Modification of Non-Metallic Inclusions in Oil-Pipeline Steels by Ca-Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Steel Production and Sampling
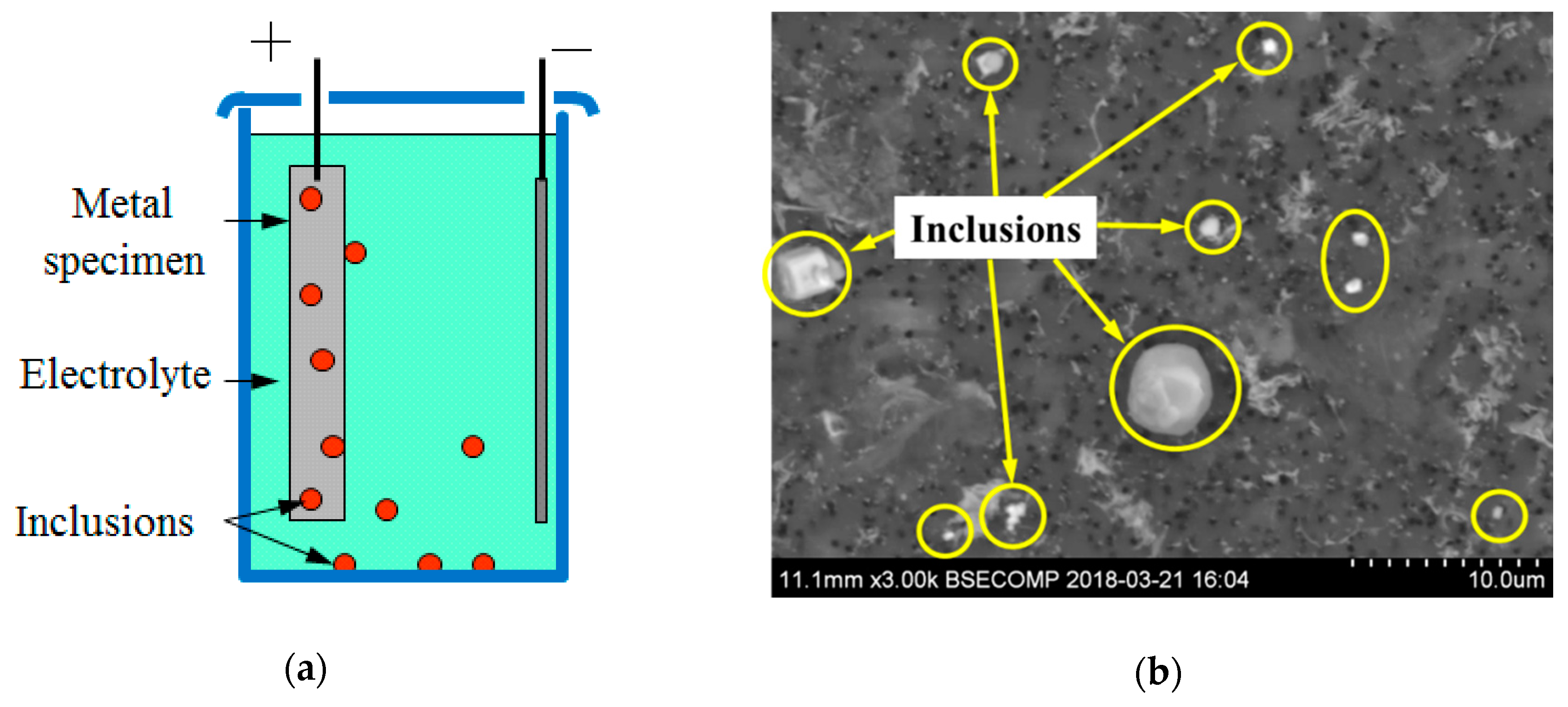
2.2. Evaluation of the Non-Metallic Inclusions and Microstructure in Steel Samples
3. Results and Discussions
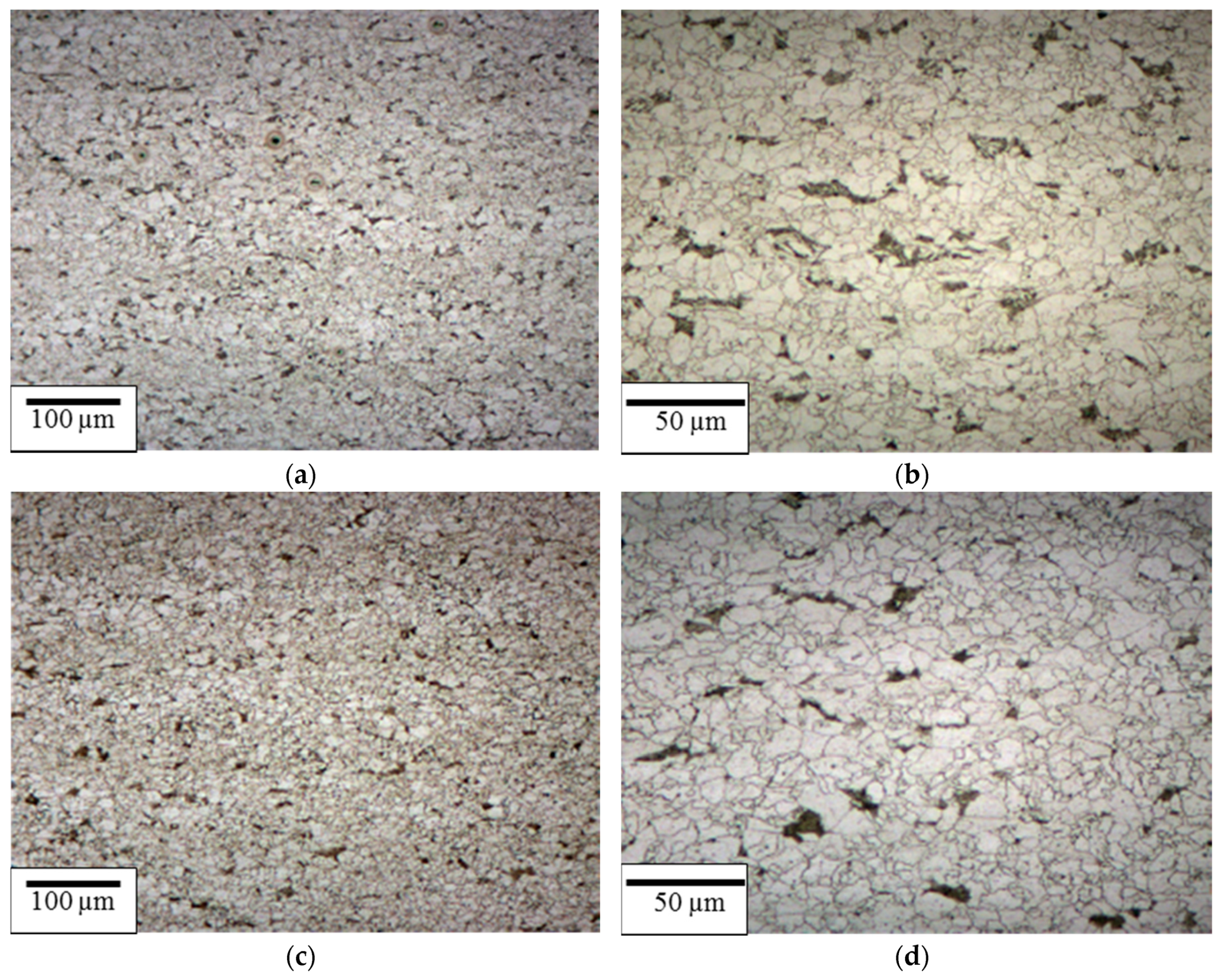
3.1. Evaluation of Composition and Microstructure of Steels
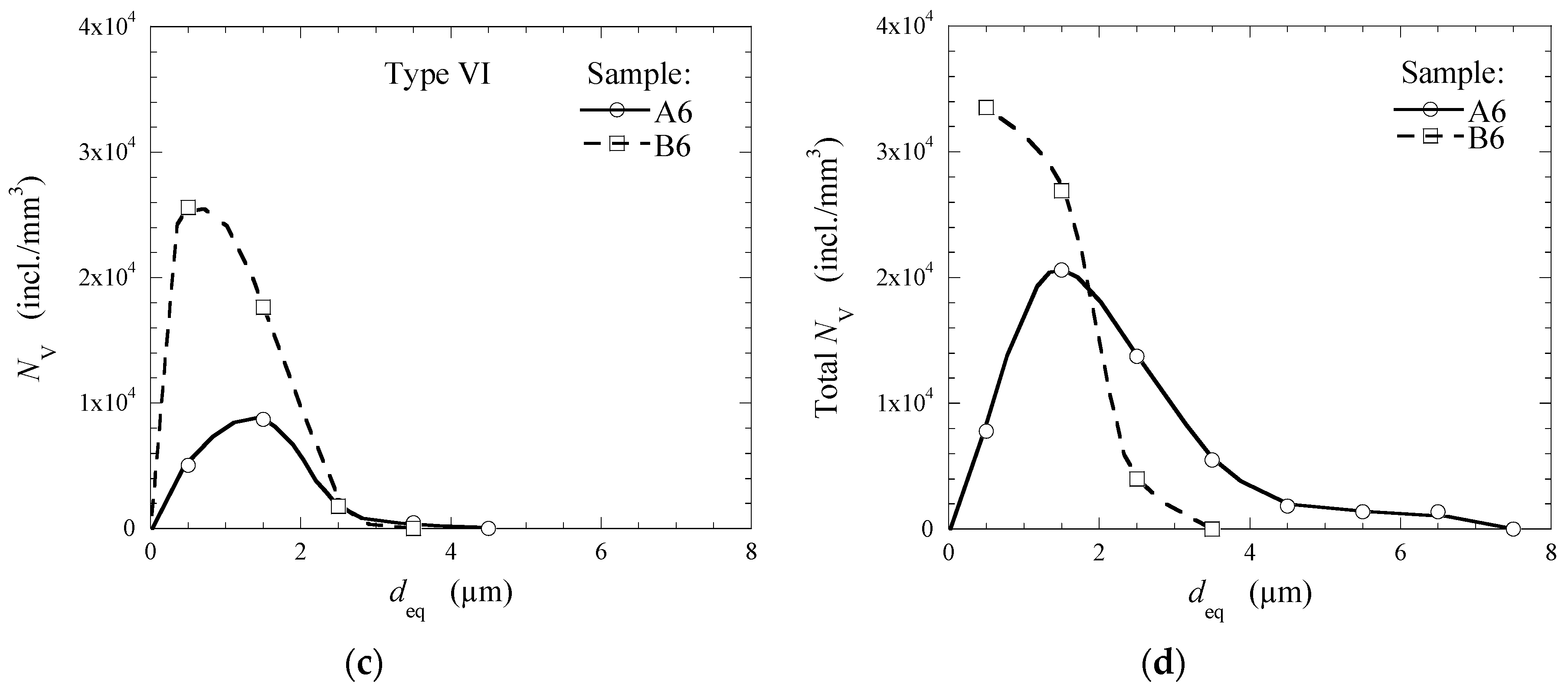
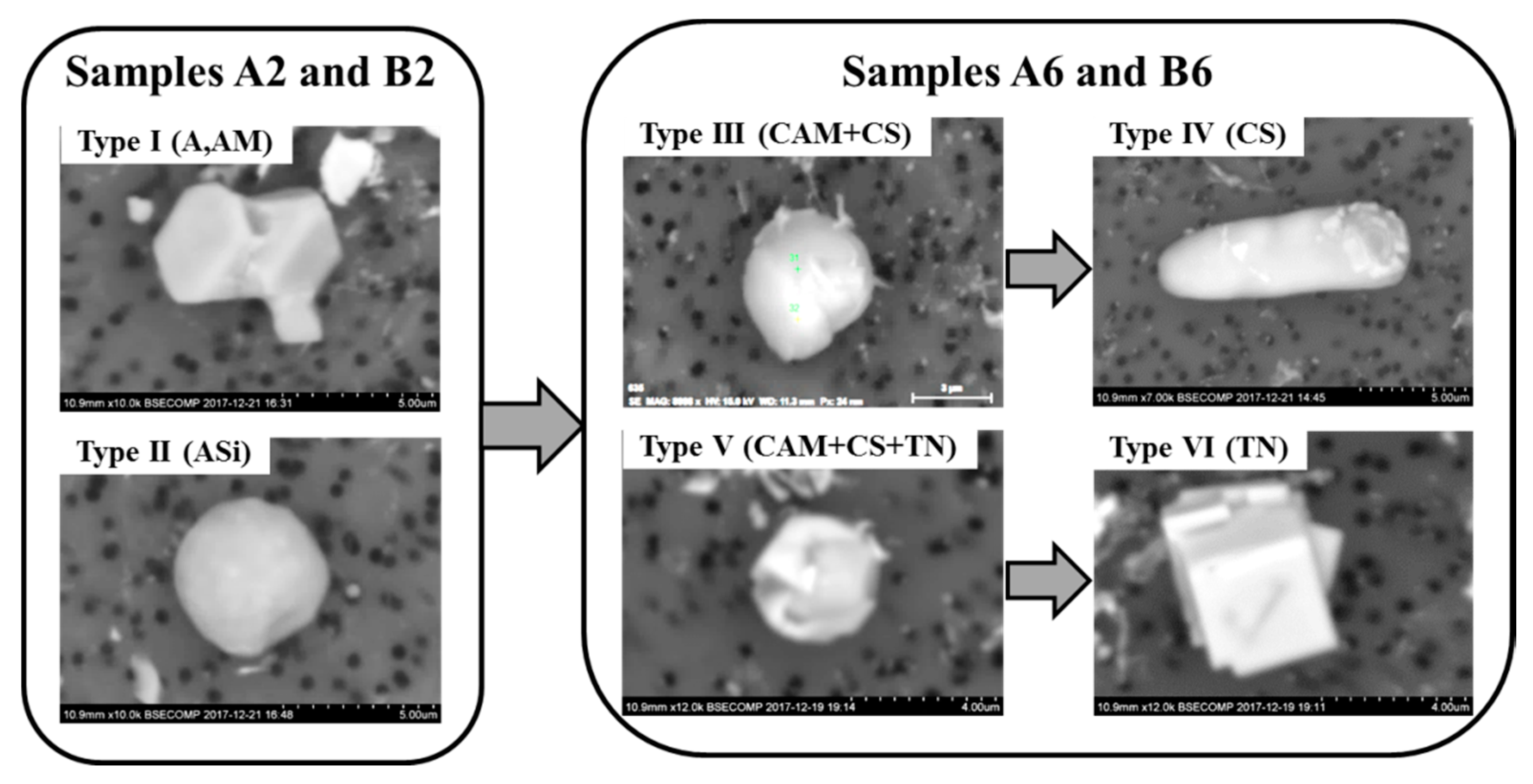
3.2. Evaluation of NMI Characteristics after Electrolytic Extraction
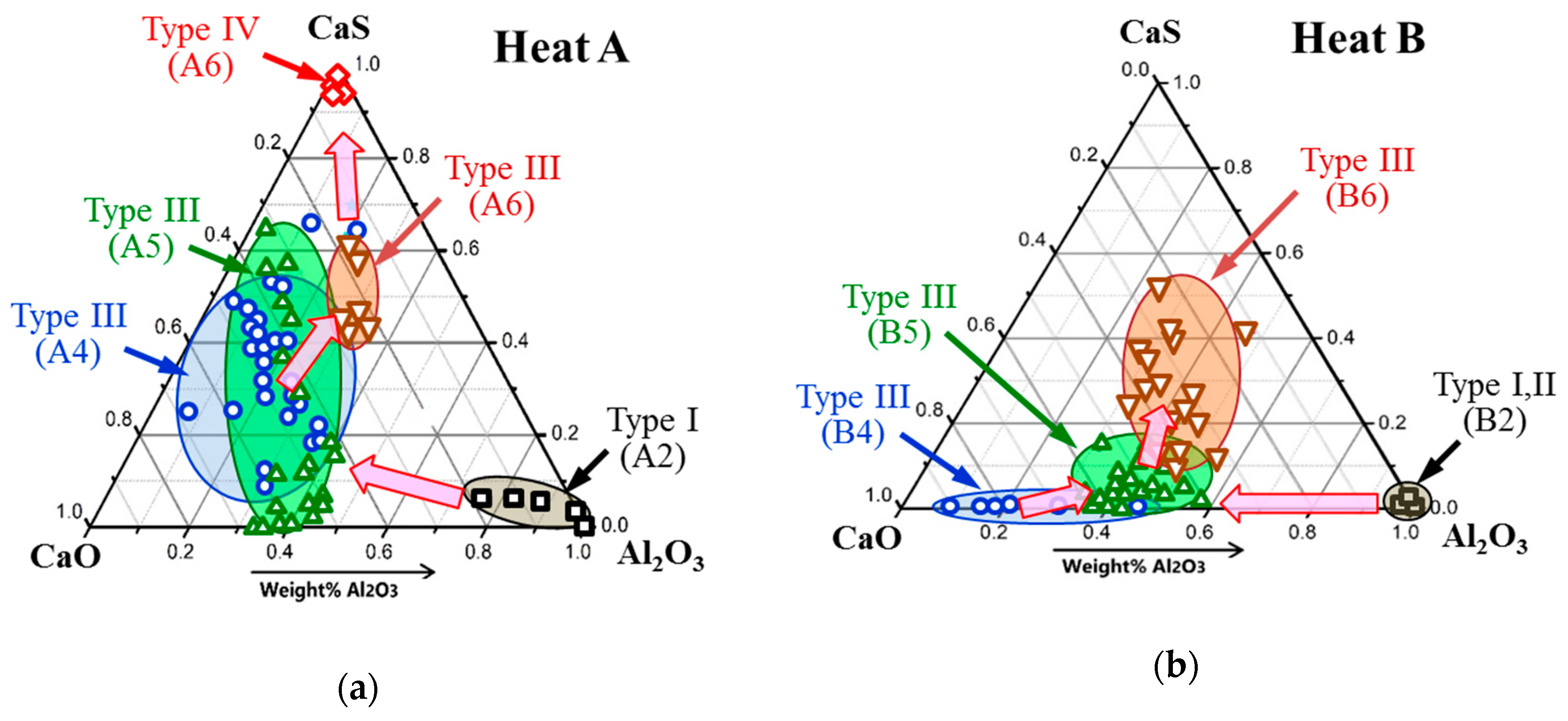
3.3. Modification of Inclusions by Ca-Treatment
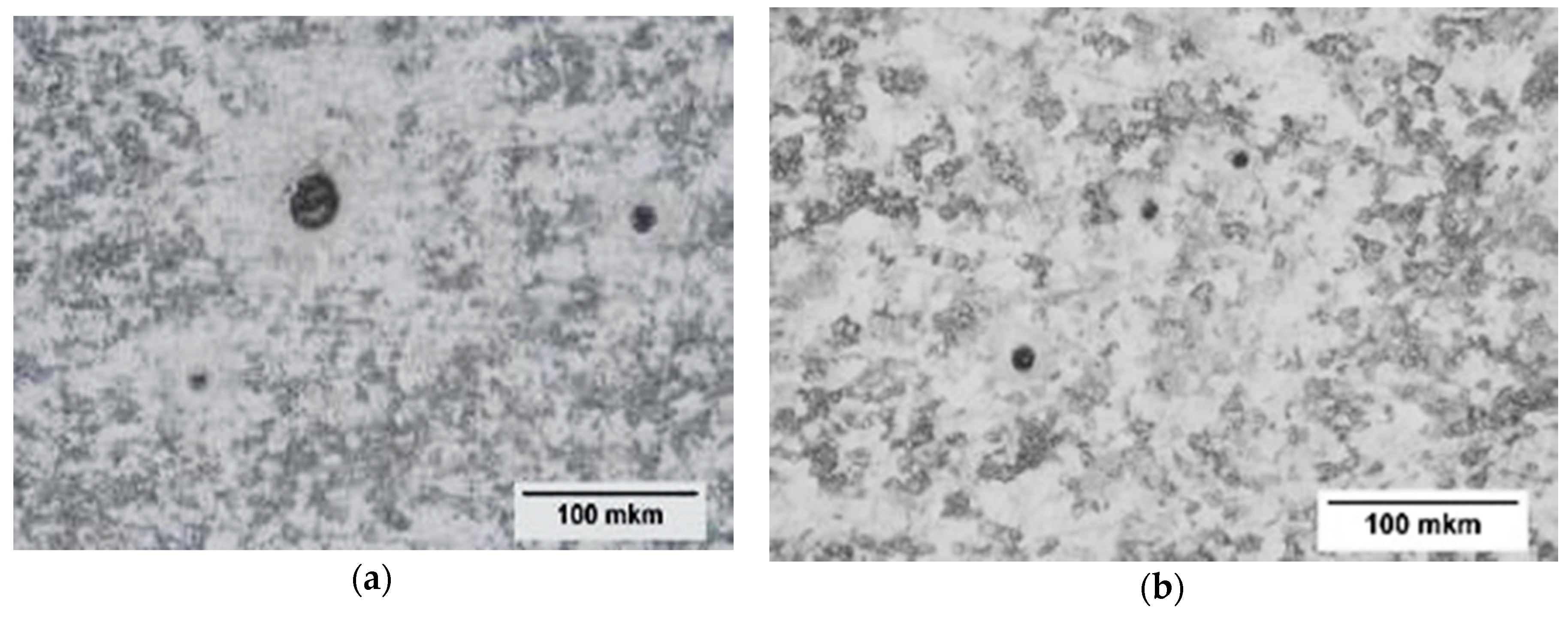
