Prediction and Knowledge Mining of Outdoor Atmospheric Corrosion Rates of Low Alloy Steels Based on the Random Forests Approach
Abstract
1. Introduction
2. Materials
2.1. Materials
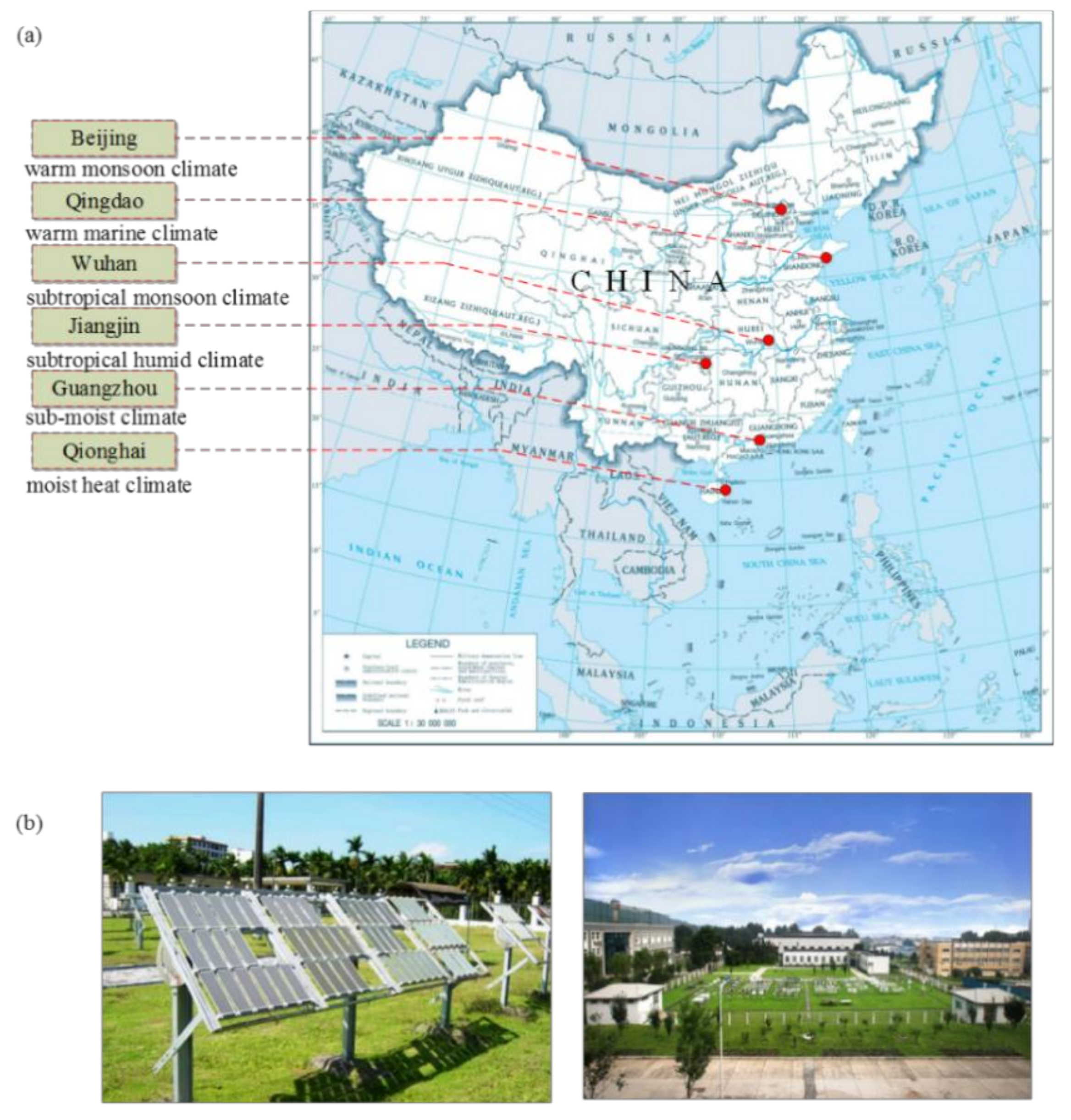
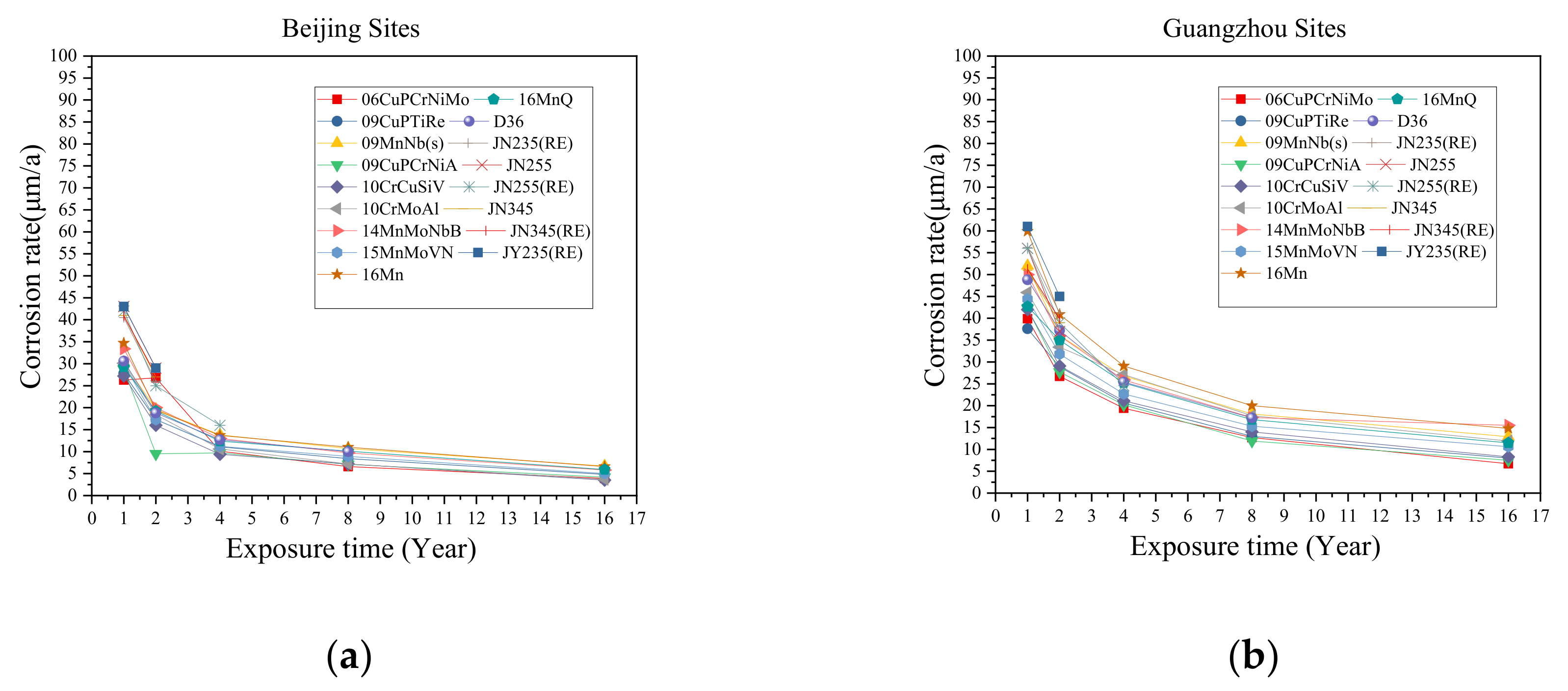
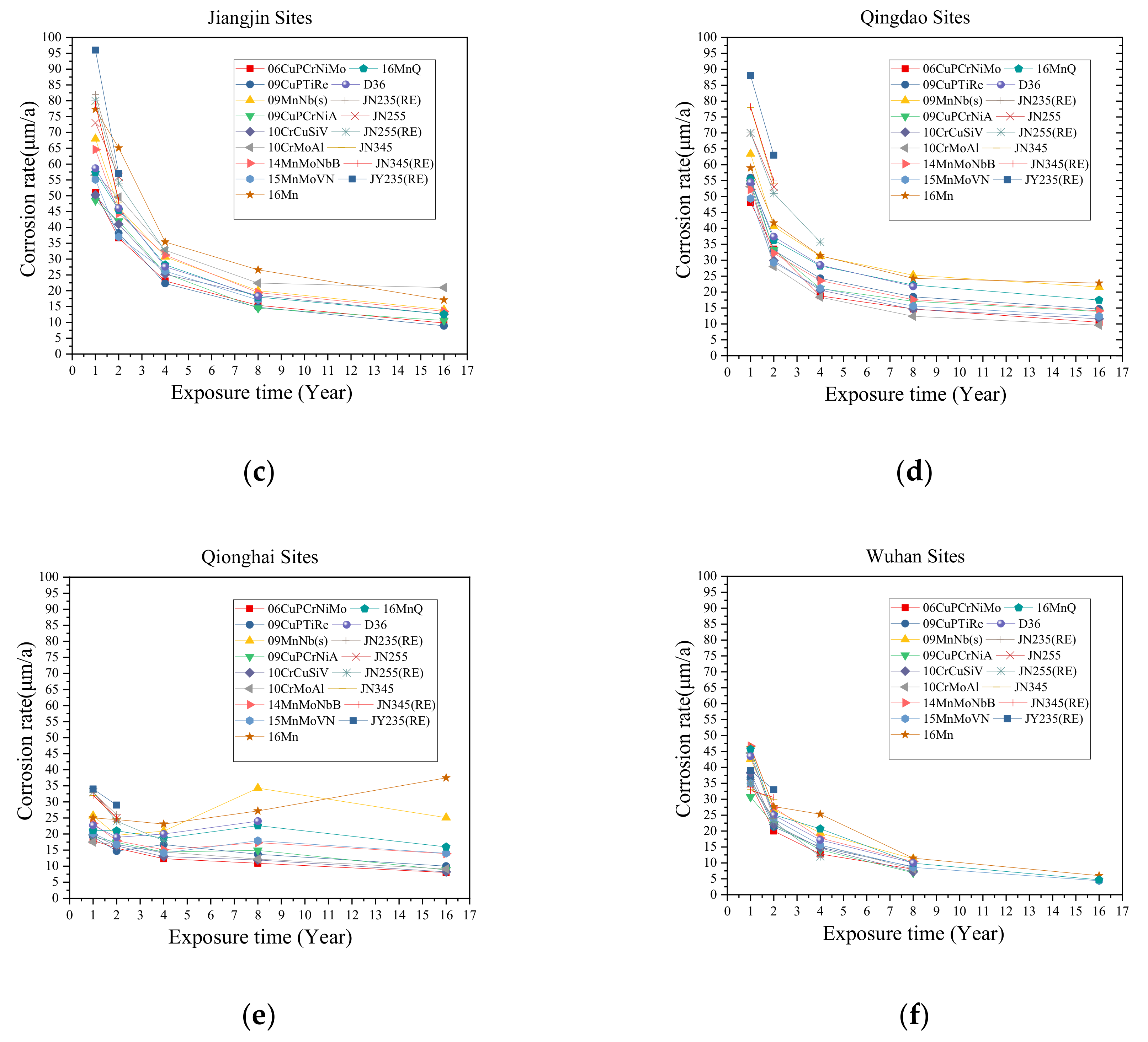
2.2. Test Sites and Environmental Factors
2.3. Corrosion Rate Measurements
3. Methods
