Kinetics of Phosphorus Transfer during Industrial Electroslag Remelting of G20CrNi2Mo Bearing Steel
Abstract
1. Introduction
2. Methodology
2.1. Experimental Procedure
2.2. Chemical Analysis
3. Kinetic Analysis of Phosphorus Transfer
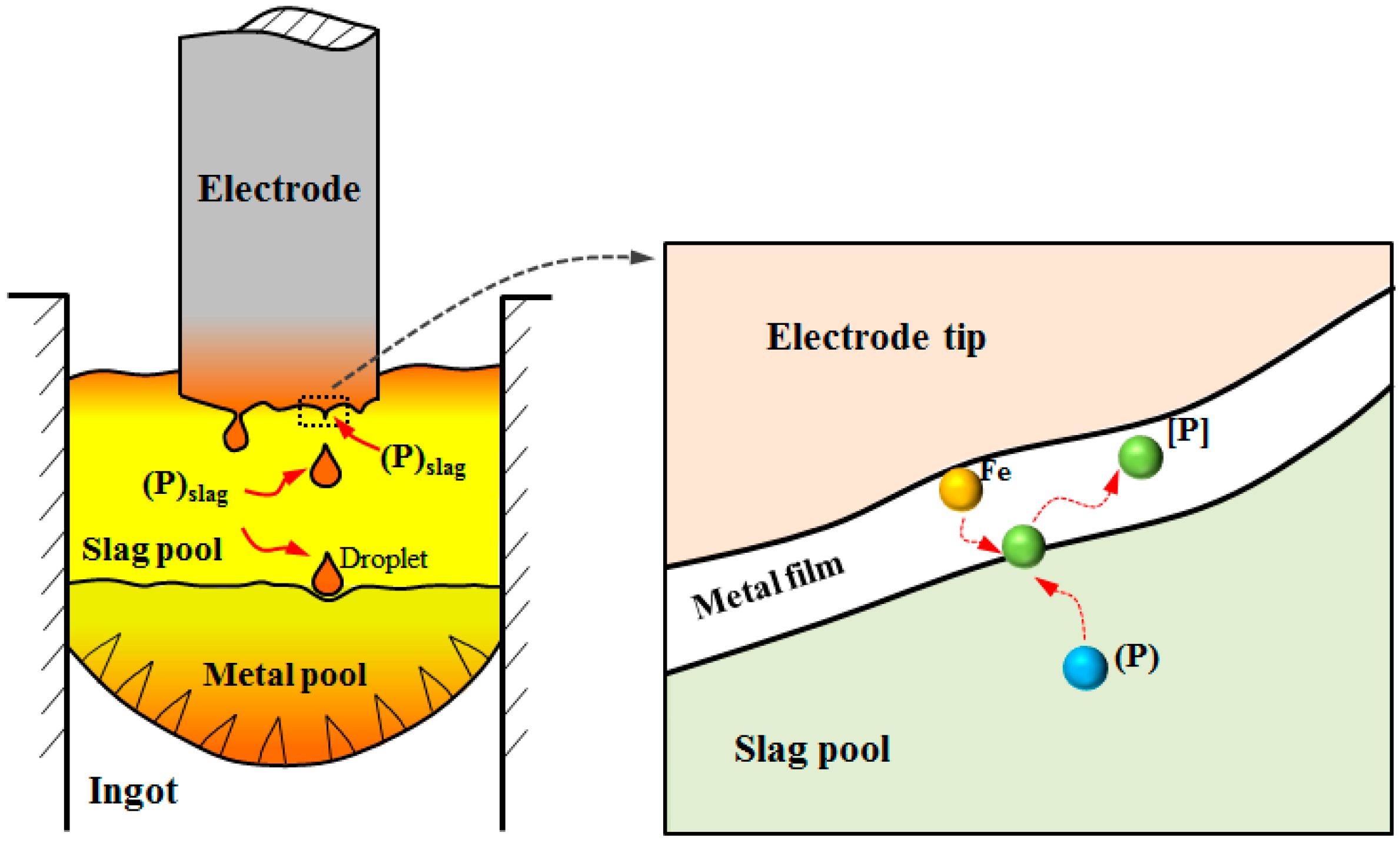
3.1. Model Assumptions
3.2. Mass Transfer Kinetics
3.3. Mass Transfer Coefficients
3.3.1. Mass Transfer Coefficient at Metal Film–Slag Interface
3.3.2. Mass Transfer Coefficient at the Droplet–Slag Interface
3.3.3. Mass Transfer Coefficient at Metal Pool–Slag Interface
3.4. Area/Volume Ratio
3.5. Modeling Solution Procedure
4. Discussion
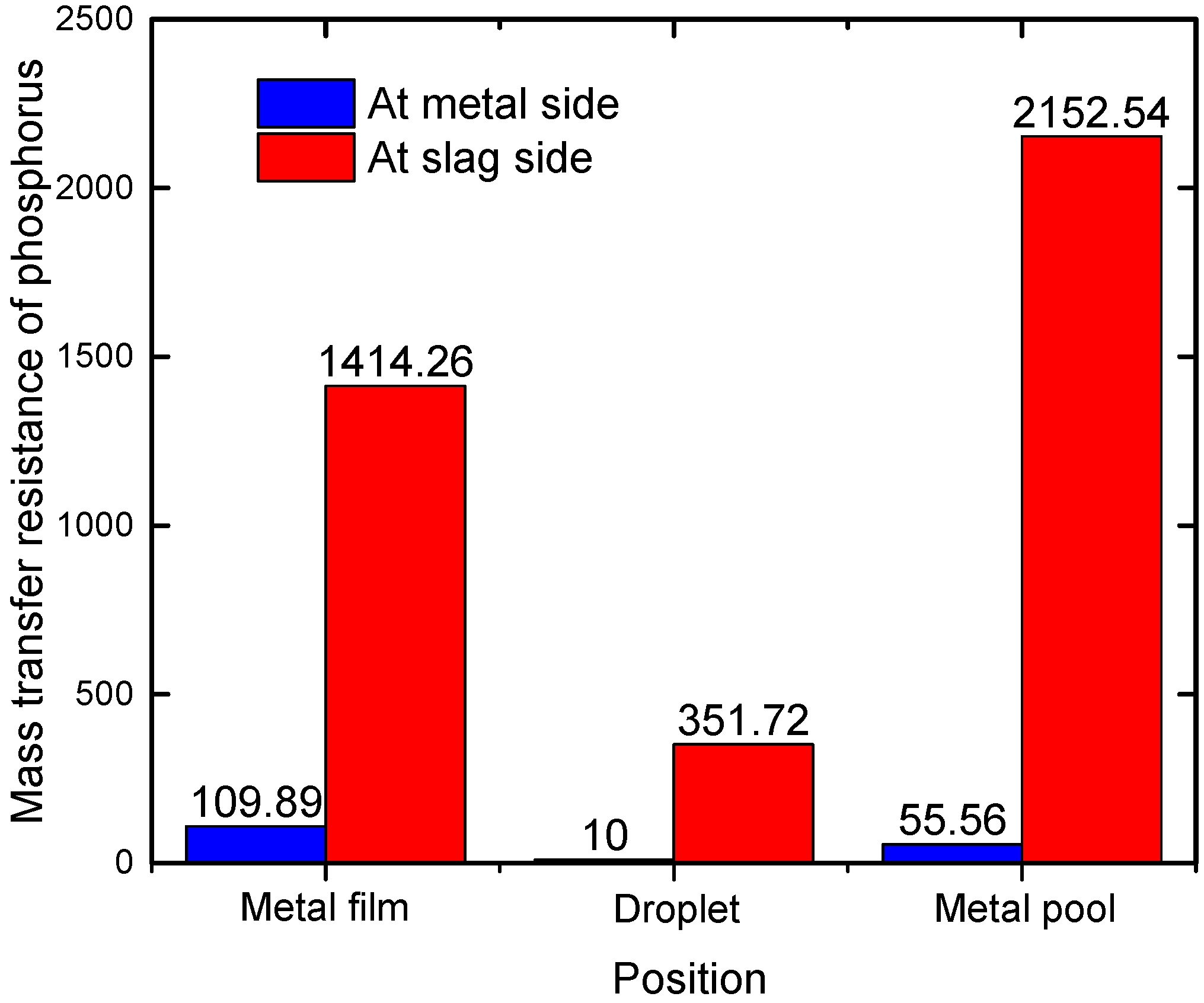
4.1. Mass Transfer Resistance of Phosphorus in Steel and Slag
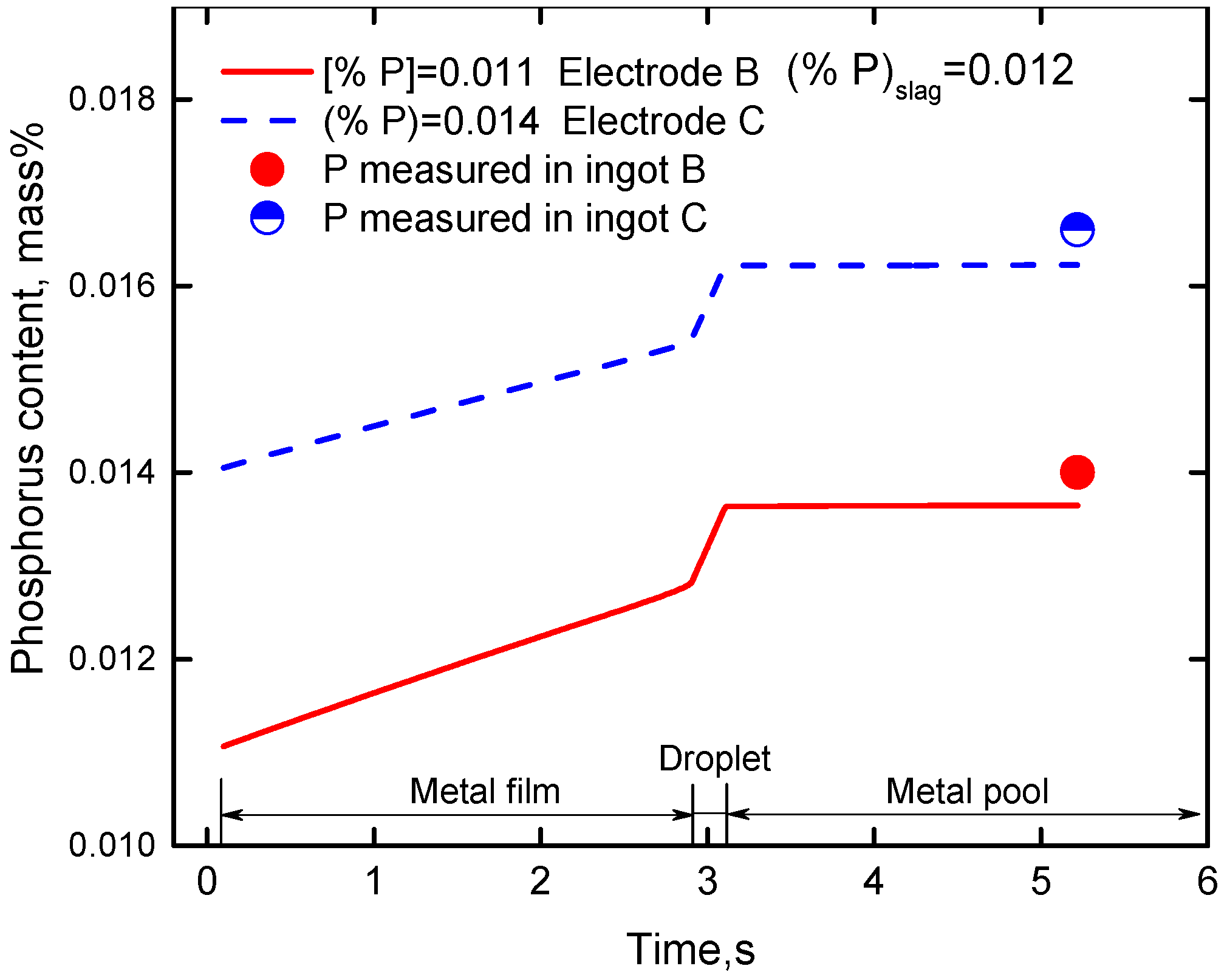
4.2. Effect of Phosphorus Content in Slag
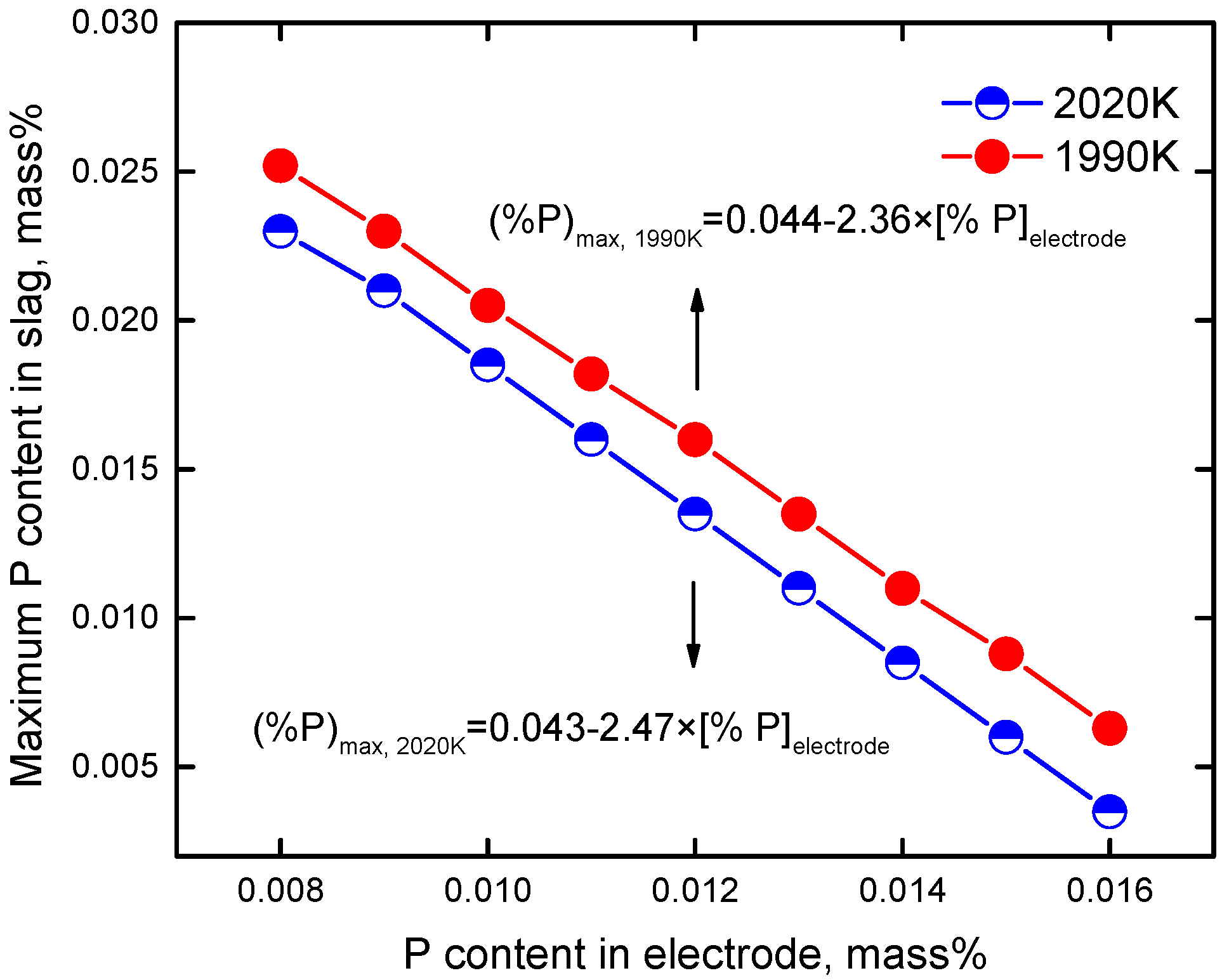
4.3. Effect of Phosphorus Content in the Electrode
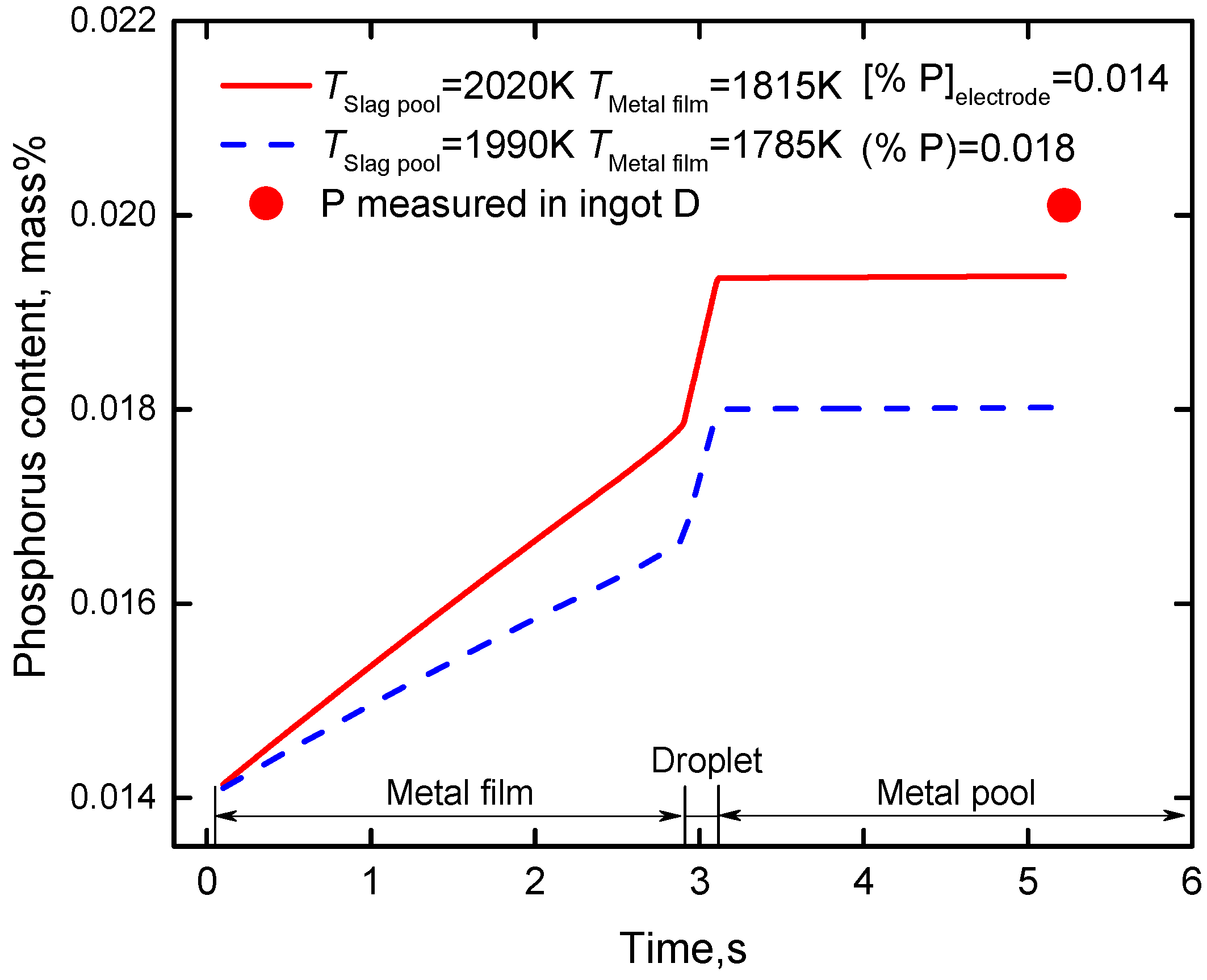
4.4. Effect of Temperature of Slag Pool
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z. A study on production process of G20CrNi2MoA carburizing bearing steel. Spe. St. Tech. 2010, 16, 10–14. [Google Scholar]