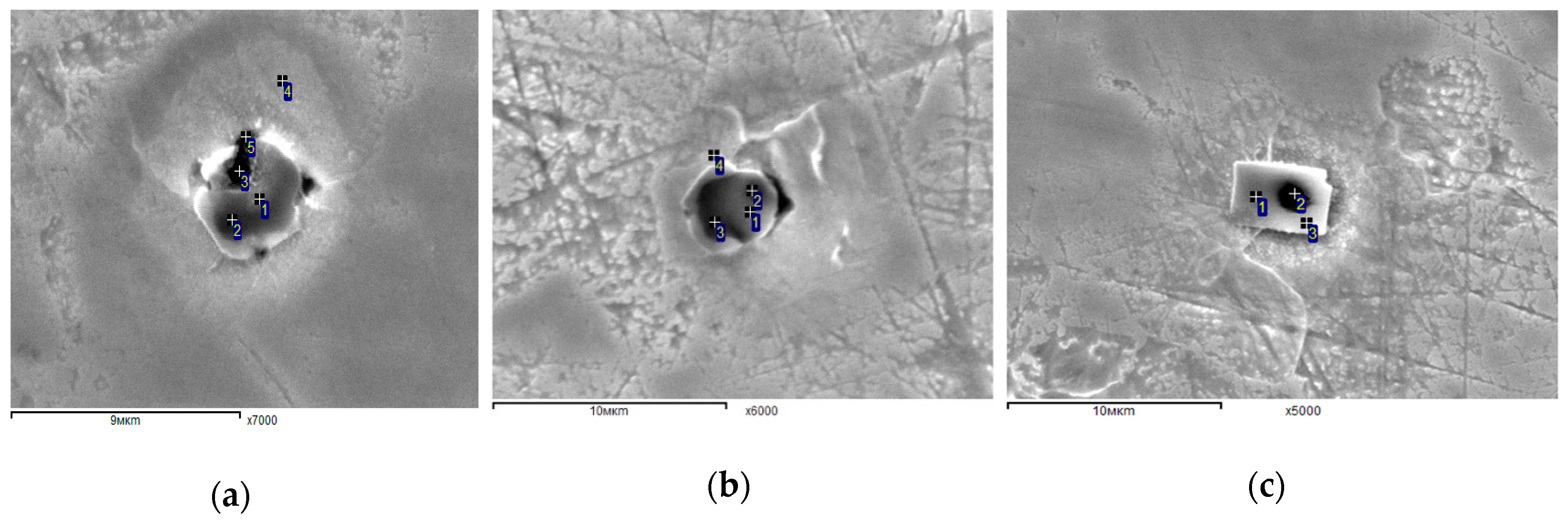
3.4. Evaluation of Corrosion Active Non-Metallic Inclusions
4. Conclusions
- After Ca-treatment, the initial oxide NMI (Type I—Al2O3/Al2O3-MgO inclusions and clusters and Type II—Al2O3-SiO2 inclusions) in steel samples A2 and B2 were modified to CaO-Al2O3-MgO inclusions. The oxide cores of Type III inclusions in steel samples taken after an addition of Ca (samples A4 and B4) contained about 40–90% of CaO in both heats. Then, the content of CaS significantly increased in the outer layer in Type III inclusions (up to 10–45% in the B6 sample and ~40–60% in the sample A6) and in the Type IV inclusions (up to 97–100% in A6 