Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Electrolyte Solution
2.3. Characterization of Corrosion Products
2.4. Corrosion Rate Measurements
2.5. Electrochemical Measurements
3. Results
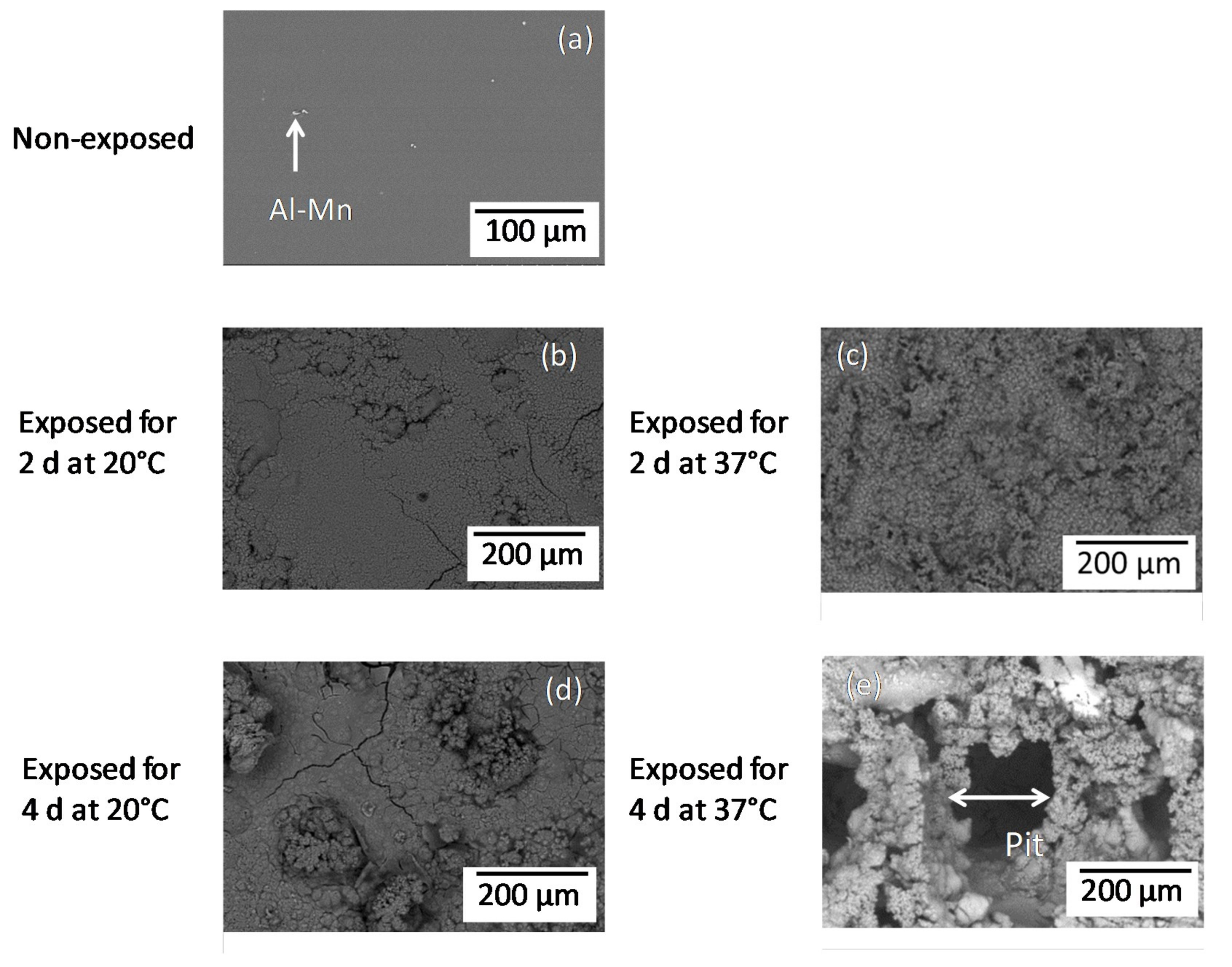
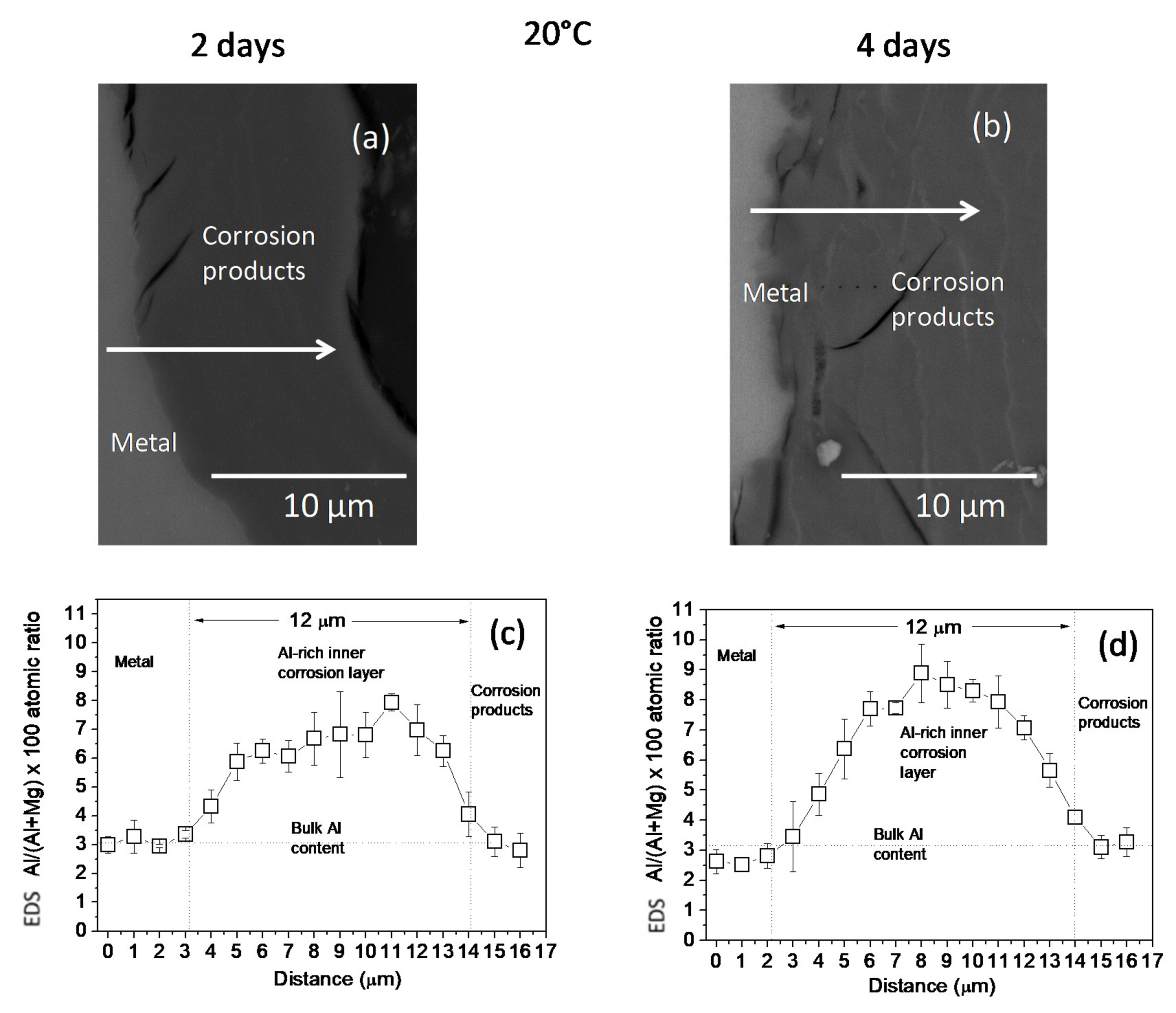
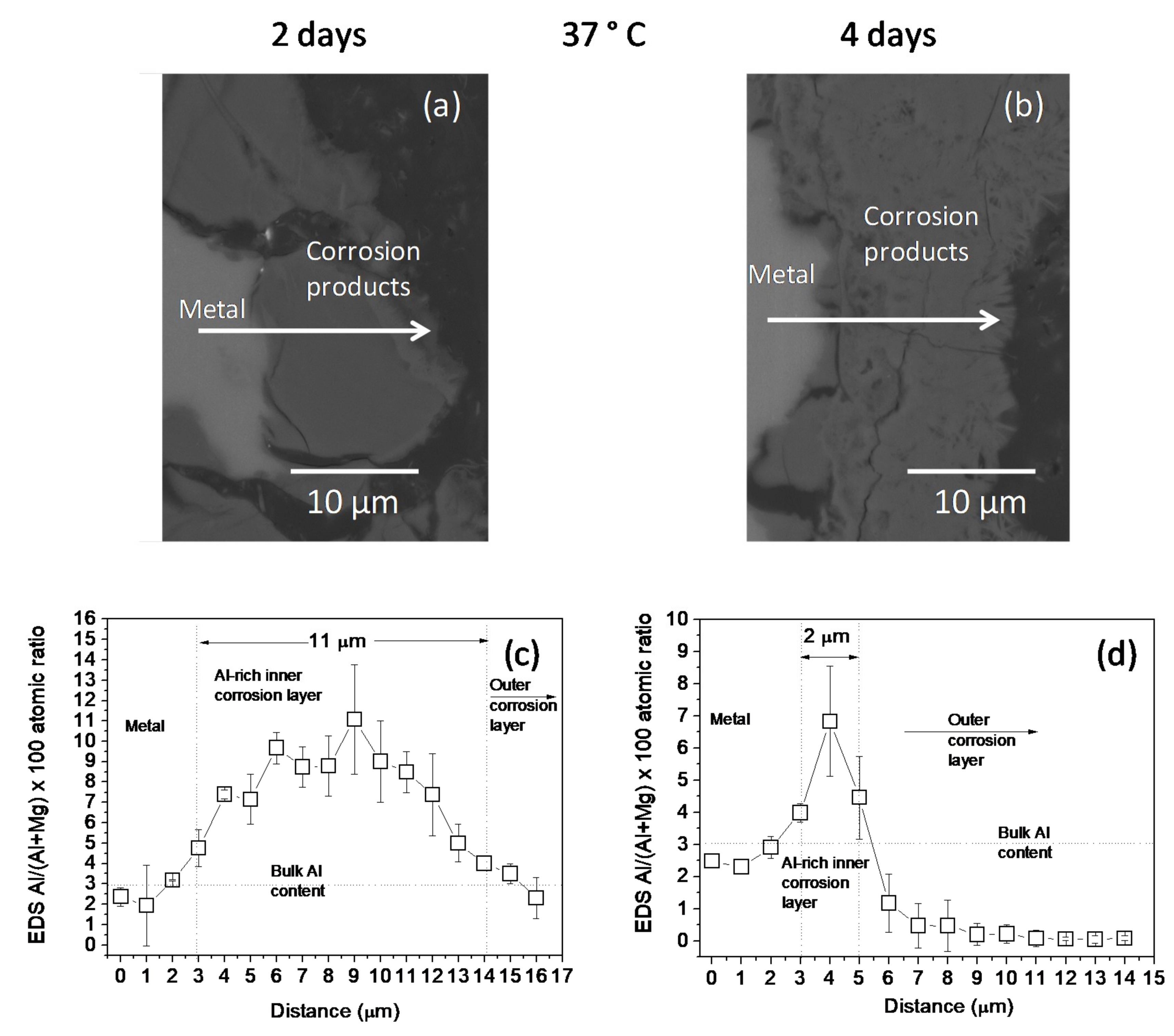
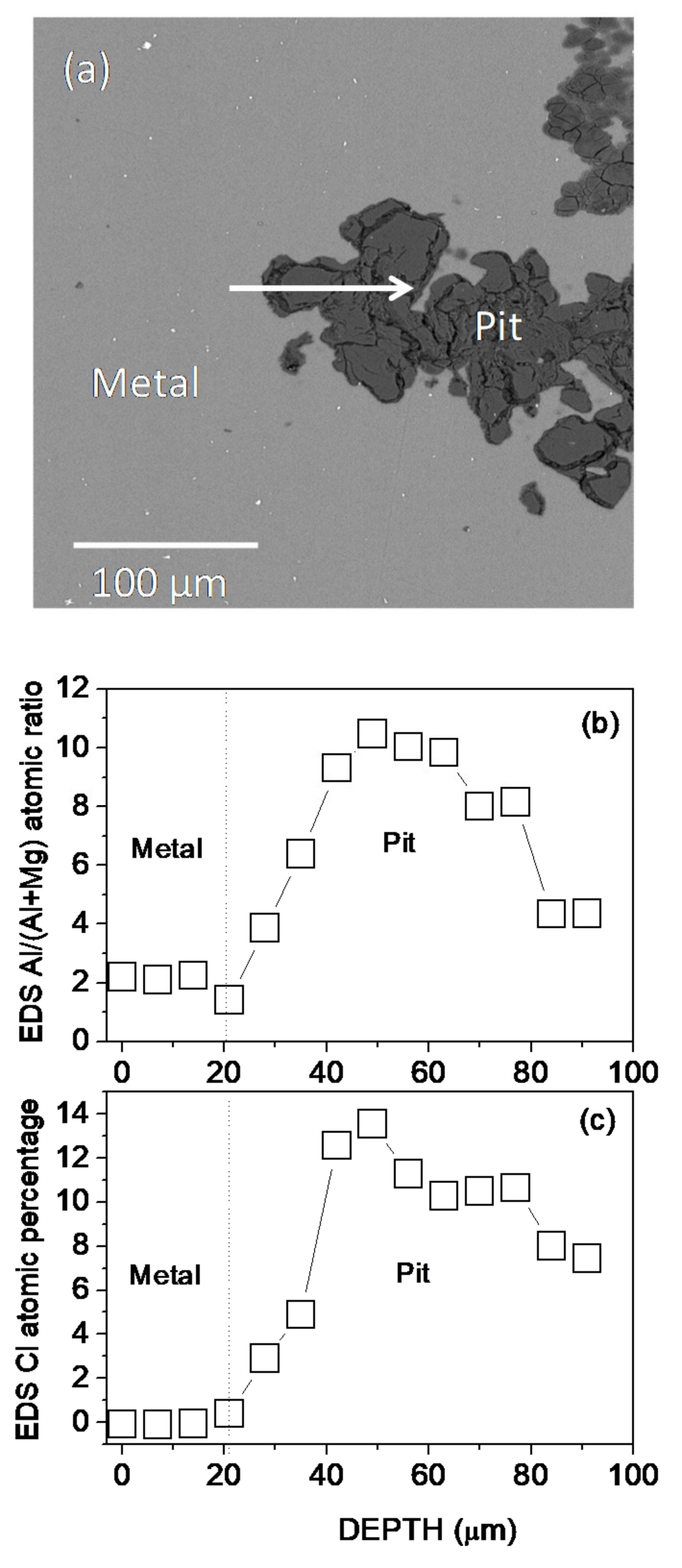
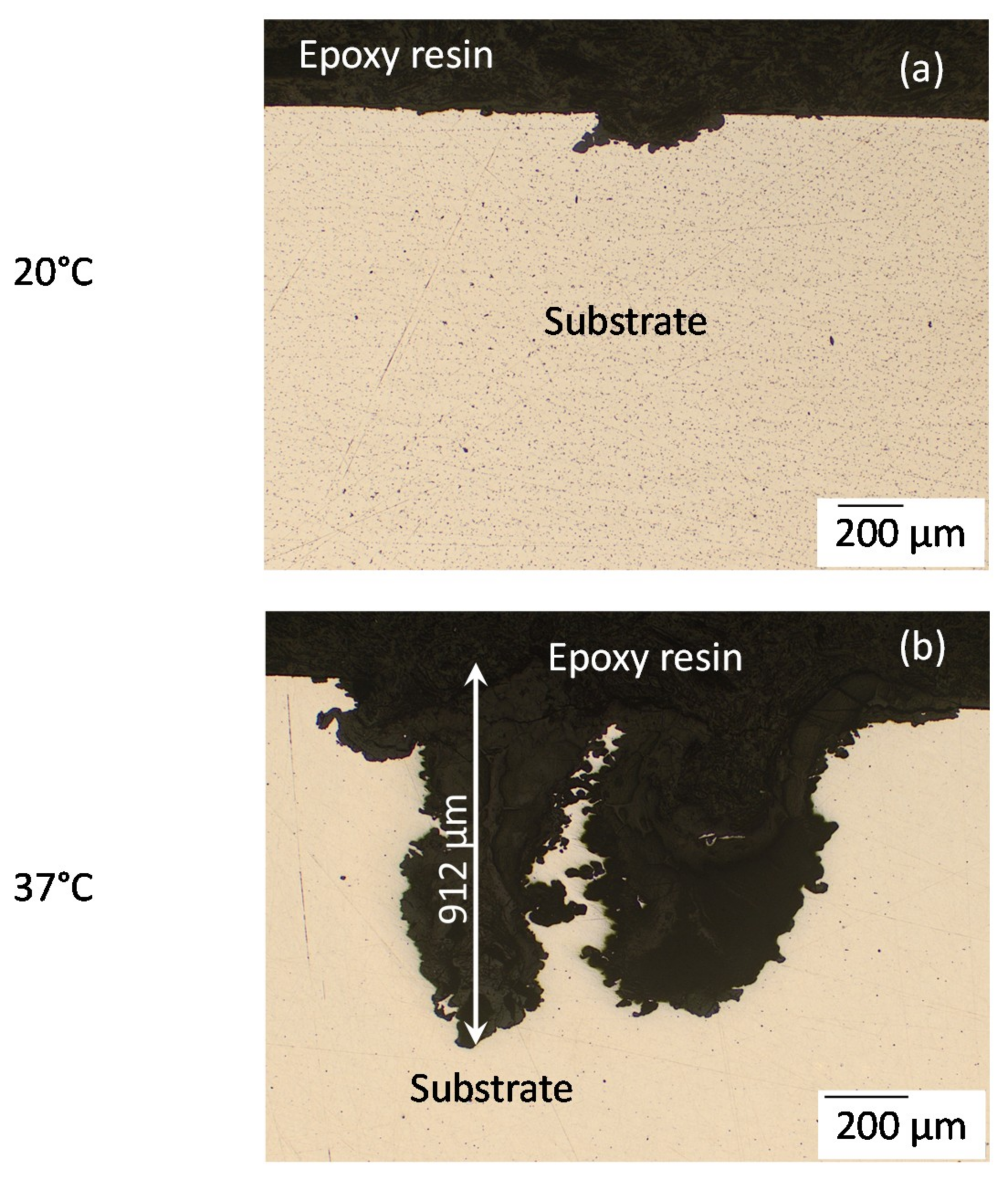
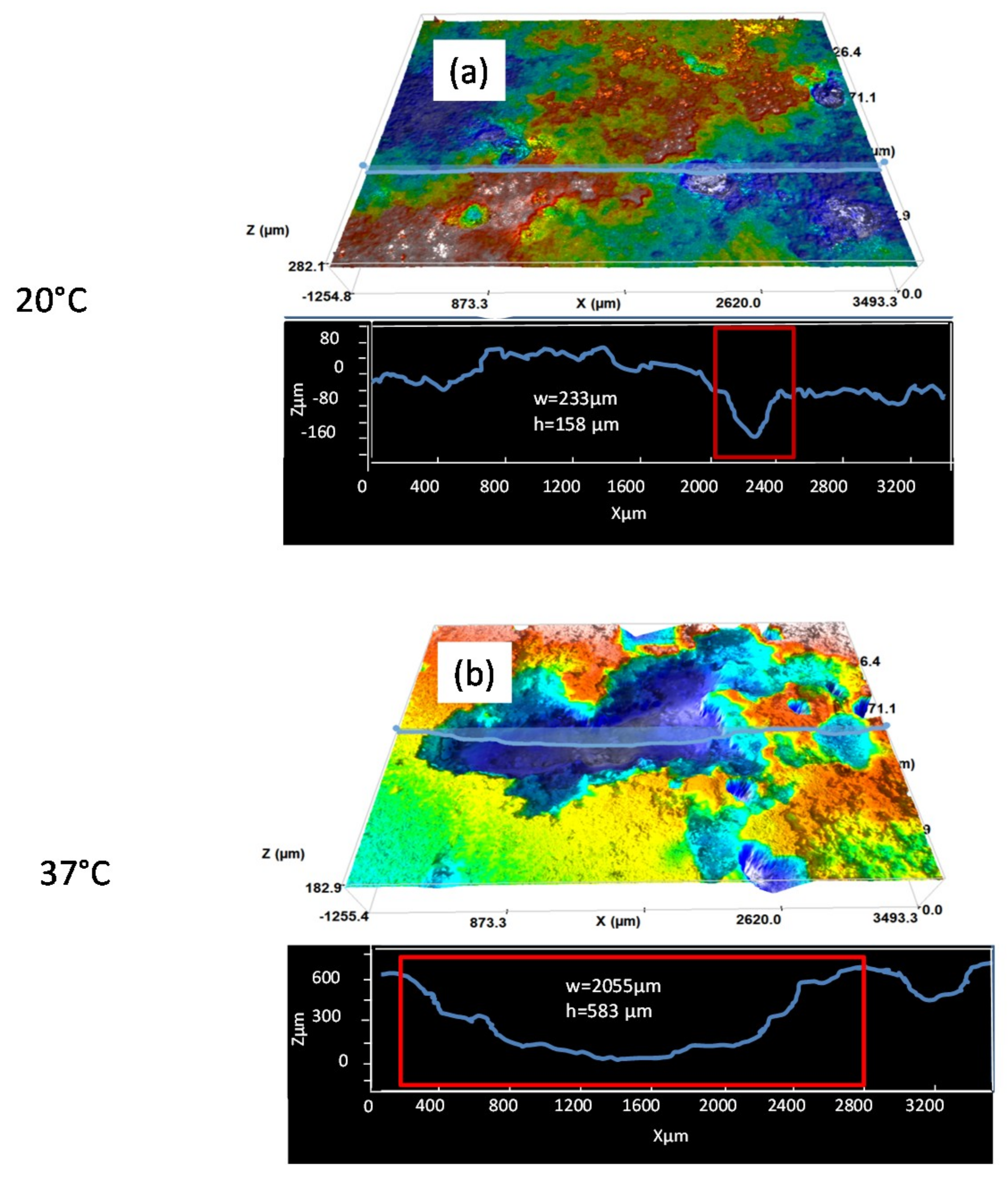
3.1. Surface and Cross-Sectional Corrosion Product Morphologies
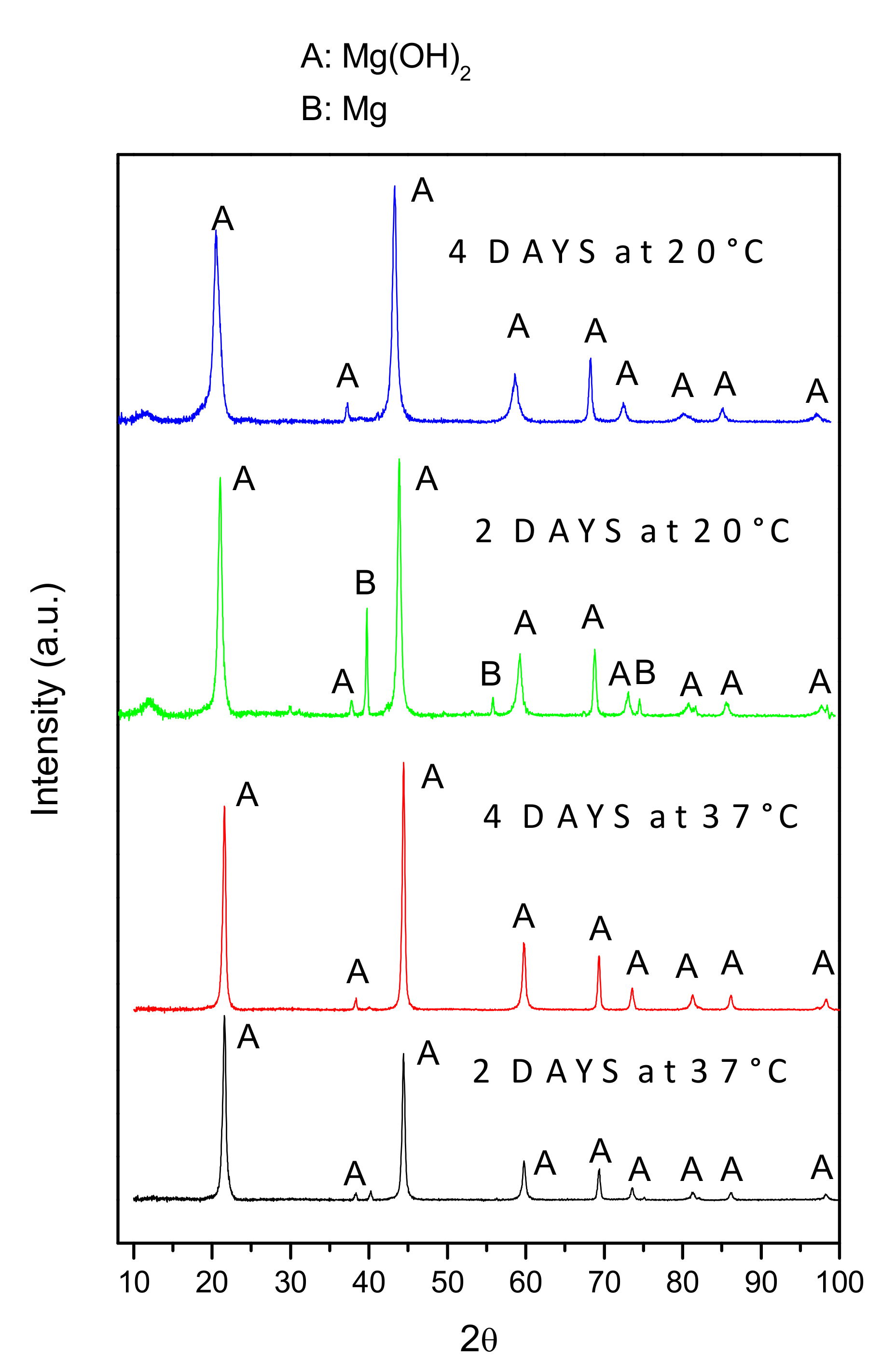
3.2. XRD Analysis
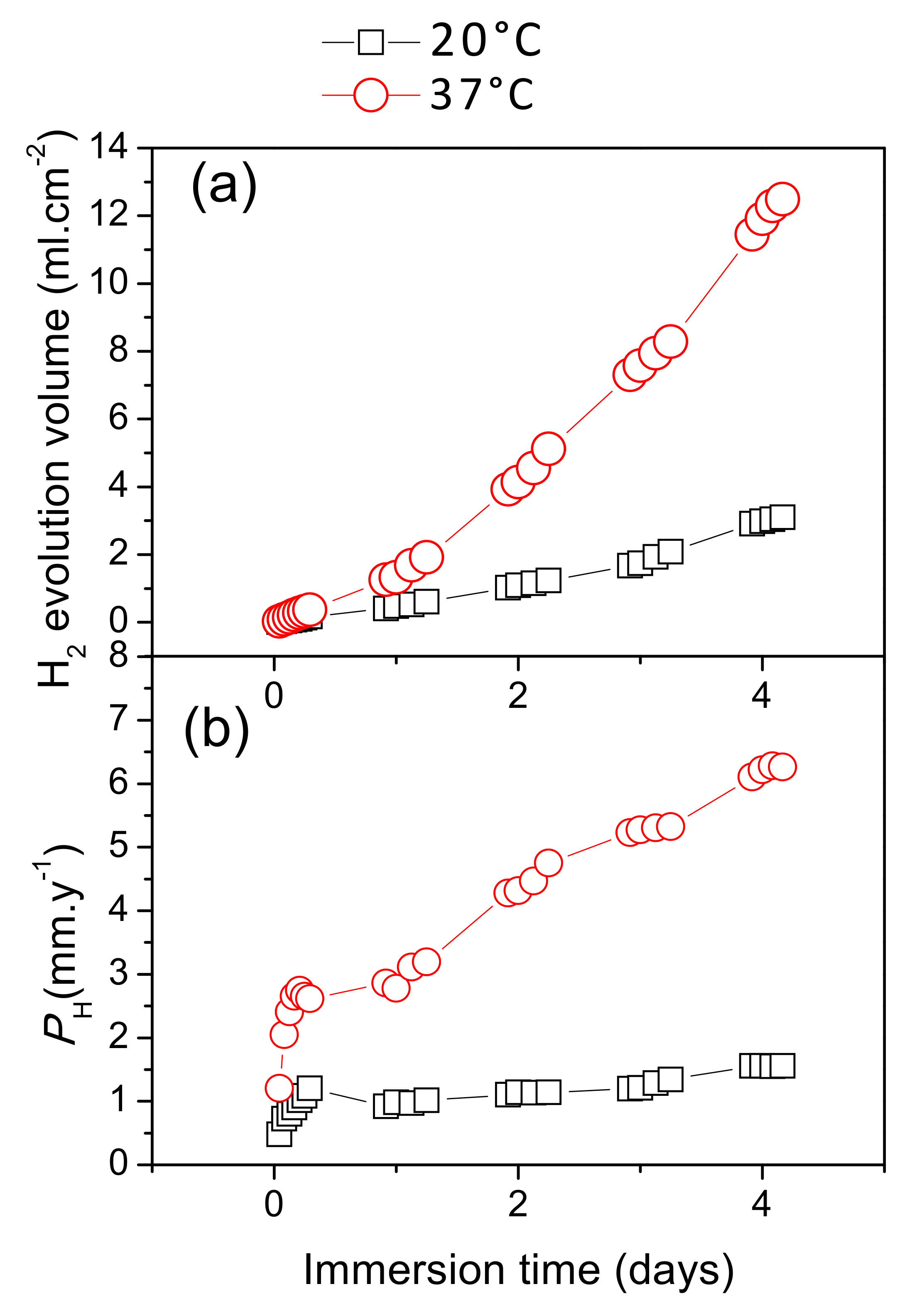
3.3. Hydrogen Evolution and Mass Loss Measurements
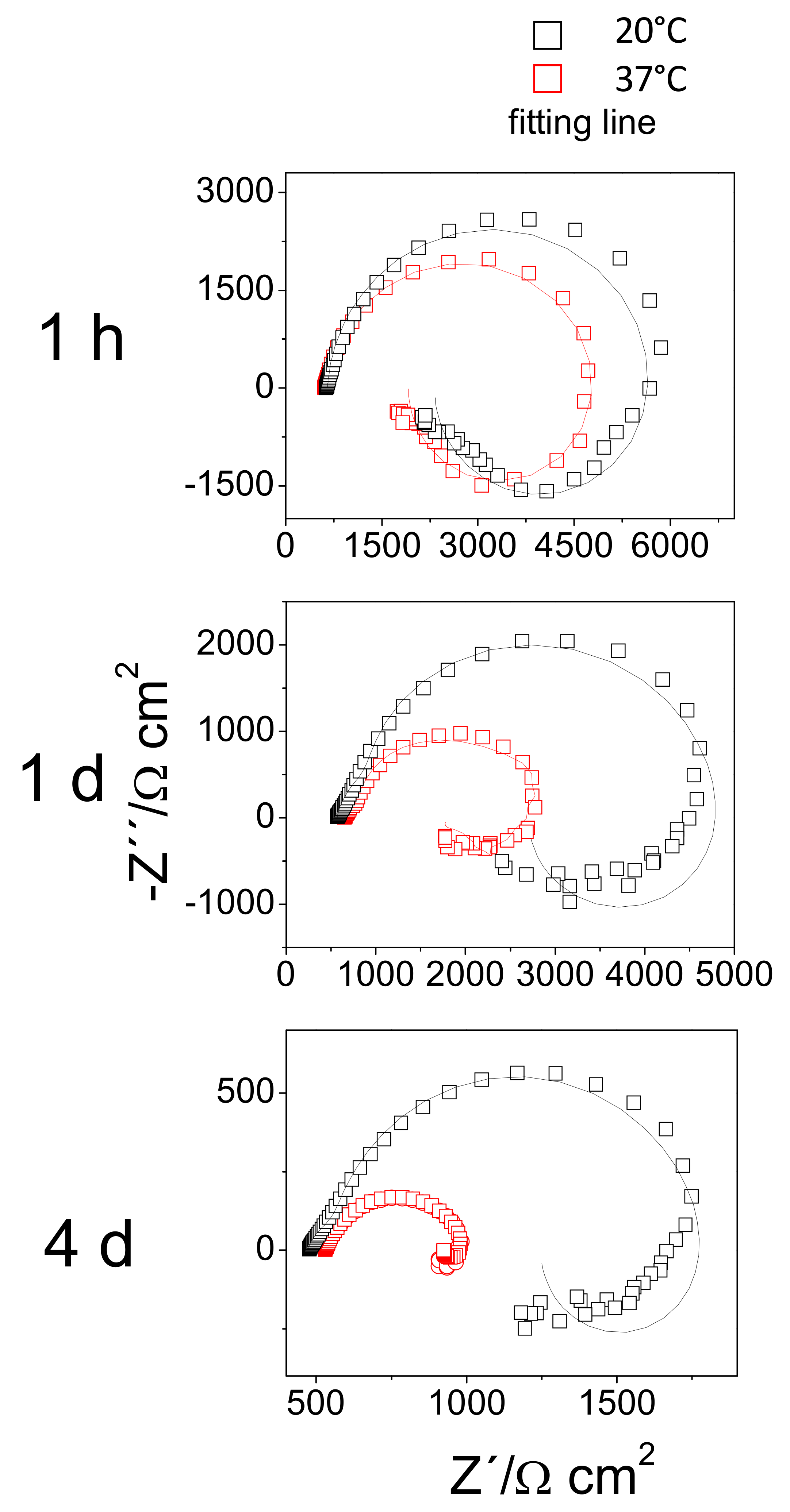
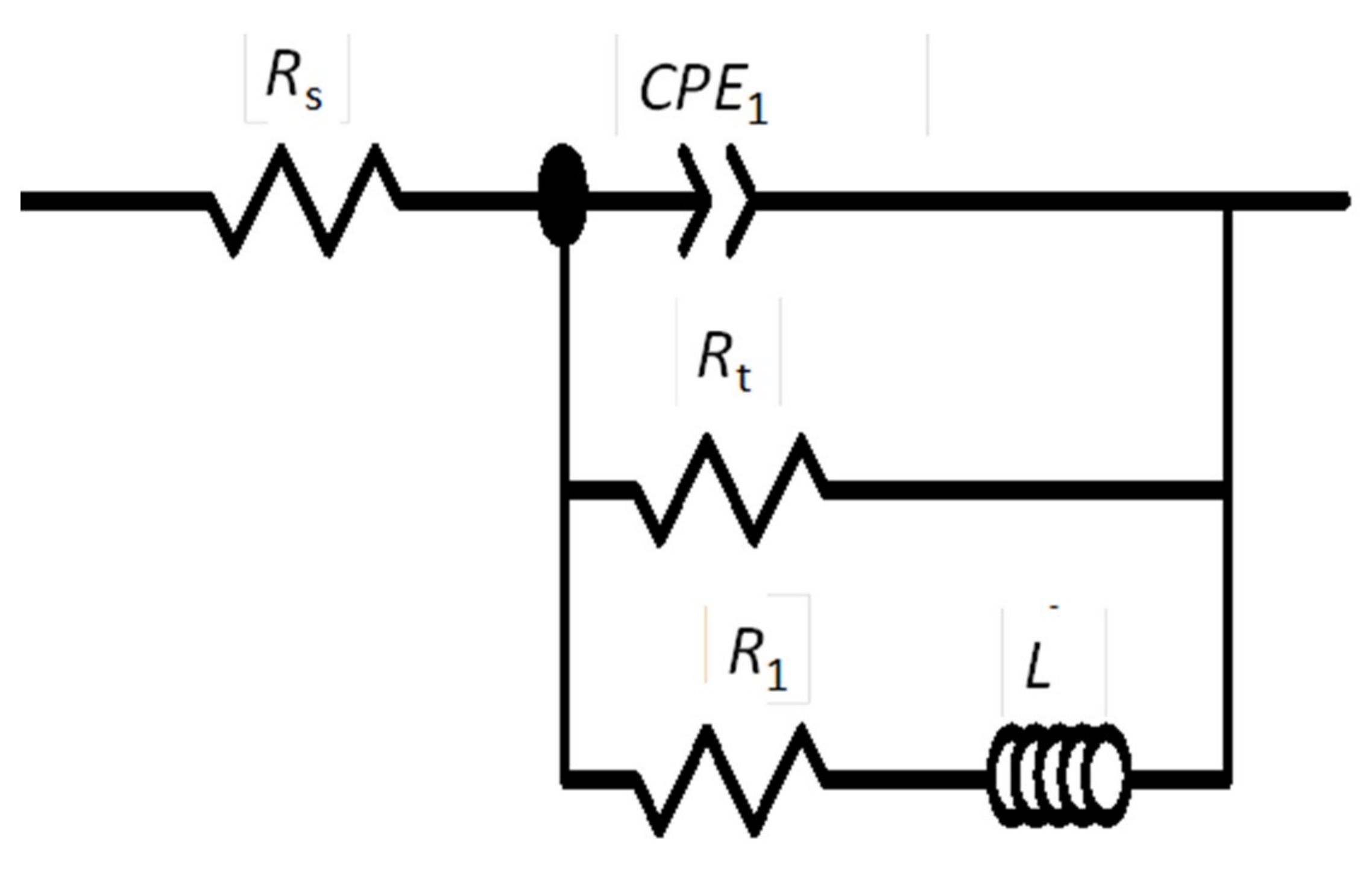
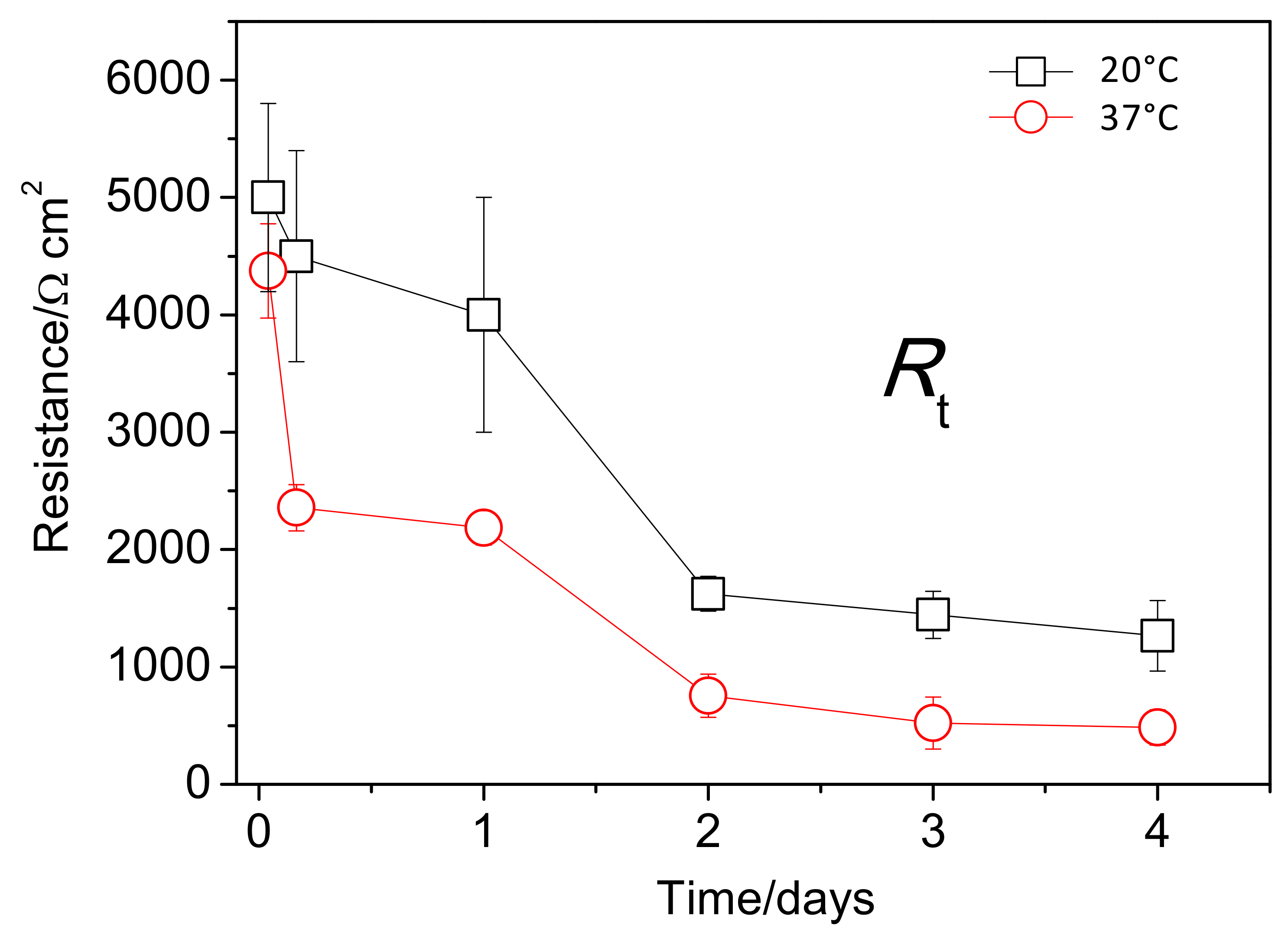
3.4. EIS Diagrams
4. Discussion
4.1. Influence of Temperature on the Composition and Thickness of the Al-Rich Corrosion Layer Formed on the Surface of the Az31b Alloy in Ringer’s Solution
