Effect of Thermomechanical Treatment on Acicular Ferrite Formation in Ti–Ca Deoxidized Low Carbon Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
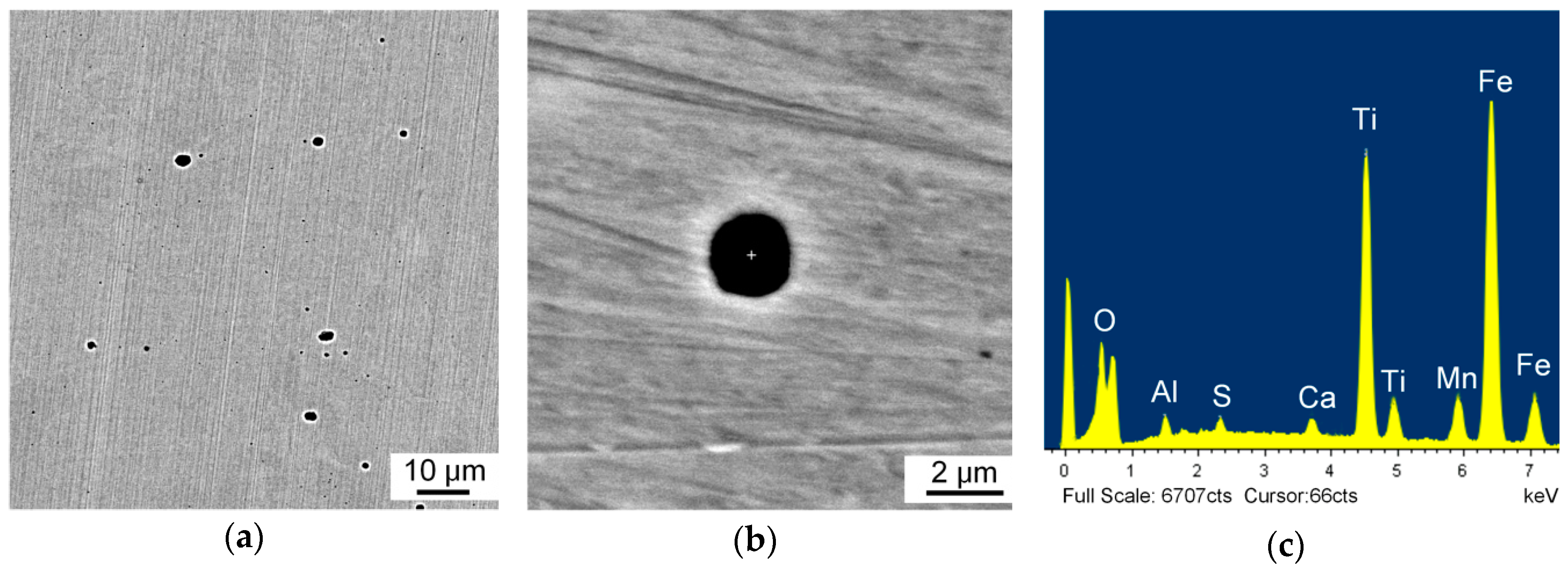
