Abstract
As mining waste, alunite is a potential resource to produce potassium salt. The decomposition of alunite is closely associated with the recovery of soluble potassium. In this study, the effect of CaO on phase transformation of alunite in the desulfation stage was examined. The results showed that CaO was beneficial to the desulfation of alunite. The decomposition temperature to obtain soluble potassium salt (K2SO4) was reduced from 800 °C to 700 °C by adding CaO. When the mass ratio of CaO/alunite was 0.1, 81% of soluble potassium was extracted by water leaching after calcination at 700 °C for 2 h. The mechanism of CaO to promote the disintegration of alunite was proposed through analyzing the phase transformation sequences. Alkaline Ca ion was inclined to bond with acidic [SO4] groups, and thus the breakage of S–O linkages between [AlO6] octahedron and [SO4] tetrahedron were improved. Monomer [SO4] tetrahedrons were released to form K2SO4 at a lower decomposition temperature. With the increase of the amount of CaO, the excess CaO bonded with neutral Al. [AlO6] tetrahedrons in alunite transformed into [AlO4] octahedrons due to the breakage of the Al–O network. Al3+ was dissociated and bonded with [SO4] tetrahedron to form soluble Al salts.
1. Introduction
As an essential source of nutrition for plant, the demand for potash fertilizer is increasing with the development of agriculture. Currently, potash fertilizer is dominantly produced from soluble K salt ores [1]. However, soluble potassium salt is a scarce resource in countries such as China. Food production is reduced due to the shortage of potash fertilizer. Thus, the exploration of using alternative potassium-bearing minerals to produce potash fertilizer has attracted great interest [2]. As an important kind of insoluble potash ore, alunite [KAl3(SO4)2(OH)6] contains an average potassium content of more than 7% [3]. Considering the abundance of alunite ore, if the potassium could be efficiently recovered, it could be a potential resource of potash fertilizer, which could then be widely used in agriculture.
K, Al and S are the primary components in natural alunite, which can be used to produce K2SO4, Al2O3, Al2(SO4)3 and KAl(SO4)2. However, alunite is difficult to dissolve in water and acids. Thermal decomposition [4,5,6,7,8,9] and hydrometallurgical leaching [10,11,12,13] are the main methods to recover valuable elements from alunite. The calcination process of alunite by thermal decomposition involves three steps, namely dehydration, dehydroxylation, and desulfurization, which occur at 50–240 °C, 450–550 °C, and 600–830 °C, respectively [7,8,9]. Küçük [4,5] used thermo-gravimetric analysis to investigate the dehydration/dehydroxylation of alunite. The conversion of alunite by dehydroxylation could reach over 99%. The kinetics study showed that the dehydroxylation reaction of alunite was first-order, and the rate-controlled step was a chemical reaction. The activation energy of dehydroxylation and desulfurization was found to be 173–303 and 220–318 kJ/mol, respectively [6]. Therefore, large energy consumption is needed for the thermal decomposition of alunite as the calcination temperature is usually higher than 700 °C.
In hydrometallurgy methods, natural alunite is first calcined to increase the solubility of K and Al. Soluble potassium and aluminum salts are formed. Then, K and Al in the calcined residue are subsequently separated and recovered by leaching and filtration [10,11,12,13]. Alkali is a common solvent to leach alunite. Mohammadi [10] used KOH solution to directly leach natural alunite. Over 80% of K and Al is recovered with the leaching temperature >80 °C. Kinetics analysis shows that the rate-controlled step of the leaching reaction is a surface chemical reaction, and the activation energy is 94.18 kJ/mol. An optimum process to extract K2O and Al2O3 was proposed by Ozacar [11,12]. Aluntie ore was pretreated by calcination at 700 °C for 2 h, and then the calcined residue was leached in NaOH solution at 110 °C for 1 h. Consequently, the leaching ratio of K was more than 90%, and Al was enriched in the leaching residue. Another kind of leaching agents that has been used to dissolve and leach alunite is inorganic acid (H2SO4 and HCl acid) [12,13]. A H2SO4 acid calcination–water leaching method was developed by Zhao [13] to recover Al and K salts simultaneously. The leaching ratios of Al and K were found to be 87.2% and 85.3%, respectively. K2SO4 and Al2(SO4)3 were extracted and effectively separated by multi-step crystallization [13]. However, incomplete dehydroxylation or desulfurization would lead to a low leaching efficiency of alunite and considerable consumption of alkali and acid for leaching.
Whether thermal decomposition or hydrometallurgy leaching is adopted, the critical step for the beneficiation of alunite is desulfurization by calcination. The disintegration of the S–O–Al framework structure in KAl3(SO4)2(OH)6 is considered as a key problem of alunite decomposition. Increasing temperature is beneficial to the decomposition of alunite. However, if the temperature is too high, the solubility of K and Al elements is reduced due to the formation of α-Al2O3 [7,8,9], leading to a lower leaching efficiency. Since [SO4] tetrahedrons in alunite are acidic groups, they may be easier to bond with alkaline compounds, leading to the disintegration of the framework structure of S–O–Al. Therefore, adding alkaline agents, e.g., alkaline earth metal oxide, should be considered as a method to promote the thermal decomposition of alunite.
