Simulation Approach for Cathodic Protection Prediction of Aluminum Fin-Tube Heat Exchanger Using Boundary Element Method
Abstract
1. Introduction
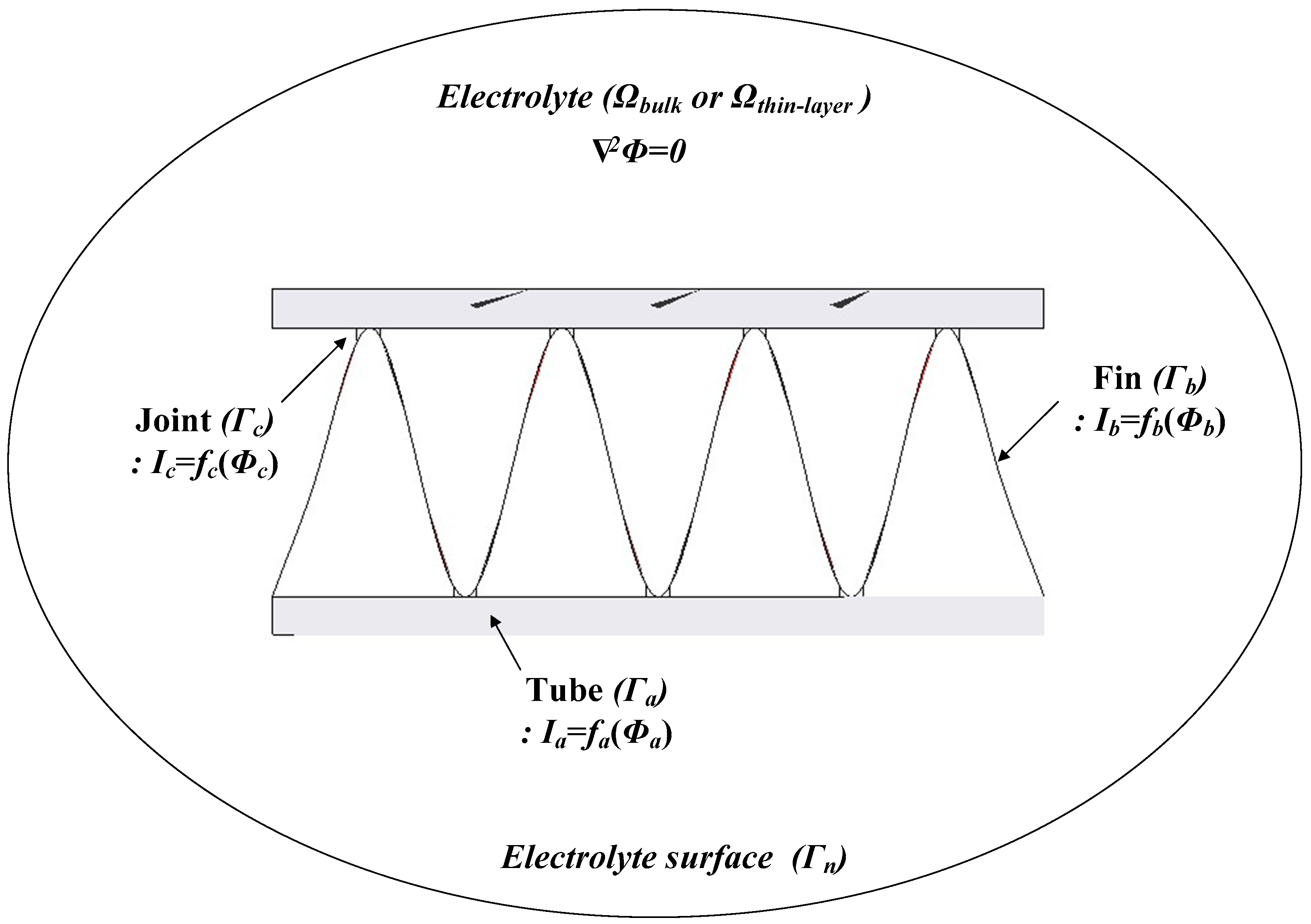
2. Boundary Element Method (BEM)
3. Experimental Procedures
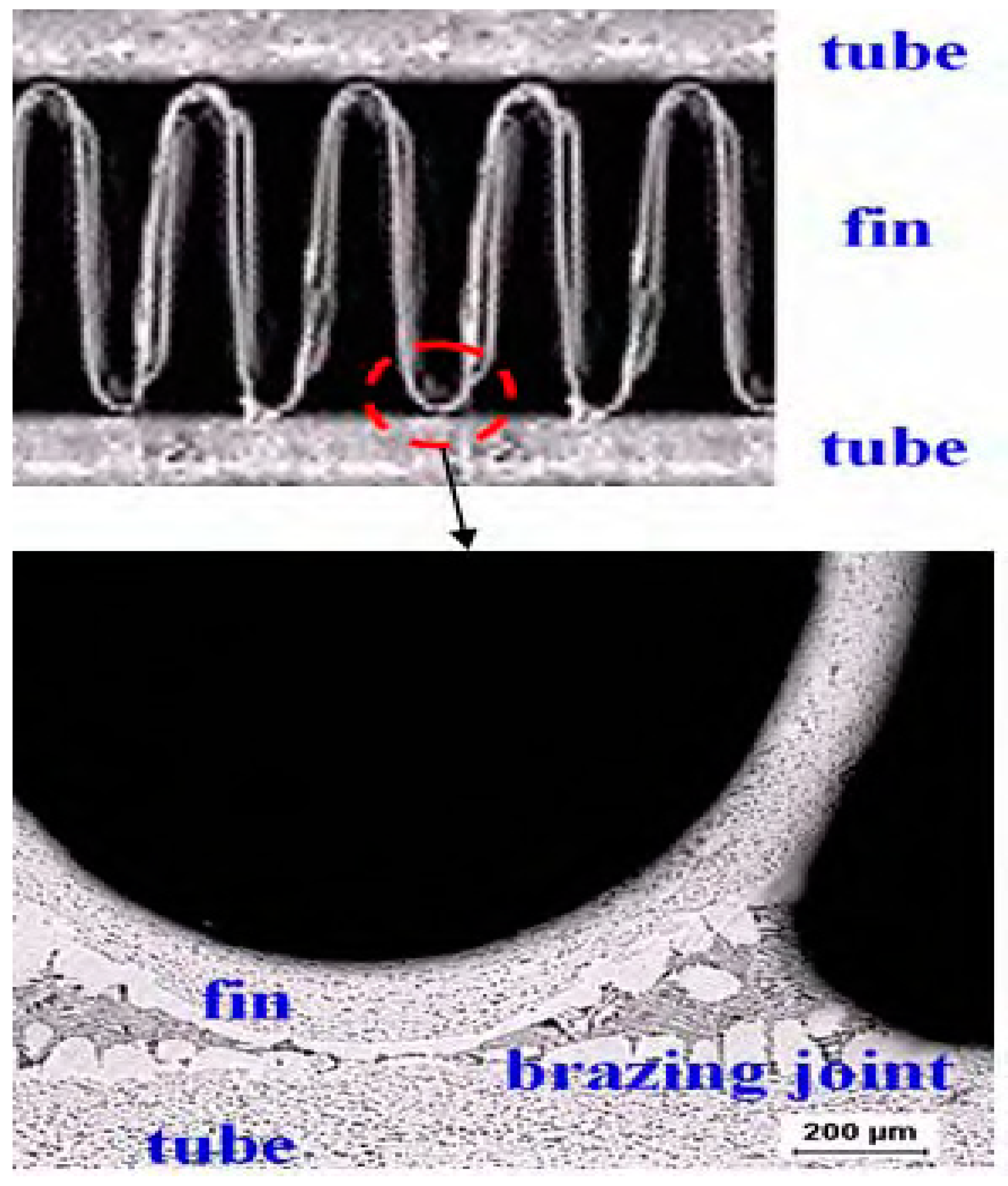
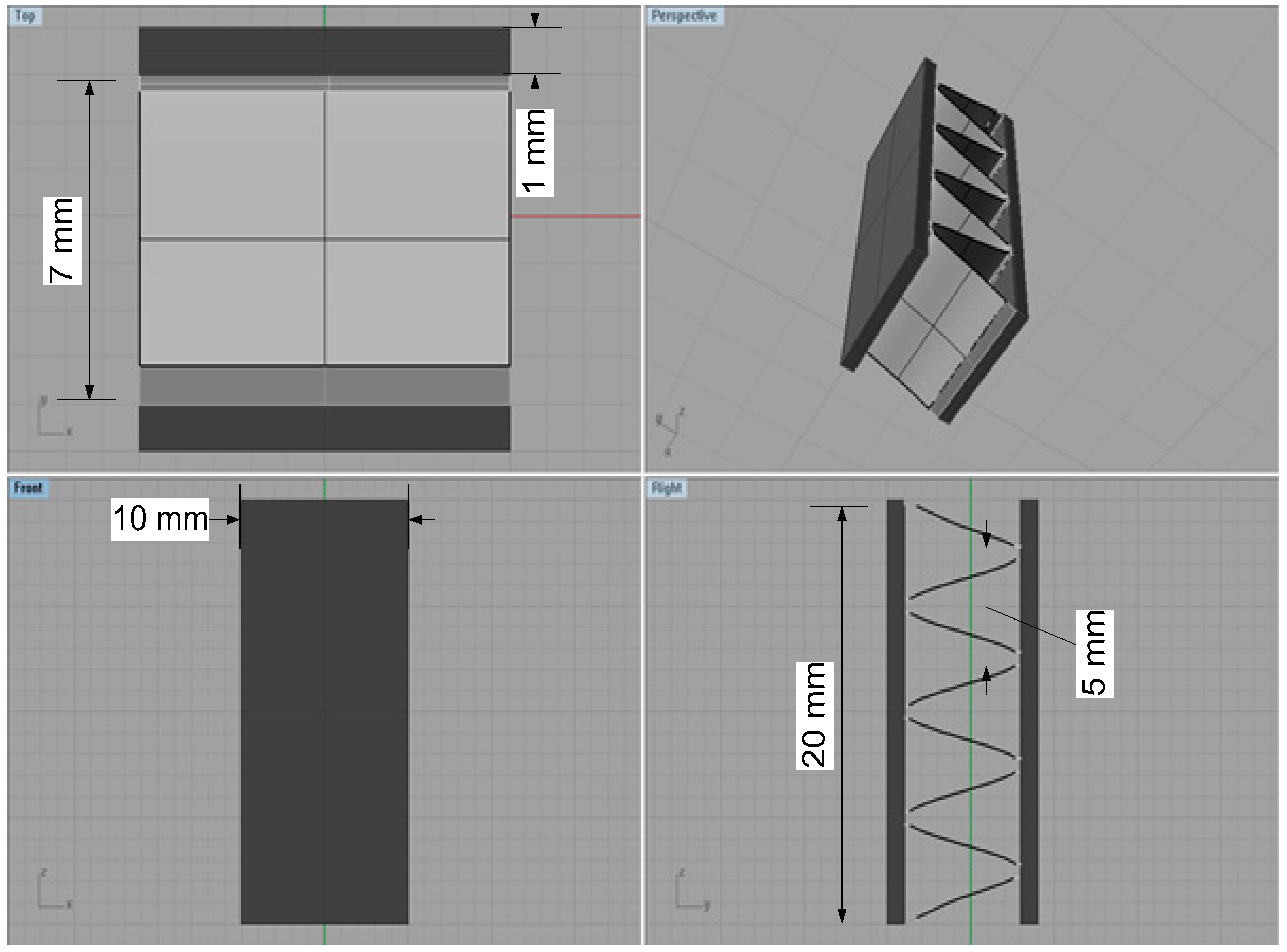
3.1. Materials and Solution
3.2. Potentiodynamic Tests
3.3. Sea Water Acetic Acid Test (SWAAT)
4. Results and Discussion
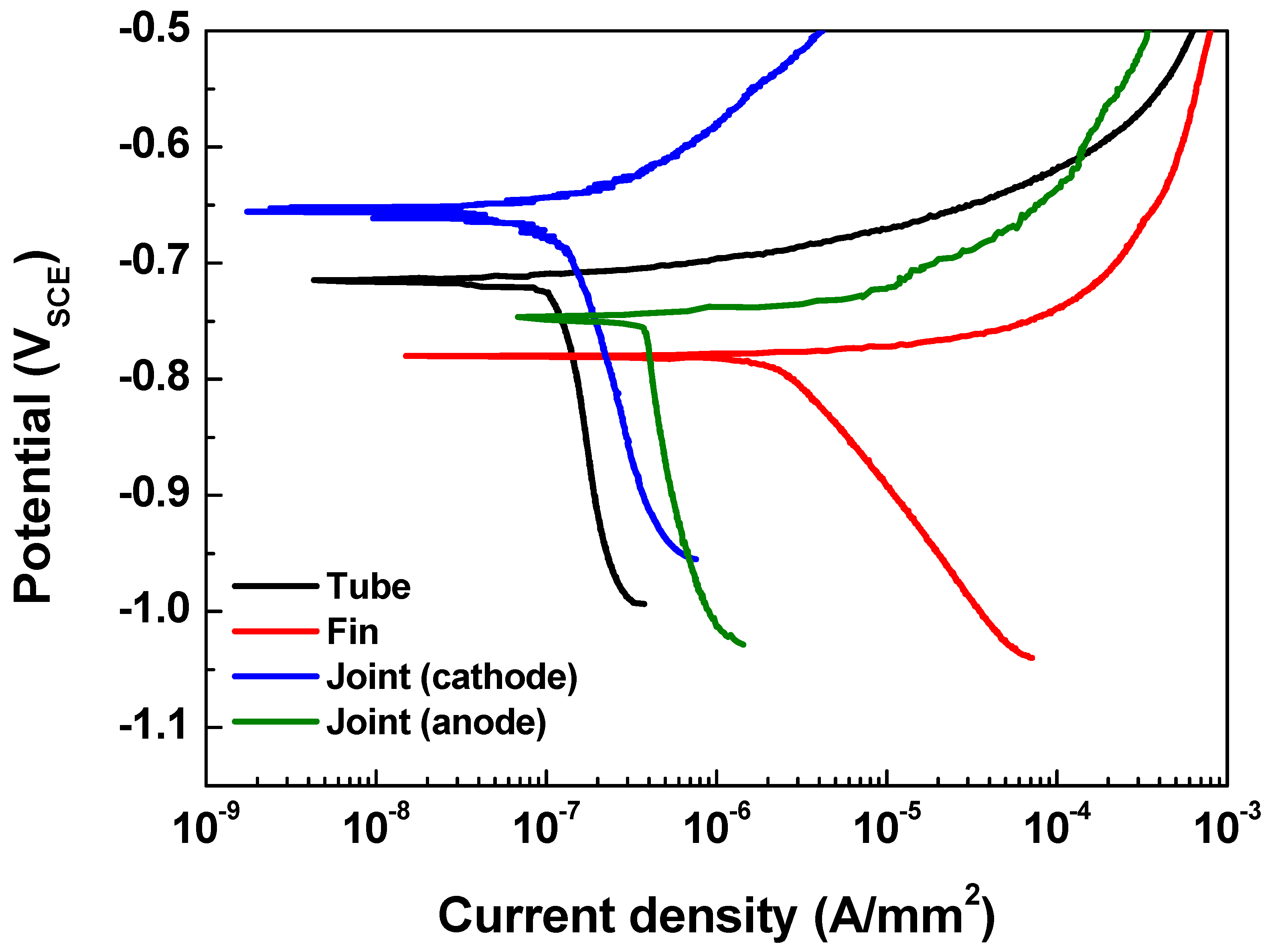
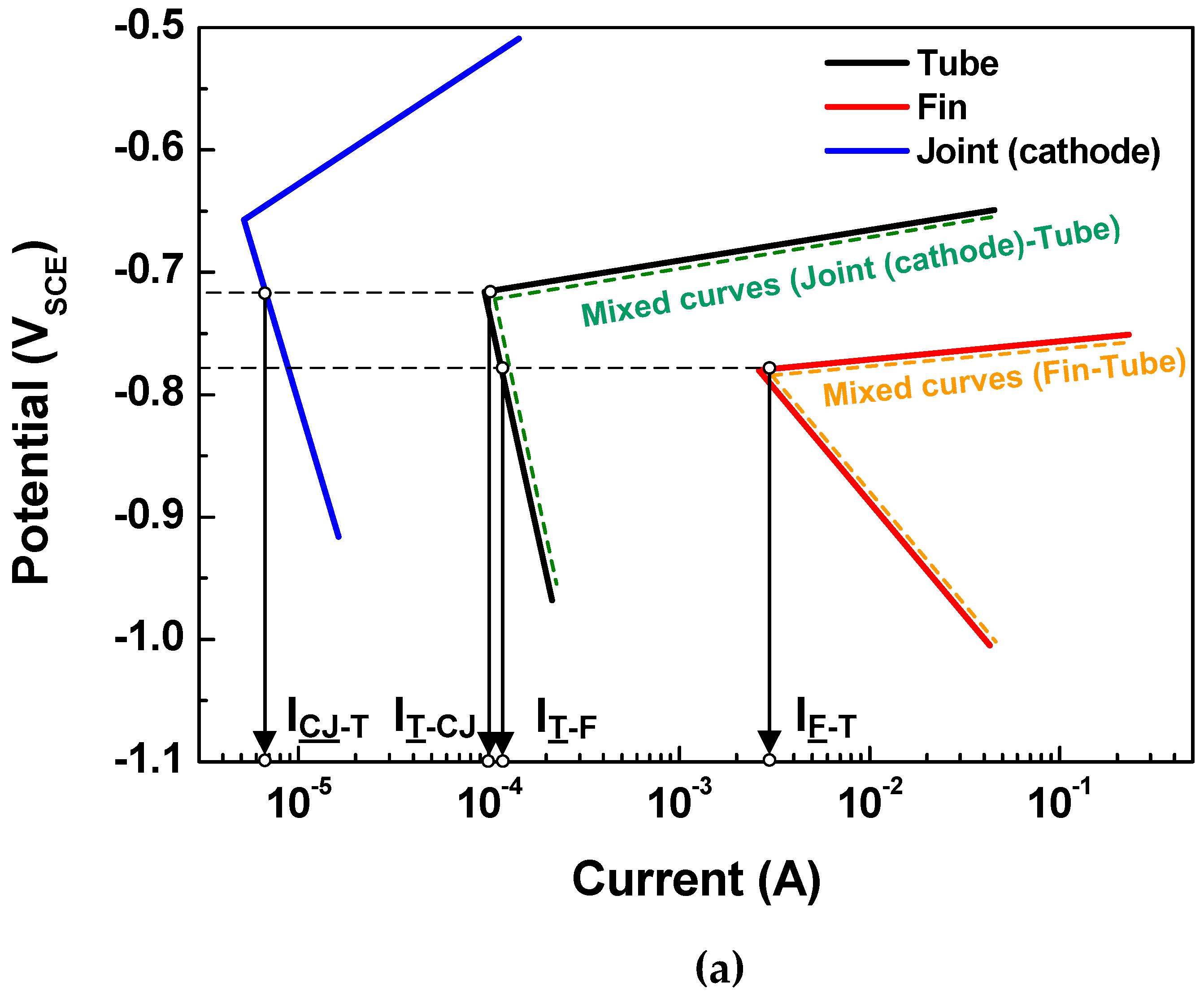
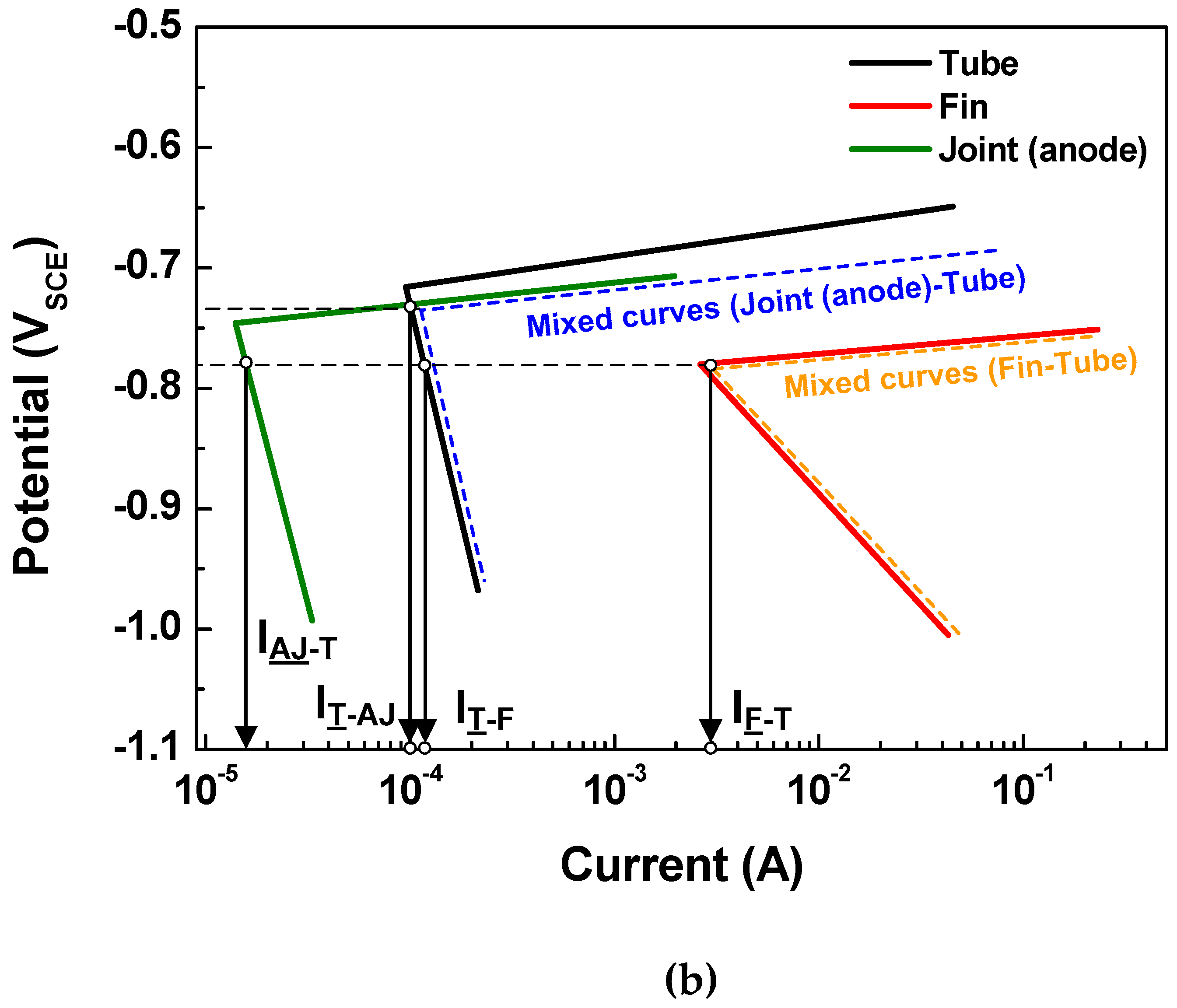
4.1. Polarization Curves
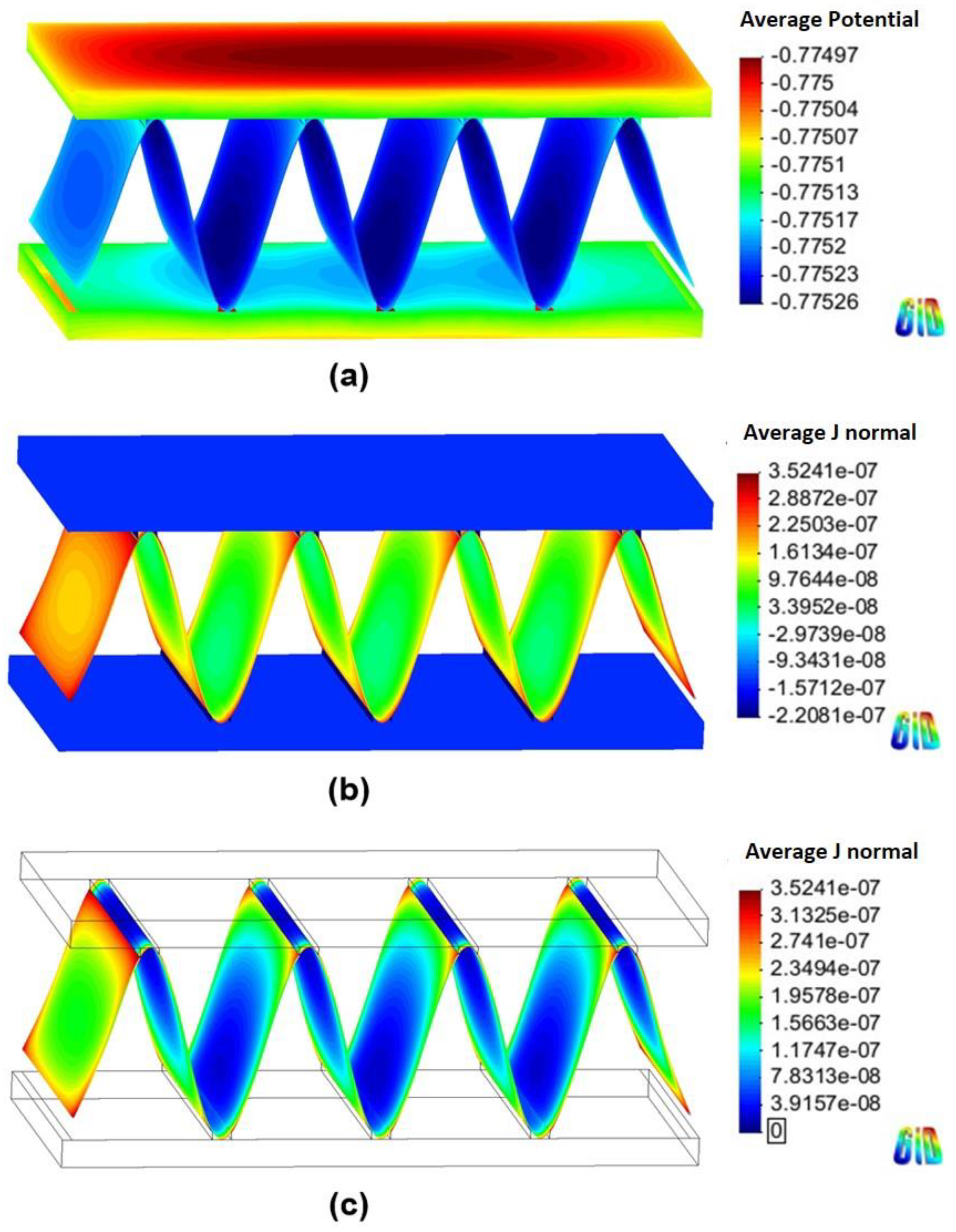
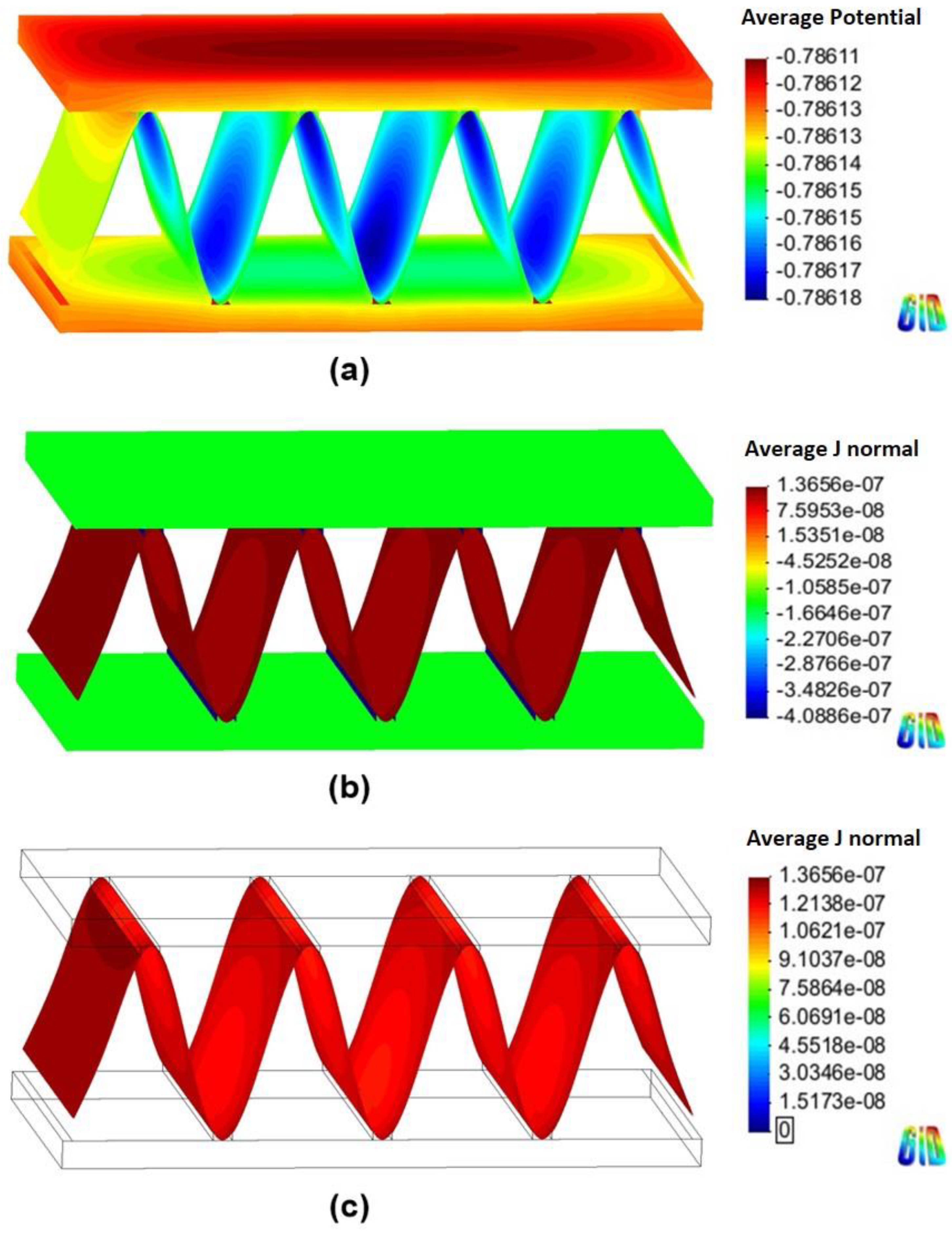
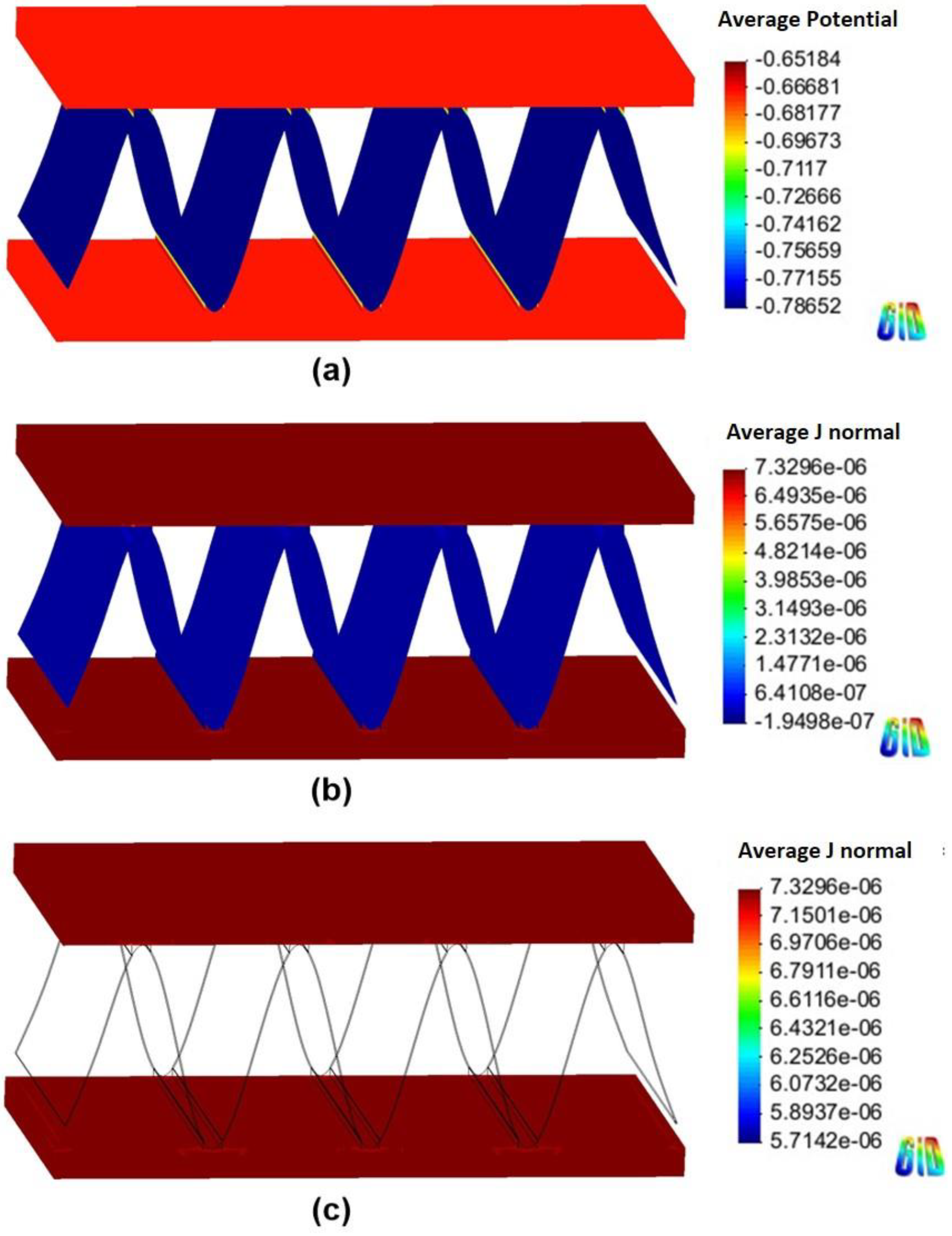
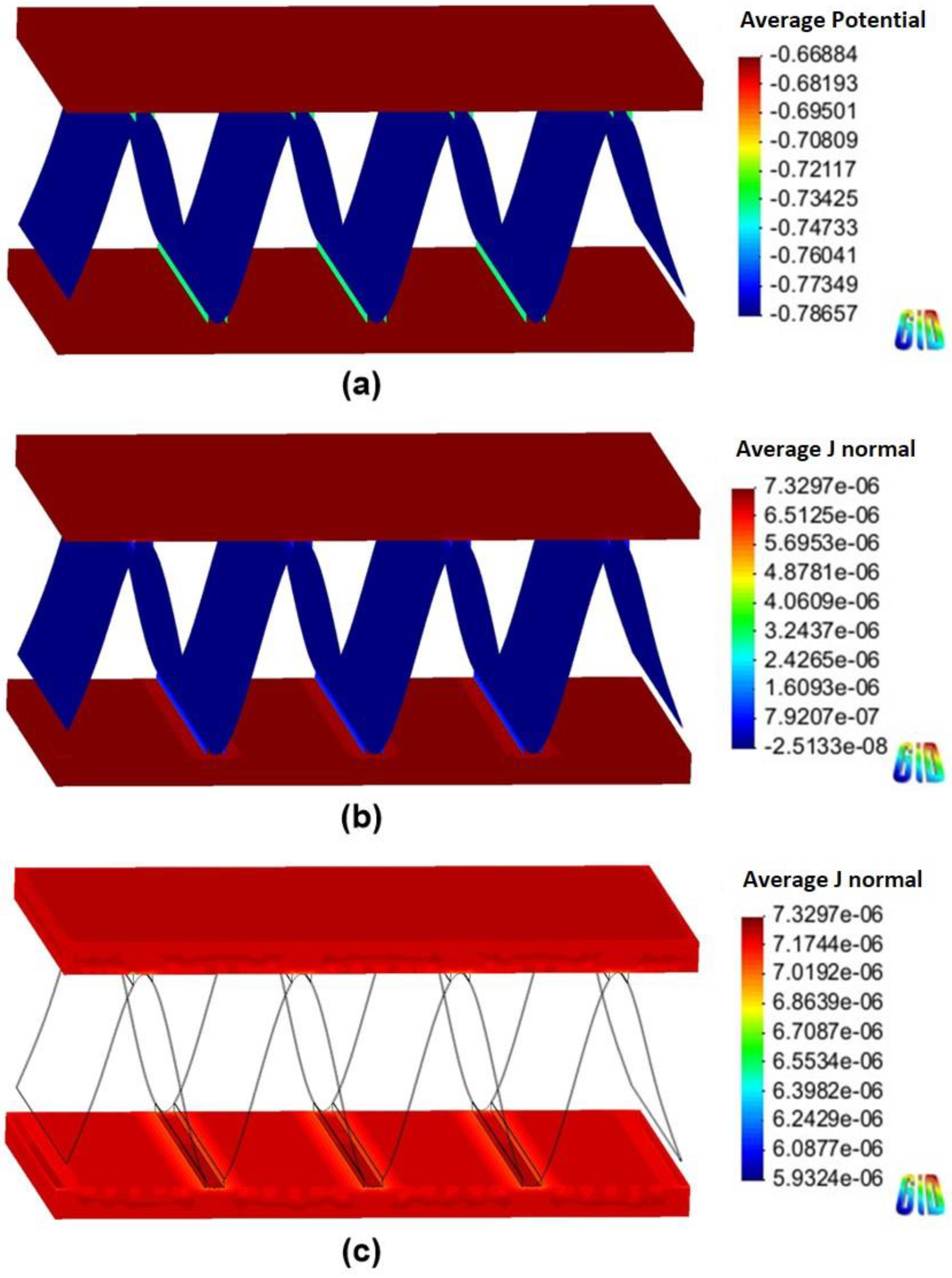
4.2. Corrosion Simulation
4.3. SWAAT Test
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| i | Current density, A/mm2 |
| V | potential, VSCE |
| icorr | corrosion current density, μA/mm2 |
| Ecorr | corrosion potential, VSCE |
| βa | anodic Tafel slope, V/decade |
| βc | cathodic Tafel slope, V/decade |
| Inet | net current, A |
Subscripts
| Ω | electrolyte domain |