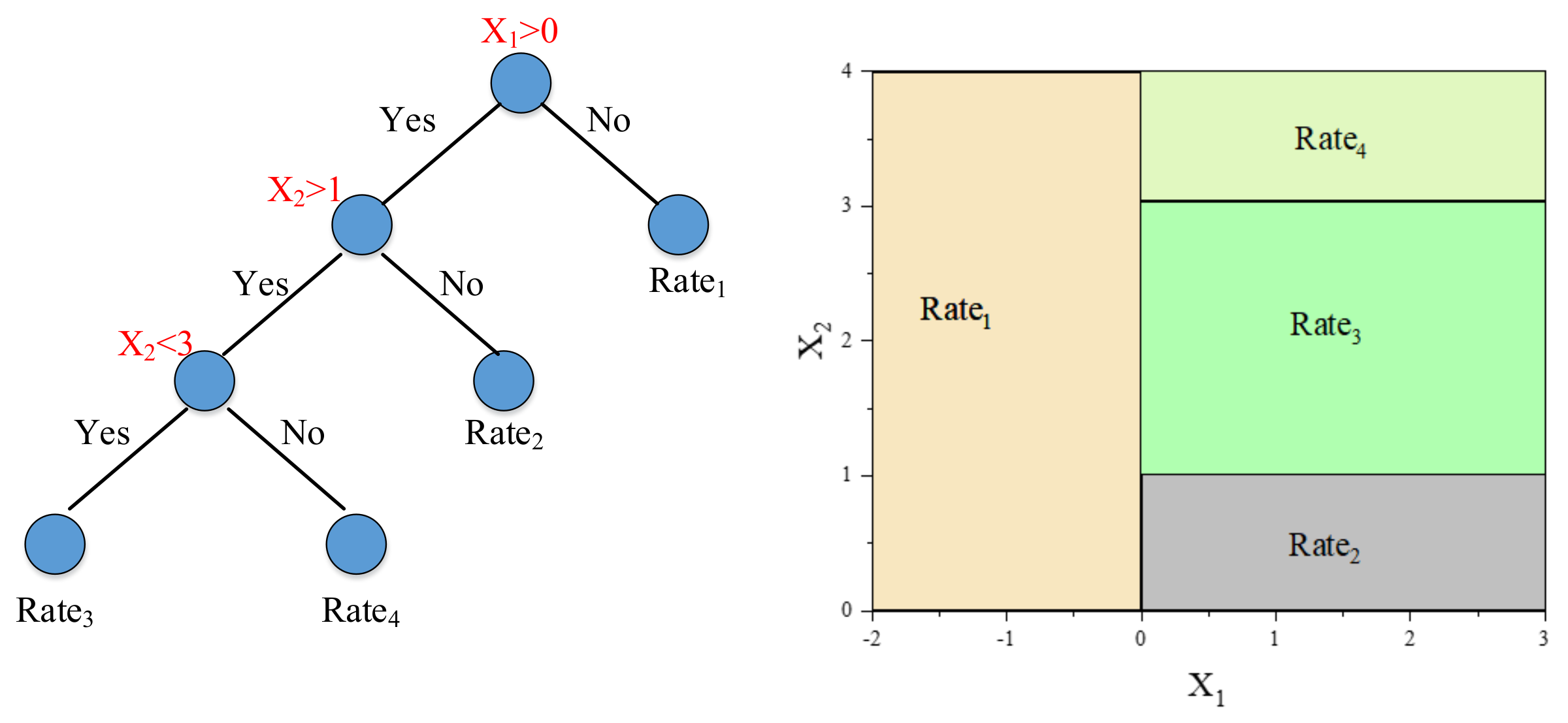
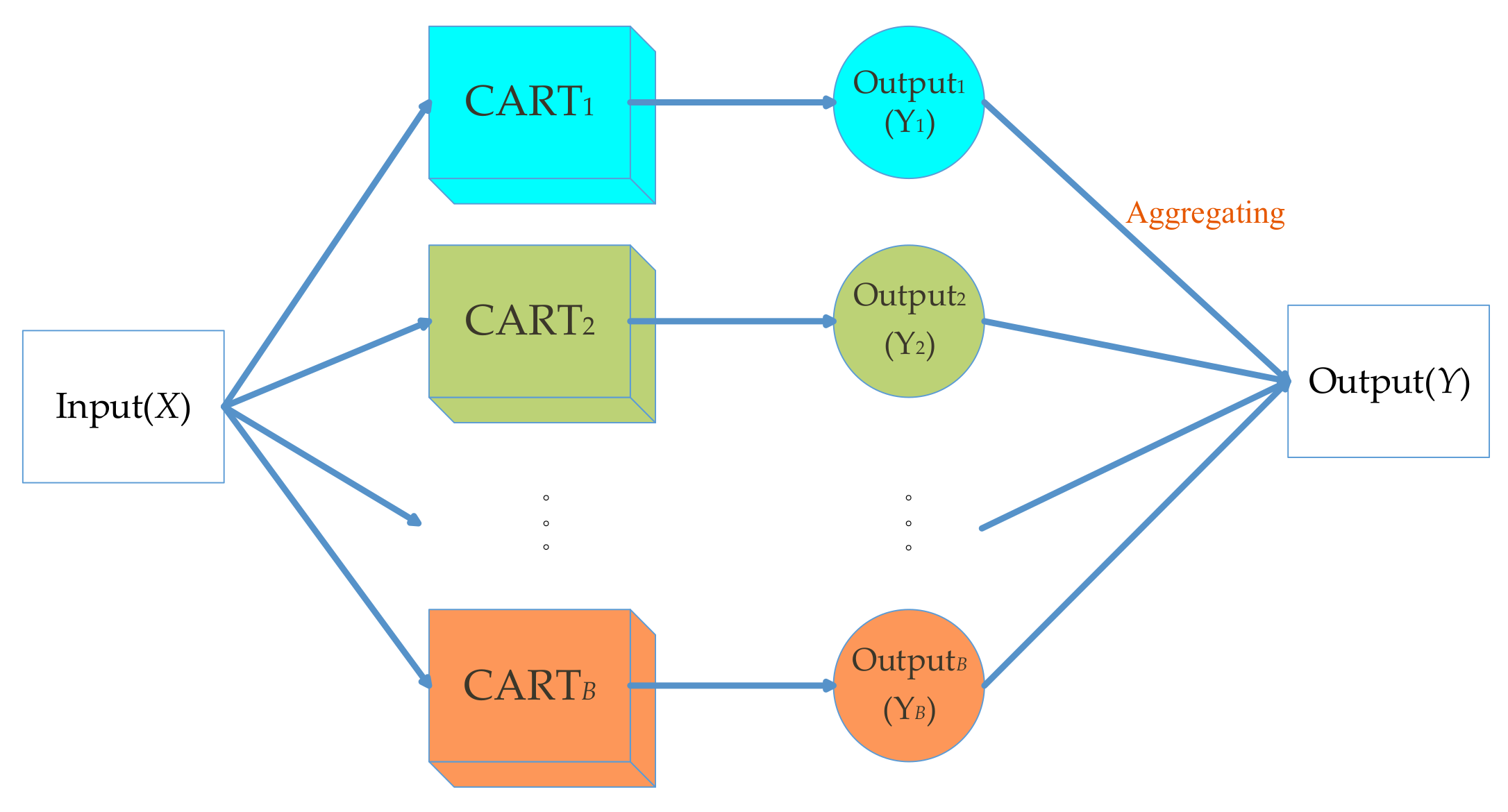
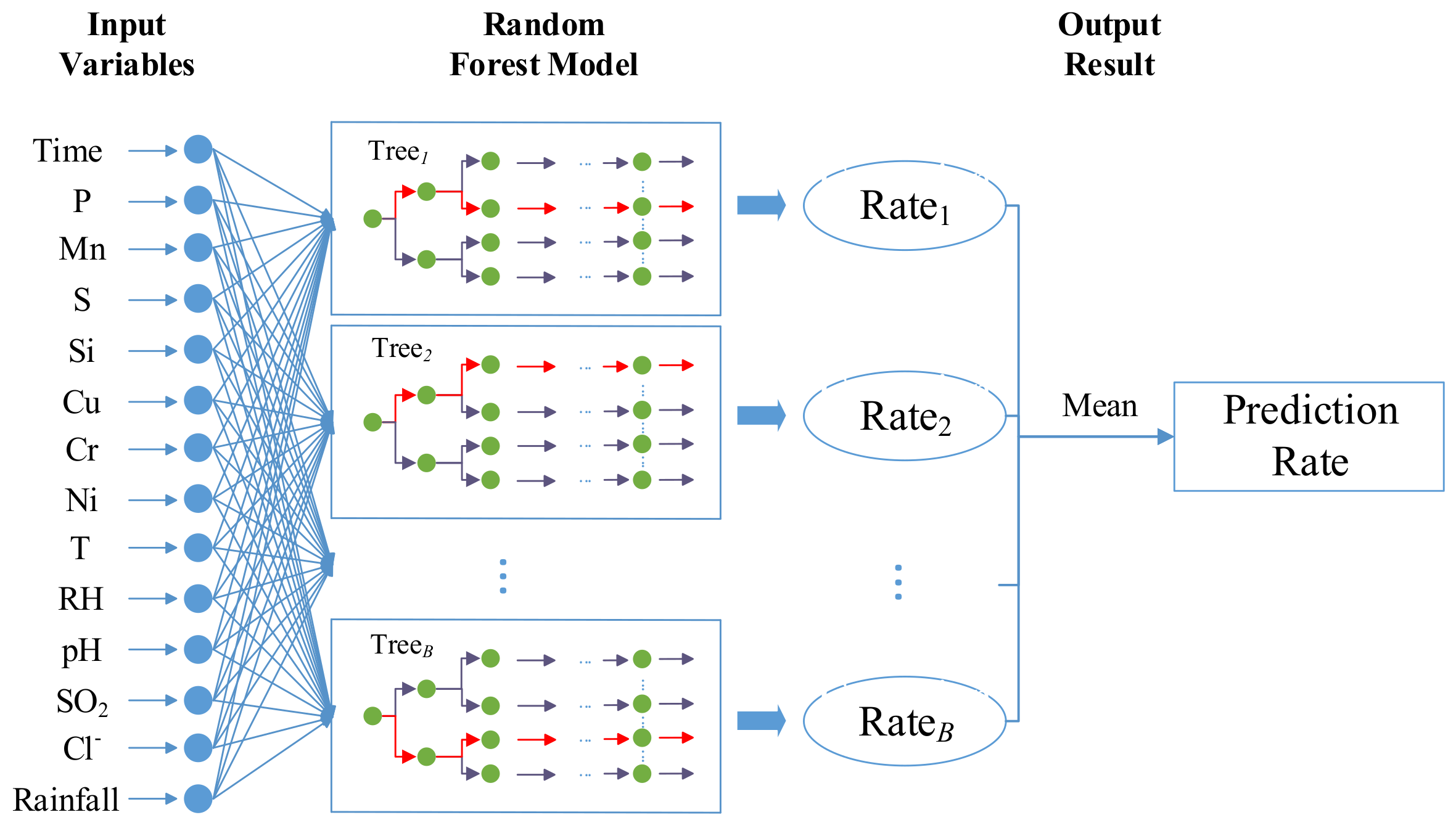
3.1. Random Forests
- For CART i, where i = 1, 2, …, B:
- ●
- Identify the selected samples for the training process of CART model and unselected samples which belong to the OOB objects.
- ●
- Estimate the OOB error εi.
- ●