4.2. Relationship Between Corrosion Resistance and the Characteristics of the Al-Rich Inner Corrosion Layer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saikrishna, N.; Reddy, G.P.K.; Munirathinam, B.; Sunil, B.R. Influence of bimodal grain size distribution on the corrosion behavior of friction stir processed biodegradable AZ31 magnesium alloy. J. Magn. Alloys. 2016, 4, 68–76. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. Biodegradable behaviors of AZ31 magnesium alloy in simulated body fluid. Mater. Sci. Eng. C 2009, 29, 1039–1045. [Google Scholar] [CrossRef]
- LeBozec, N.; Jonsson, M.; Thierry, D. Atmospheric corrosion of magnesium alloys: Influence of temperature, relative humidity, and chloride deposition. Corrosion 2004, 60, 356–361. [Google Scholar] [CrossRef]
- Merino, M.C.; Pardo, A.; Arrabal, R.; Merino, S.; Casajus, P.; Mohedano, M. Influence of chloride ion concentration and temperature on the corrosion of Mg–Al alloys in salt fog. Corros. Sci. 2010, 52, 1696–1704. [Google Scholar] [CrossRef]
- Melchers, R.E. Effect of temperature on the marine immersion corrosion of carbon steels. Corrosion 2002, 58, 768–782. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N. Magnesium Biomaterials: Design, Testing, and Best Practice; Springer New York: New York, NY, USA, 2014; pp. 40–43. [Google Scholar]
- Cai, Y.K.; Zhao, Y.; Ma, X.B.; Zhou, K.; Chen, Y. Influence of environmental factors on atmospheric corrosion in dynamic environment. Corros. Sci. 2018, 137, 163–175. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Abidin, N.I.Z.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in Hank’s solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M. The effect of temperature on the nucleation of corrosion pits on titanium in Ringer’s physiological solution. Biomaterials 2005, 26, 245–256. [Google Scholar] [CrossRef]
