Effect of Thermodynamic Melt Formation Characteristics on Liquid Phase Fluidity of Iron Ore in the Sintering Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
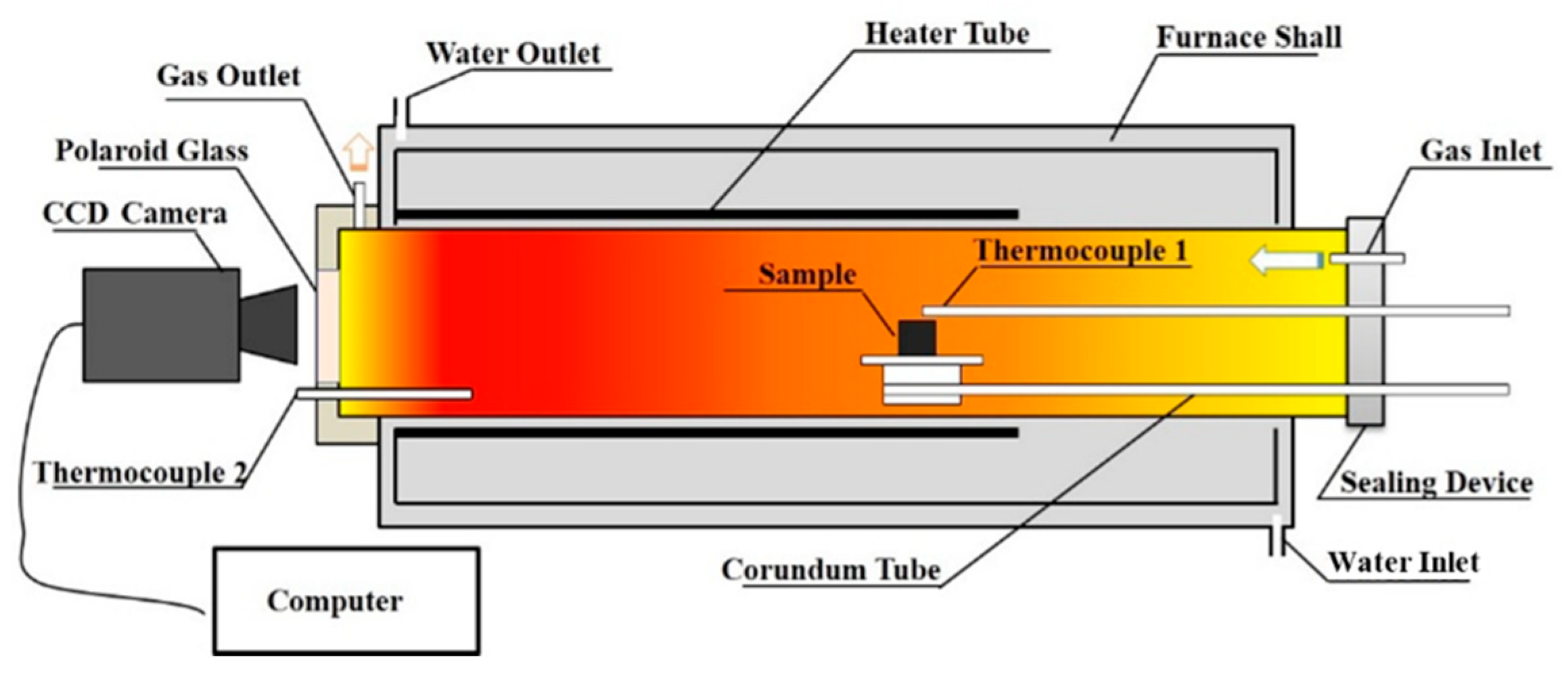
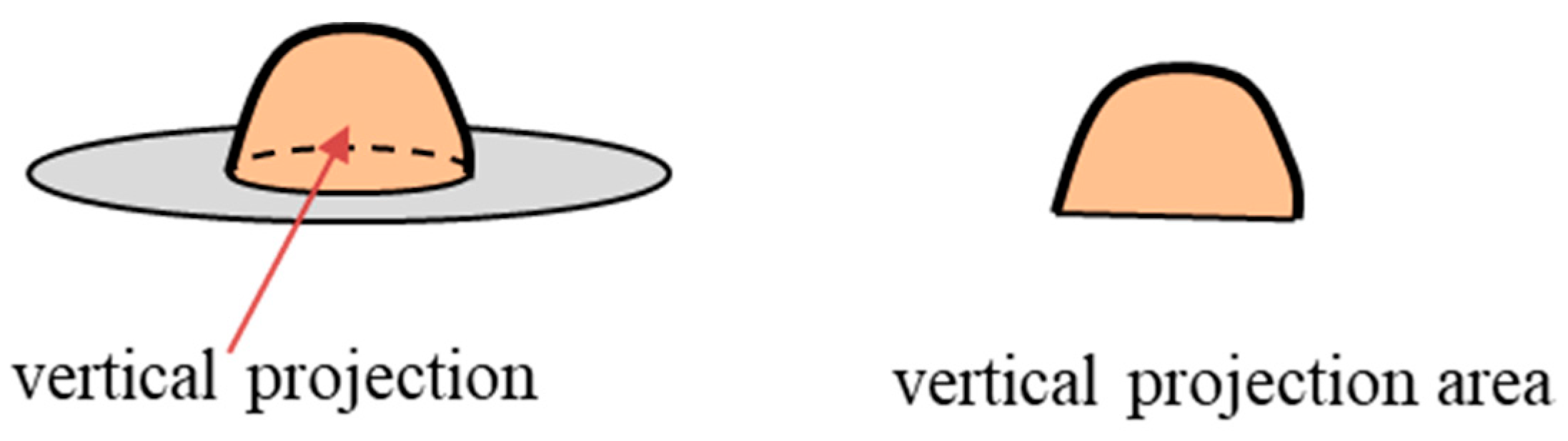
2.2. Experimental Procedure
3. Results
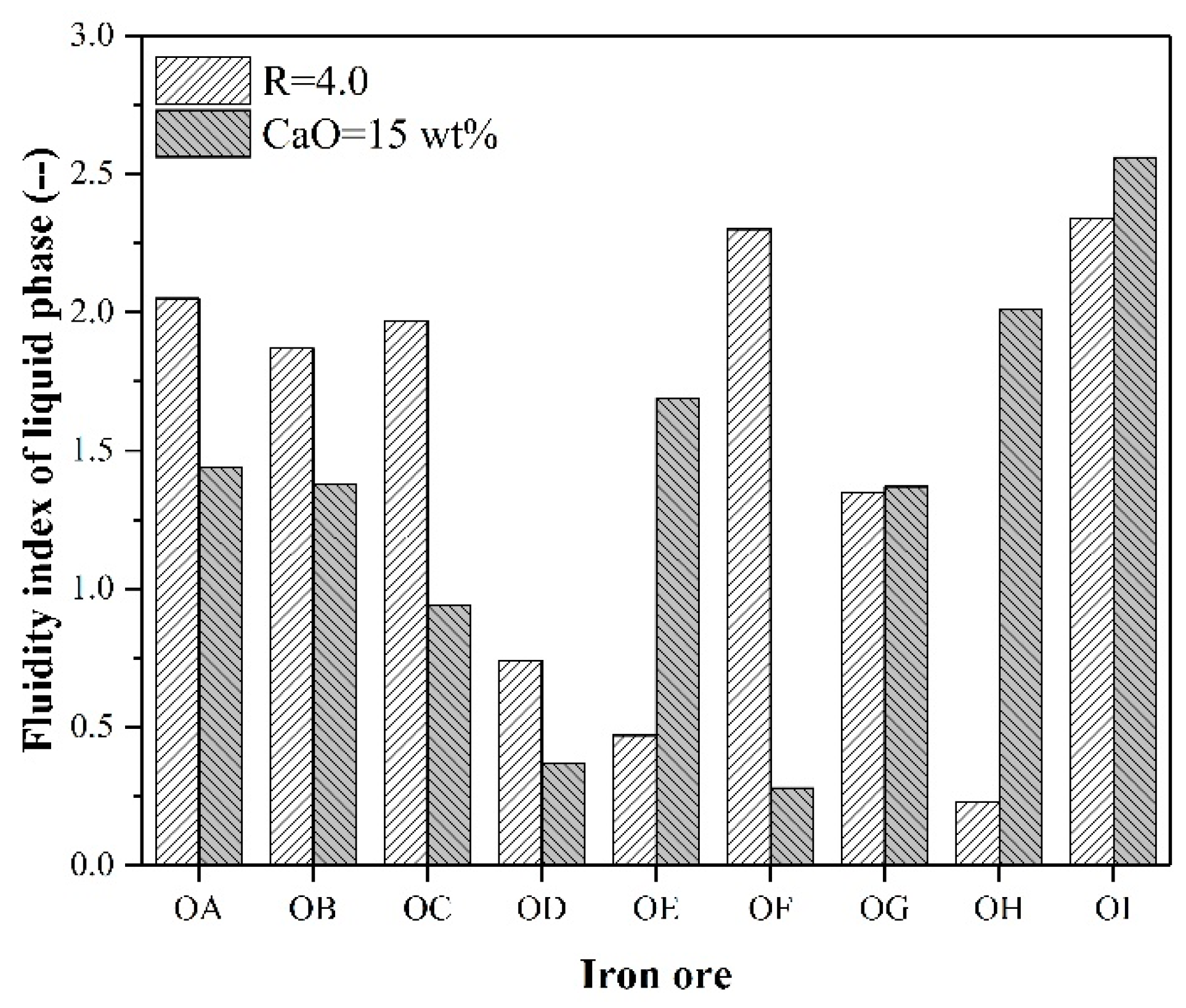
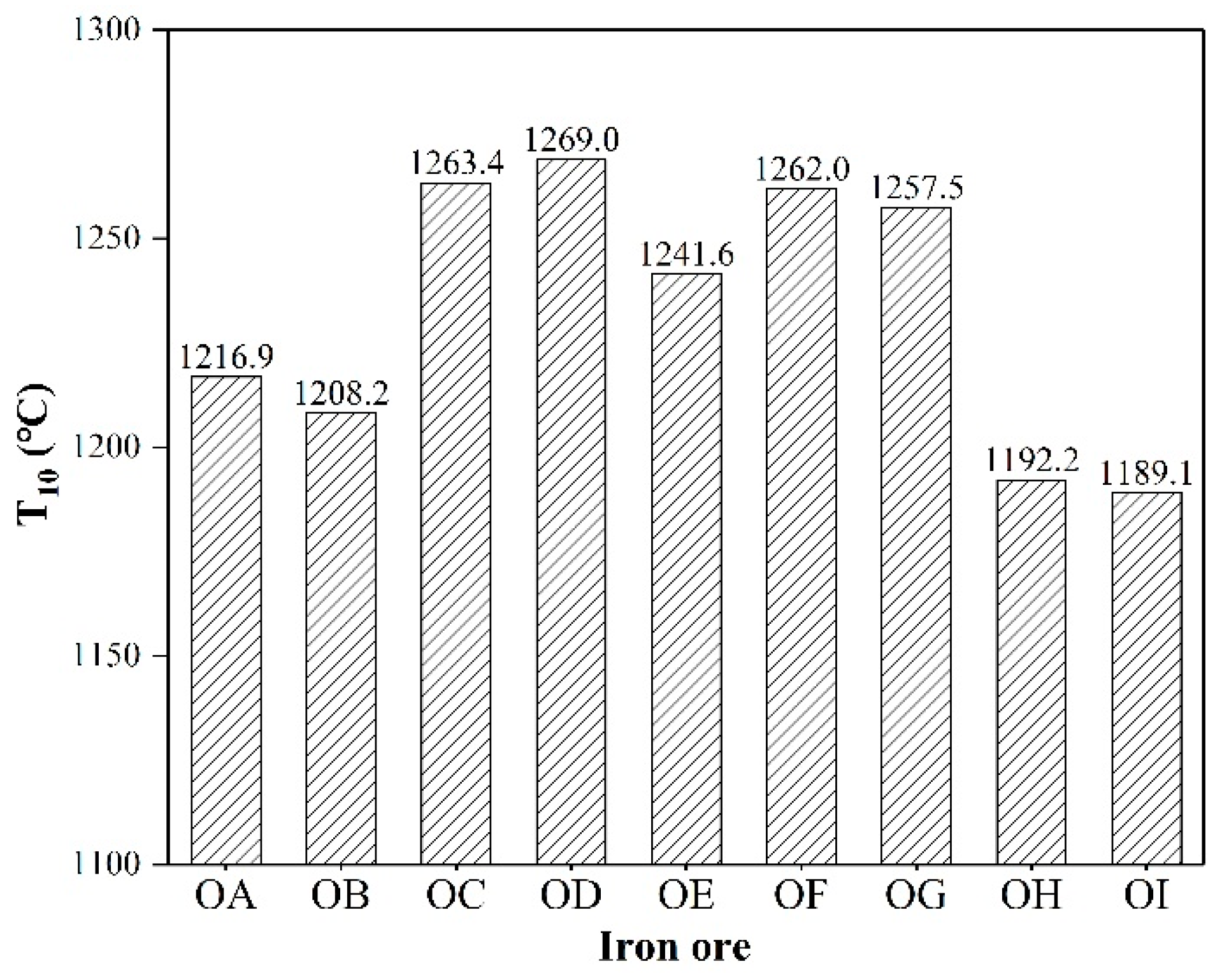
3.1. Liquid Phase Fluidity and Generation Temperature of Effective Liquid Phase
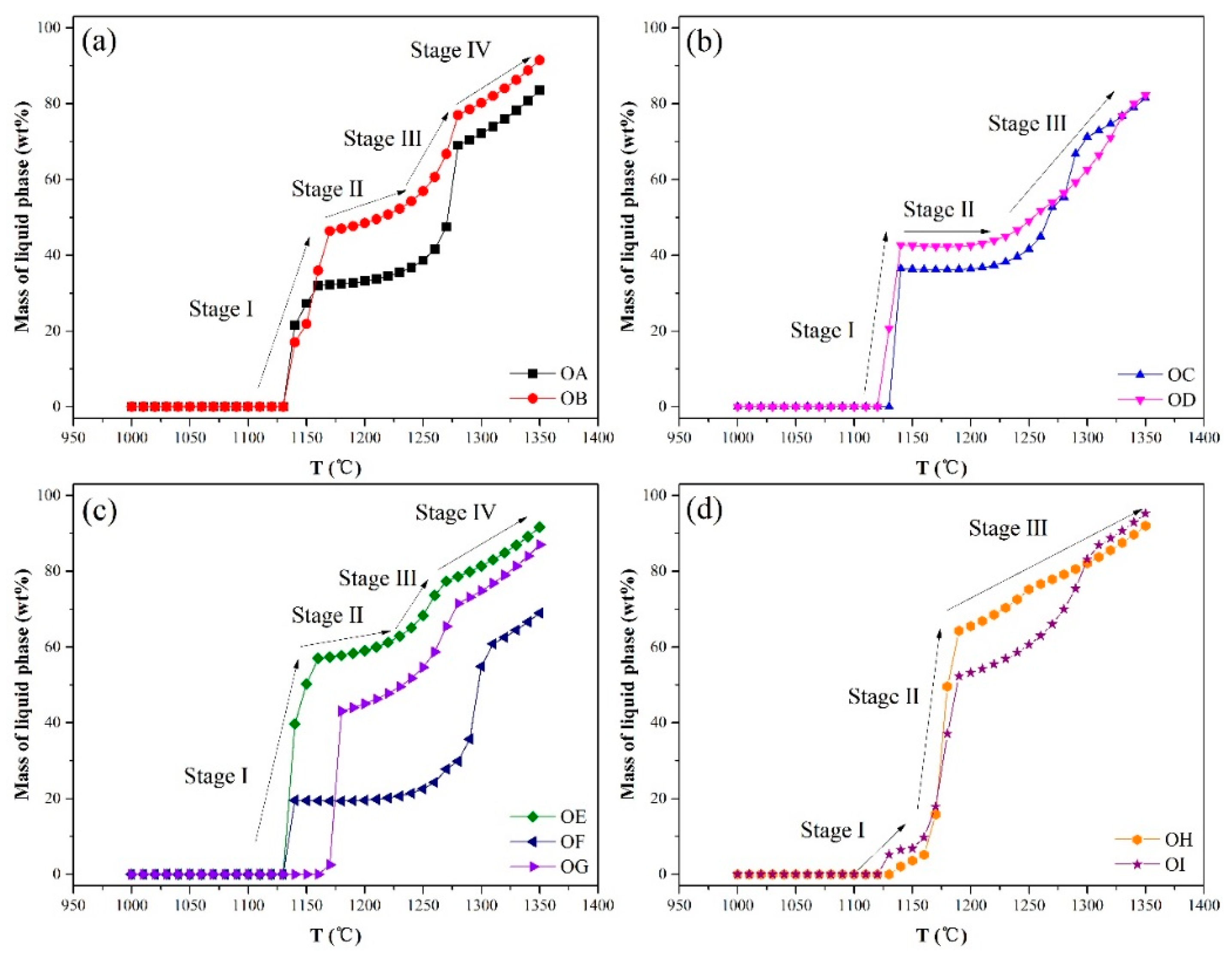
3.2. Thermodynamic Equilibrium and Viscosity Calculations
4. Discussion
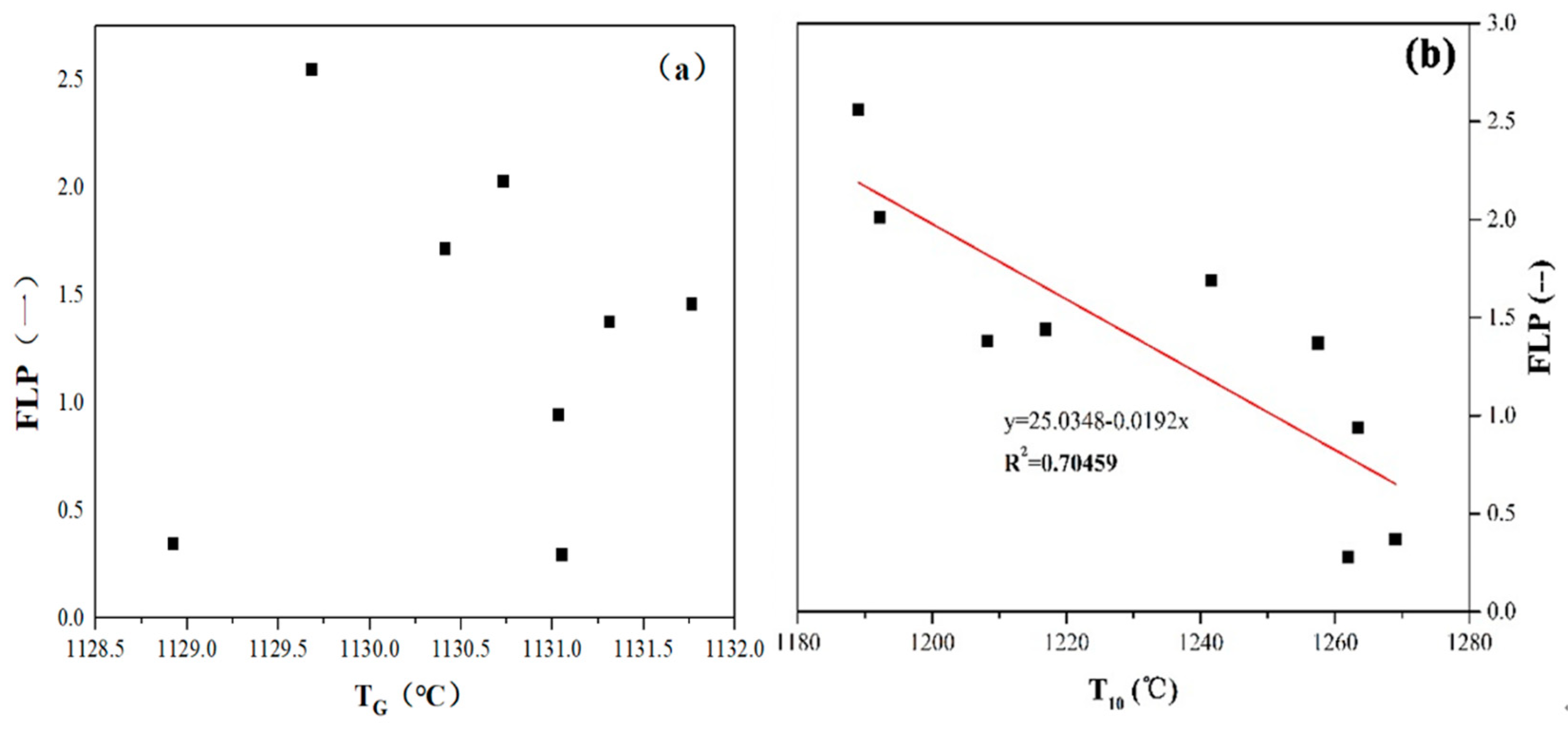
4.1. The Effect of the Melt Formation Temperature on FLP
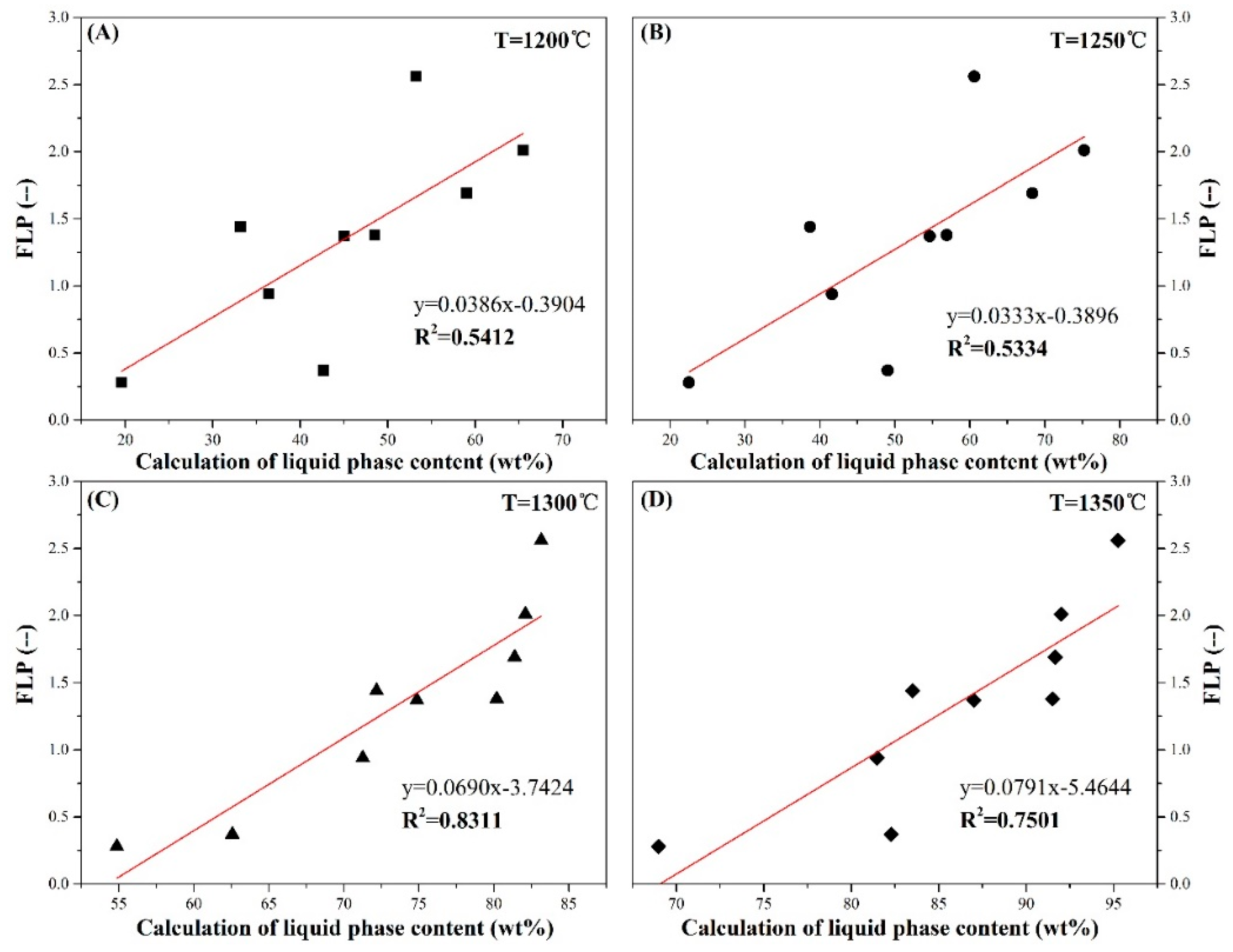
4.2. The Relationship between Liquid Phase Content and FLP
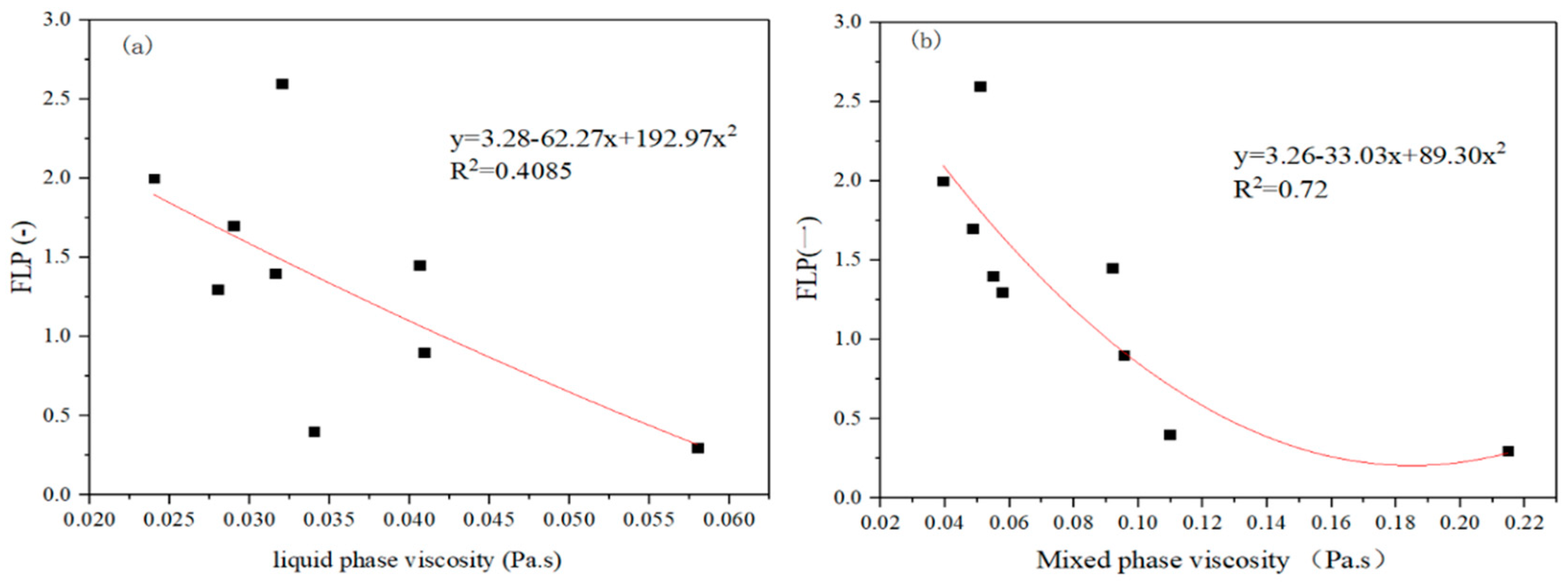
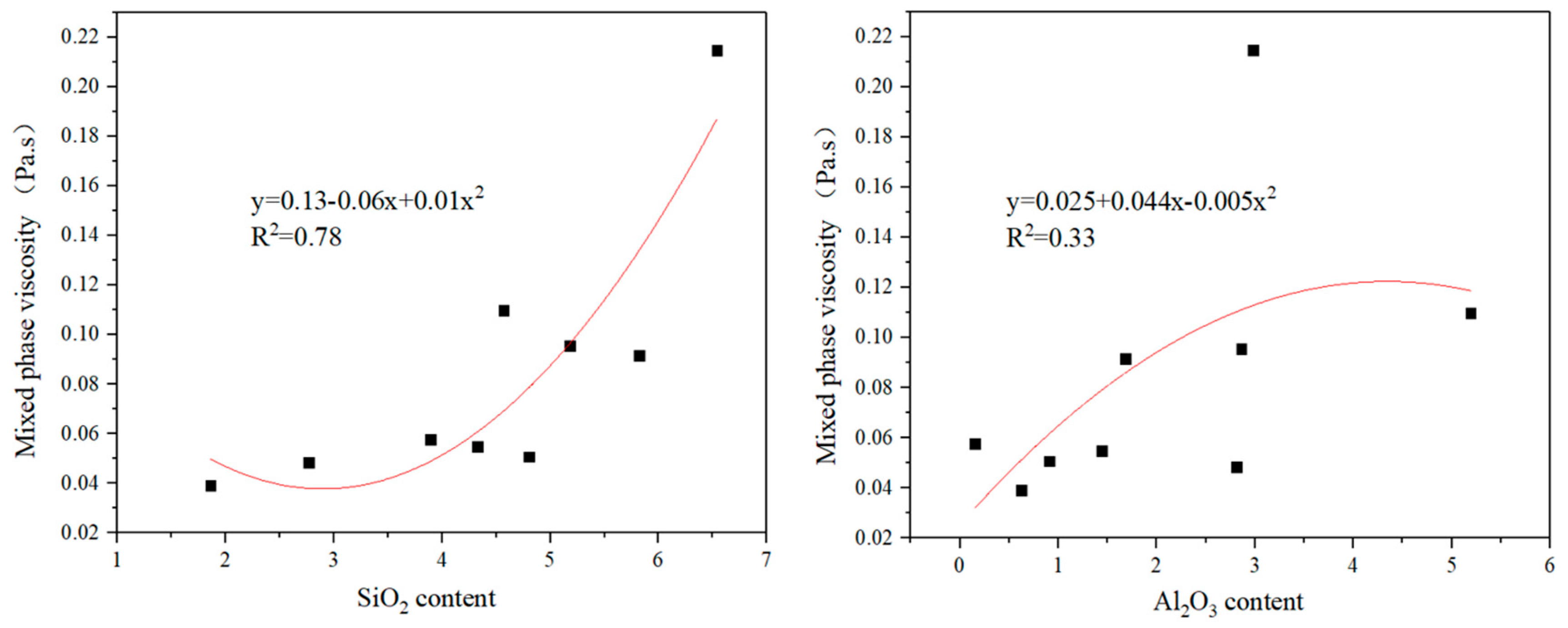
4.3. The Effect of the Liquid Phase Viscosity on FLP
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C. Silico-ferrite of calcium and aluminum (SFCA) iron ore sinter bonding phases: New insights into their formation during heating and cooling. Metall. Mater. Trans. B 2012, 43, 1344–1357. [Google Scholar] [CrossRef]
