Duplex and Superduplex Stainless Steels: Microstructure and Property Evolution by Surface Modification Processes
Abstract
1. Duplex and Super Duplex Stainless Steels
2. Surface Modification Technologies
3. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvarez-Armas, I.; Degallaix-Moreuil, S. Duplex Stainless Steels; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lo, K.H.; Kwok, C.T.; Chan, W.K.; Kuan, H.C.; Lai, K.K.; Wang, K.Y. Duplex Stainless Steels. In Encyclopedia of Iron, Steel, and Their Alloys; CRC Press: Boca Raton, FL, USA, 2016; pp. 1150–1160. [Google Scholar]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Gunn, R. Duplex Stainless Steels: Microstructure, Properties and Applications; Woodhead Publishing: Cambridge, UK, 1997. [Google Scholar]
- Nilsson, J.O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Solomon, H.D.; Devine, T.M., Jr. Duplex Stainless Steels—A Tale of Two Phases; American Society for Metals: Metals Park, OH, USA, 1982; pp. 693–656. [Google Scholar]
- Kangas, P.; Chai, G. Use of advanced austenitic and duplex stainless steels for applications in Oil & Gas and Process industry. Century Stainl. Steels 2013, 794, 645–669. [Google Scholar]
- Chail, G.; Kangas, P. Super and hyper duplex stainless steels: Structures, properties and applications. Procedia Struct. Integr. 2016, 2, 1755–1762. [Google Scholar] [CrossRef]
- Salvio, F.; da Silva, B.R.S. On the Role of HISC on Super and Hyper Duplex Stainless Steel Tubes. In Proceedings of the Offshore Technology Conference, Rio de Janeiro, Brazil, 29–31 October 2013. [Google Scholar]
- Wan, J.; Ruan, H.; Wang, J.; Shi, S. The Kinetic diagram of sigma phase and its precipitation hardening effect on 15Cr-2Ni duplex stainless steel. Mater. Sci. Eng. A 2017, 711, 571–578. [Google Scholar] [CrossRef]
- Atamert, S.; King, J.E. Sigma-phase formation and its prevention in duplex stainless steels. J. Mater. Sci. Lett. 1993, 12, 1144–1147. [Google Scholar] [CrossRef]
- Chen, T.H.; Weng, K.L.; Yang, J.R. The effect of high-temperature exposure on the microstructural stability and toughness property in a 2205 duplex stainless steel. Mater. Sci. Eng. A 2002, 338, 259–270. [Google Scholar] [CrossRef]