| Ωbulk | bulk electrolyte domain |
| Ωthin-layer | thin-layer electrolyte domain |
| Γn | surface of the electrolyte |
| Γa | surface of the tube part |
| Γb | surface of the fin part |
| Γc | surface of the joint part |
| Φ | electrical potential |
| fa(Φa) | non-linear functions of the tube surface |
| fb(Φb) | non-linear functions of the tube fin |
| fc(Φc) | non-linear functions of the tube joint |
| A1, A2, and A3 | three metals |
| EA1, EA2, and EA3 | corrosion potential of three metals, V |
| IA-B | net current of A in galvanic condition between A and B |
Abbreviation
| Aluminum | Al |
| heating, ventilation, air conditioning, and refrigeration | HVACR |
| sulphur oxides | SOx |
| sea water acetic acid test | SWAAT |
| saturated calomel electrode | SCE |
| 3 dimension | 3D |
| American Society for Testing and Materials | ASTM |
| nitrogen | N2 |
| open-circuit potential | OCP |
References
- Andreatta, F.; Anzytti, A.; Fedrizzi, L. Corrosion behaviour of AA 8xxx aluminium fins in heat exchanger. Surf. Interface Anal. 2016, 48, 789–797. [Google Scholar] [CrossRef]
- Tierce, S.; Pébère, N.; Blanc, C.; Casenave, C.; Mankowski, G.; Robidou, H. Corrosion behaviour of brazing material AA 4343. Electrochim. Acta 2006, 52, 1092–1100. [Google Scholar] [CrossRef]
- Adams, T.M.; Dowling, M.F.; Abdel-Khalik, S.I.; Jeter, S.M. Applicability of traditional turbulent single-phase forced convection correlations to noncircular microchannels. Int. J. Heat Mass Trans. 1999, 42, 4411–4415. [Google Scholar] [CrossRef]
- Akers, W.W.; Rosson, H.F. Condensation inside a horizontal tube. Chem. Eng. Prog. S Ser. 1960, 56, 145–150. [Google Scholar]
- Cavallini, A.; Col, D.D.; Doretti, L.; Matkovic, M.; Rosetto, L.; Zilio, C. Condensation in horizontal smooth tubes: A new heat transfer model for heat exchanger design. Heat Transf. Eng. 2006, 27, 31–38. [Google Scholar] [CrossRef]
- Abdulstaar, M.; Mhaede, M.; Wagner, L.; Wollmann, M. Corrosion behaviour of Al 1050 severely deformed by rotary swaging. Mater. Design 2014, 57, 325–329. [Google Scholar] [CrossRef]
- Hong, M.S.; Park, I.J.; Kim, J.G. Alloying effect of copper concentration on the localized corrosion of aluminum alloy for heat exchanger tube. Met. Mater. Int. 2017, 23, 708–714. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Q.; Wang, B.; Du, Z. Microstructure evolution and tensile mechanical properties of thixoformed high performance Al–Zn–Mg–Cu alloy. Met. Mater. Int. 2015, 21, 897–906. [Google Scholar] [CrossRef]
- ASM International, Materials Park. Metals Handbook: Welding and Brazing, 8th ed.; ASM International, Materials Park: Novelty, OH, USA, 1971; Volume 6. [Google Scholar]
- Tierce, S.; Pébère, N.; Blanc, C.; Mankowski, G.; Robidou, H.; Vaumousse, D.; Lacaze, J. Solidification and phase transformations in brazed aluminum alloys used in automotive heat exchangers. Int. J. Cast. Met. Res. 2005, 18, 370–376. [Google Scholar] [CrossRef]
- Lacaze, J.; Tierce, S.; Lafont, M.C.; Thebault, Y.; Pébère, N.; Mankowski, G.; Blanc, C.; Robidou, H.; Vanmosse, D.; Daloz, D. Study of the microstructure resulting from brazed aluminium materials used in heat exchangers. Mater. Sci. Eng. A 2005, 413, 317–321. [Google Scholar] [CrossRef]
- Sekulić, D.P. Molten aluminum equilibrium membrane formed during controlled atmosphere brazing. Int. J. Eng. Sci. 2001, 39, 229–241. [Google Scholar] [CrossRef]