- Sinha, M.; Nistala, S.H.; Chandra, S.; Mankhand, T.R.; Ghose, A.K. Correlating mechanical properties of sinter phases with their chemistry and its effect on sinter quality. Ironmak. Steelmak. 2017, 44, 100–107. [Google Scholar] [CrossRef]
- Umadevi, T.; Brahmacharyulu, A.; Sah, R.; Mahapatra, P.C.; Prabhu, M. Optimisation of MgO addition in low and high silica iron ore sinter to improve sinter reducibility at JSW Steel Limited. Ironmak. Steelmak. 2014, 41, 270–278. [Google Scholar] [CrossRef]
- Wu, S.L.; Liu, Y.; Du, J.X.; Mi, K.; Lin, H. Experiment study of assimilation ability between iron ores and CaO. J. Univ. Sci. Technol. Beijing 2002, 24, 254–257. (In Chinese) [Google Scholar] [CrossRef]
- Wu, S.L.; Du, J.X.; Ma, H.B.; Tian, Y.Q.; Xu, H.F. Fluidity of liquid phase in iron ores during sintering. J. Univ. Sci. Technol. Beijing 2005, 27, 291–293. (In Chinese) [Google Scholar] [CrossRef]
- Wu, S.L.; Pei, Y.D.; Chen, H.; Peng, P.; Yang, F. Evaluation on liquid phase fluidity of iron ore in sintering. J. Univ. Sci. Technol. Beijing 2008, 30, 1095–1100. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, Z.X.; Chen, L.K.; Yang, Y.D.; Li, X.C.; Barati, M. Effect of Coarse-grain and Low-grade Iron Ores on Sinter Properties. ISIJ Int. 2017, 57, 795–804. [Google Scholar] [CrossRef]
- Wu, S.L.; Oliveira, D.; Dai, Y.M.; Xu, J. Ore-blending optimization model for sintering process based on characteristics of iron ores. Int. J. Miner. Metall. Mater. 2012, 19, 217–224. [Google Scholar] [CrossRef]
- Wu, S.L.; Zhang, G.L.; Chen, S.G.; Su, B. Influencing factors and effects of assimilation characteristic of iron ores in sintering process. ISIJ Int. 2014, 54, 582–588. [Google Scholar] [CrossRef]
- Lv, X.W.; Bai, C.G.; Deng, Q.Y.; Huang, X.B.; Qiu, G.B. Behavior of liquid phase formation during iron ores sintering. ISIJ Int. 2011, 51, 722–727. [Google Scholar] [CrossRef]
- Okazaki, J.; Higuchi, K.; Hosotani, Y.; Shinagawa, K. Influence of iron ore characteristics on penetrating behavior of melt into ore layer. ISIJ Int. 2003, 43, 1384–1392. [Google Scholar] [CrossRef]
- Wu, S.L.; Bian, M.L.; Wang, Q.F.; Zhang, G.L.; Du, Y. Fusion characteristics of iron ore fines and its evaluation method. J. Univ. Sci. Technol. Beijing 2010, 32, 1526–1531. (In Chinese) [Google Scholar] [CrossRef]