- Miao, X.; Yu, C.; Shi, C.; Du, J.; Zhu, H.; Cheng, G. Formation and control of calcium aluminates in bearing steel. J. Univ. Sci. Tech. Beijing 2008, 29, 771–775. [Google Scholar]
- Weber, V.; Jardy, A.; Dussoubs, B.; Ablitzer, D.; Ryberon, S.; Schmitt, V.; Hans, S.; Poisson, H. A comprehensive model of the electroslag remelting process: Description and validation. Metall. Mater. Trans. B 2009, 40, 271–280. [Google Scholar] [CrossRef]
- Rückert, A.; Pfeifer, H. Mathematical modelling of the flow field, temperature distribution, melting and solidification in the electroslag remelting process. Magnetohydrodynamics 2009, 45, 527–533. [Google Scholar]
- Matity, S.; Ballal, N.; Goldhahn, G.; Kwaalla, R. Development of ultrahigh strength low alloy steel through electroslag refining process. ISIJ Int. 2009, 49, 902–910. [Google Scholar] [CrossRef]
- Briant, C.; Banerji, S. Tempered martensite embrittlement in a high purity steel. Metall. Mater. Trans. A 1979, 10, 1151–1155. [Google Scholar] [CrossRef]
- Bloom, T.; Foshnacht, D.; Haezebrouck, D. The influence of phosphorus on the properties of sheet steel products and methods used to control steel phosphorus levels in steel product manufacturing. Iron Steelmak. 1990, 17, 35–41. [Google Scholar]