The aim of this study was to investigate the effect of CaO on the recovery of soluble potassium from alunite by thermal decomposition. The phase and structure transformation was characterized to clarify the disintegration mechanisms of alunite in the desulfurization stage when CaO was added.
2. Experimental Section
2.1. Materials
In this study, natural alunite ore was provided by the Zijin Mining Group from Fujian Province, China. The average particle size of the alunite samples was <74 μm. The mineral phase and chemical composition of alunite ore are listed in Figure S1 and Table S1, respectively. The mass fractions of K and Al elements were 7.49 and 17.91 wt %, respectively. CaO was analytical grade (purity >99%) and provided by the Beijing Chemical Reagents Company. Before sample preparation, alunite and CaO were dried at 110 °C for 24 h.
2.2. Methods
2.2.1. Sample Preparation
The powder samples of alunite and CaO were firstly mixed and milled in an agate mortar. A total of 1.0 g of alunite was used. The mass ratio of CaO/alunite was selected as 0, 0.1, 0.2, 0.4, and 0.6. Then, the CaO/alunite mixture was pressed into a tablet. The molding pressure was 8 MPa.
2.2.2. Thermal Decomposition Experiment
The desulfurization of alunite by thermal decomposition was conducted in a tube furnace at temperatures from 600 to 900 °C. The atmosphere was inert N2 (>99.9%). When the desired temperature (i.e., 600, 700, 800, and 900 °C) was reached, the samples were placed in the furnace and were calcined for 2 h. After cooling at room temperature, the calcined samples with an average particle size of <74 μm were collected by crushing and sieving. Then, X-ray diffraction (XRD, X’Pert PRO MPD, the Netherlands) was employed to identify the lattice structure and phase composition. Fourier transform infrared spectrometry (FTIR, Nicolet-470, NICOLET, Milwaukee, WI, USA) was used to analyze the chemical bonding and phase structure of alunite during the thermal decomposition process. Scanning electron microscopy (SEM, JSM-6700F, JEOL, Tokyo, Japan) and energy dispersive spectrometry (EDS, Noran Systemsix, USA) were performed to analyze the elemental distribution and solid–solid reaction interface.
2.2.3. Water Leaching and Lixivium Crystallization
To determine the contents of the soluble potassium and aluminum after desulfurization, the calcined samples were leached by deionized water at 30 °C for 1 h. The solid–liquid ratio was 1:10 (g/mL). Then, the lixivium containing the soluble K and Al salts were separated by filtration. The K and Al concentrations in the lixivium were measured by inductively coupled plasma–atomic emission spectrometry (ICP-AES, Perkine-Elmer OPTIMA 3000, PerkinElmer, Akron, OH, USA). Finally, the recovery ratios of K (ηK) and Al (ηAl) were calculated by the following equations:
where CK and CAl are the concentrations of K and Al in the lixivium (g/L), respectively; VF is the volume of the lixivium (L); ma is the mass of alunite (g); and ωK and ωAl are the mass fractions of K and Al in alunite (wt %), respectively.
The lixivium and the leaching residue were separated by filtration. The leaching residue was dried at 110 °C for 24 h. The lixivium was concentrated and crystallized by evaporation at 80 °C, and the crystallized product was collected. The mineral phase and chemical compositions of the crystallized product and the leaching residual were analyzed by X-ray fluorescence (XRF, AXIOSmAX, PANalytical, Etten-Leur, The Netherlands), XRD, and ICP-AES.
3. Results and Discussion
3.1. Recovery of the Soluble Potassium from Alunite
3.1.1. Effect of CaO Addition on the Recovery Ratio
The effect of CaO addition on the recovery ratio of K was studied at temperatures from 600 to 900 °C (Figure 1). When the calcination temperature was 600 °C, the recovery ratio of K increased from 5% to 32%, while the mass ratio of CaO/alunite increased from 0 to 0.4. However, when the mass ratio of CaO/alunite was >0.4, the recovery ratio of K did not change significantly. The results indicate that the desulfurization of alunite was incomplete, although the thermal composition of alunite was enhanced by CaO. The reason for this was that the S–O–Al bonds were not broken at lower temperatures, and CaO was not fully activated. Similarly, when the calcination temperature was 700 °C, the recovery ratio of K increased notably from 27% to 81%, while the mass ratio of CaO/alunite increased from 0 to 0.1. However, when the mass ratio of CaO/alunite was >0.2, the recovery ratio of K deceased slightly. Since [SO4] tetrahedrons in alunite were acidic groups, it was inferred that CaO bonded with [SO4] in the desulfurization process. CaO migrated into the sulfoaluminate lattice and destroyed the S–O–Al framework structure of alunite. As a result, increasing the amount of CaO was beneficial to the desulfurization of alunite. When the calcination temperature was >800 °C, the recovery ratio of K almost did not vary, maintaining an approximately constant value of about 82%. The S–O–Al bonds were completely broken at >800 °C, and thus the recovery ratio did not change remarkably, regardless of adding CaO. The effect of temperature was greater than that of CaO. Therefore, considering the energy consumption, the calcination temperature of 700 °C was suggested.