sample). Also, some amounts of TiN were found to precipitate in the outer layer of Type V (up to 53%) and Type VI (78–100%) inclusions during the solidification of steel.
- The frequency of Type III oxy-sulfide inclusions in the steel samples after Ca-treatment decreases drastically from ~98% to 36% in Heat A and from ~89% to 20% in Heat B, while the frequency of Types V and VI inclusions containing TiN increased significantly up to values between 59% and 80% in the steel samples A6 and B6, respectively.
- Although the total number of inclusions in the Heat B is larger than that in the Heat A, the average and maximum sizes of the observed inclusions in Heat B are significantly smaller than those in Heat A. Especially, the average size of the CaS inclusions of Type IV (~5.3 µm), which were only observed in the final product of Heat A, is significantly larger compared to the other types of NMI (1.1–2.8 µm) in both heats.
- The final product of the Heat A has larger amounts (~2.4 times) of the discovered corrosion-active non-metallic inclusions in the size range of 5–7 μm compared to the level in Heat B. Also, most of the corrosion-active non-metallic inclusions correspond to large size inclusions of Types III, IV, V, and VI, which were observed in both heats.
Author Contributions
Funding
Conflicts of Interest
References
- Carneiro, R.A.; Ratnapuli, R.C.; Lins, V.F.C. The Influence of Chemical Composition and Microstructure of API Linepipe Steels on Hydrogen Induced Cracking and Sulfide Stress Corrosion Cracking. Mater. Sci. Eng. A 2003, 357, 104–110. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Characterization of Inclusions of X80 Pipeline Steel and its Correlation with Hydrogen-induced Cracking. Corros. Sci. 2011, 53, 1201–1208. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Deng, X.X.; McLean, A. Effects of Non-Metallic Inclusions and Their Shape Modification on the Properties of Pipeline Steel. In Proceedings of the AISTech Conference Proceedings—7th International Conference on the Science and Technology of Ironmaking, Cleveland, OH, USA, 4–7 May 2015; PR-368-358-2015. pp. 3388–3406. [Google Scholar]
- Tetelman, A.S.; Robertson, W.D. The Mechanism of Hydrogen Embrittlement Observed in Iron-Silicon Single Crystals; Ft. Belvoir Defense Technical Information Center: Fort Belvoir, VA, USA, 1961.
- Oriani, R.A.; Josephic, P.H. Hydrogen-enhanced Load Relaxation in a Deformed Medium-carbon Steel. Acta Metall. 1979, 27, 997–1005. [Google Scholar] [CrossRef]
- Herring, D.H. Hydrogen Embrittlement. Wire Forming Technology International. 2010. Available online: http://www.heat-treat-doctor.com/documents/Hydrogen%20Embrittlement.pdf (accessed on 17 December 2018).