- Basu, S.; Lahiri, A.K.; Seetharaman, S. Phosphorus partition between liquid steel and CaO-SiO2-FeOx-P2O5-MgO slag containing 15 to 25 Pct FeO. Metall. Mater. Trans. B 2007, 38, 623–630. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R.; Takada, M. Phosphorus distribution between liquid iron and MgO saturated slags of the system CaO-MgO-FeOx-SiO2. Tetsu-to-Hagane 1981, 67, 2645–2654. [Google Scholar] [CrossRef]
- Muraki, M.; Fukushima, H.; Sano, N. Phosphorus distribution between CaO-CaF2-SiO2 melts and carbon-saturated iron. Trans. ISIJ 1985, 25, 1025–1030. [Google Scholar] [CrossRef]
- Shirota, Y.; Katohgi, K.; Klein, K.; Klein, K.; Engell, H.; Janke, D. Phosphate capacity of FeO-Fe2O3-CaO-P2O5 and FeO-Fe2O3-CaO-CaF2-P2O5 slags by levitation melting. Trans. ISIJ 1985, 25, 1132–1140. [Google Scholar] [CrossRef]
- Im, J.; Morita, K.; Sano, N. Phosphorus distribution ratios between CaO-SiO2-FetO slags and carbon-saturated iron at 1573 K. ISIJ Int. 1996, 36, 517–521. [Google Scholar] [CrossRef]
- Turkdogan, E. Assessment of P2O5 activity coefficients in molten slags. ISIJ Int. 2000, 40, 964–970. [Google Scholar] [CrossRef]
- Lee, C.; Fruehan, R. Phosphorus equilibrium between hot metal and slag. Iron Steelmak. 2005, 32, 503–508. [Google Scholar] [CrossRef]