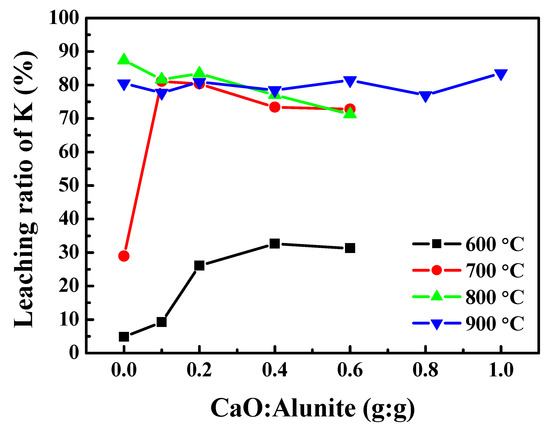
Figure 1.
The effect of mass ratio of CaO/alunite on the recovery ratio of potassium.
The effect of CaO addition on the recovery ratio of Al was investigated (Figure 2). The recovery ratios of Al increased with increasing amount of CaO at temperatures from 600 to 900 °C. When the mass ratio of CaO/alunite was lower than 0.2, the recovery ratios of Al were below 1%, indicating that little soluble Al was formed. However, when the mass ratio of CaO/alunite was higher than 0.2, the recovery ratios of Al increased up to 4%, 10%, and 15% at 700, 800, and 900 °C, respectively. The results indicate that adding CaO was beneficial to the formation of soluble Al salt, especially at high temperatures. In the process of desulfurization without CaO, the product of Al was insoluble Al2O3 due to the breakage of S–O bonds between [SO4] and [AlO6] [14]. Since the alkalinity of CaO was strong, it was inferred that the Al–O bonds were broken to form soluble [AlSO4]. Because the alums (KAl(SO4)2·12H2O) formed during crystallization, K and Al were difficult to separate if the concentration of Al in the solution was high [12,13]. Thus, the mass ratio of CaO/alunite should be lower than 0.2 to recover a higher purity of potassium salt by crystallization.
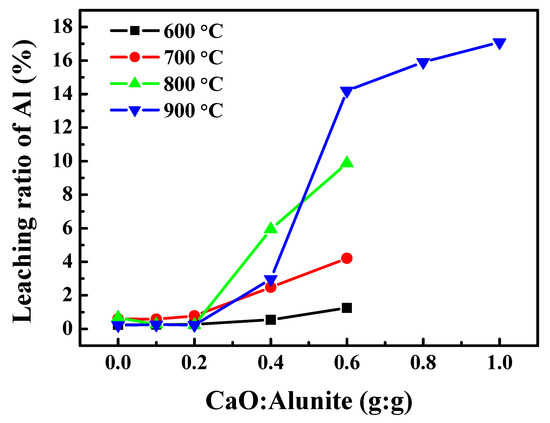
Figure 2.
The effect of mass ratio of CaO/alunite on the recovery ratio of aluminum.
3.1.2. Effect of CaO Addition on Water Leaching Product
To investigate the existing state of the soluble salts, the calcined products (CaO/alunite = 0.2 at 700 °C) were leached by deionized water at 30 °C. The lixivium was crystallized by evaporation to obtain the crystallized product. As shown in Figure 3, the phase of K2Ca2(SO4)3 (syngenite) existed in the calcined products, whereas the peaks of K2Ca2(SO4)3 vanished in the leaching residue. The result shows the soluble potassium salt in the calcined products was in the form of K2Ca2(SO4)3 when CaO was added. The syngenite decomposed in the water, and K was dissolved after leaching:
K2Ca2(SO4)3 = K2SO4 + 2CaSO4 ↓
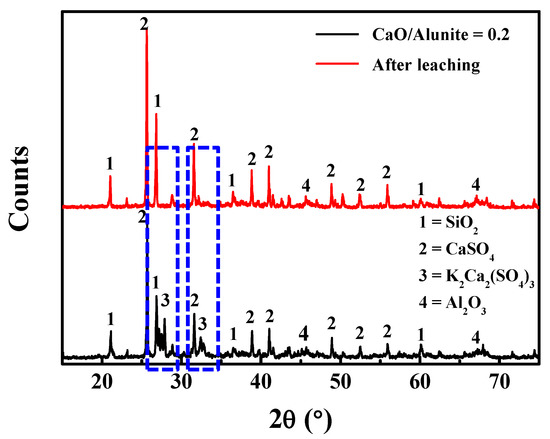
Figure 3.
