 558–563. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Han, X.; Liu, Y. Corrosion mechanical properties of hot extrusion treated AM50GdX magnesium alloy. J. Chin. Soc. Rare Earths 2016, 34, 425–431. [Google Scholar]
- Atren, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Wu, W.; Li, X.; Du, C.; Jiang, B. Stress corrosion cracking behavior of ZK60 magnesium alloy under different conditions. Inter. J. Hydrogen Energ. 2017, 42, 26162–26174. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, A.; Jiang, J.; Cheng, Z.; Song, D.; Yuan, Y.; Liu, H. Stress corrosion cracking behavior of fine-grained AZ61 magnesium alloys processed by equal-channel angular pressing. Metals 2017, 7, 343. [Google Scholar] [CrossRef]
- Padekar, B.S.; Raman, R.S.; Raja, V.S.; Paul, L. Stress corrosion cracking of a recent rare-earth containing magnesium alloy, EV31A, and a common Al-containing alloy, AZ91E. Corro. Sci. 2013, 71, 1–9. [Google Scholar] [CrossRef]
- Kannan, M.B.; Dietzel, W. Pitting-induced hydrogen embrittlement of magnesium–aluminium alloy. Mater. Des. 2012, 42, 321–326. [Google Scholar] [CrossRef]







| Al | Gd | Mn | Zn | Si | Cu | Ni | Mg |
|---|---|---|---|---|---|---|---|
| 4.8266 | 0.9304 | 0.2749 | 0.1299 | 0.0144 | 0.0018 | 0.0010 | Bal. |
| Type | UTS (MPa) | Elongation (%) |
|---|---|---|
| As-cast | 213 ± 10 | 2.75 ± 0.2 |
| Hot-extrusion | 315 ± 12 | 8.1 ± 0.3 |
| Solution Environment | Strength (Mpa) | Corrosion Current Density (mA/cm2) | Corrosion Potential (V) |
|---|---|---|---|
| 0.5 mol/L NaCl | 0 | 1.775 × 10−4 | −1.65 |
| 0.5 mol/L NaCl | 100 | 5.374 × 10−4 | −1.67 |
| 0.5 mol/L Na2SO4 | 0 | 2.718 × 10−4 | −1.71 |
| 0.5 mol/L Na2SO4 | 100 | 5.423 × 10−4 | −1.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Liu, X.; Zhang, Z.; Song, Y. Stress Corrosion Behavior of AM50Gd Magnesium Alloy in Different Environments. Metals 2019, 9, 616. https://doi.org/10.3390/met9050616
Yang M, Liu X, Zhang Z, Song Y. Stress Corrosion Behavior of AM50Gd Magnesium Alloy in Different Environments. Metals. 2019; 9(5):616. https://doi.org/10.3390/met9050616
Chicago/Turabian StyleYang, Miao, Xiaobo Liu, Zhiyi Zhang, and Yulai Song. 2019. "Stress Corrosion Behavior of AM50Gd Magnesium Alloy in Different Environments" Metals 9, no. 5: 616. https://doi.org/10.3390/met9050616
APA StyleYang, M., Liu, X., Zhang, Z., & Song, Y. (2019). Stress Corrosion Behavior of AM50Gd Magnesium Alloy in Different Environments. Metals, 9(5), 616. https://doi.org/10.3390/met9050616





