Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
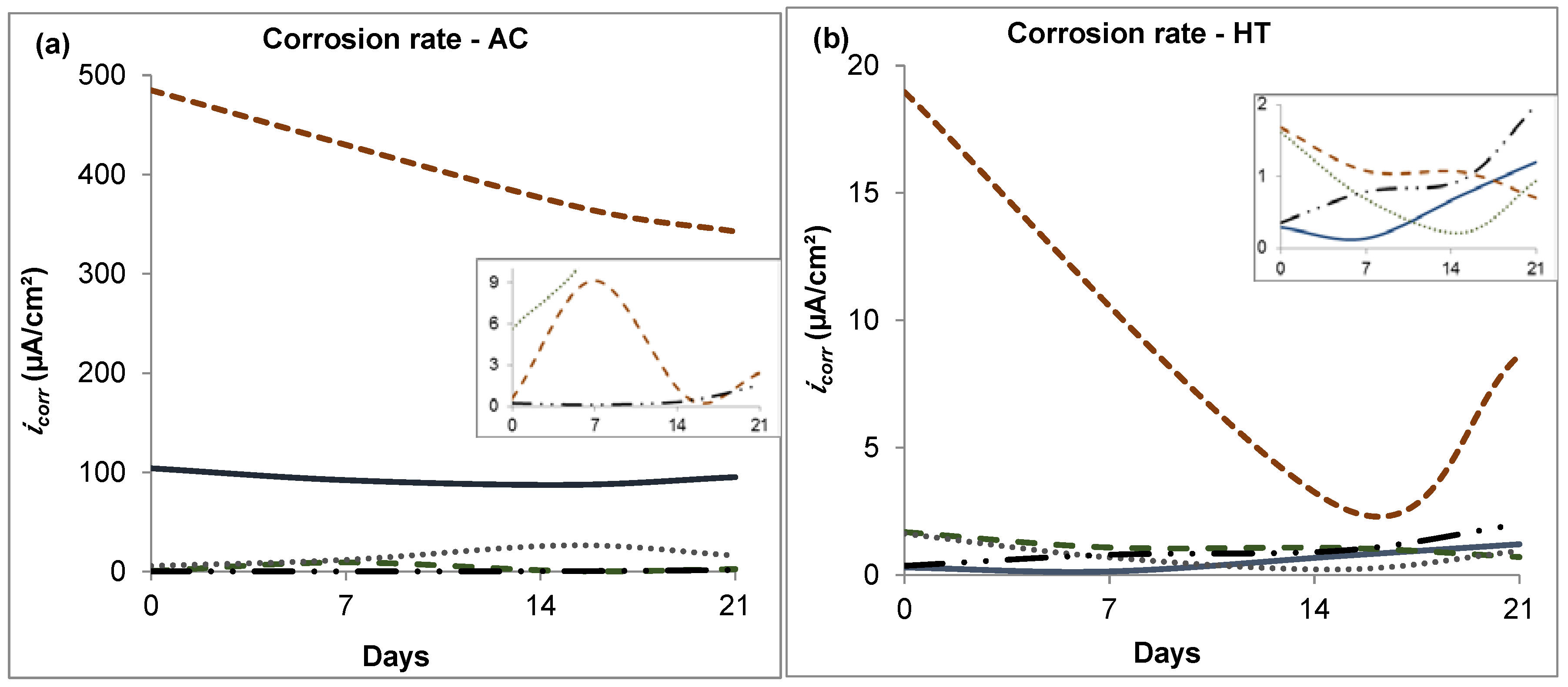
3.1. Linear Polarization Resistance
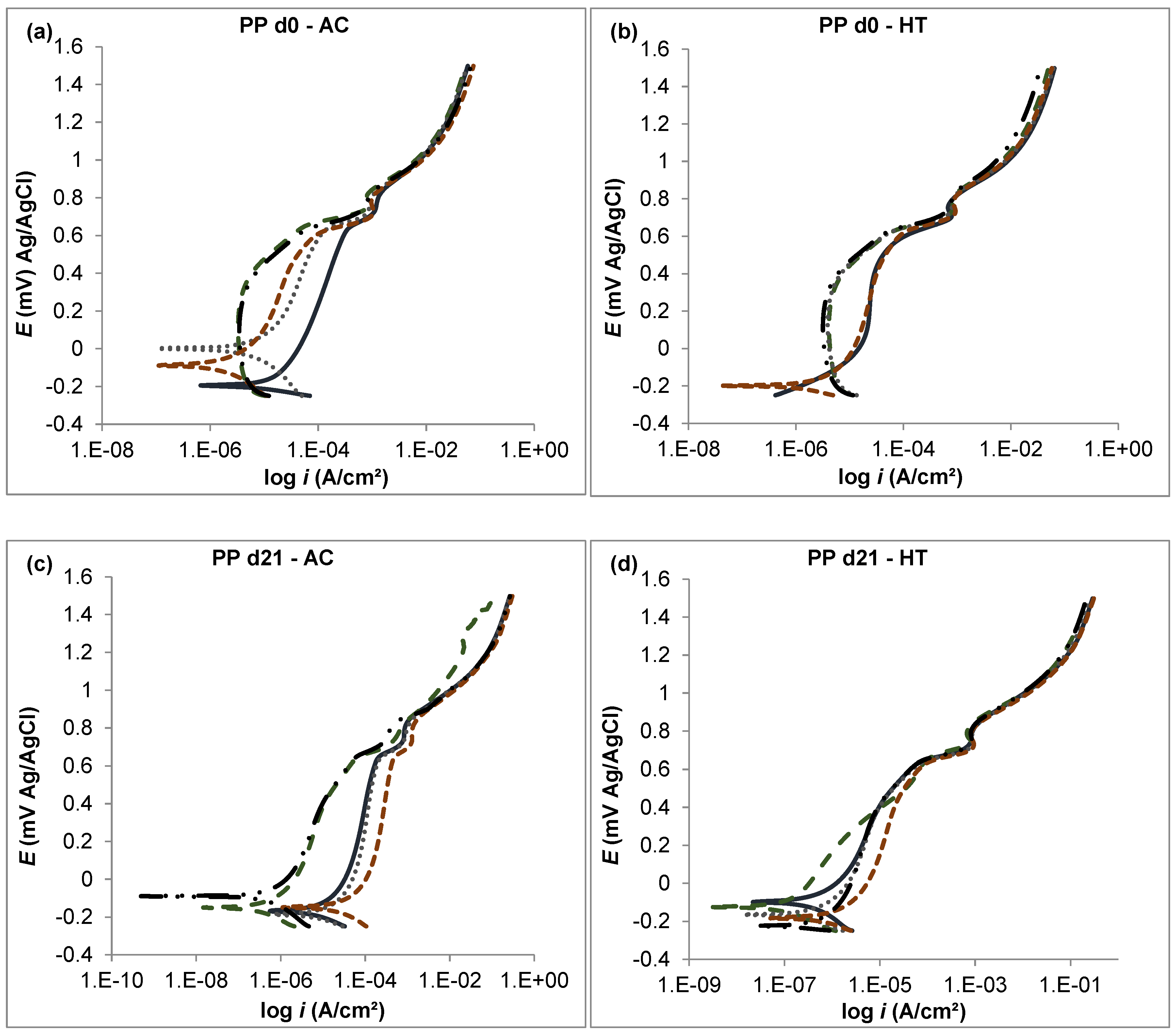