- Huang, F.; Liu, J.; Deng, Z.; Cheng, J.; Lu, Z.; Li, X. Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel. Mater. Sci. Eng. A 2010, 527, 6997–7001. [Google Scholar] [CrossRef]
- Kim, W.K.; Koh, S.U.; Yang, B.Y.; Kim, K.Y. Effect of Environmental and Metallurgical Factors on Hydrogen Induced Cracking of HSLA Steels. Corros. Sci. 2008, 50, 3336–3342. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.J.; Lee, C. Role of Ca Treatment in Hydrogen Induced Cracking of Hot Rolled API Pipeline Steel in Acid Sour Media. Met. Mater. Int. 2013, 19, 45–48. [Google Scholar] [CrossRef]
- Lube, I.I.; Pecheritsa, A.A.; Neklyudov, I.V.; Rodionova, I.G.; Zaitsev, A.I.; Marchenko, L.G.; Emel’yanov, A.V.; Stolyarov, V.I. Study of the effect of process parameters in steel production on the content of corrosion-active nonmetallic inclusions in corrosion-resistant pipes. Metallurgist 2005, 49, 269–275. [Google Scholar] [CrossRef]
- Mitrofanov, A.V.; Petrova, M.V.; Kirillov, I.E.; Rodionova, I.G.; Udod, K.A.; Endel’, N.I. Factors affecting housing and utilities pipe corrosion resistance. Metallurgist 2016, 60, 76–80. [Google Scholar] [CrossRef]
- Karasev, A.V.; Suito, H. Analysis of Size Distributions of Primary Oxide Inclusions in Fe-10 mass pct Ni-M (M = Si, Ti, Al, Zr, and Ce) Alloy. Metall. Mater. Trans. B 1999, 30, 259–270. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Characteristics of Particle Size Distribution of Deoxidation Products with Mg, Zr, Al, Ca, Si/Mn and Mg/Al in Fe-10mass% Ni Alloy. ISIJ Int. 2006, 46, 14–21. [Google Scholar] [CrossRef]
- Doostmohammadi, H.; Karasev, A.; Jönsson, P.G. A Comparison of a Two-Dimensional and a Three-Dimensional Method for Inclusion Determinations in Tool Steel. Steel Res. Int. 2010, 81, 398–406. [Google Scholar] [CrossRef]
- Kanbe, Y.; Karasev, A.; Todoroki, H.; Jönsson, P.G. Analysis of Largest Sulfide Inclusions in Low Carbon Steel by Using Statistics of Extreme Values. Steel Res. Int. 2011, 82, 313–322. [Google Scholar] [CrossRef]
- Kanbe, Y.; Karasev, A.; Todoroki, H.; Jönsson, P.G. Application of Extreme Value Analysis for Two- and Three-Dimensional Determinations of the Largest Inclusion in Metal Samples. ISIJ Int. 2011, 51, 593–602. [Google Scholar] [CrossRef]