- For each variable . We first permute the values of xj randomly. Then, estimate the model error εij by the OOB samples with permuted values. We obtain the difference dij = εij – εi.
- After the operation of the above process. For variable xj, we can obtain the mean value and the corresponding standard deviation, σj.
- Compute the importance estimation of the variable xj by .
- Normalize the results of importance estimation to range [0, 1] by .
3.2. Evaluation Criteria
- (1)
- Mean absolute percentage error (MAPE)
- (2)
- Root mean square error (RMSE)
- (3)
- Determination coefficients (R2)
4. Results and Discussion
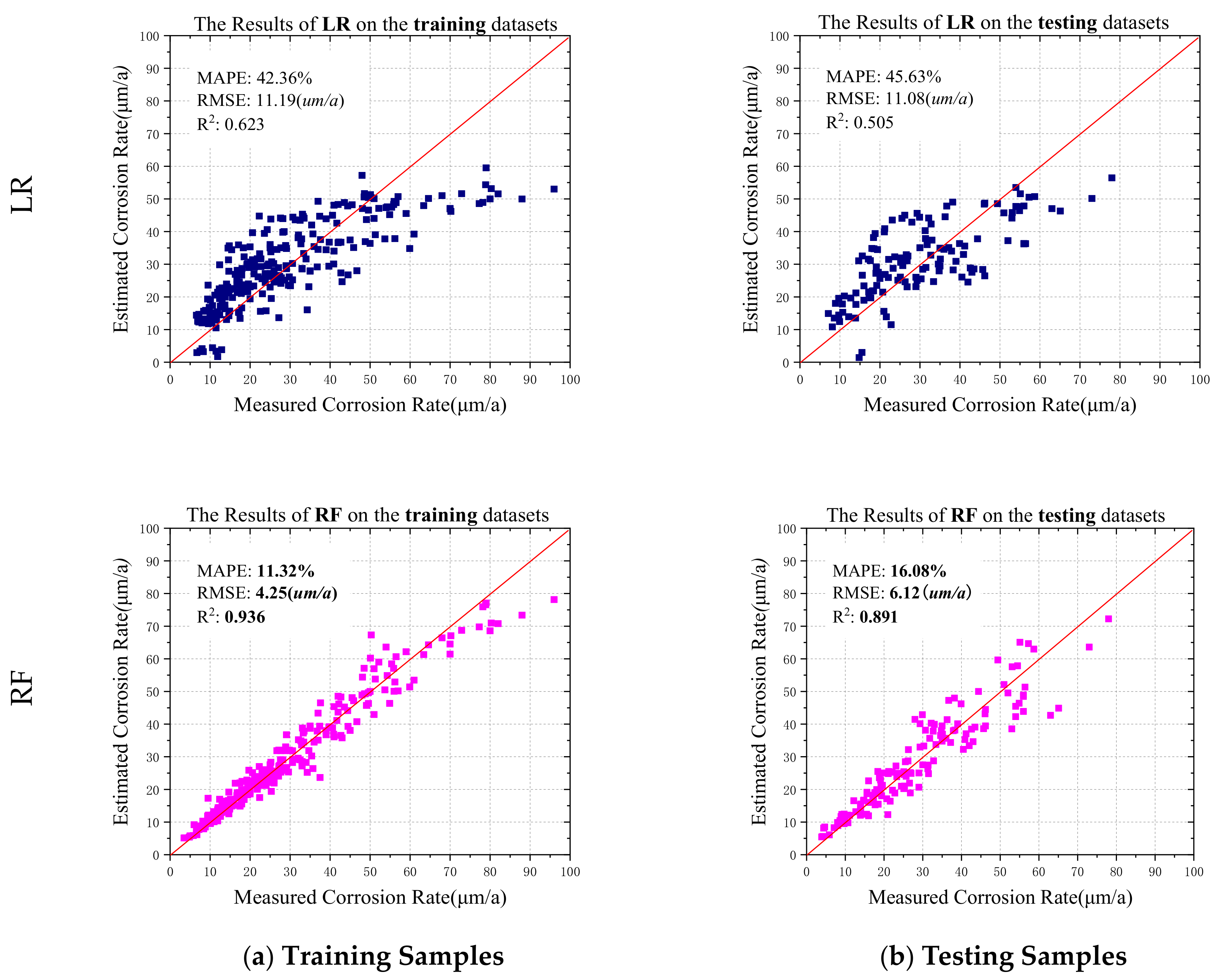
4.1. Modeling of Random Forests and Application on Corrosion Dataset
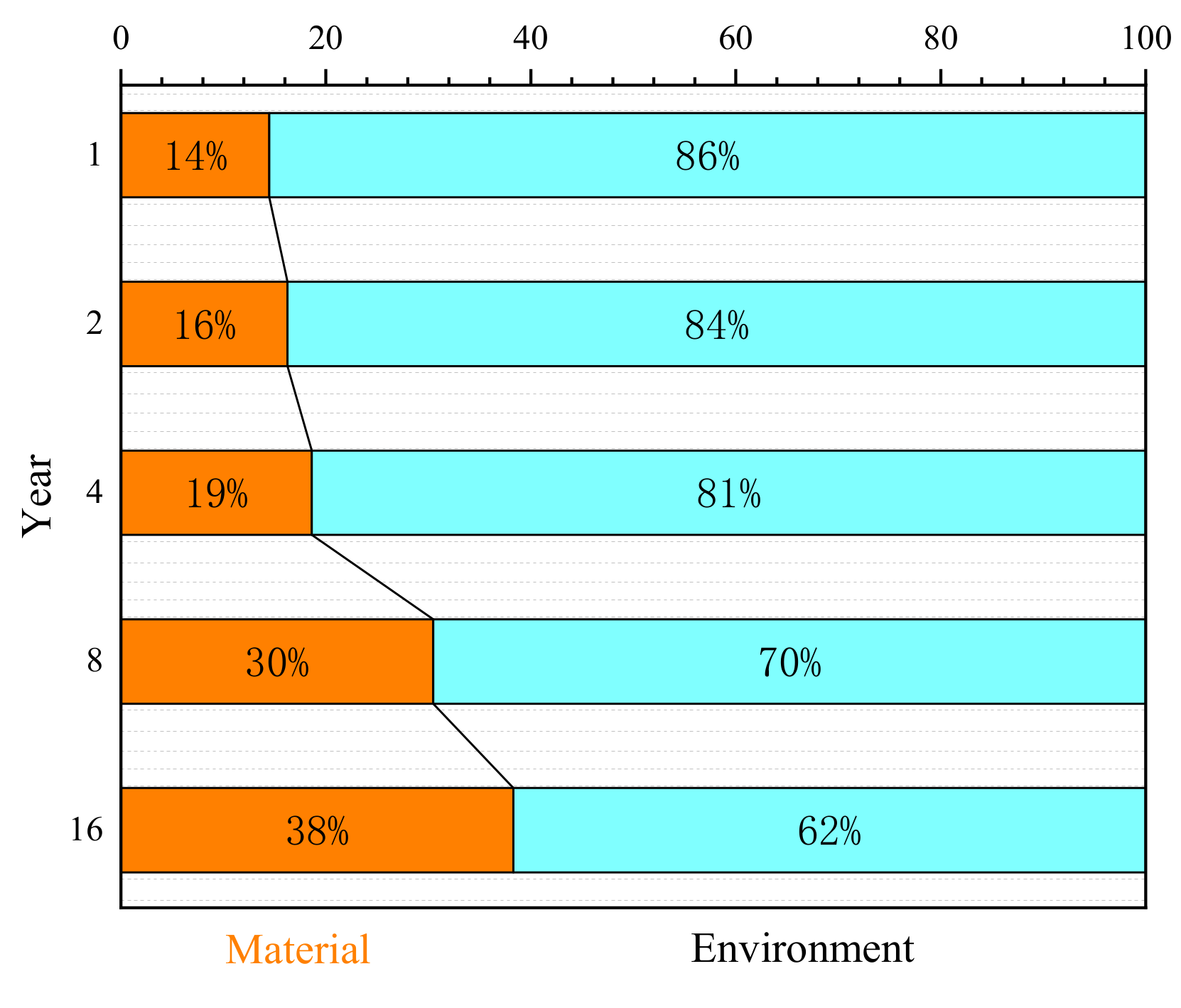
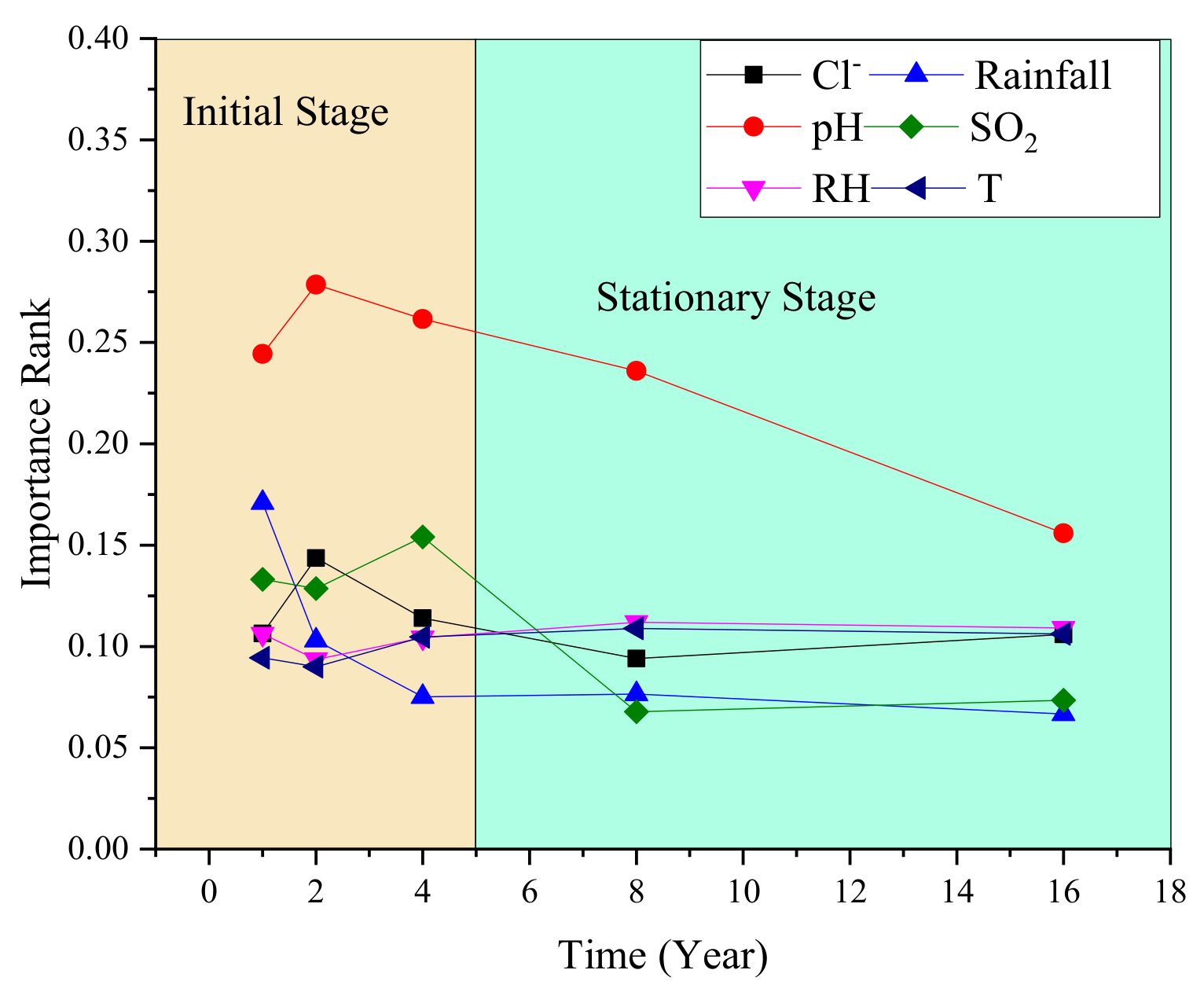
4.2. Variable Importance Ranks According to the Model
- Figure 9 shows that pH always plays the most important role at the two corrosion stages. This is because low pH in rainfall can help to dissolve the corrosion products to iron ions and form non-aggregated “flowing” rusts [33]. With lower pH, the more dissolved product ions resulted in a larger corrosion rate [33,34,35].