3.2. Potentiodynamic Polarization Curves
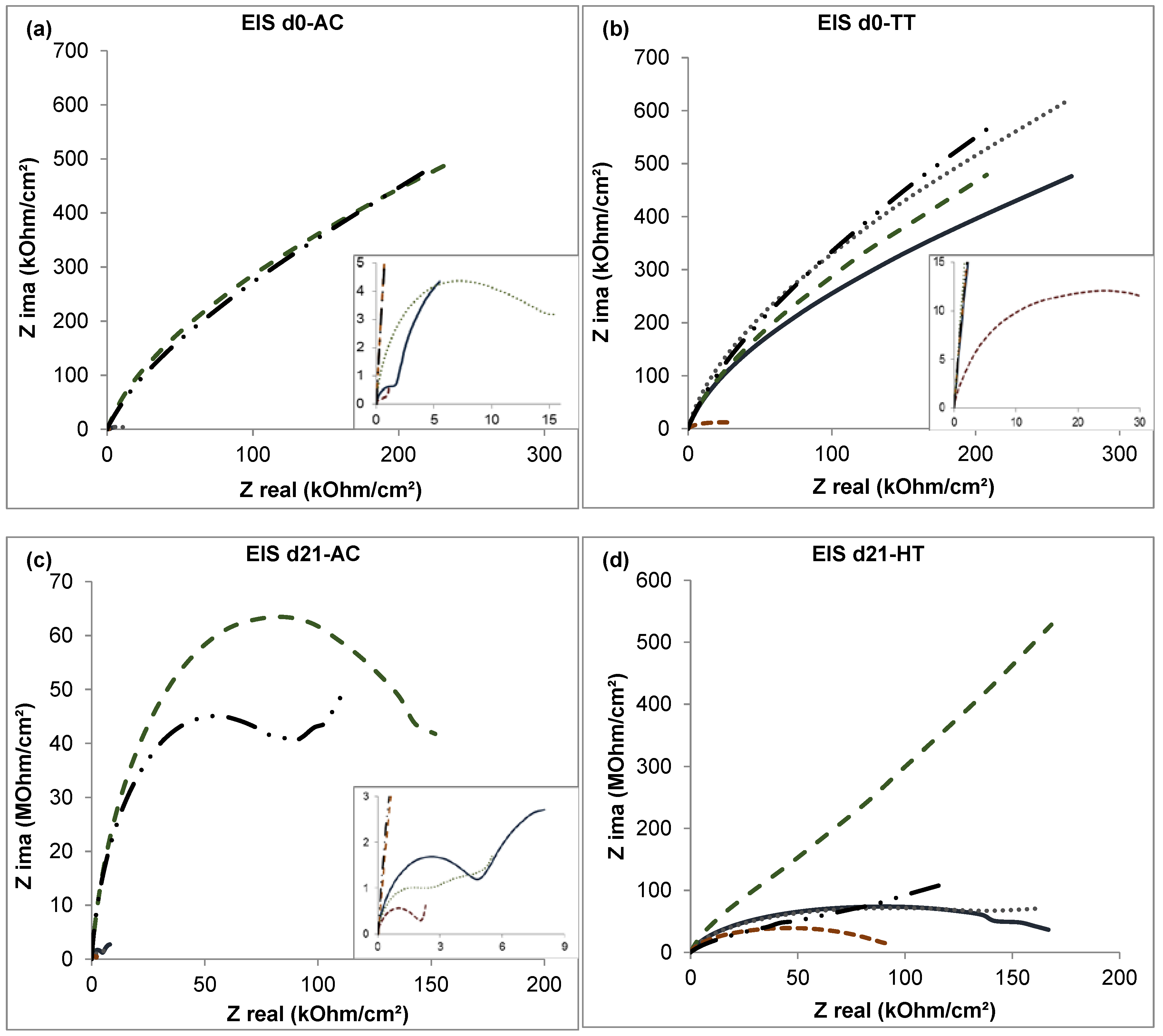
3.3. Electrochemical Impedance Spectroscopy
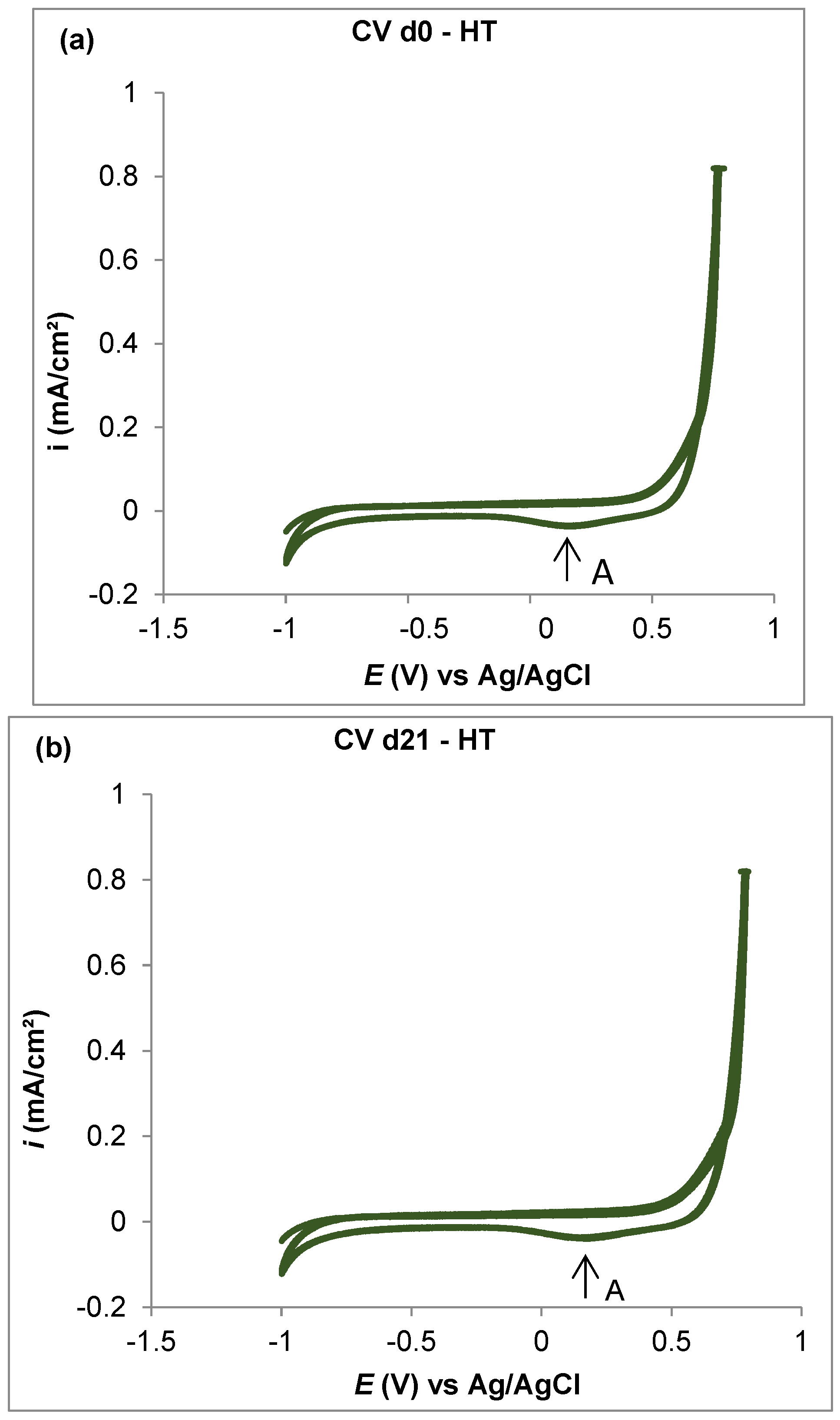
3.4. Cyclic Voltammetry
4. Conclusions
- The corrosion resistance in the analyzed CoCrMo alloys was improved by both boron addition and heat treatment.