- Basu, S.; Lahiri, A.; Seetharaman, S. Phosphorus partition between liquid steel and CaO-SiO2 -P2O5-MgO slag containing low FeO. Metall. Mater. Trans. B 2007, 38, 357–366. [Google Scholar] [CrossRef]
- Yang, X.; Duan, J.; Shi, C.; Zhang, M.; Zhang, Y.; Wang, J. A thermodynamic model of phosphorus distribution ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags and molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Assis, A.; Tayeb, M.; Sridhar, S.; Fruehan, R. Phosphorus equilibrium between liquid iron and CaO-SiO2 -MgO-Al2O3-FeO-P2O5 Slag Part 1: literature review, methodology, and BOF Slags. Metall. Mater. Trans. B 2015, 46, 2255–2263. [Google Scholar] [CrossRef]
- Kitamura, S.; Kitamura, T.; Shibata, K.; Mizukam, Y.; Mukaw, S.; Nakagawa, J. Effect of stirring energy, temperature and flux composition on hot metal dephosphorization kinetics. ISIJ Int. 1991, 31, 1322–1328. [Google Scholar] [CrossRef]
- Wei, P.; Sano, M.; Hirasawa, M.; Mori, K. Phosphorus reaction between molten iron of high carbon concentration and slag containing FeO and Fe2O3. Tetsu-to-Hagane 1990, 76, 878–885. [Google Scholar] [CrossRef]