- Virtanen, S.; Milosev, I.; Gomez-Barrena, E.; Trebse, R.; Salo, J.; Konttinen, Y.T. Special modes of corrosion under physiological and simulated physiological conditions. Acta Biomater. 2008, 4, 468–476. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting corrosion of metals a review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Esmaily, M.; Blucher, D.B.; Svensson, J.E.; Halvarsson, M.; Johansson, L.G. New insights into the corrosion of magnesium alloys—the role of aluminum. Scripta Mater. 2016, 115, 91–95. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Phillips, R.C.; Kish, J.R. Nature of Surface Film on Matrix Phase of Mg Alloy AZ80 Formed in Water. Corrosion 2013, 69, 813–820. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion behavior of magnesium/aluminum alloys in 3.5wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Nordlien, J.H.; Nisancioglu, K.; Ono, S.; Masuko, N. Morphology and Structure of Oxide Films Formed on MgAl Alloys by Exposure to Air and Water. J. Electrochem. Soc. 1996, 143, 2564–2572. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Ge, F.; Lin, Y.; Wang, L.W.; Lei, L.; Tian, H.Y.; Yu, M.D.; Wang, X. Corrosion behavior of AZ31 magnesium alloy in the chloride solution containing ammonium nitrate. Electrochim. Acta 2018, 278, 421–437. [Google Scholar] [CrossRef]
- Jang, Y.; Collins, B.; Sankar, J.; Yun, Y. Effect of biologically relevant ions on the corrosion products formed on alloy AZ31B: An improved understanding of magnesium corrosion. Acta Biomater. 2013, 9, 8761–8770. [Google Scholar] [CrossRef]
- Liao, J.S.; Hotta, M. Corrosion products of field-exposed Mg-Al series magnesium alloys. Corros. Sci. 2016, 112, 276–288. [Google Scholar] [CrossRef]
- Delgado, M.G.; Garcia-Galvan, F.R.; Llorente, I.; Perez, P.; Adeva, P.; Feliu Jr, S. Influence of aluminum enrichment in the near-surface region of commercial twin-roll cast AZ31 alloys on their corrosion behavior. Corros. Sci. 2017, 123, 182–196. [Google Scholar] [CrossRef]