- The alloys with precipitated boron carbides showed good corrosion resistance.
- The base alloy and most of the boron alloys exhibited a slight tendency to increased corrosion rates after several immersion days.
- The highest corrosion rate was observed in alloys with the highest carbon content.
- The heat-treated alloy with 0.06% boron added exhibited the best corrosion resistance during the immersion time (21 days) with respect to the other tested alloys. This was attributed to the combined effects of boron, which stabilized the carbon, and HT, which homogenized the microstructure.
Author Contributions
Conflicts of Interest
References
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Hanawa, T. Evaluation techniques of metallic biomaterials in vitro. Sci. Technol. Adv. Mater. 2002, 3, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M.; Hanawa, T.; Narushima, T. Japanese research and development on metallic biomedical, dental, and healthcare materials. JOM 2005, 57, 18–24. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Antunes Altobelli, R.; de Oliveira Lopes, M.C. Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomater. 2012, 8, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Bumgardner, J.D. Changes in the surface oxide composition of Co-Cr-Mo implant alloy by macrophage cells and their released reactive chemical species. Biomaterials 2004, 25, 1233–1238. [Google Scholar] [CrossRef]
- Dobbs, H.S.; Robertson, J.L.M. Heat treatment of cast Co-Cr-Mo for orthopaedic implant use. J. Mater. Sci. 1983, 18, 391–401. [Google Scholar] [CrossRef]
- Diabb, J.; Juárez-Hernandez, A.; Colas, R.; Castillo, A.G.; García-Sanchez, E.; Hernandez-Rodriguez, M.A.L. Boron influence on wear resistance in nickel-based alloys. Wear 2009, 267, 550–555. [Google Scholar] [CrossRef]
- Bermingham, M.; Bermingham, M.J.; Mcdonald, S.D.; Stjohn, D.H.; Dargusch, M.S. The effect of boron on the refinement of microstructure in cast cobalt alloys. J. Mater. Res. 2011, 26, 951–956. [Google Scholar] [CrossRef]
- Opiekun, Z. Kinetics of secondary carbide precipitation in boron-modified cobalt alloys of MAR-M509 type. J. Mater. Sci. 1991, 26, 3386–3391. [Google Scholar] [CrossRef]
- Bocchini, P.J.; Sudbrack, C.K.; Noebe, R.D.; Dunand, D.C.; Seidman, D.N. Microstructural and creep properties of boron- and zirconium-containing cobalt-based superalloys. Mater. Sci. Eng. A 2017, 682, 260–269. [Google Scholar] [CrossRef]
- Kolb, M.; Freund, L.P.; Fischer, F.; Povstugar, I.; Makineni, S.K.; Gault, B.; Raabe, D.; Müller, J.; Spiecker, E.; Neumeier, S.; et al. On the grain boundary strengthening effect of boron in γ/γ′ Cobalt-base superalloys. Acta Mater. 2018, 145, 247–254. [Google Scholar] [CrossRef]
- Zhuang, L.Z.; Langer, E.W. Effects of alloy additions on the microstructures and tensile properties of cast Co-Cr-Mo alloy used for surgical implants. J. Mater. Sci. 1989, 24, 4324–4330. [Google Scholar] [CrossRef]