- Monaghan, B.; Pomfret, R.; Coley, K. The kinetics of dephosphorization of carbon-saturated iron using an oxidizing slag. Metall. Mater. Trans. B 1998, 29, 111–118. [Google Scholar] [CrossRef]
- Mori, K.; Fukami, Y.; Kawai, Y. Rate of dephosphorization of liquid iron-carbon alloys by molten slags. Trans. ISIJ 1988, 28, 315–318. [Google Scholar] [CrossRef][Green Version]
- Manning, C.; Fruehan, R. The rate of the phosphorous reaction between liquid iron and slag. Metall. Mater. Trans. B 2013, 44, 37–44. [Google Scholar] [CrossRef]
- Sasabe, M.; Kinoshita, Y. Permeability of oxygen through molten CaO-SiO2-Al2O3 system with Fe2O3 or CaF2. Tetsu-to-Hagane 1979, 65, 1727–1736. [Google Scholar] [CrossRef][Green Version]
- Shibata, E.; Sun, H.; Mori, K. Transfer rate of oxygen from gas into liquid iron through molen slag. Tetsu-to-Hagane 1999, 85, 27–33. [Google Scholar] [CrossRef]
- Kawai, Y.; Nakamura, H.; Kawakami, K.; Toyoda, T.; Ishizaka, A.; Ebisawa, T. Thermodynamics and kinetics of hot metal dephosphorization by CaO based slag containing CaF2. Tetsu-to-Hagane 1983, 69, 1755–1762. [Google Scholar] [CrossRef][Green Version]
- Suito, H.; Inoue, R. Effect of calcium fluoride on phosphorus distribution between MgO-saturated slags of the system CaO-MgO-FeOx-SiO2 and liquid iron. Tetsu-to-Hagane 1982, 68, 1541–1550. [Google Scholar] [CrossRef]