- Magnabosco, R.; Santos, D.C.d. Intermetallic Phases Formation During Short Aging between 850 °C and 950 °C of a Superduplex Stainless Steel. J. Mater. Res. Technol. 2012, 26. [Google Scholar] [CrossRef]
- He, Y.-L.; Zhu, N.-Q.; Lu, X.-G.; Li, L. Experimental and computational study on microstructural evolution in 2205 duplex stainless steel during high temperature aging. Mater. Sci. Eng. A 2010, 528, 721–729. [Google Scholar] [CrossRef]
- Escriba, D.M.; Materna-Morris, E.; Plaut, R.L.; Padilha, A.F. Chi-phase precipitation in a duplex stainless steel. Mater. Charact. 2009, 60, 1214–1219. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2006, 58, 65–71. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Sigma phase precipitation in duplex stainless steel 2205. Mater. Sci. Eng. A 2007, 444, 271–276. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Liu, P. Aging at 400–600 °C of submerged arc welds of 22Cr–3Mo–8Ni duplex stainless steel and its effect on toughness and microstructure. Mater. Sci. Technol. 1991, 7, 853–862. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chumbley, L.S.; Gleeson, B. Determination of isothermal transformation diagrams for sigma-phase formation in cast duplex stainless steels CD3MN and CD3MWCuN. Metall. Mater. Trans. A 2004, 35, 3377–3386. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Karlsson, L.; Andersson, J.O. Secondary austenite for mation and its relation to pitting corrosion in duplex stainless steel weld metal. Mater. Sci. Technol. 1995, 11, 276–283. [Google Scholar] [CrossRef]
- Kuwahara, H.; Matsuoka, H.; Takada, J.; Kikuchi, S.; Tomii, Y.; Tamura, I. Plasma nitriding of Fe-18Cr-9Ni in the range of 723?823 K. Oxid. Met. 1991, 36, 143–156. [Google Scholar] [CrossRef]
- Flis, J.; Mańkowski, J.; Roliński, E. Corrosion Behaviour of Stainless Steels Aner Plasma and Ammonia Nitriding. Surf. Eng. 1989, 5, 151–157. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and Corrosion resistance of plasma nitrided Stainless Steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- Bielawski, J.; Baranowska, J. Formation of nitrided layers on duplex steel—influence of multiphase substrate. Surf. Eng. 2010, 26, 299–304. [Google Scholar] [CrossRef]