- At the stationary stage, the influence of SO2 is much less than Cl−, T, and RH. Figure 9 indicated that Cl−, T, and RH played a second important role on corrosion at this time. Relevant results were also proved in previous work [36,37,38]. As for the influence change of SO2, it is because SO2 could promote the phase transformation to a more stable structure of α-FeOOH [37]. Therefore, the SO2 plays a major role at the initial stage, but has a lower effect on corrosion at the stationary stage.
- Rainfall had a secondary important effect on corrosion in the first year, but the influence would rapidly decrease as time increased. From the 4th year, the influence of rainfall always is the lowest.
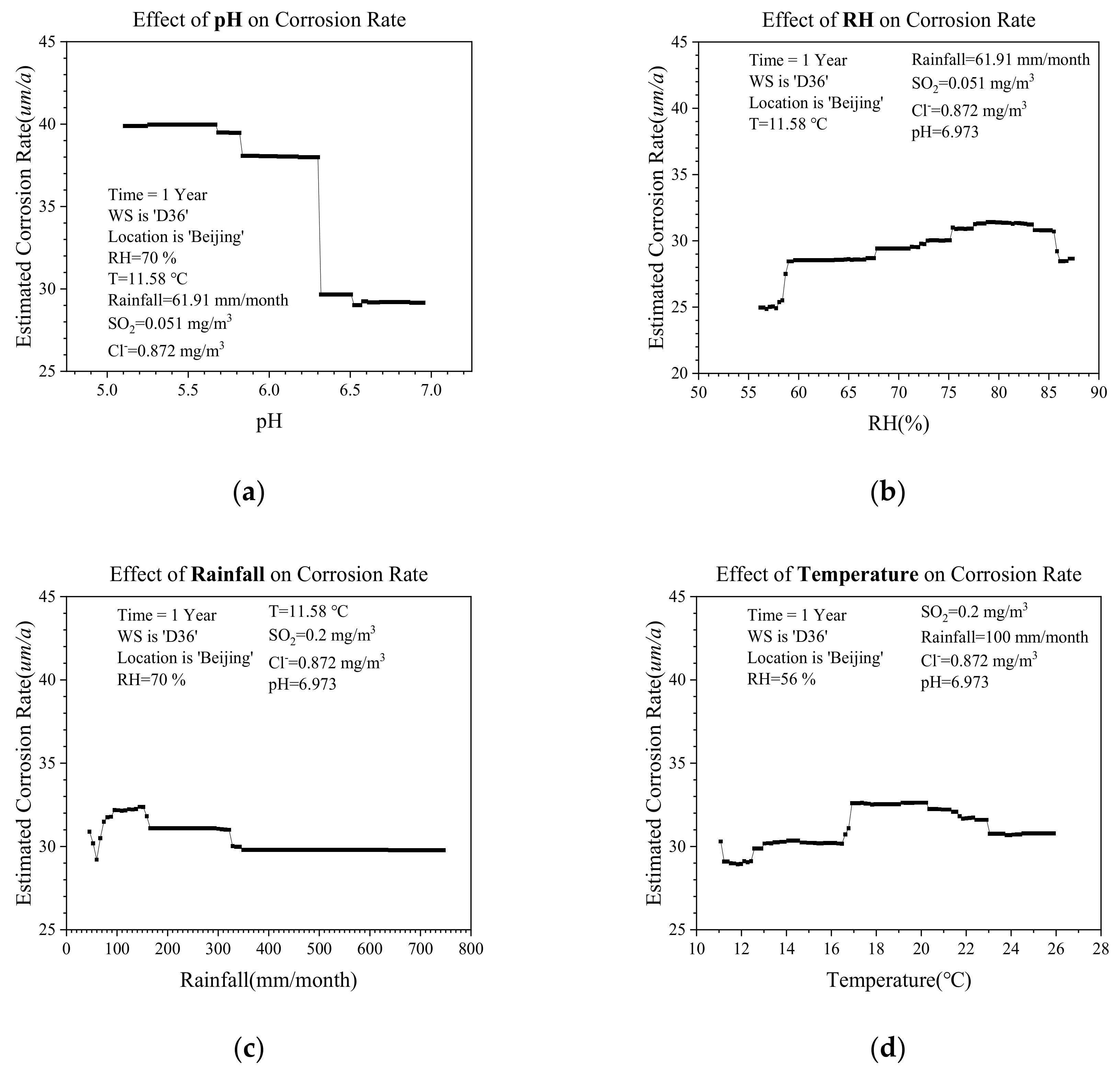
4.3. The Effect of Environmental Factors
- Figure 10a demonstrated that with a lower pH value, LAS had a higher corrosion rate. This is because the lower pH would generate more hydrogen ions, which can promote more severe dissolution of corrosion products [39]. Therefore, lower pH resulted in more damages to the products, which otherwise can play a protective role for the material [33,34,35]. According to the results in Figure 10a, it can be seen that when the value of pH crossed over the threshold around 6.29, there is a sudden change in the corrosion rate. For this threshold value, we explain that for the atmospheric corrosion of ‘D36’ in ‘Beijing’, when the pH of rain is lower than about 6.29, the hydrogen ion dissolves the metal, which accelerates the corrosion rate and may lead to part of the rust-protective layer being completely dissolved. This means that the corresponding area of ‘D36’ is not protected by the rust-layer, and it would lead to the sudden change of the corrosion rate.
- Figure 10b shows that there is a large increase in corrosion rate when the RH reaches about 59%. In the range of 55–85%, the corrosion rate and RH increase simultaneously. However, when the value of RH is larger than 85%, the corrosion rate decreases conversely.
- When the value of rainfall ranges from 100 (mm/month) to 147 (mm/month), the corresponding corrosion rate is the largest. After that, the corrosion rate gradually decreases as the rainfall increases.