- Li, S.; Cheng, G.; Miao, Z.; Chen, L.; Li, C.; Jiang, X. Kinetic analysis of aluminum and oxygen variation of G20CrNi2Mo bearing steel during industrial electroslag remelting process. ISIJ Int. 2017, 57, 2148–2156. [Google Scholar] [CrossRef]
- Fraser, M.; Mitchell, A. Mass transfer in the electroslag process. Pt. 2. Mass-transfer coefficients. Iron Steelmak. 1976, 3, 288–301. [Google Scholar]
- Wei, J.; Mitchell, A. Changes in composition during A.C. ESR- I. Theoretical development. Act. Metall. Sin. 1984, 20, 261–279. [Google Scholar]
- Etienne, M. Loss of reactive elements during electroslag processing of iron-base alloys. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1970. [Google Scholar]
- Shi, C.; Chen, X.; Guo, H.; Zhu, Z.; Ren, H. Assessment of oxygen control and its effect on inclusion characteristics during electroslag remelting of die steel. Steel Res. Int. 2012, 83, 472–486. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Shi, C.; Geng, R.; Zhang, J. Effect of directional solidification in electroslag remelting on the microstructure and cleanliness of an austenitic hot-work die steel. ISIJ Int. 2018, 58, 1275–1284. [Google Scholar] [CrossRef]