- Liu, D.M.; Loo, C.E.; Pinson, D.; Burgess, S.; Evans, G.; Lucas, J. Understanding coalescence in iron ore sintering using two bench-scale techniques. ISIJ Int. 2014, 54, 2179–2188. [Google Scholar] [CrossRef]
- FactSage official Chinese website. Available online: http://www.factsage.com/ (accessed on 3 May 2018).
- Kasai, E.; Sakano, Y.; Kawaguchi, T. Influence of iron ore properties on the flow of melt formed in the sintering process. Tetsu Hagané 2000, 86, 139–145. [Google Scholar] [CrossRef]
- Zhang, G.L.; Wu, S.L.; Chen, S.G.; Su, B.; Hou, C.G. Influence of gangue existing states of iron ore on formation and flow of liquid phase in sintering process. Int. J. Miner. Metall. Mater. 2014, 10, 962–968. [Google Scholar] [CrossRef]
- Sinha, M.; Nistala, S.H.; Chandra, S.; Mankhand, T.R. Thermodynamic study of evolution of sinter phases at different alumina level. Ironmak. Steelmak. 2017, 44, 92–99. [Google Scholar] [CrossRef]
- Sukenaga, S.; Gonda, Y.; Yoshimura, S.; Saito, N.; Nakshima, K. Penetration behavior of calcium ferrite melts into hematite substrate. ISIJ Int. 2010, 50, 195–199. [Google Scholar] [CrossRef]
- Machida, S.; Nushiro, K.; Ichikawa, K.; Nada, H.; Sdakai, H. Experimental evaluation of chemical composition and viscosity of melts during iron ore sintering. ISIJ Int. 2005, 45, 513–521. [Google Scholar] [CrossRef]
- Schonhorn, H.; Frisch, H.L.; Kwei, T.K. Kinetics of wetting of surfaces by polymer melts. J. Appl. Phys. 1966, 37, 4967–4973. [Google Scholar] [CrossRef]
- Yin, L.; Murray, B.T.; Singler, T.J. Dissolutive wetting in the Bi-Sn system. Acta Mater. 2006, 54, 3561–3574. [Google Scholar] [CrossRef]
- Long, H.M.; Wu, X.J.; Chun, T.J.; Di, Z.X. Assimilation Behavior of Calcium Ferrite and Calcium Diferrite with Sintered Al2O3 and MgO. Metall. Mater. Trans. B 2016, 47, 2830–2836. [Google Scholar] [CrossRef]
- Liu, W.X. (Ed.) Metallurgy of Iron and Steel, 2nd ed.; Metallurgical Industry, Publishing House: Beijing, China, 2010; pp. 113–116. (In Chinese) [Google Scholar]

| Iron Ore | TFe | FeO | CaO | SiO2 | Al2O3 | MgO | LOI |
|---|---|---|---|---|---|---|---|
| OA | 51.26 | 0.13 | 0.11 | 6.21 | 1.79 | 0.35 | 10.72 |