X-ray diffraction (XRD) patterns of the calcined products at 700 °C (CaO/alunite = 0.2) and leaching residual.
This result is different from that of previous works [4,5,6,7,8,14], where the soluble potassium salt is K2SO4 when calcined without CaO.
As shown in Figure 4, the primary components of the crystallized product were K2SO4, K2Ca2(SO4)3, and CaSO4. The syngenite decomposed into CaSO4, which was slightly soluble in water. When the concentration ratio of Ca2+/SO42− reached a certain value, K2SO4 and CaSO4 can form double salts in aqueous solution system [15]. Thus, the K and Ca was precipitated with the formation of K2Ca2(SO4)3 by crystallization. XRF result shows that the mass fraction of potassium in the crystallized product was 40.42% (calculated by K2O). The crystallized product reached a qualified grade (40%) used for the production of potassium fertilizer [16] (Table 1). The purity of K salt was lower than that prepared by calcination without CaO (45.41%) [14]. Therefore, although the desulfurization of alunite was enhanced by CaO, the purity of the crystallized product was reduced due to the formation of K2Ca2(SO4)3.
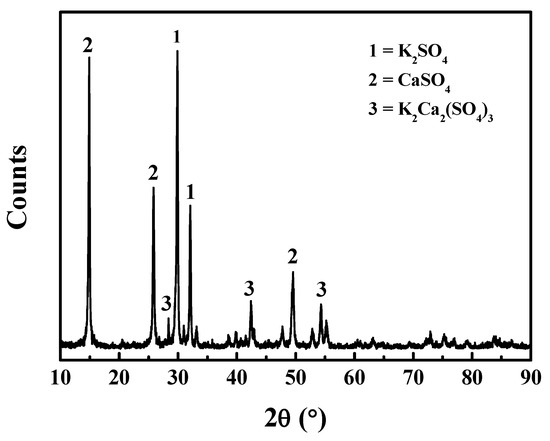
Figure 4.
XRD patterns of the crystallized product of lixivium from the calcined products at 700 °C (CaO/alunite = 0.2).

Table 1.
X-ray fluorescence (XRF) analysis of the crystallized product of lixivium from the calcined products (CaO/alunite = 0.2) (in oxide form).
3.2. Effect of CaO on the Desulfurization of Alunite
3.2.1. X-ray Diffraction (XRD) Analysis
To investigate the desulfurization mechanisms of alunite with CaO, the phase transformation of the calcined samples was identified by XRD. The XRD patterns of the alunite samples with different additions of CaO calcined at 600, 700, and 800 °C for 2 h are shown in Figure 5.
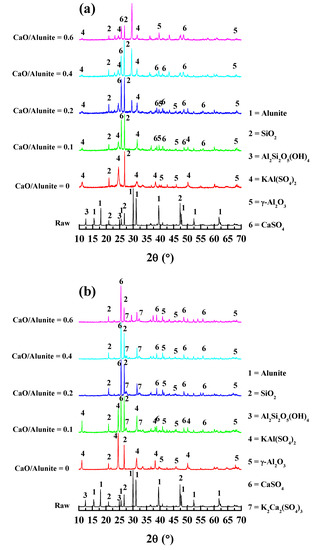
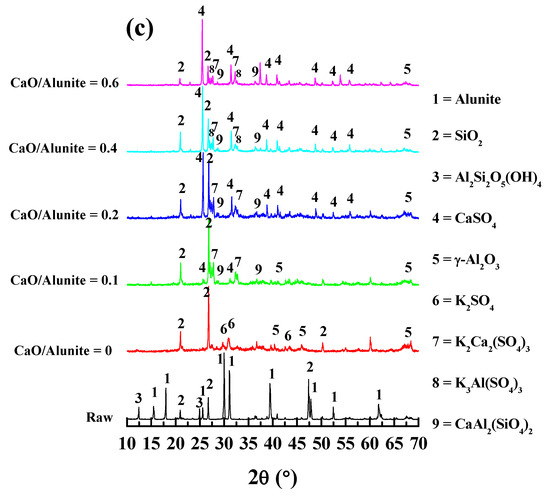
Figure 5.
XRD patterns of the alunite–CaO system at different decomposition temperatures: (a) 600 °C; (b) 700 °C; and (c) 800 °C.