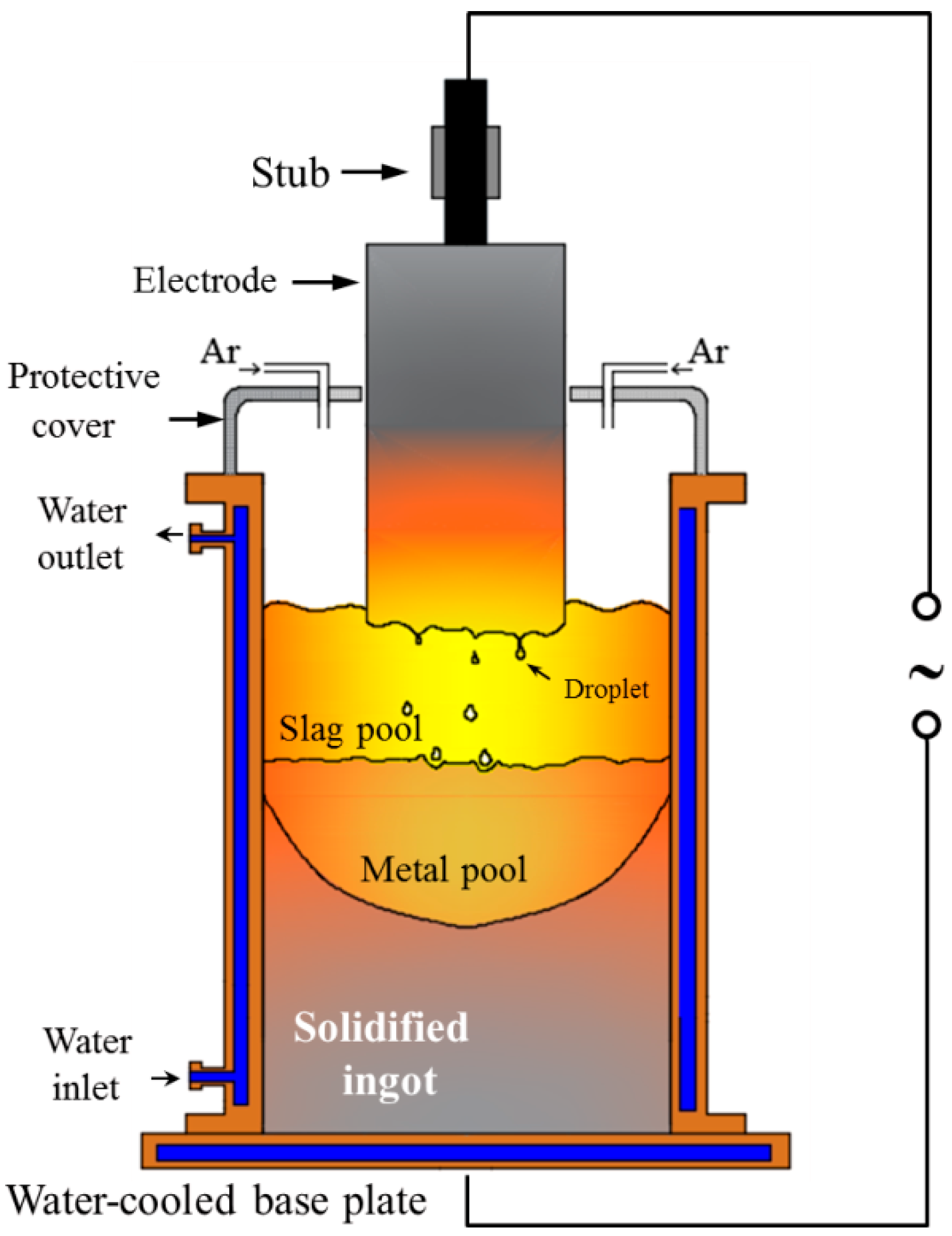
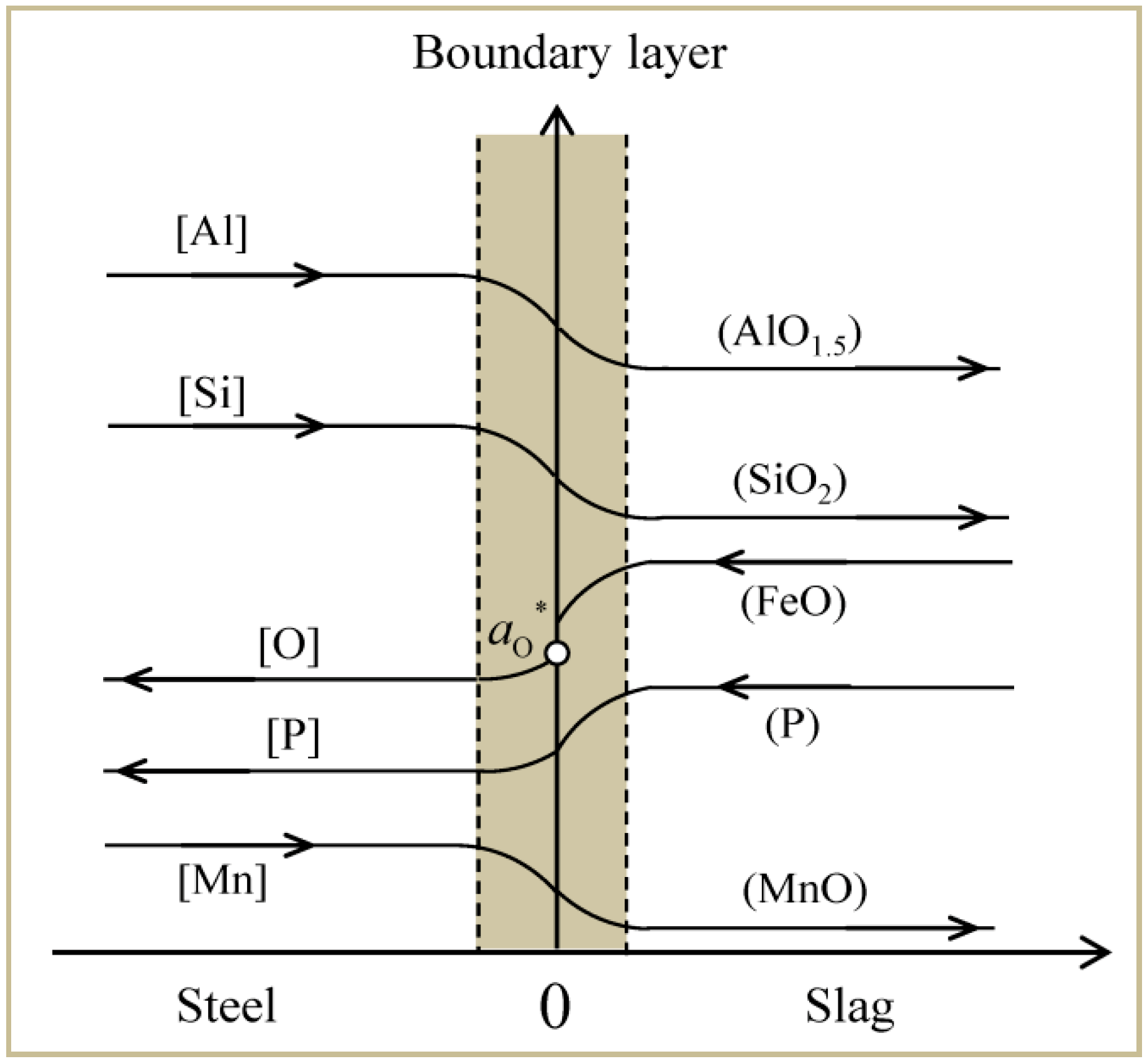


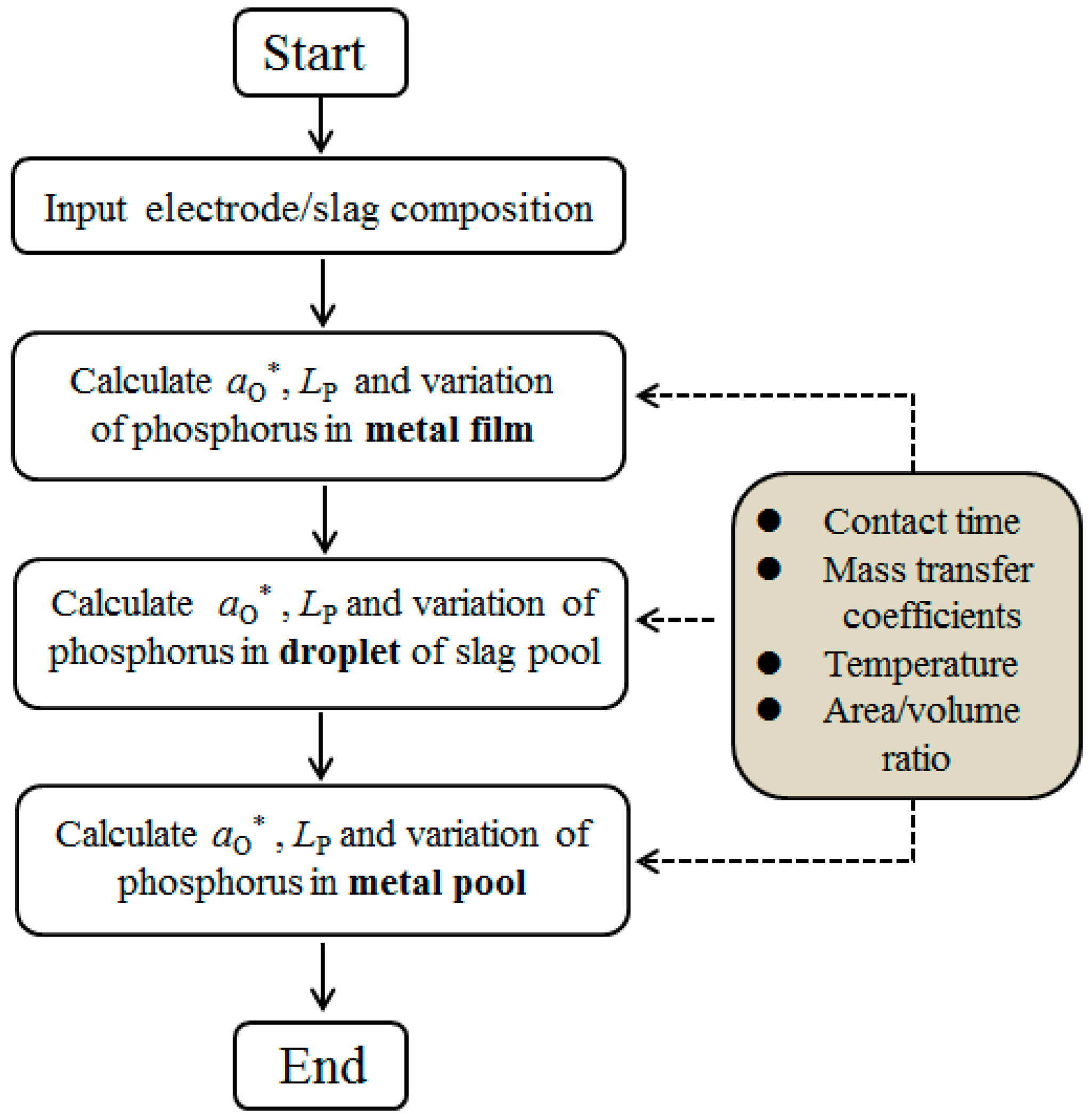
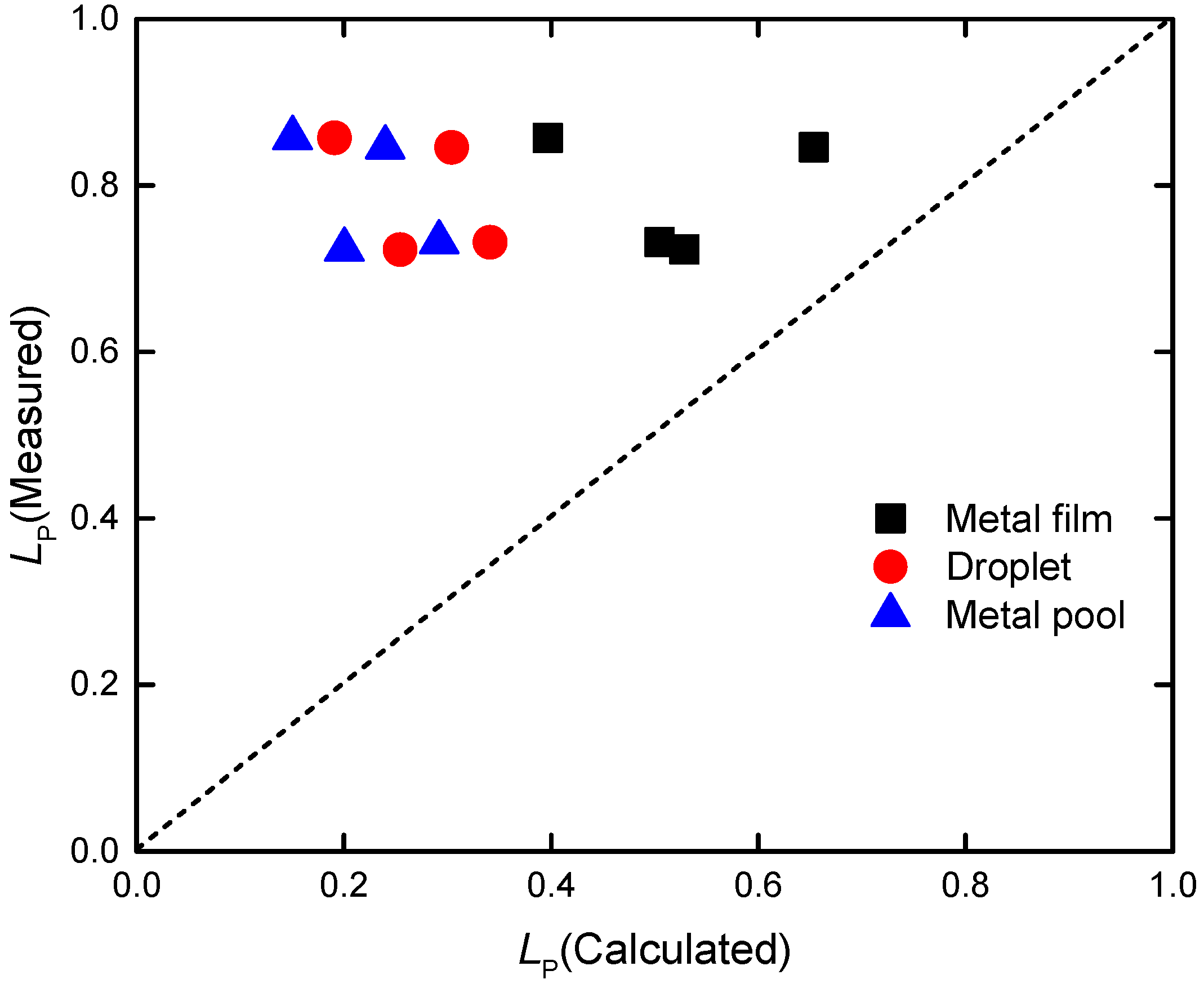

| Exp. | Voltage (V) | Current (A) | Temperature (K/°C) | Melting Rate (kg/min) |
|---|---|---|---|---|
| A | 55 | 7000–9000 | 2005 (1732) | 6.30 |
| B | 55 | 7000–9000 | 1997 (1724) | 6.20 |
| C | 54 | 7000–9000 | 1993 (1720) | 6.15 |
| D | 53 | 7000–9000 | 1988 (1715) | 6.12 |
| Exp. | CaF2 | Al2O3 | CaO | MgO | SiO2 | MnO | FeO | P |
|---|---|---|---|---|---|---|---|---|
| A | 30.89 | 31.80 | 31.76 | 5.39 | 1.83 | 0.10 | 0.27 | 0.009 |
| B | 38.53 | 43.83 | 13.07 | 3.98 | 1.41 | 0.12 | 0.32 | 0.012 |
| C | 55.72 | 35.89 | 7.38 | 0.23 | 1.45 | 0.16 | 0.38 | 0.012 |
| D | 62.15 | 32.58 | 4.42 | 0.10 | 1.20 | 0.18 | 0.43 | 0.017 |
| Exp. | Compositions in Electrode | Compositions at Bottom of Ingot | ||||||
|---|---|---|---|---|---|---|---|---|
| [Als] | [Si] | [Mn] | [P] | [Als] | [Si] | [Mn] | [P] | |
| A | 0.040 | 0.33 | 0.62 | 0.011 | 0.034 | 0.22 | 0.61 | 0.0123 |
| B | 0.040 | 0.33 | 0.62 | 0.011 | 0.029 | 0.28 | 0.60 | 0.0140 |
| C | 0.040 | 0.32 | 0.62 | 0.014 | 0.020 | 0.30 | 0.58 | 0.0166 |
| D | 0.040 | 0.32 | 0.62 | 0.014 | 0.017 | 0.31 | 0.59 | 0.0201 |