| OB | 52.03 | 0.11 | 0.10 | 4.60 | 1.53 | 0.31 | 10.97 |
| OC | 58.32 | 0.27 | 0.11 | 5.77 | 3.18 | 0.33 | 5.69 |
| OD | 58.69 | 0.22 | 0.06 | 5.13 | 5.82 | 0.35 | 4.80 |
| OE | 64.14 | 0.18 | 0.11 | 3.08 | 3.12 | 0.12 | 3.05 |
| OF | 63.62 | 0.26 | 0.10 | 7.51 | 3.43 | 0.79 | 2.19 |
| OG | 65.64 | 4.45 | 0.02 | 4.48 | 0.17 | 0.02 | 0.30 |
| OH | 68.59 | 29.02 | 0.4 | 2.17 | 0.72 | 0.77 | 1.05 |
| OI | 57.51 | 18.38 | 3.42 | 5.17 | 0.98 | 1.17 | 5.31 |
| Sample | Fe2O3 | Fe3O4 | SiO2 | CaO | Al2O3 | MgO |
|---|---|---|---|---|---|---|
| OA | 75.44 | 0.12 | 5.82 | 16.61 | 1.68 | 0.33 |
| OB | 77.20 | 0.10 | 4.33 | 16.64 | 1.44 | 0.29 |
| OC | 75.55 | 0.24 | 5.18 | 15.87 | 2.86 | 0.30 |
| OD | 74.03 | 0.20 | 4.57 | 15.69 | 5.19 | 0.31 |
| OE | 78.65 | 0.16 | 2.77 | 15.50 | 2.81 | 0.11 |
| OF | 74.19 | 0.23 | 6.54 | 15.37 | 2.98 | 0.69 |
| OG | 77.04 | 3.86 | 3.89 | 15.06 | 0.15 | 0.02 |
| OH | 1.08 | 80.30 | 1.86 | 15.48 | 0.62 | 0.66 |
| OI | 19.38 | 54.95 | 4.80 | 18.88 | 0.91 | 1.09 |
| Iron Ore | TG (°C) | Liquid Phase Content (wt%) | Viscosity (Pa∙s) | ||||
|---|---|---|---|---|---|---|---|