- Jasinski, J.J.; Fraczek, T.; Kurpaska, L.; Lubas, M.; Sitarz, M. Investigation of nitrogen transport in active screen plasma nitriding processes—Uphill diffusion effect. J. Mol. Struct. 2018, 1164, 37–44. [Google Scholar] [CrossRef]
- Paijan, L.H.; Berhan, M.N.; Adenan, M.S.; Yusof, N.F.M.; Haruman, E. Structural Development of Expanded Austenite on Duplex Stainless Steel by Low Temperature Thermochemical Nitriding Process. Adv. Mater. Res. 2012, 576, 260–263. [Google Scholar] [CrossRef]
- Dong, H. S-phase surface engineering of Fe–Cr, Co–Cr and Ni–Cr alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- De Oliveira, W.R.; Kurelo, B.C.E.S.; Ditzel, D.G.; Serbena, F.C.; Foerster, C.E.; de Souza, G.B. On the S-phase formation and the balanced plasma nitriding of austenitic-ferritic super duplex stainless steel. Appl. Surf. Sci. 2018, 434, 1161–1174. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Bell, T. Structural characteristics of low temperature plasma carburised austenitic stainless steel. Mater. Sci. Technol. 1999, 15, 1171–1178. [Google Scholar] [CrossRef]
- Mingolo, N.; Tschiptschin, A.P.; Pinedo, C.E. On the formation of expanded austenite during plasma nitriding of an AISI 316L austenitic stainless steel. Surf. Coat. Technol. 2006, 201, 4215–4218. [Google Scholar] [CrossRef]
- Borgioli, F.; Fossati, A.; Galvanetto, E.; Bacci, T. Glow-discharge nitriding of AISI 316L austenitic stainless steel: Influence of treatment temperature. Surf. Coat. Technol. 2005, 200, 2474–2480. [Google Scholar] [CrossRef]
- Fewell, M.P.; Mitchell, D.R.G.; Priest, J.M.; Short, K.T.; Collins, G.A. The nature of expanded austenite. Surf. Coat. Technol. 2000, 131, 300–306. [Google Scholar] [CrossRef]
- Blawert, C.; Kalvelage, H.; Mordike, B.L.; Collins, G.A.; Short, K.T.; Jiraskova, Y.; Schneeweiss, O. Nitrogen and carbon expanded austenite. Surf. Coat. Technol. 2001, 136, 181–187. [Google Scholar] [CrossRef]
- Ichii, T.; Fujimura, K.; Takase, K. Structure of the ion-nitrided layer of 18-8 stainless steel. Tech. Rep. Kansai Univ. 1986, 27, 134–144. [Google Scholar]
- Leylanda, A.; Lewis, D.B.; Stevenson, P.R.; Matthewsa, A. Low temperature plasma diffusion treatment of stainless steels for improved wear resistance. Surf. Coat. Technol. 1993, 62, 608–617. [Google Scholar] [CrossRef]