| Parameters | Metal Film | Droplet in Slag Pool | Metal Pool |
|---|---|---|---|
| kP,m cm/s | 0.0091 | 0.10 | 0.018 |
| kP,s cm/s | 0.0037 | 0.022 | 0.0042 |
| Temperature, (K/°C) | 1815 (1542) | 2020 (1747) | 1957 (1684) |
| Am/Vm, cm−1 | 70 | 24 | 0.15 |
| Residence time, s | 2.89 | 0.23 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Cheng, G.; Huang, Y.; Dai, W.; Miao, Z. Kinetics of Phosphorus Transfer during Industrial Electroslag Remelting of G20CrNi2Mo Bearing Steel. Metals 2019, 9, 467. https://doi.org/10.3390/met9040467
Li S, Cheng G, Huang Y, Dai W, Miao Z. Kinetics of Phosphorus Transfer during Industrial Electroslag Remelting of G20CrNi2Mo Bearing Steel. Metals. 2019; 9(4):467. https://doi.org/10.3390/met9040467
Chicago/Turabian StyleLi, Shijian, Guoguang Cheng, Yu Huang, Weixing Dai, and Zhiqi Miao. 2019. "Kinetics of Phosphorus Transfer during Industrial Electroslag Remelting of G20CrNi2Mo Bearing Steel" Metals 9, no. 4: 467. https://doi.org/10.3390/met9040467
APA StyleLi, S., Cheng, G., Huang, Y., Dai, W., & Miao, Z. (2019). Kinetics of Phosphorus Transfer during Industrial Electroslag Remelting of G20CrNi2Mo Bearing Steel. Metals, 9(4), 467. https://doi.org/10.3390/met9040467