- As for the temperature effect on corrosion, Figure 10d illustrated that when temperature values range from 11–17 °C, the higher temperature could promote the corrosion, and the largest corrosion rate happens when the temperature is in ranges from 17–20 °C; afterwards, the corrosion rate would decrease, though the temperature still increases. For this case, the explanation is that when the temperature in Beijing was higher than 20 °C, there existed a severe evaporation process. Therefore, on the surface of LAS, it is harder to form the electrolyte film, which is a necessity for corrosion.
- Figure 10e shows that SO2 can promote the corrosion process, i.e., the higher SO2 corresponds to the higher corrosion rate. Besides, we also give a threshold of 0.61 (mg/cm3), which can lead to the sudden change of the corrosion rate.
- The effect of Cl− in Figure 10f illustrated that the threshold of Cl− was about 0.89 (mg/cm3). The higher Cl− will accelerate the corrosion rate. Figure 10f also shows that the influence of Cl− concentration on the corrosion rate is not larger. For this result, we explain it from two aspects: Firstly, the exposure time in Figure 10f is ‘1 year’, and according to the results from previous sections, it can be seen that the importance of Cl− is lower than the pH, rainfall, and SO2. In addition, the paper used Cl− concentration in the air to represent the chloride ion. However, there are works saying that the Cl− deposition/concentration in microclimate around the specimen may have an important influence on corrosion [5]. In this regard, we will design a new outdoor experiment to collect the microclimate around the specimen and to improve the results of this part in future works.
5. Conclusions
- The paper analyzed the characteristics of various LAS in various environments and built the prediction model using the random forests, artificial neural network, support vector regression, and logistic regression methods. The comparison results show that the Random Forests model can obtain the best fitting results on training samples and generalization results on testing samples. It indicated that the random forests can better implement the mapping relationship between corrosion factors and rates.
- By building five RF models with different exposure times, the paper obtained the effect changes of six environmental factors on corrosion with time. The results show that pH always plays the most important role at the entire corrosion process; SO2 and Cl− were more important for corrosion than T and RH at the initial stage; and Cl−, T, RH, and pH had a major influence on corrosion at the stationary stage, while rainfall just plays a major role in the 1st year.
- After the modeling, the paper forecasted the corrosion rate when one of six environmental factors changed, and other variables were constant. According to the forecast results, the paper can give qualitative and quantitative analysis of the influences of six environmental factors on corrosion.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; De la Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef]
- Kihira, H.; Senuma, T.; Tanaka, M.; Nishioka, K.; Fujii, Y.; Sakata, Y. A corrosion prediction method for weathering steels. Corros. Sci. 2005, 47, 2377–2390. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rustlayer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar]
- Gao, X.; Zhu, M.; Sun, C.; Fu, G. Dynamic Recrystallization Behavior and Microstructure Evolution of Bridge Weathering Steel in Austenite Region. Steel Res. Int. 2013, 84, 377–386. [Google Scholar] [CrossRef]
- Krivy, V.; Kuzmova, M.; Kreislova, K.; Urban, V. Characterization of corrosion products on weathering steel bridges influenced by chloride deposition. Metals 2017, 7, 336. [Google Scholar] [CrossRef]
- Li, H.; Chai, F.; Yang, C.F.; Li, C.; Luo, X.B. Corrosion behavior of low alloy steel for cargo oil tank under upper deck conditions. J. Iron Steel Res. Int. 2018, 25, 120–130. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Du, L.; Li, J.; Zhou, X.; Wang, X.; Wang, Y.; Liu, C.; Xu, G.; Misra, R.D.K. Hydrogen assisted cracking and CO2 corrosion behaviors of low-alloy steel with high strength used for armor layer of flexible pipe. Appl. Surf. Sci. 2018, 440, 974–991. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef]
- Kamrunnahar, M.; Urquidi-Macdonald, M. Prediction of corrosion behavior using neural network as a data mining tool. Corros. Sci. 2010, 52, 669–677. [Google Scholar] [CrossRef]
- Kamrunnahar, M.; Urquidi-Macdonald, M. Prediction of corrosion behaviour of Alloy 22 using neural network as a data mining tool. Corros. Sci. 2011, 53, 961–967. [Google Scholar] [CrossRef]
- Jiang, G.; Keller, J.; Bond, P.L.; Yuan, Z. Predicting concrete corrosion of sewers using artificial neural network. Water Res. 2016, 92, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.Z.; Mohammadi, Z. A hybrid intelligent model combining ANN and imperialist competitive algorithm for prediction of corrosion rate in 3C steel under seawater environment. Neural Comput. Appl. 2017, 28, 3455–3464. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Macdonald, D.D. Prediction of primary water stress corrosion crack growth rates in Alloy 600 using artificial neural networks. Corros. Sci. 2015, 92, 217–227. [Google Scholar] [CrossRef]
- Fang, S.F.; Wang, M.P.; Qi, W.H.; Zheng, F. Hybrid genetic algorithms and support vector regression in forecasting atmospheric corrosion of metallic materials. Comput. Mater. Sci. 2008, 44, 647–655. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I. Prediction of first-year corrosion losses of carbon steel and zinc in continental regions. Materials 2017, 10, 422. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Nikolaeva, L.A.; Kovtanyuk, V.V.; Igonin, T.N.; Andryushchenko, T.A. Comparative estimation of long-term predictions of corrosion losses for carbon steel and zinc using various models for the Russian territory. Corros. Eng. Sci. Technol. 2017, 52, 149–157. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, D.; Zhou, X.; Yang, T.; Zhi, Y.; Pei, Z.; Zhang, D.; Shao, L. Data mining to online galvanic current of zinc/copper Internet atmospheric corrosion monitor. Corros. Sci. 2018, 133, 443–450. [Google Scholar] [CrossRef]