- Makishi, T.; Nakata, K. Surface Hardening of Nickel Alloys by Means of Plasma Nitriding. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35, 227–238. [Google Scholar] [CrossRef]
- Williamson, D.L.; Davis, J.A.; Wilbur, P.J. Effect of austenitic stainless steel composition on low-energy, high-flux, nitrogen ion beam processing. Surf. Coat. Technol. 1998, 104, 178–184. [Google Scholar] [CrossRef]
- Li, X.Y.; Habibi, N.; Bell, T.; Dong, H. Microstructural characterisation of a plasma carburised low carbon Co–Cr alloy. Surf. Eng. 2007, 23, 45–51. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Hu, X.; Dong, H. Surface & Coatings Technology Surface modi fi cation of a medical grade Co–Cr–Mo alloy by low-temperature plasma surface alloying with nitrogen and carbon. Surf. Coat. Technol. 2013, 232, 906–911. [Google Scholar]
- Williamson, D.L.; Wei, R.; Wilbur, P.J. Metastable phase formation and enhanced diffusion in f. c. c. alloys under high dose, high flux nitrogen implantation at high and low ion energies. Surf. Coat. Technol. 1994, 65, 15–23. [Google Scholar] [CrossRef]
- Foerster, C.E.; Souza, J.F.P.; Silva, C.A.; Ueda, M.; Kuromoto, N.K.; Serbena, F.C.; Silva, S.L.R.; Lepienski, C.M. Effect of cathodic hydrogenation on the mechanical properties of AISI 304 stainless steel nitrided by ion implantation, glow discharge and plasma immersion ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 257, 727–731. [Google Scholar] [CrossRef]
- Manova, D.; Mändl, S.; Neumann, H.; Rauschenbach, B. Formation of metastable diffusion layers in Cr-containing iron, cobalt and nickel alloys after nitrogen insertion. Surf. Coat. Technol. 2017, 312, 81–90. [Google Scholar] [CrossRef]
- Stinville, J.C.; Villechaise, P.; Templier, C.; Rivière, J.P.; Drouet, M. Lattice rotation induced by plasma nitriding in a 316L polycrystalline stainless steel. Acta Mater. 2010, 58, 2814–2821. [Google Scholar] [CrossRef]
- Dearnley, P.; Dahm, K.L.; Dearnley, P.A. On the Nature, Properties and Wear Response of S-phase (nitrogen-alloyed Stainless Steel) Coatings on Aisi 316l. J. Mater. Des. Appl. 2000, 21, 181–198. [Google Scholar]
- Blawert, C.; Mordike, B.L.; Rensch, U.; Schreiber, G.; Oettel, H. Nitriding Response of Chromium Containing Ferritic Steels on Plasma Immersion Ion Implantation at Elevated Temperature. Surf. Eng. 2002, 18, 249–254. [Google Scholar] [CrossRef]