| 1200 °C | 1250 °C | 1300 °C | 1350 °C | Pure Liquid | Mixed Phase | ||
| OA | 1131.76 | 33.18 | 38.67 | 72.17 | 83.52 | 0.0406 | 0.0918 |
| OB | 1131.31 | 48.51 | 56.92 | 80.20 | 91.51 | 0.0316 | 0.0549 |
| OC | 1131.03 | 36.42 | 41.61 | 71.25 | 81.49 | 0.0409 | 0.0955 |
| OD | 1128.92 | 42.68 | 49.05 | 62.56 | 82.28 | 0.0340 | 0.1098 |
| OE | 1130.41 | 59.05 | 68.34 | 81.38 | 91.66 | 0.0290 | 0.0486 |
| OF | 1131.05 | 19.58 | 22.54 | 54.86 | 68.99 | 0.0580 | 0.2148 |
| OG | 1168.34 | 45.06 | 54.63 | 74.87 | 87.00 | 0.0280 | 0.0577 |
| OH | 1130.73 | 65.50 | 75.22 | 82.10 | 92.00 | 0.0240 | 0.0393 |
| OI | 1129.68 | 53.23 | 60.57 | 83.15 | 95.25 | 0.0320 | 0.0508 |
| Iron Ore | Temperature (°C) | Phase Composition (wt%) | |||
|---|---|---|---|---|---|
| SLAGA | SPINA | C2SA | Mel_A | ||
| OC | 1200 | 36.42 | 47.17 | 13.61 | 1.09 |
| 1250 | 41.61 | 43.02 | 10.21 | 3.33 | |
| 1300 | 71.25 | 26.88 | 0.00 | 0.00 | |
| OD | 1200 | 42.68 | 38.29 | 4.59 | 12.96 |
| 1250 | 49.05 | 33.57 | 0.81 | 14.94 | |
| 1300 | 62.56 | 27.05 | 0.00 | 8.61 | |
| OH | 1200 | 65.50 | 32.74 | 2.90 | 0.00 |