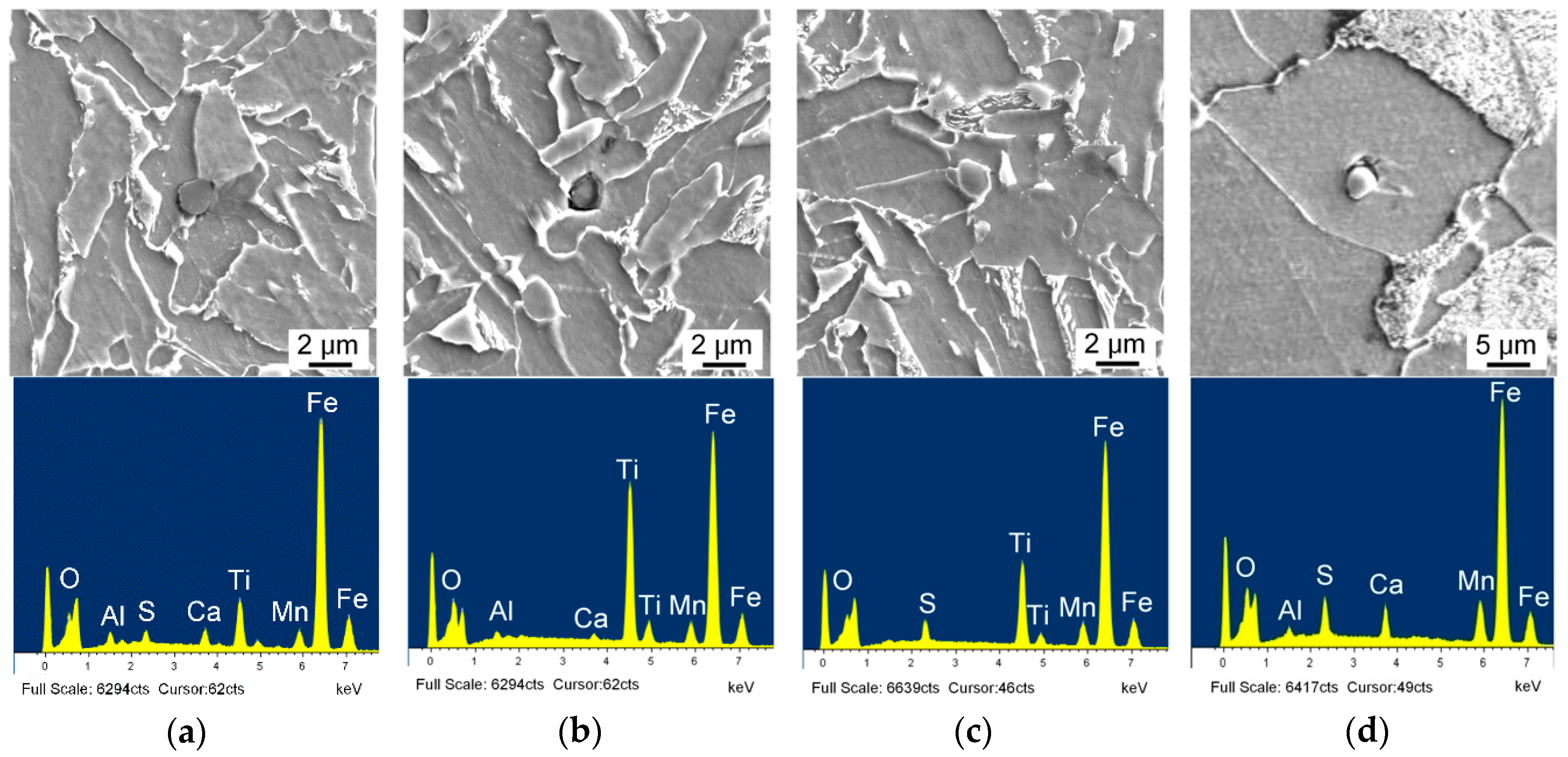
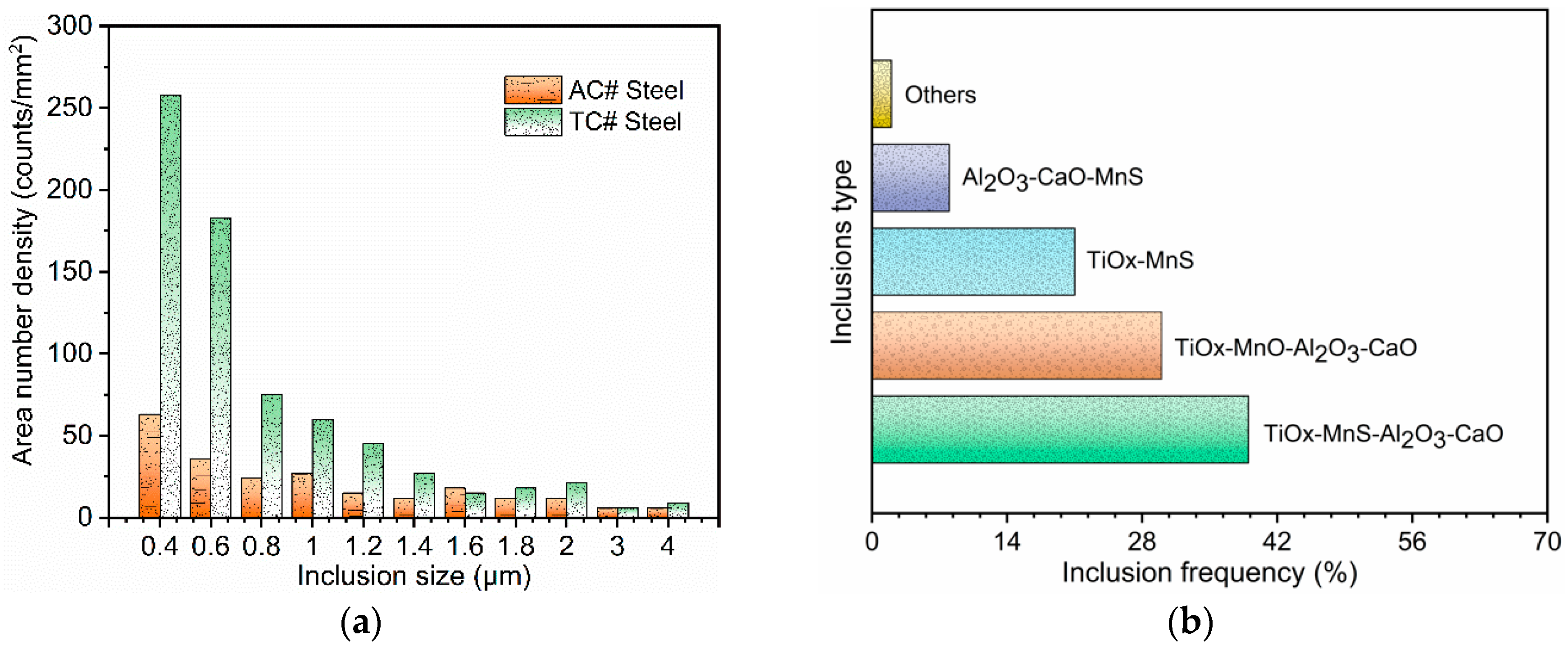