When the calcination temperature was 600 °C, the main phase compositions of the calcined sample were KAl(SO4)2 and SiO2 without adding CaO (Figure 5a). This indicates that alunite decomposed to form KAl(SO4)2, and the desulfurization was not complete at 600 °C. Since the solubility of KAl(SO4)2 was low, only a small amount of the soluble potassium was dissolved by water leaching. Therefore, the recovery ratio of K at 600 °C was low (5%). As the amount of CaO increased, the phase of CaSO4 was clearly identified. S in CaSO4 was from the [SO4] groups in KAl3(SO4)2(OH)6. Thus, the formation of CaSO4 was attributed to the desulfurization of KAl3(SO4)2(OH)6. It was inferred that alkaline Ca ion was inclined to bond with acidic S and neutral Al, and thus the breakage of S–O linkages was enhanced. Therefore, the recovery ratio of K was increased slightly, to 26–32%, by adding CaO.
When the calcination temperature was 700 °C, the phases of KAl(SO4)2 and SiO2 were detected obviously without adding CaO, which is similar to what was detected at 600 °C (Figure 5b). Thus, the amount of soluble K was still small. As the amount of CaO increased, the peaks of K2Ca2(SO4)3 began to appear. The formation of K2Ca2(SO4)3 was attributed to the reaction between K2SO4 and CaSO4. The formation of K2SO4 and CaSO4 originated from the release of [SO4] groups in alunite. This indicates that the reaction degree of desulfurization was increased. K2Ca2(SO4)3 easily decomposed into K2SO4 and CaSO4 in aqueous solution [15]. The recovery ratio of K remarkably increased, to 72–82%, due to the high solubility of K2SO4 in water.
When the calcination temperature was 800 °C, the phase including K element was K2SO4 without adding CaO (Figure 5c). This indicates that KAl(SO4)2 further decomposed, and the desulfurization of KAl3(SO4)2(OH)6 was complete [14]. As the mass ratio of CaO/alunite increased from 0.1 to 0.2, the peaks of K2SO4 disappeared, while the phase of K2Ca2(SO4)3 was clearly identified. Both K2SO4 and K2Ca2(SO4)3 are soluble in water. Thus, the recovery ratio of K maintained a high value (76–83%), regardless of adding CaO. However, when the mass ratio of CaO/alunite was higher than 0.4, the phase of K3Al(SO4)3 appeared, while the peaks of K2Ca2(SO4)3 gradually weakened. It was inferred that Al2(SO4)3 was generated and reacted with K2SO4 to form K3Al(SO4)3. Although alkaline CaO bound preferentially to acidic S–O, the excess CaO may bond with neutral Al3+ due to its extreme alkalinity when the desulfurization was complete. Consequently, the Al–O–Al bonds were broken, and Al3+ in the framework of alunite was released and reacted with [SO4] tetrahedron groups to form Al2(SO4)3. K2Ca2(SO4)3 easily decomposed into K2SO4 and CaSO4 in aqueous solution [15]. Since Al2(SO4)3 was soluble in water, the recovery ratio of Al increased significantly, from 1% to 10%.
3.2.2. Fourier-transform Infrared Spectrometry (FTIR) Results
FTIR experiments were performed to characterize the evolution of the S–O–Al structures in KAl3(SO4)2(OH)6 in the desulfurization process (Figure 6). When the calcination temperature was 600 °C, the band at 1200–1280 cm−1 was assigned to the in-plane bending vibration of the S–O bond in a SO4 tetrahedron of alunite [14,17,18] (Figure 6a). The peaks at 600, 620 and 680 cm−1 belonged to the bending vibration of SO4 tetrahedrons in CaSO4 [17,18]. As the mass ratio of CaO/alunite increased, peaks at 600–700 cm−1 appeared, indicating the formation of CaSO4. The band at 1200–1280 cm−1 still existed, although its intensity was gradually weakened. This indicates that the structure of [SO4] tetrahedrons in alunite was not destroyed completely by adding CaO at 600 °C. The desulfurization was attributed to the S–O linkages between [AlO6] octahedron and [SO4] tetrahedron in alunite [14]. Thus, it was evidence that the desulfurization of alunite was incomplete.
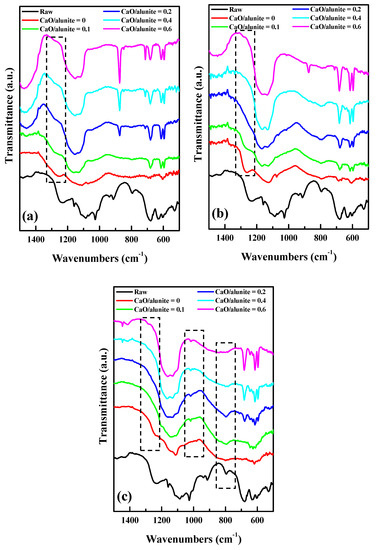
Figure 6.
Fourier-transform infrared spectrometry (FTIR) absorption spectra of the alunite–CaO system (CaO/alunite = 0.4) at different decomposition temperatures: (a) 600 °C; (b) 700 °C; and (c) 800 °C.