- Karasev, A.; Jönsson, P.G. Assessment of Non-Metallic Inclusions in Different Industrial Steel Grades by Using the Electrolytic Extraction Method. In Proceedings of the 5th International Conference on Process Development in Iron and Steelmaking SCANMET-V, Luleå, Sweden, 12–15 June 2016; pp. 1–7. [Google Scholar]


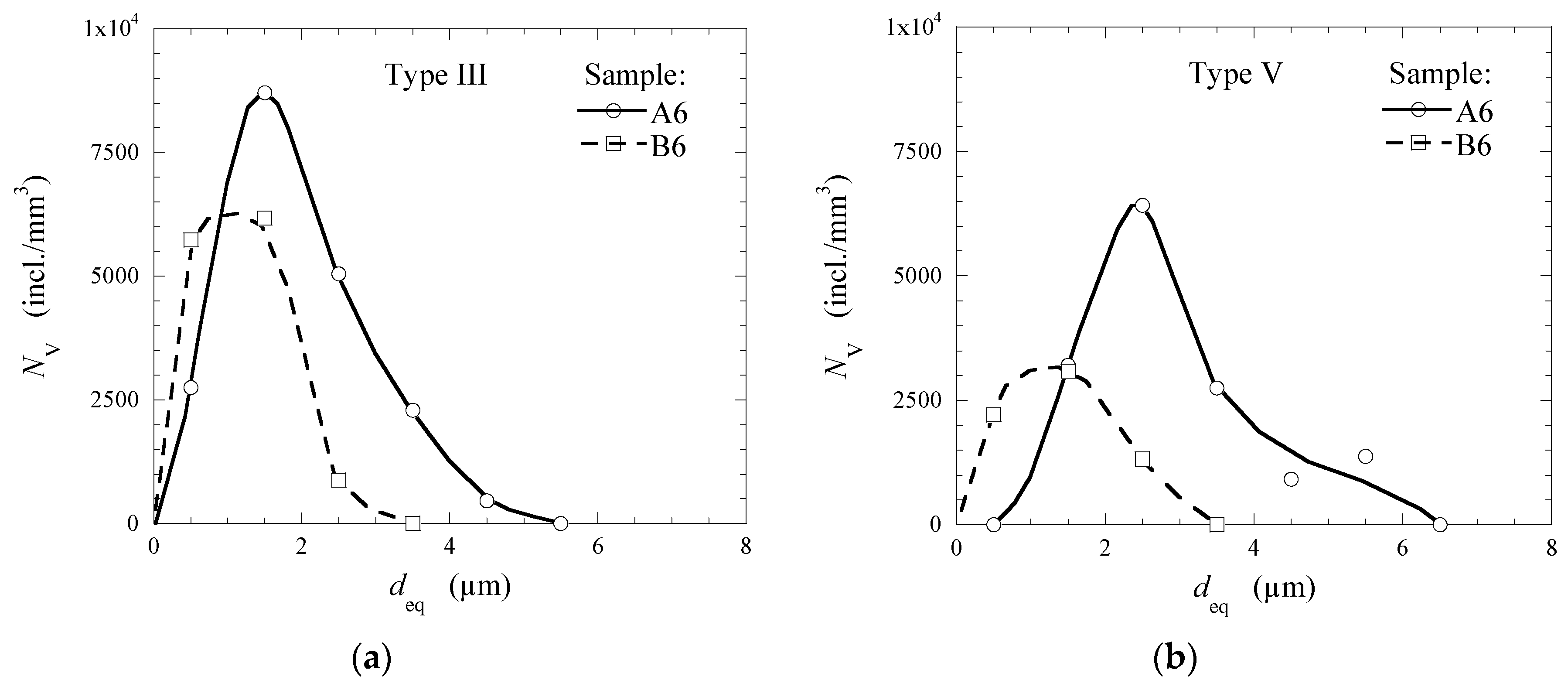
| Steel | C | Si | Mn | Cr | Ni | Cu | Al | Ti | Ca | S | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.06 | 0.24 | 0.63 | 0.43 | 0.17 | 0.33 | 0.025 | 0.019 | 0.0020 | 0.002 | 0.005 |
| B | 0.05 | 0.23 | 0.67 | 0.43 | 0.18 | 0.35 | 0.022 | 0.021 | 0.0014 | 0.001 | 0.007 |
| Type of NMI 1 | Composition | Size (µm) | Sample |
|---|---|---|---|
 | Al2O3—75–84%, MgO—0–19%, CaO—0–17%, CaS—0–5%. | 0.5–7.9 | A2, B2 |
 | Al2O3—5–78%, SiO2—21–94%, MgO—0–2%, CaO—0–4%, CaS—0–5%. | 0.7–5.6 | B2 |
 | CaO—9–69%, Al2O3—2–54%, MgO—0–22%, SiO2—0–7%, CaS—9–62%. | 0.9–5.2 | A4–A6 B4–B6 |
 | CaS—97–100%, Al2O3—0–2%, MgO—0–1%, CaO—0–2%. | 2.5–7.4 | A6 |
 | CaO—4–45%, Al2O3—4–45%, MgO—0–19%, SiO2—1–5%, CaS—0–50%, TiN—1–53%. | 1.1–5.0 | A4–A6, B5–B6 |
 | TiN—78–100%, Al2O3—0–5%, MgO—0–5%, CaO—0–5%, CaS—0–13%. | 0.7–4.3 | A4–A6, B4–B6 |
| Steel | NA of Corrosion Active NMI (incl./mm2) |
|---|---|
| A | 8.5 |
| B | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, E.; Karasev, A.V.; Kuznetsov, D.; Jönsson, P.G. Modification of Non-Metallic Inclusions in Oil-Pipeline Steels by Ca-Treatment. Metals 2019, 9, 391. https://doi.org/10.3390/met9040391
Sidorova E, Karasev AV, Kuznetsov D, Jönsson PG. Modification of Non-Metallic Inclusions in Oil-Pipeline Steels by Ca-Treatment. Metals. 2019; 9(4):391. https://doi.org/10.3390/met9040391
Chicago/Turabian StyleSidorova, Elena, Andrey V. Karasev, Denis Kuznetsov, and Pär G. Jönsson. 2019. "Modification of Non-Metallic Inclusions in Oil-Pipeline Steels by Ca-Treatment" Metals 9, no. 4: 391. https://doi.org/10.3390/met9040391
APA StyleSidorova, E., Karasev, A. V., Kuznetsov, D., & Jönsson, P. G. (2019). Modification of Non-Metallic Inclusions in Oil-Pipeline Steels by Ca-Treatment. Metals, 9(4), 391. https://doi.org/10.3390/met9040391