- Sekulic, D.P.; Zellmer, B.J.; Nigro, N. Influence of joint topology on the formation of brazed joints. Model. Simul. Mater. Sci. Eng. 2001, 9, 357–370. [Google Scholar] [CrossRef]
- Zellmer, B.J.; Nigro, N.; Sekulic, D.P. Numerical modelling and experimental verification of the formation of 2D and 3D brazed joints. Model. Simul. Mater. Sci. Eng. 2001, 9, 339–355. [Google Scholar] [CrossRef]
- Marshall, G.J.; Bolingbroke, R.K.; Gray, A. Microstructural control in an aluminum core alloy for brazing sheet applications. Metall. Trans. A 1993, 24A, 1935–1942. [Google Scholar] [CrossRef]
- Al-Kharafi, F.M.; Badawy, W.A. Corrosion and passivation of Al and Al-Si alloys in nitric-acid solutions II-Effect of chloride ions. Electrochim. Acta 1995, 40, 1811–1817. [Google Scholar] [CrossRef]
- Abdel Rehim, S.S.; Hassan, H.H.; Amin, M.A. Chronoamperometric studies of pitting corrosion of Al and (Al-Si) alloys by halide ions in neutral sulphate solution. Corros. Sci. 2004, 46, 1921–1938. [Google Scholar] [CrossRef]
- Amaya, K.; Aoki, S. Effective boundary element methods in corrosion analysis. Eng. Anal. Bound. Elem. 2003, 27, 507–519. [Google Scholar] [CrossRef]