When the calcination temperature was 700 °C, the peaks at 600–700 cm−1 assigned to CaSO4 were observed, which is similar to what was observed at 600 °C (Figure 6b). As the mass ratio of CaO/alunite increased from 0 to 0.1, the band at 1200–1280 cm−1 appeared. However, when the mass ratio of CaO/alunite was higher than 0.1, the band at 1200–1280 cm−1 began to disappear, indicating that the S–O linkages between [AlO6] octahedron and [SO4] tetrahedron were broken. Consequently, the monomer [SO4] tetrahedrons were released and bonded with K+ and Ca2+ to form K2SO4 and CaSO4, which was in accordance with the XRD results. According to previous studies [4,14], the [SO4] groups of alunite were fully disintegrated at 800 °C. However, the desulfurization of alunite was completed at 700 °C by adding CaO. Therefore, the decomposition temperature of alunite to obtain soluble potassium salt was reduced.
When the calcination temperature was 800 °C, no peaks were observed at 1200–1280 cm−1, regardless of adding CaO (Figure 6c). This indicates that the desulfurization of alunite was complete at 800 °C. The peaks at 790–810 cm−1 were assigned to the stretching vibration of the Al–O bond in a [AlO4] tetrahedron [19,20,21]. As the mass ratio of CaO/alunite increased from 0 to 0.2, the intensity of the peaks at 790–810 cm−1 increased. This indicates that [AlO6] octahedron (six-fold-coordination Al3+) in alunite transformed to tetrahedron [AlO4] (four-fold-coordination Al3+). The [AlO4] tetrahedron acted as a network former rather than a network modifier, as with the [AlO6] octahedron [22,23]. The increase in the number of [AlO4] indicated that the Al–O framework in KAl(SO4)2 and KAl3(SO4)2(OH)6 was broken. However, as the mass ratio of CaO/alunite was higher than 0.2, the peaks at 790–810 cm−1 were gradually weakened and vanished. This result indicates that the Al–O linkages of [AlO4] in KAl(SO4)2 and KAl3(SO4)2(OH)6 were further disintegrated. Consequently, Al3+ was dissociated and released from the Al–O network due to the breakage of the Al–O linkages. Al3+ bonded with the [SO4] tetrahedron to form Al2(SO4)3. Simultaneously, the peaks at 1108 cm−1 appeared and were enhanced with the increase of temperature, which belonged to the bending vibration of the [SO4] tetrahedron in sulfoaluminate [24]. Therefore, it was inferred that KAl(SO4)2 was further decomposed into K3Al(SO4)3 by adding CaO at 800 °C.
3.2.3. Phase Structure Evolution
To further clarify the phase structure evolution and the product layer formation during thermal decomposition, the sample calcined at 800 °C for 30 min was investigated by SEM and EDS analysis. From the inner core to the outer layer of the calcined sample, three regions were observed (Figure 7). According to EDS spot analysis (Table 2), the region at Point 1 was the unreacted KAl3(SO4)2(OH)6, where the atomic ratio of K:S:Al was approximately 1:1:3. In the region at Point 2, the elemental composition mainly included K, Ca, and S, with a little Al. Combined with the XRD results, the phases at Point 2 mainly consisted of K2Ca2(SO4)3 and K3Al(SO4)3. This region was considered as the product layer. The region at Point 3 was the mixture of CaSO4 and CaO, which only contained Ca, S, and O. From Point 1 to Point 3 of the calcined particle, the content of K and S increased. This result suggests that the S–O–Al bonds were broken and the framework structure of alunite was gradually destroyed as Ca2+ diffused into it. Consequently, large amounts of K+ were released and migrated towards the reaction boundary. Meanwhile, monomer [SO4] migrated into the product layer to bond with K+ and Ca2+. Therefore, K, in the form of K2Ca2(SO4)3, was enriched in the product layer. From Point 2 to Point 3 of the calcined particle, the content of S increased, while the content of K decreased. It was inferred that S in the form of gaseous SO3 reacted with CaO. The diffusion of SO3 was much faster than that of solid K+. Thus, the product layer of CaSO4 was thicker than that of K2Ca2(SO4)3. The SEM/EDS analyses were in accordance with XRD and FTIR results.
Figure 7.
Scanning electron microscopy (SEM) observation and energy dispersive spectrometry (EDS) mappings of the sample calcinated at 800 °C for 30 min (CaO: alunite = 0.4).

Table 2.
Energy dispersive spectrometry (EDS) spot analysis of the calcinated sample in Figure 7.
3.3. Effect of CaO on Thermal Behavior of Alunite
To investigate the effect of CaO on the thermal behavior of alunite, thermo-gravimetric (TG) analysis was carried out. The TG curves of alunite ore with different amounts of CaO at a heating rate of 10 °C/min are shown in Figure 8. Two mass losses appeared in TG curves, indicating that the decomposition process can be divided into two stages. In Stage I, the first mass loss appeared at 500–650 °C. This was due to the release of H2O (dehydroxylation) and the transformation of alunite to KAl(SO4)2 [14].