- Zhuang, L.Z.; Langer, E.W. Effects of alloy additions on the fatigue properties of cast Co-Cr-Mo alloy used for surgical implants. J. Mater. Sci. 1990, 25, 683–689. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Bravo-Bárcenas, D.; Cimenoglu, H.; Figueroa-López, U.; Flores-Jiménez, M.; Meydanoglu, O. The boriding process in CoCrMo alloy: Fracture toughness in cobalt boride coatings. Surf. Coat. Technol. 2014, 260, 362–368. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Bravo-Bárcenas, D.; Meneses-Amador, A.; Ortiz-Dominguez, M.; Cimenoglu, H.; Figueroa-López, U.; Andraca-Adame, J. Growth kinetics and mechanical properties of boride layers formed at the surface of the ASTM F-75 biomedical alloy. Surf. Coat. Technol. 2013, 237, 402–414. [Google Scholar] [CrossRef]
- Rodríguez-Castro, G.A.; Reséndiz-Calderon, C.D.; Jiménez-Tinoco, L.F.; Meneses-Amador, A.; Gallardo-Hernández, E.A.; Campos-Silva, I.E. Micro-abrasive wear resistance of CoB/Co2B coatings formed in CoCrMo alloy. Surf. Coat. Technol. 2015, 284, 258–263. [Google Scholar] [CrossRef]
- Mu, D.; Shen, B.; Zhao, X. Effects of boronizing on mechanical and dry-sliding wear properties of CoCrMo alloy. Mater. Des. 2010, 31, 3933–3936. [Google Scholar] [CrossRef]
- Bedolla-Gil, Y.; Hernandez-Rodriguez, M.A.L. Tribological Behavior of a Heat-Treated Cobalt-Based Alloy. J. Mater. Eng. Perform. 2013, 22, 541–547. [Google Scholar] [CrossRef]