- ISO 16428, Implants for Surgery—Test Solutions and Environmental Conditions for Static and Dynamic Corrosion Tests on Implantable Materials and Medical Devices. 2005. Available online: https://www.iso.org/standard/30280.html (accessed on 5 April 2019).
- Johnston, S.; Shi, Z.M.; Dargusch, M.S.; Atrens, A. Influence of surface condition on the corrosion of ultra-high-purity Mg alloy wire. Corros. Sci. 2016, 108, 66–75. [Google Scholar] [CrossRef]
- Veleva, L.; Fernandez-Olaya, M.G.; Feliu, S. Initial stages of AZ31B magnesium alloy degradation in ringer’s solution: Interpretation of EIS, mass loss, hydrogen evolution data and scanning electron microscopy observations. Metals 2018, 8, 933. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 2, 63–72. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Cao, M.; Liu, L.; Fan, L.; Yu, Z.F.; Li, Y.; Oguzie, E.E.; Wang, F.H. Influence of Temperature on Corrosion Behavior of 2A02 Al Alloy in Marine Atmospheric Environments. Materials 2018, 11, 235. [Google Scholar] [CrossRef]
- Kish, J.R.; Hu, Y.; Li, J.; Zheng, W.; McDermid, J.R. Technical note: Examination of focused ion beam-sectioned surface films formed on AM60 B Mg alloy in an aqueous saline solution. Corrosion 2012, 68, 468–474. [Google Scholar] [CrossRef]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. A physical corrosion model for bioabsorbable metal stents. Acta Biomater. 2014, 10, 2313–2322. [Google Scholar] [CrossRef]

| Element | Mg | Al | Zn | Mn |
|---|---|---|---|---|
| wt% | 95.8 | 3.0 | 1.0 | 0.2 |
| Exposure Temperature | O (at.%) | Mg (at.%) | Al (at.%) | Cl (at.%) | Zn (at.%) |
|---|---|---|---|---|---|
| 20 °C | 64 ± 4 | 33 ± 4 | 2.3 ± 0.2 | 0.0 ± 0.1 | 0.2 ± 0.2 |
| 37 °C | 47 ± 2 | 39 ± 3 | 4.2 ± 0.6 | 8.1 ± 1 | 1.8 ± 0.4 |
| Temperature | Average Pit Depth (µm) | Average Pit Width (µm) |
|---|---|---|
| 20 °C | 105 ± 57 | 256 ± 45 |
| 37 °C | 470 ± 325 | 1857 ± 405 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliu, S., Jr.; Veleva, L.; García-Galvan, F. Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution. Metals 2019, 9, 591. https://doi.org/10.3390/met9050591
Feliu S Jr., Veleva L, García-Galvan F. Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution. Metals. 2019; 9(5):591. https://doi.org/10.3390/met9050591
Chicago/Turabian StyleFeliu, Sebastian, Jr., Lucien Veleva, and Federico García-Galvan. 2019. "Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution" Metals 9, no. 5: 591. https://doi.org/10.3390/met9050591
APA StyleFeliu, S., Jr., Veleva, L., & García-Galvan, F. (2019). Effect of Temperature on the Corrosion Behavior of Biodegradable AZ31B Magnesium Alloy in Ringer’s Physiological Solution. Metals, 9(5), 591. https://doi.org/10.3390/met9050591