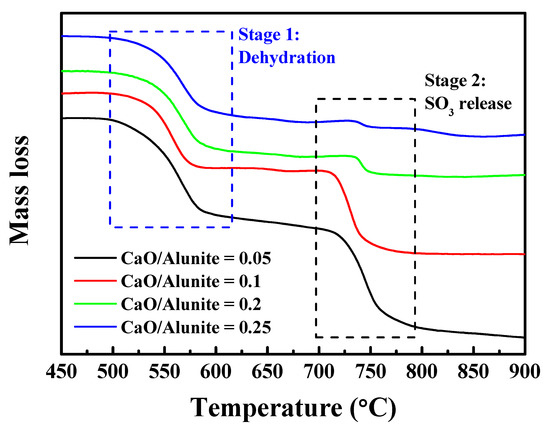
Figure 8.
Thermo-gravimetric (TG) curves of the alunite samples with different CaO/alunite ratios during thermal decomposition.
As the amount of CaO increased, the change of the TG curves at 500–650 °C was slight, indicating that the dehydroxylation of alunite was not promoted significantly by CaO. Despite the discharge of H2O gas, –OH groups might bond with CaO to form Ca(OH)2, although Ca(OH)2 peaks were not detected in XRD patterns (Figure 5). However, Ca(OH)2 readily decomposes into CaO and H2O at temperatures <500 °C [25].
–OH in alunite was released in the form of H2O gas, regardless of adding CaO. Thus, the mass loss in the stage of dehydroxylation hardly changed.
In Stage II, the second mass loss occurred at 650–850 °C. This was attributed to the emission of SO3 (desulfation) and the transformation of KAl(SO4)2 to K2SO4 [14].
As the amount of CaO increased, the mass loss at 650–680 °C was reduced, indicating that the emission of SO3 was eliminated. When the mass ratio of CaO/alunite was 0.25, no mass loss was observed in the desulfation stage. This indicates that SO3 reacted with CaO to form CaSO4, which is in accordance to XRD results.
CaSO4 had difficulty decomposing into CaO and SO3 if the temperature was lower than 850 °C [26]. S was fixed in solid calcined products in the form of CaSO4, rather than gaseous SO3. Thus, the sample mass did not decrease during desulfation. Since SO3 is acidic and toxic, CaO can be considered as a sulfur-capturing agent to avoid air pollution and equipment corrosion.
4. Conclusions
In this paper, the effect of CaO on the desulfation of alunite was investigated. The decomposition mechanisms were analyzed. The results show that adding CaO was beneficial to the decomposition of alunite. The recovery ratio of K was 81% after calcination at 700 °C for 2 h when the mass ratio of CaO/alunite was 0.1. In the desulfation of alunite, alkaline Ca ion was inclined to bond with acidic [SO4] groups. At 600 °C, the breakage of S–O bonds was not complete, and thus KAl(SO4)2 was formed, rather than K2SO4. At 700 °C, the breakage of S–O linkages between [AlO6] octahedron and [SO4] tetrahedron was improved. Monomer [SO4] tetrahedrons were released to form K2SO4. At 800 °C, when the mass ratio of CaO/alunite was >0.2, the excess CaO bonded with neutral Al due to its extreme alkalinity, except for reacting with [SO4]. Thus, the Al–O network was broken, and [AlO6] tetrahedrons in alunite transformed into [AlO4] octahedrons. Consequently, Al3+ was dissociated and bonded with [SO4] tetrahedron to form K3Al(SO4)3 or Al2(SO4)3.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4701/9/3/337/s1, Figure S1: The mineral phase analysis of the alunite ore used in this study, Table S1: Elements analysis of the alunite ore used in this paper (in oxide form).