- Clemow, A.J.T.; Daniell, B.L. Solution treatment behavior of Co-Cr-Mo alloy. J. Biomed. Mater. Res. 1979, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Montero-Ocampo, C.; Salinas Rodriguez, A. Effect of carbon content on the resistance to localized corrosion of as-cast cobalt-based alloys in an aqueous chloride solution. J. Biomed. Mater. Res. 1995, 29, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Valero-Vidal, C.; Casabán-Julián, L.; Herraiz-Cardona, I.; Igual-Muñoz, A. Influence of carbides and microstructure of CoCrMo alloys on their metallic dissolution resistance. Mater. Sci. Eng. C 2013, 33, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Metikoš-Huković, M.; Pilić, Z.; Babić, R.; Omanović, D. Influence of alloying elements on the corrosion stability of CoCrMo implant alloy in Hank’s solution. Acta Biomater. 2006, 2, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- Kocijan, A.; Milošev, I.; Merl, D.K.; Pihlar, B. Electrochemical Study of Co-Based Alloys in Simulated Physiological Solution. J. Appl. Electrochem. 2004, 34, 517–524. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.-O.A.; Mischler, S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
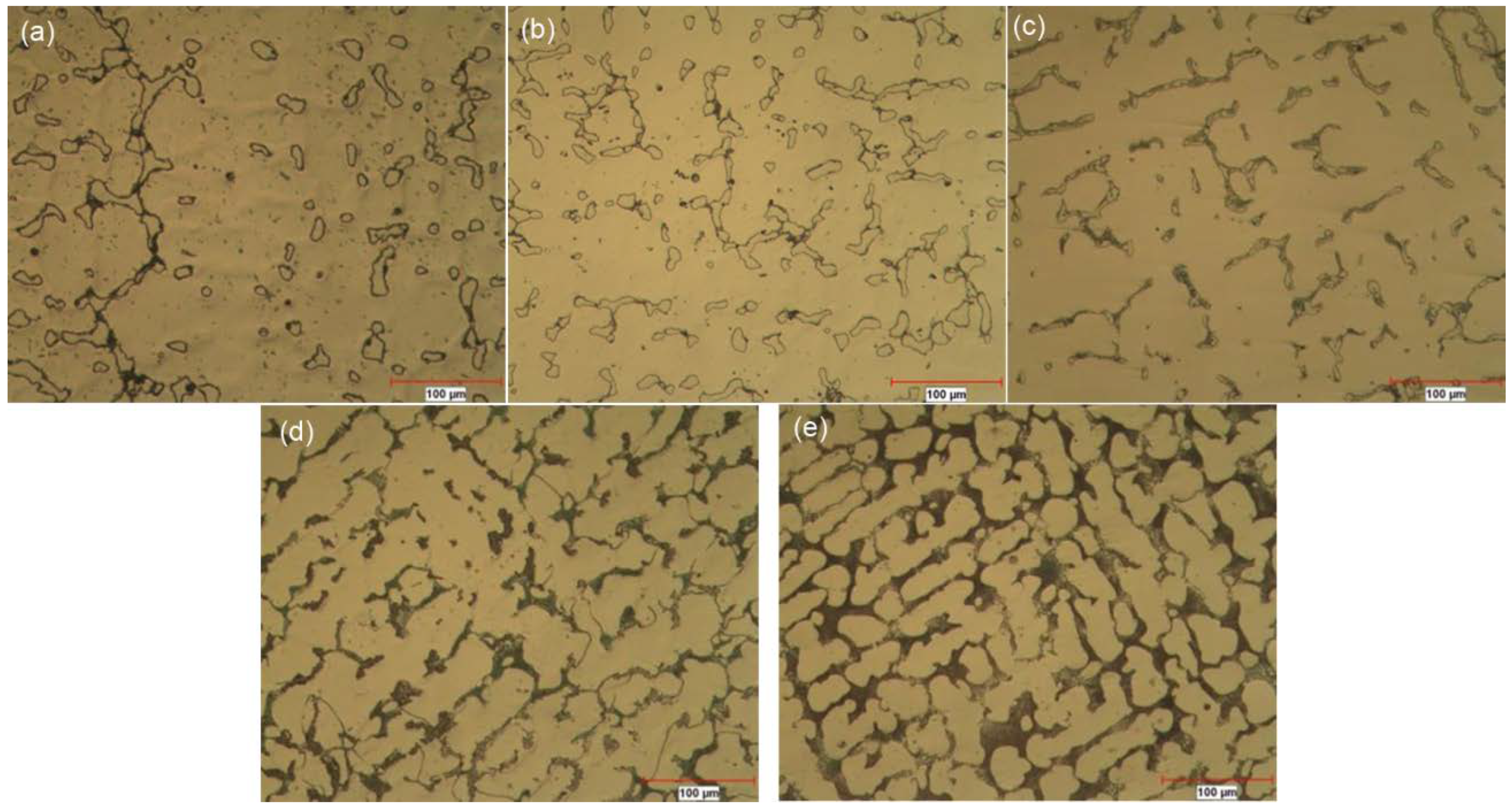
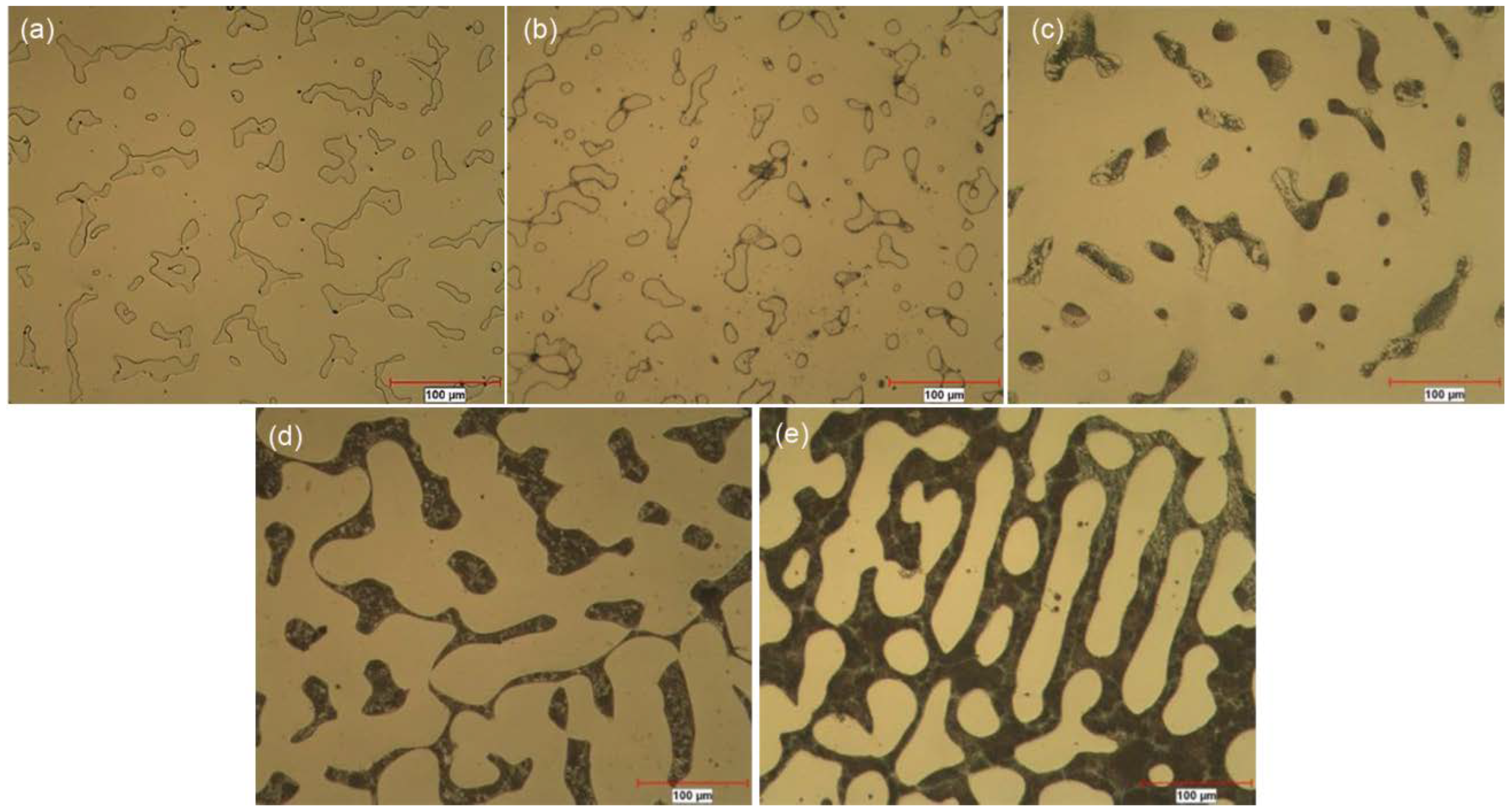
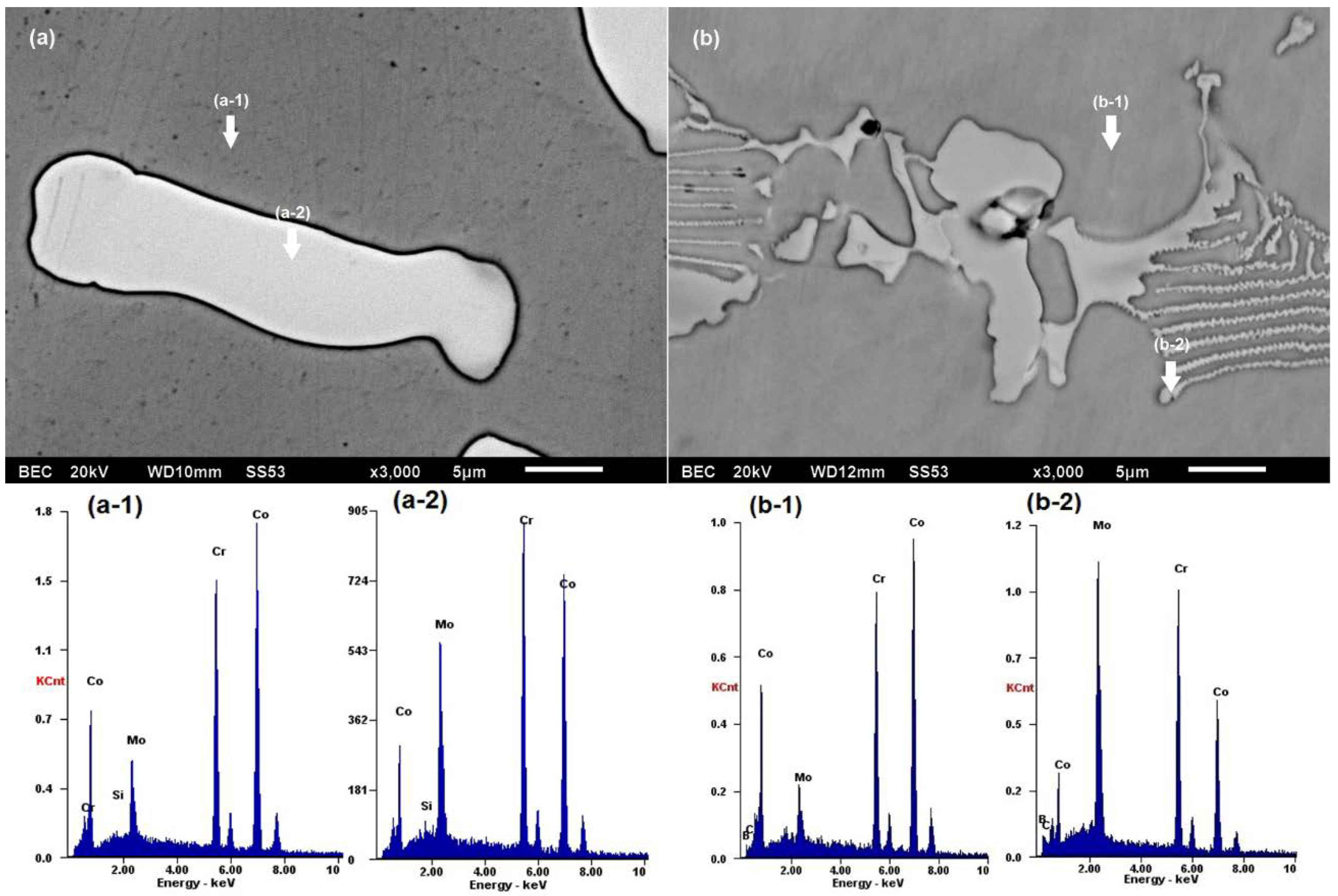
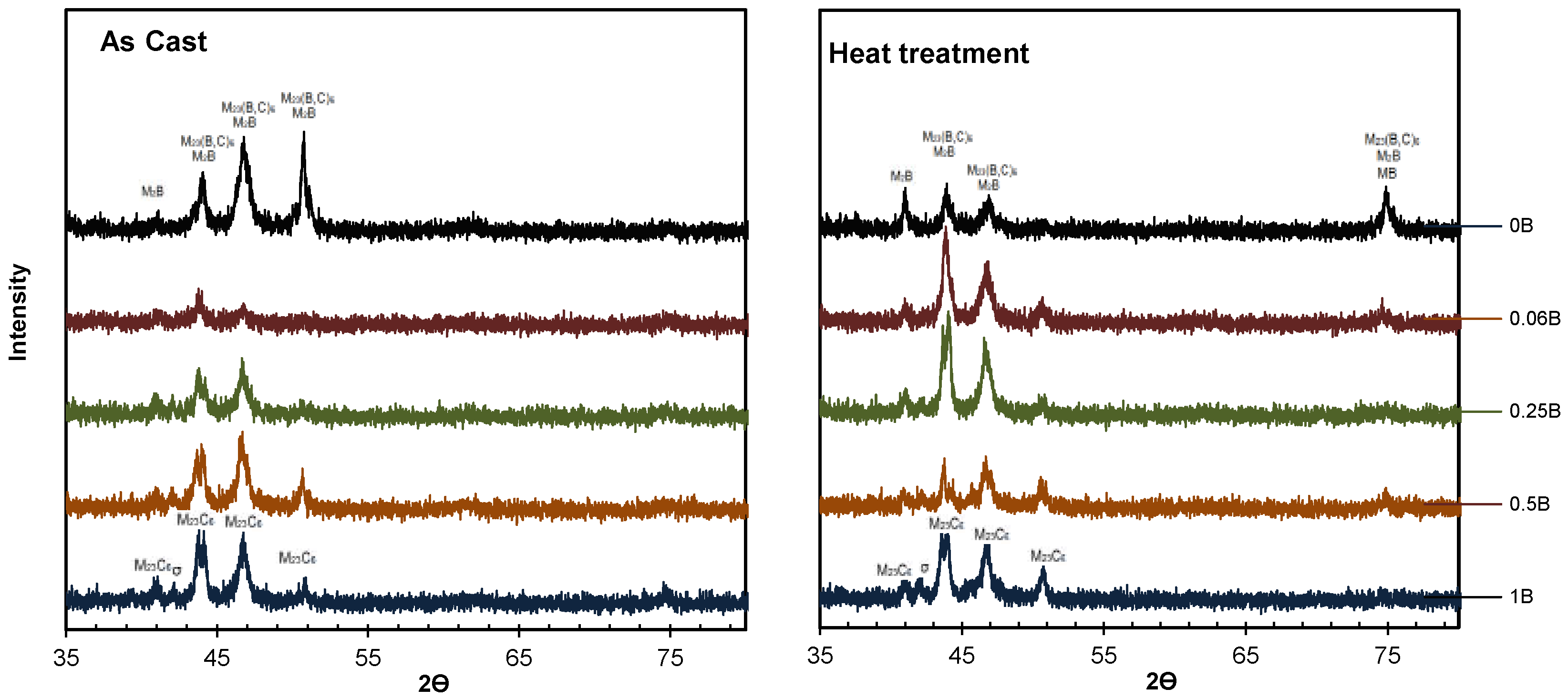


| Alloy | Cr | Mo | C | Si | Fe | B | Co |
|---|---|---|---|---|---|---|---|
| 0B-AC | 27 | 9.6 | 0.084 | 0.27 | 0.60 | 0.00 | Bal. |