- Larisch, B.; Brusky, U.; Spies, H.-J. Plasma nitriding of stainless steels at low temperatures. Surf. Coat. Technol. 1999, 116–119, 205–211. [Google Scholar] [CrossRef]
- Gontijo, L.C.; Machado, R.; Casteletti, L.C.; Kuri, S.E.; Nascente, P.A.P. X-ray diffraction characterisation of expanded austenite and ferrite in plasma nitrided stainless steels. Surf. Eng. 2010, 26, 265–270. [Google Scholar] [CrossRef]
- Panicaud, B.; Chemkhi, M.; Roos, A.; Retraint, D. Theoretical modelling of iron nitriding coupled with a nanocrystallisation treatment. Application to numerical predictions for ferritic stainless steels. Appl. Surf. Sci. 2012, 258, 6611–6620. [Google Scholar] [CrossRef]
- Pinedo, C.E.; Varela, L.B.; Tschiptschin, A.P. Low-temperature plasma nitriding of AISI F51 duplex stainless steel. Surf. Coat. Technol. 2013, 232, 839–843. [Google Scholar] [CrossRef]
- Alphonsa, J.; Raja, V.S.; Mukherjee, S. Study of plasma nitriding and nitrocarburizing for higher corrosion resistance and hardness of 2205 duplex stainless steel. Corros. Sci. 2015, 100, 121–132. [Google Scholar] [CrossRef]
- Maleque, M.A.; Lailatul, P.H.; Fathaen, A.A.; Norinsan, K.; Haider, J. Nitride alloy layer formation of duplex stainless steel using nitriding process. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 012015. [Google Scholar] [CrossRef]
- Blawert, C.; Weisheit, A.; Mordike, B.L.; Knoop, R.M. Plasma immersion ion implantation of stainless steel: Austenitic stainless steel in comparison to austenitic-ferritic stainless steel. Surf. Coat. Technol. 1996, 85, 15–27. [Google Scholar] [CrossRef]
- Chiu, L.H.; Su, Y.Y.; Chen, F.S.; Chang, H. Microstructure and Properties of Active Screen Plasma Nitrided Duplex Stainless Steel. Mater. Manuf. Process. 2010, 25, 316–323. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Varela, L.B.; Pinedo, C.E.; Li, X.Y.; Dong, H. Development and microstructure characterization of single and duplex nitriding of UNS S31803 duplex stainless steel. Surf. Coat. Technol. 2017, 327, 83–92. [Google Scholar] [CrossRef]
- Li, X.; Dou, W.; Tian, L. Combating the Tribo-Corrosion of LDX2404 Lean Duplex Stainless Steel by Low Temperature. Lubricants 2018, 6, 93. [Google Scholar] [CrossRef]