- Melchers, R.E. Effect of small compositional changes on marine immersion corrosion of low alloy steels. Corros. Sci. 2004, 46, 1669–1691. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Zhou, Z.H. Ensemble Methods: Foundations and Algorithms. Chapman and Hall/CRC: Boca Raton, FL, USA, 2012. [Google Scholar]
- Yang, B.; Cao, J.M.; Jiang, D.P.; Lv, J.D. Facial expression recognition based on dual-feature fusion and improved random forest classifier. Multimed. Tools Appl. 2018, 77, 20477–20499. [Google Scholar] [CrossRef]
- Rahmati, O.; Pourghasemi, H.R.; Melesse, A.M. Application of GIS-based data driven random forest and maximum entropy models for groundwater potential mapping: A case study at Mehran Region, Iran. Catena 2016, 137, 360–372. [Google Scholar] [CrossRef]
- Quintana, D.; Sáez, Y.; Isasi, P. Random Forest Prediction of IPO Underpricing. Appl. Sci. 2017, 7, 636–651. [Google Scholar] [CrossRef]
- Yuk, E.; Park, S.; Park, C.S.; Baek, J.G. Feature-learning-based printed circuit board inspection via speeded-up robust features and random forest. Appl. Sci. 2018, 8, 932–945. [Google Scholar] [CrossRef]
- Park, S.; Im, J.; Jang, E.; Rhee, J. Drought assessment and monitoring through blending of multi-sensor indices using machine learning approaches for different climate regions. Agric. For. Meteorol. 2016, 216, 157–169. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–493. [Google Scholar] [CrossRef]
- Bengio, Y. Learning deep architectures for AI. Found. Trends in Mach. Learn. 2009, 2, 1–127. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Feng, J. Deep forest: Towards an alternative to deep neural networks. In proceeding of the 26th International Joint Conference on Artificial Intelligence, Melbourne, Australia, 19–25 August 2017. [Google Scholar]
- Hou, Y.; Aldrich, C.; Lepkova, K.; Machuca, L.L.; Kinsella, B. Analysis of electrochemical noise data by use of recurrence quantification analysis and machine learning methods. Electrochim. Acta 2017, 256, 337–347. [Google Scholar] [CrossRef]
- Hou, Y.; Aldrich, C.; Lepkova, K.; Kinsella, B. Identifying corrosion of carbon steel buried in iron ore and coal cargoes based on recurrence quantification analysis of electrochemical noise. Electrochim. Acta 2018, 283, 212–220. [Google Scholar] [CrossRef]
- Ng, D.P.; Wu, D.; Wood, B.L.; Fromm, J.R. Computer-aided detection of rare tumor populations in flow cytometry: An example with classic Hodgkin lymphoma. Am. J. Clin. Pathol. 2015, 144, 517–524. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Igonin, T.N.; Kovtanyuk, V.V.; Nikolaeva, L.A. Long-term forecast of corrosion mass losses of technically important metals in various world regions using a power function. Corros. Sci. 2014, 88, 306–316. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Wicke, D.; Cochrane, T.A.; O’Sullivan, A.D.; Cave, S.; Derksen, M. Effect of age and rainfall pH on contaminant yields from metal roofs. Water Sci. Technol. 2014, 69, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Wu, L.; Han, R.; Sun, Y. Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- Mendoza, A.R.; Corvo, F. Outdoor and indoor atmospheric corrosion of non-ferrous metals. Corros. Sci. 2000, 42, 1123–1147. [Google Scholar] [CrossRef]
- Dan, Z.; Muto, I.; Hara, N. Effects of environmental factors on atmospheric corrosion of aluminium and its alloys under constant dew point conditions. Corros. Sci. 2012, 57, 22–29. [Google Scholar] [CrossRef]
- Hu, Y.B.; Dong, C.F.; Sun, M.; Xiao, K.; Zhong, P.; Li, X.G. Effects of solution pH and Cl- on electrochemical behavior of an aermet100 ultra-high strength steel in acidic environments. Corros. Sci. 2011, 53, 4159–4165. [Google Scholar] [CrossRef]



| Alloy | Element Compositions, wt. % | |||||||
|---|---|---|---|---|---|---|---|---|
| Mn | S | P | Si | Cr | Cu | Ni | Fe | |
| 06CuPCrNiMo | 0.4 | 0.023 | 0.05 | 0.17 | - | 0.17 | - | Balance |
| 09CuPCrNiA | 0.4 | 0.023 | 0.015 | 0.26 | 0.1 | 0.05 | 0.02 | Balance |
| 09CuPTiRe | 0.4 | 0.019 | 0.08 | 0.28 | - | 0.29 | - | Balance |
| 09MnNb(s) | 1.18 | 0.024 | 0.027 | 0.2 | 0.1 | 0.05 | 0.1 | Balance |
| 10CrCuSiV | 0.31 | 0.002 | 0.01 | 0.62 | 0.83 | 0.25 | 0.1 | Balance |
| 10CrMoAl | 0.45 | 0.002 | 0.012 | 0.35 | 0.98 | 0.09 | - | Balance |
| 14MnMoNbB | 1.53 | 0.01 | 0.022 | 0.34 | 0.1 | 0.05 | - | Balance |
| 15MnMoVN | 1.52 | 0.004 | 0.026 | 0.4 | 0.1 | 0.05 | - | Balance |
| 16Mn | 1.4 | 0.025 | 0.009 | 0.36 | 0.1 | 0.05 | - | Balance |
| 16MnQ | 1.37 | 0.023 | 0.03 | 0.3 | 0.1 | 0.07 | 0.05 | Balance |
| D36 | 1.4 | 0.018 | 0.022 | 0.39 | 0.05 | 0.05 | - | Balance |
| JN235(RE) | 0.516 | 0.025 | 0.03 | 0.3 | 0.1 | 0.07 | - | Balance |
| JN255 | 0.67 | 0.006 | 0.016 | 0.07 | 0.02 | 0.05 | 0.05 | Balance |
| JN255(RE) | 0.39 | 0.005 | 0.01 | 0.62 | 0.83 | 0.25 | 0.1 | Balance |
| JN345 | 0.39 | 0.005 | 0.11 | 0.05 | 0.9 | 0.4 | 0.65 | Balance |
| JN345(RE) | 0.36 | 0.011 | 0.089 | 0.28 | - | 0.29 | - | Balance |
| JY235(RE) | 0.27 | 0.01 | 0.089 | 0.28 | - | 0.29 | - | Balance |
| Factors | Unit | Average | Minimum | Maximum |
|---|---|---|---|---|
| Average Relative Humidity (RH) | % | 75.09 | 56.17 | 87.71 |
| Average Temperature (T) | °C | 17.58 | 11.08 | 26.05 |
| Rainfall | mm/month | 159.74 | 45.64 | 753 |
| SO2 concentration (SO2) | mg/cm3 | 0.089 | 0.015 | 0.302 |
| pH of rain (pH) | - | 6.14 | 5.11 | 6.97 |
| Chloride concentration (Cl−) | mg/cm3 | 0.221 | 0.0001 | 1.967 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, Y.; Fu, D.; Zhang, D.; Yang, T.; Li, X. Prediction and Knowledge Mining of Outdoor Atmospheric Corrosion Rates of Low Alloy Steels Based on the Random Forests Approach. Metals 2019, 9, 383. https://doi.org/10.3390/met9030383
Zhi Y, Fu D, Zhang D, Yang T, Li X. Prediction and Knowledge Mining of Outdoor Atmospheric Corrosion Rates of Low Alloy Steels Based on the Random Forests Approach. Metals. 2019; 9(3):383. https://doi.org/10.3390/met9030383
Chicago/Turabian StyleZhi, Yuanjie, Dongmei Fu, Dawei Zhang, Tao Yang, and Xiaogang Li. 2019. "Prediction and Knowledge Mining of Outdoor Atmospheric Corrosion Rates of Low Alloy Steels Based on the Random Forests Approach" Metals 9, no. 3: 383. https://doi.org/10.3390/met9030383
APA StyleZhi, Y., Fu, D., Zhang, D., Yang, T., & Li, X. (2019). Prediction and Knowledge Mining of Outdoor Atmospheric Corrosion Rates of Low Alloy Steels Based on the Random Forests Approach. Metals, 9(3), 383. https://doi.org/10.3390/met9030383