- McCafferty, E. Distribution of potential and current in circular corrosion cells having unequal polarization parameters. J. Electrochem. Soc. 1997, 124, 1869–1878. [Google Scholar] [CrossRef]
- Adey, R.A.; Niku, S.M. Computer modeling of galvanic corrosion. ASTM Spec. Tech. Publ. 1998, 978, 96–117. [Google Scholar]
- Ridha, M.; Amaya, K.; Aoki, S. Boundary element simulation for identification of steel corrosion in concrete using magnetic field measurement. Corrosion 2005, 61, 784–791. [Google Scholar] [CrossRef]
- Jafarian, F.; Umbrello, D.; Jabbaripour, B. Indentification of new material model for machining simulation of Inconel 718 ally and the effect of tool edge geometry on microstructure changes. Simul. Model. Pract. Theory 2016, 66, 273–284. [Google Scholar] [CrossRef]
- Gastli, A.; Metwally, I.A. Computation of eddy-current density on ESP motor and well casings under different operating conditions. Simul. Model. Pract. Theory 2008, 16, 483–493. [Google Scholar] [CrossRef]
- Nisançiöglu, K. Electrochemical behavior of aluminum-base intermetallics containing iron. J. Electrochem. Soc. 1990, 137, 69–77. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Hu, Q.F.; Zhang, T.; Geng, S.J.; Wang, F.H. A method for determining the polarization state of the metal with the middle potential in order to calculate the corrosion rate of multi-metals complicated galvanic couple. Mater. Corros. 2017, 68, 935–942. [Google Scholar] [CrossRef]
| Parts | Chemical Composition (wt%) | ||||
|---|---|---|---|---|---|
| Cu | Fe | Si | Zn | Mn | |
| Tube (AA 1100) | 0.001 | 0.200 | 0.010 | - | - |
| Fin (AA 3003) | 0.002 | 0.210 | 0.220 | 0.150 | 0.640 |
| Cathodic joint (AA 4343) | 0.110 | 0.120 | 5.380 | 0.080 | 0.010 |
| Anodic joint (modified AA 4343) | 0.120 | 0.120 | 7.730 | 0.480 | 0.010 |
| Parts | βa (V/decade) | βc (V/decade) | icorr (μA/mm2) | Ecorr (VSCE) |
|---|---|---|---|---|
| Tube (AA 1100) | 0.021 0.003 | 0.886 0.01 | 0.109 0.05 | −0.716 0.02 |
| Fin (AA 3003) | 0.010 0.005 | 0.223 0.01 | 2.351 0.25 | −0.792 0.02 |
| Cathodic joint (AA 4343) | 0.109 0.03 | 0.748 0.09 | 0.129 0.06 | −0.657 0.01 |
| Anodic joint (modified AA 4343) | 0.018 0.002 | 0.859 0.05 | 0.349 0.09 | −0.746 0.03 |
| Polarization State FROM SIMULATION | Leakage Time (day) | ||
|---|---|---|---|
| High conductivity | Low conductivity | ||