| 0B-HT | 30 | 8.3 | 0.060 | 0.17 | 0.58 | 0.00 | Bal. |
| 0.06B-AC | 27 | 9.4 | 0.098 | 0.74 | 0.73 | 0.06 | Bal. |
| 0.06B-HT | 27 | 10.4 | 0.080 | 0.53 | 0.56 | 0.06 | Bal. |
| 0.25B-AC | 28 | 10.0 | 0.125 | 0.57 | 0.36 | 0.25 | Bal. |
| 0.25B-HT | 28 | 10.2 | 0.065 | 0.19 | 0.79 | 0.25 | Bal. |
| 0.5B-AC | 28 | 10.8 | 0.223 | 0.73 | 0.61 | 0.50 | Bal. |
| 0.5B-HT | 28 | 8.9 | 0.260 | 0.58 | 0.56 | 0.50 | Bal. |
| 1B-AC | 27 | 9.0 | 0.078 | 0.21 | 0.45 | 1.00 | Bal. |
| 1B-HT | 27 | 9.7 | 0.087 | 0.50 | 0.62 | 1.00 | Bal. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Rodriguez, M.A.L.; Laverde-Cataño, D.A.; Lozano, D.; Martinez-Cazares, G.; Bedolla-Gil, Y. Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy. Metals 2019, 9, 307. https://doi.org/10.3390/met9030307
Hernandez-Rodriguez MAL, Laverde-Cataño DA, Lozano D, Martinez-Cazares G, Bedolla-Gil Y. Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy. Metals. 2019; 9(3):307. https://doi.org/10.3390/met9030307
Chicago/Turabian StyleHernandez-Rodriguez, Marco A.L., Dionisio A. Laverde-Cataño, Diego Lozano, Gabriela Martinez-Cazares, and Yaneth Bedolla-Gil. 2019. "Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy" Metals 9, no. 3: 307. https://doi.org/10.3390/met9030307
APA StyleHernandez-Rodriguez, M. A. L., Laverde-Cataño, D. A., Lozano, D., Martinez-Cazares, G., & Bedolla-Gil, Y. (2019). Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy. Metals, 9(3), 307. https://doi.org/10.3390/met9030307