- Mesa, D.H.; Pinedo, C.E.; Tschiptschin, A.P. Improvement of the cavitation erosion resistance of UNS S31803 stainless steel by duplex treatment. Surf. Coat. Technol. 2010, 205, 1552–1556. [Google Scholar] [CrossRef]
- Bobadilla, M.; Tschiptschin, A. On the Nitrogen Diffusion in a Duplex Stainless Steel. Mater. Res. 2015, 18, 390–394. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; Biserova-Tahchieva, A.; Lopez-Jimenez, I.; Calliari, I.; Cabrera, J.M.; Roca, A. Influence of severe plastic deformation in phase transformation of superduplex stainless steels. J. Mater. Sci. 2019, 54, 2648–2657. [Google Scholar] [CrossRef]
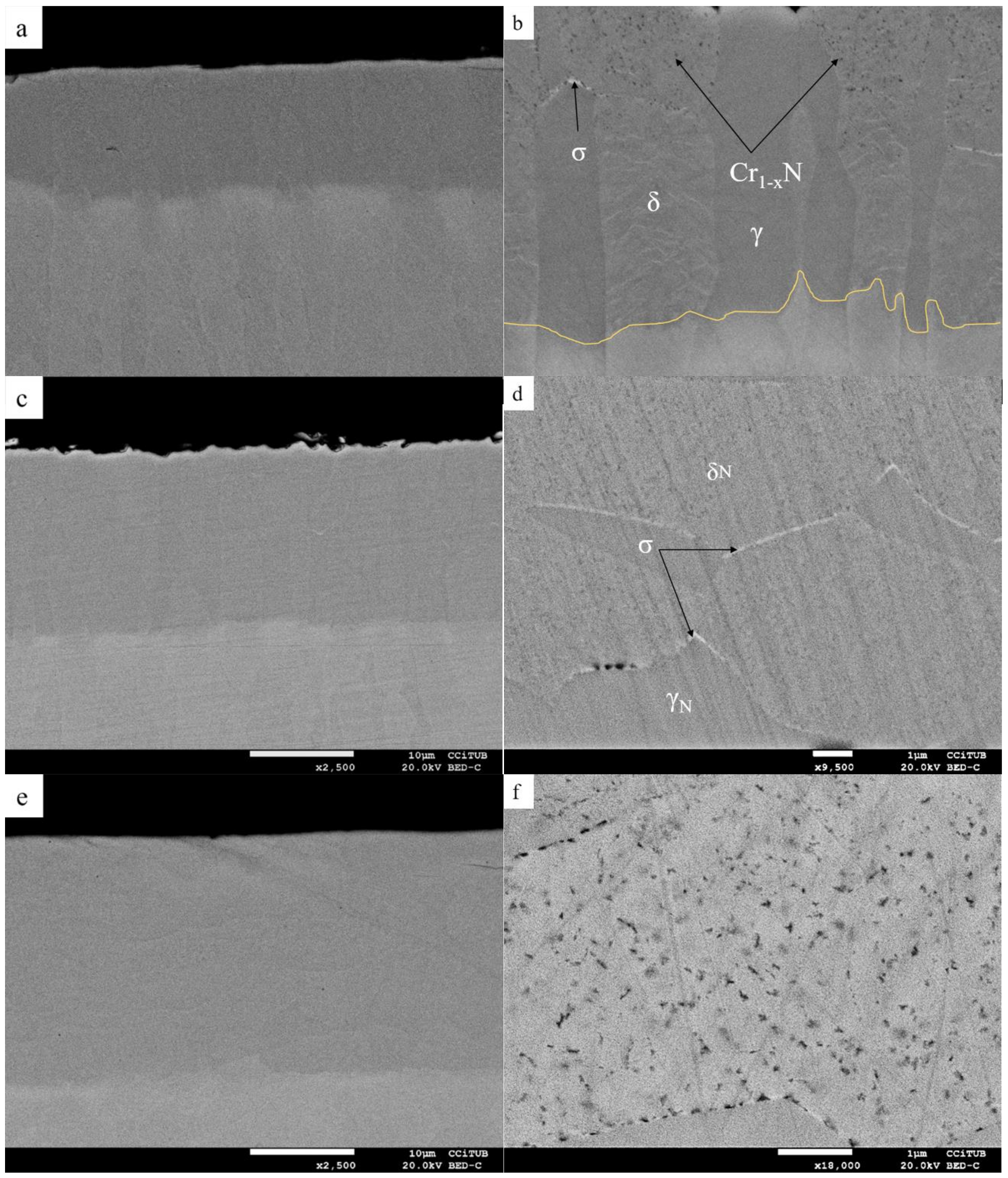
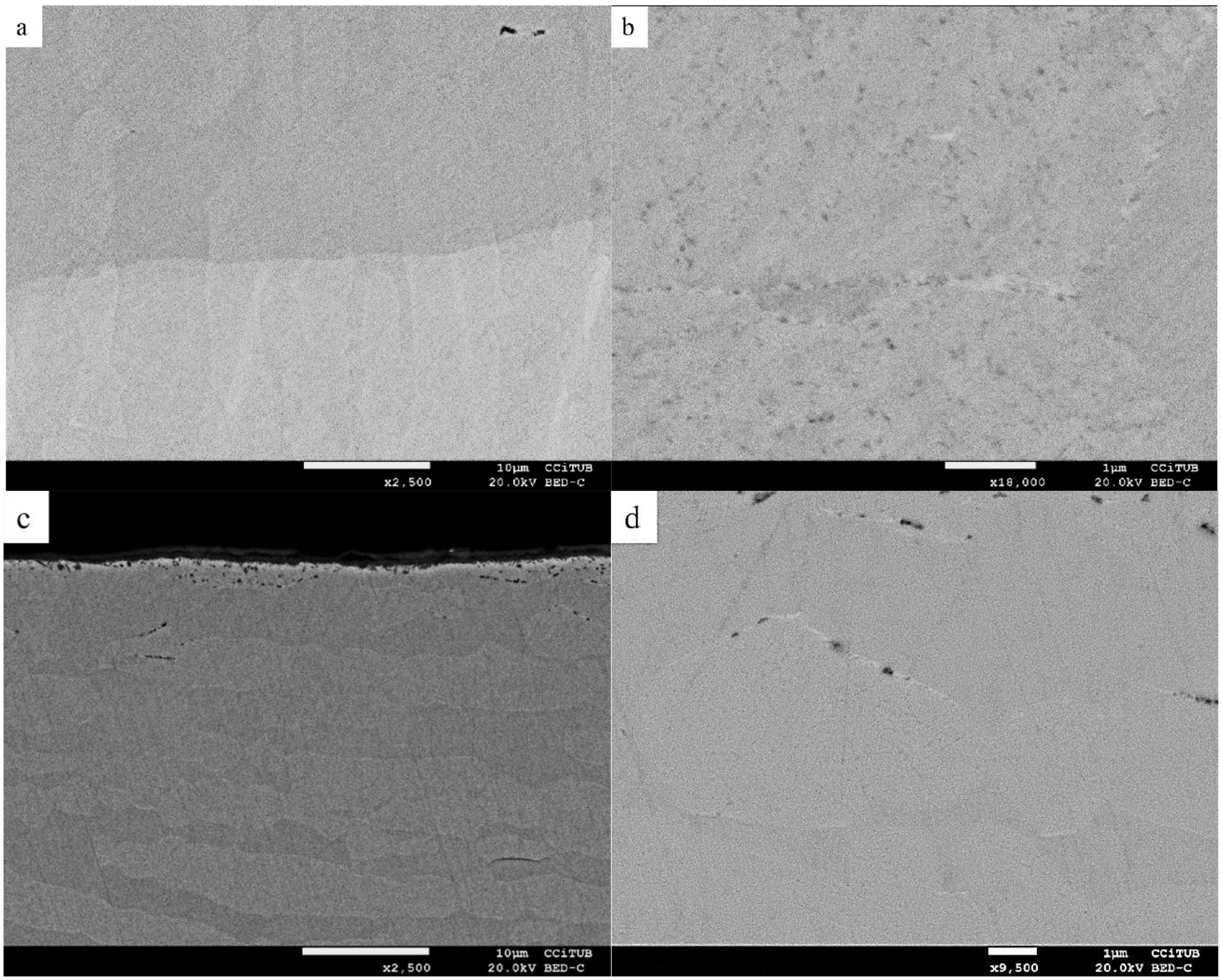
| Sample Identification | Chemical Composition wt % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UNS | C | Si | Mn | P | S | Cr | Ni | Mo | N | Cu |
| S32205 | 0.015 | 0.4 | 1.5 | 0.018 | 0.001 | 22.49 | 5.77 | 3.21 | 0.184 | 0.18 |
| S32750 | 0.018 | 0.26 | 0.84 | 0.019 | 0.001 | 25.08 | 6.880 | 3.82 | 0.294 | 0.17 |
| Material | HV0.3 | Thickness Layer |
|---|---|---|
| Super Duplex S32750 tube | 1263 ÷ 1393 | 13.7 ± 0.0006 µm |
| Super Duplex S32750 plate | 1379 ÷ 1402 | 16.5 ± 0.0004 µm |
| Duplex S32205 plate | 1263 ÷ 1329 | 23.5 ± 0.0003 µm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahchieva, A.B.; Llorca-Isern, N.; Cabrera, J.-M. Duplex and Superduplex Stainless Steels: Microstructure and Property Evolution by Surface Modification Processes. Metals 2019, 9, 347. https://doi.org/10.3390/met9030347
Tahchieva AB, Llorca-Isern N, Cabrera J-M. Duplex and Superduplex Stainless Steels: Microstructure and Property Evolution by Surface Modification Processes. Metals. 2019; 9(3):347. https://doi.org/10.3390/met9030347
Chicago/Turabian StyleTahchieva, Alisiya Biserova, Núria Llorca-Isern, and José-María Cabrera. 2019. "Duplex and Superduplex Stainless Steels: Microstructure and Property Evolution by Surface Modification Processes" Metals 9, no. 3: 347. https://doi.org/10.3390/met9030347
APA StyleTahchieva, A. B., Llorca-Isern, N., & Cabrera, J.-M. (2019). Duplex and Superduplex Stainless Steels: Microstructure and Property Evolution by Surface Modification Processes. Metals, 9(3), 347. https://doi.org/10.3390/met9030347