Author Contributions
Y.Z. wrote the paper and analyzed the data; X.Q. performed the experiments; and J.G. and Z.G. designed the experiments and contributed materials.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2016YFB0601304), the National Natural Science Foundation of China (Nos. 51604020 and 91534121) and the Fundamental Research Funds for the Central Universities (No. FRF-TP-17-040A2).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2013–2017; International Fertilizer Industry Association (IFA): Paris, France, 2013. [Google Scholar]
- Ma, H.; Yang, J.; Su, S.; Liu, M.; Zheng, H.; Wang, Y.; Qi, H.; Zhang, P.; Yao, W. 20 years advances in preparation of potassium salts from potassic rocks: A review. Acta Geol. Sin. 2015, 89, 2058–2071. [Google Scholar]
- Stoffregen, R.E.; Alpers, C.N.; Jambor, J.L. Alunite-jarosite crystallography, thermodynamics and geochronology. Rev. Mineral. Geochem. 2000, 40, 453–479. [Google Scholar] [CrossRef]
- Küçük, A.; Gülaboǧlu, M.Ş. Thermal decomposition of Şaphane alunite ore. Ind. Eng. Chem. Res. 2002, 41, 6028–6032. [Google Scholar] [CrossRef]
- Küçük, A.; Gülaboǧlu, M.Ş.; Bayrakcüeken, S. Dehydration kinetics of Şebinkarahisar (Gedehor) alunite ore in a fluidized-bed reactor. Ind. Eng. Chem. Res. 2004, 43, 962–968. [Google Scholar] [CrossRef]
- Küçük, F.; Yildiz, K. The decomposition kinetics of mechanically activated alunite. Thermochim. Acta 2006, 448, 107–110. [Google Scholar] [CrossRef]
- Frost, R.L.; Wain, D.L.; Wills, R.A.; Musemeci, A.; Martens, W. A thermogravimetric study of the alunites of sodium, potassium and ammonium. Thermochim. Acta 2006, 443, 56–61. [Google Scholar] [CrossRef]
- Frost, R.L.; Wain, D. A thermogravimetric and infrared emission spectroscopic study of alunite. J. Therm. Anal. Calorim. 2008, 91, 267–274. [Google Scholar] [CrossRef]
- Kristóf, J.; Frost, R.L.; Palmer, S.J.; Horváth, E.; Jakab, E. Thermoanalytical studies of natural potassium, sodium and ammonium alunites. J. Therm. Anal. Calorim. 2010, 100, 961–966. [Google Scholar] [CrossRef]
- Mohammadi, M.; Salarirad, M.M. Kinetics of direct leaching of natural alunite in KOH. Ind. Eng. Chem. Res. 2013, 52, 14359–14365. [Google Scholar] [CrossRef]
- Ozacar, M.; Sengil, I. Optimum conditions for leaching calcined alunite ore in strong NaOH. Can. Metall. Q. 1999, 38, 249–255. [Google Scholar] [CrossRef]
- Ozdemir, M.; Cetisli, H. Extraction kinetics of alunite in sulfuric acid and hydrochloric acid. Hydrometallurgy 2005, 76, 217–224. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, X.; Zhong, S.; Zhu, Y.; Yang, X.; Yi, L.; Li, G.; Song, J.; Yu, H.; Ruan, R.; et al. Extraction of Al and K salts from associated alunite tailings by an acid calcination-water leaching method. J. Clean. Prod. 2015, 107, 786–792. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, J.; Meng, L.; Guo, Z. Phase transformation and non-isothermal kinetics studies on thermal decomposition of alunite. J. Alloys Compd. 2017, 710, 182–190. [Google Scholar] [CrossRef]
- Clarke, L.; Partridge, E.P. Potassium sulfate from syngenite by high-temperature extraction with water. Ind. Eng. Chem. Res. 1934, 26, 897–903. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, J.; Chen, P.; Guo, Z. Recovery of potassium from K-feldspar by thermal decomposition with flue gas desulfurization gypsum and CaCO3: Analysis of mechanism and kinetics. Energy Fuels 2017, 31, 699–707. [Google Scholar] [CrossRef]
- Wen, L.; Liang, W.X.; Zhang, Z.G.; Huang, J.C. The Infrared Spectroscopy of Minerals; Chongqing University Press: Chongqing, China, 1988. [Google Scholar]
- Lane, M.D. Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. Am. Mineral. 2007, 92, 1–18. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Priya, G.K.; Padmaja, P.; Warrier, K.G.K.; Damodaran, A.D.; Aruldhas, G. Dehydroxylation and high temperature phase formation in sol-gel boehmite characterized by fourier transform infrared spectroscopy. J. Mater. Sci. Lett. 1997, 16, 1584–1587. [Google Scholar] [CrossRef]
- Aronne, A.; Esposito, S.; Pernice, P. FTIR and DTA study of lanthanum aluminosilicate glasses. Mater. Chem. Phys. 1997, 51, 163–168. [Google Scholar] [CrossRef]
- Navrotsky, A.; Geisinger, K.L.; McMillan, P.F.; Gibbs, G.V. The tetrahedral framework in glasses and melts: Influence from molecular orbital calculations and implications for structure, thermodynamics and physical properties. Phys. Chem. Miner. 1985, 11, 284–298. [Google Scholar] [CrossRef]
- Barashkov, M.V.; Komyak, A.I.; Shashkov, S.N. Vibrational spectra and structure of potassium alum. J. Appl. Spectrosc. 2004, 71, 328–333. [Google Scholar] [CrossRef]
- Criado, J.; Morales, J. On the thermal decomposition mechanism for dehydroxylation of alkaline-earth hydroxides. J. Thermal Anal. 1976, 10, 103–110. [Google Scholar] [CrossRef]
- Ma, L.; Ning, P.; Zheng, S.; Niu, X.; Zhang, W.; Du, Y. Reaction mechanism and kinetic analysis of the decomposition of phosphogypsum via a solid-state reaction. Ind. Eng. Chem. Res. 2010, 49, 3597–3602. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).