| 1250 | 75.22 | 25.65 | 0.17 | 0.00 | |
| 1300 | 82.10 | 18.75 | 0.00 | 0.00 | |
| OI | 1200 | 53.23 | 35.17 | 12.00 | 0.00 |
| 1250 | 60.57 | 29.72 | 10.01 | 0.00 | |
| 1300 | 83.15 | 15.92 | 1.16 | 0.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Li, H.; Zhang, W.; Su, B. Effect of Thermodynamic Melt Formation Characteristics on Liquid Phase Fluidity of Iron Ore in the Sintering Process. Metals 2019, 9, 404. https://doi.org/10.3390/met9040404
Wu S, Li H, Zhang W, Su B. Effect of Thermodynamic Melt Formation Characteristics on Liquid Phase Fluidity of Iron Ore in the Sintering Process. Metals. 2019; 9(4):404. https://doi.org/10.3390/met9040404
Chicago/Turabian StyleWu, Shengli, Heping Li, Weili Zhang, and Bo Su. 2019. "Effect of Thermodynamic Melt Formation Characteristics on Liquid Phase Fluidity of Iron Ore in the Sintering Process" Metals 9, no. 4: 404. https://doi.org/10.3390/met9040404
APA StyleWu, S., Li, H., Zhang, W., & Su, B. (2019). Effect of Thermodynamic Melt Formation Characteristics on Liquid Phase Fluidity of Iron Ore in the Sintering Process. Metals, 9(4), 404. https://doi.org/10.3390/met9040404