| Cathodic joint | Cathode | Cathode | 56 days |
| Tube | Cathode | Anode | |
| Fin | Anode | Anode | |
| Anodic joint | Cathode | Anode/Cathode | 80 days |
| Tube | Cathode | Anode | |
| Fin | Anode | Anode | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Park, I.-J.; Kim, J.-G. Simulation Approach for Cathodic Protection Prediction of Aluminum Fin-Tube Heat Exchanger Using Boundary Element Method. Metals 2019, 9, 376. https://doi.org/10.3390/met9030376
Kim Y-S, Park I-J, Kim J-G. Simulation Approach for Cathodic Protection Prediction of Aluminum Fin-Tube Heat Exchanger Using Boundary Element Method. Metals. 2019; 9(3):376. https://doi.org/10.3390/met9030376
Chicago/Turabian StyleKim, Yong-Sang, In-Jun Park, and Jung-Gu Kim. 2019. "Simulation Approach for Cathodic Protection Prediction of Aluminum Fin-Tube Heat Exchanger Using Boundary Element Method" Metals 9, no. 3: 376. https://doi.org/10.3390/met9030376
APA StyleKim, Y.-S., Park, I.-J., & Kim, J.-G. (2019). Simulation Approach for Cathodic Protection Prediction of Aluminum Fin-Tube Heat Exchanger Using Boundary Element Method. Metals, 9(3), 376. https://doi.org/10.3390/met9030376