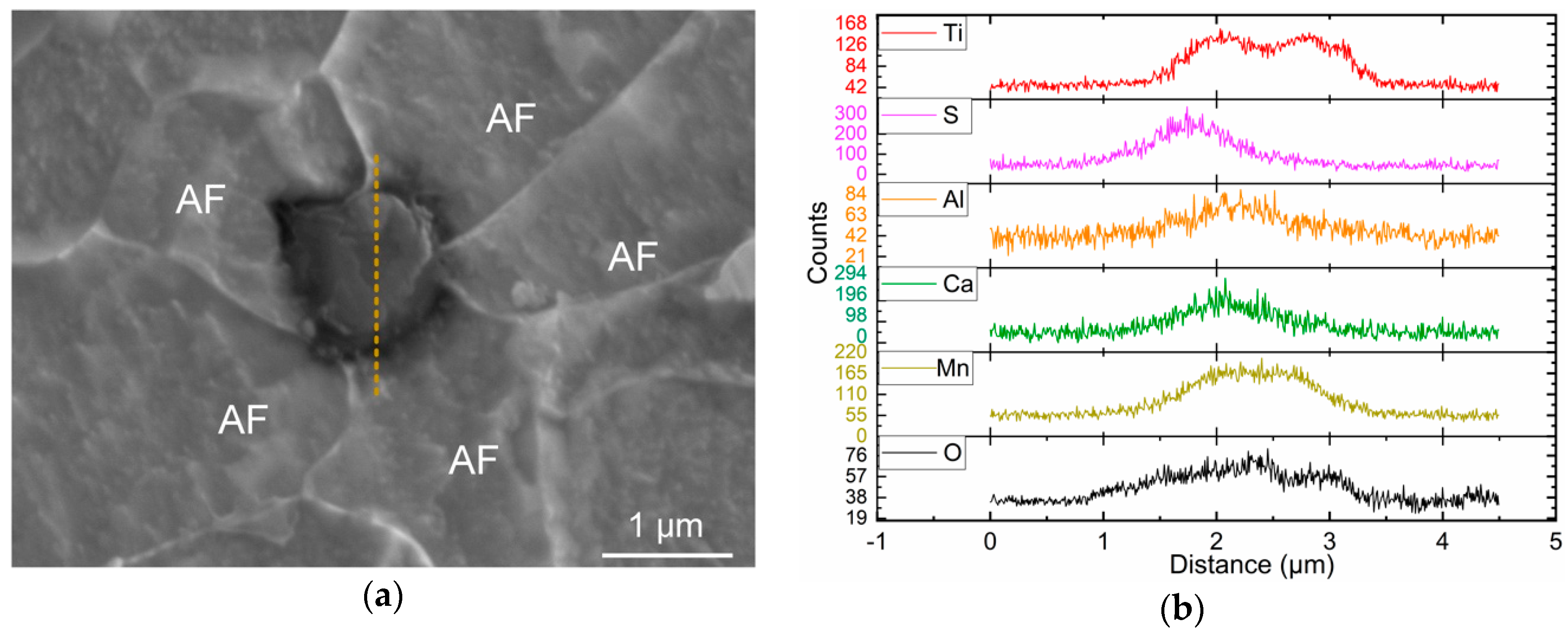
3.1. Inclusion Characterization
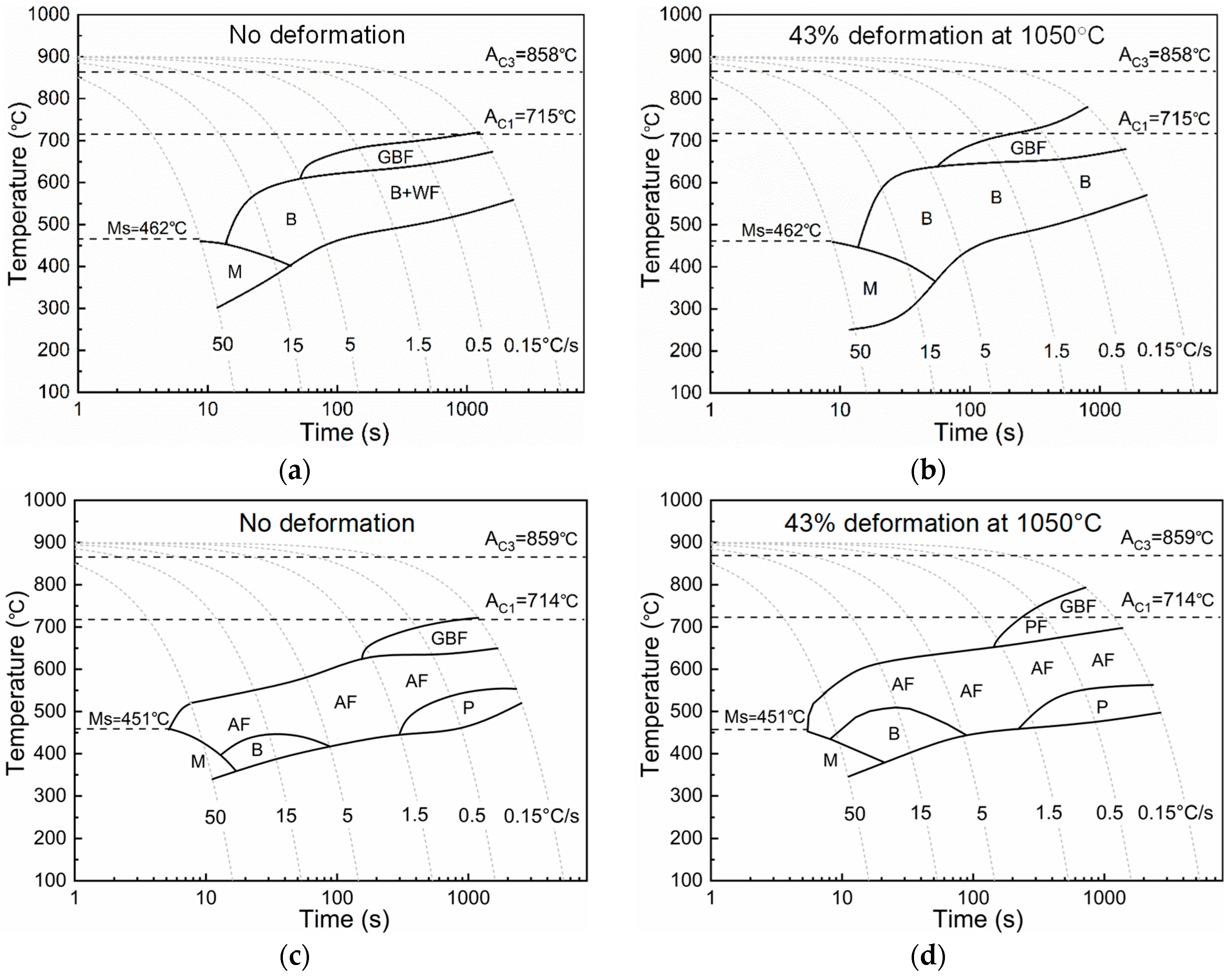
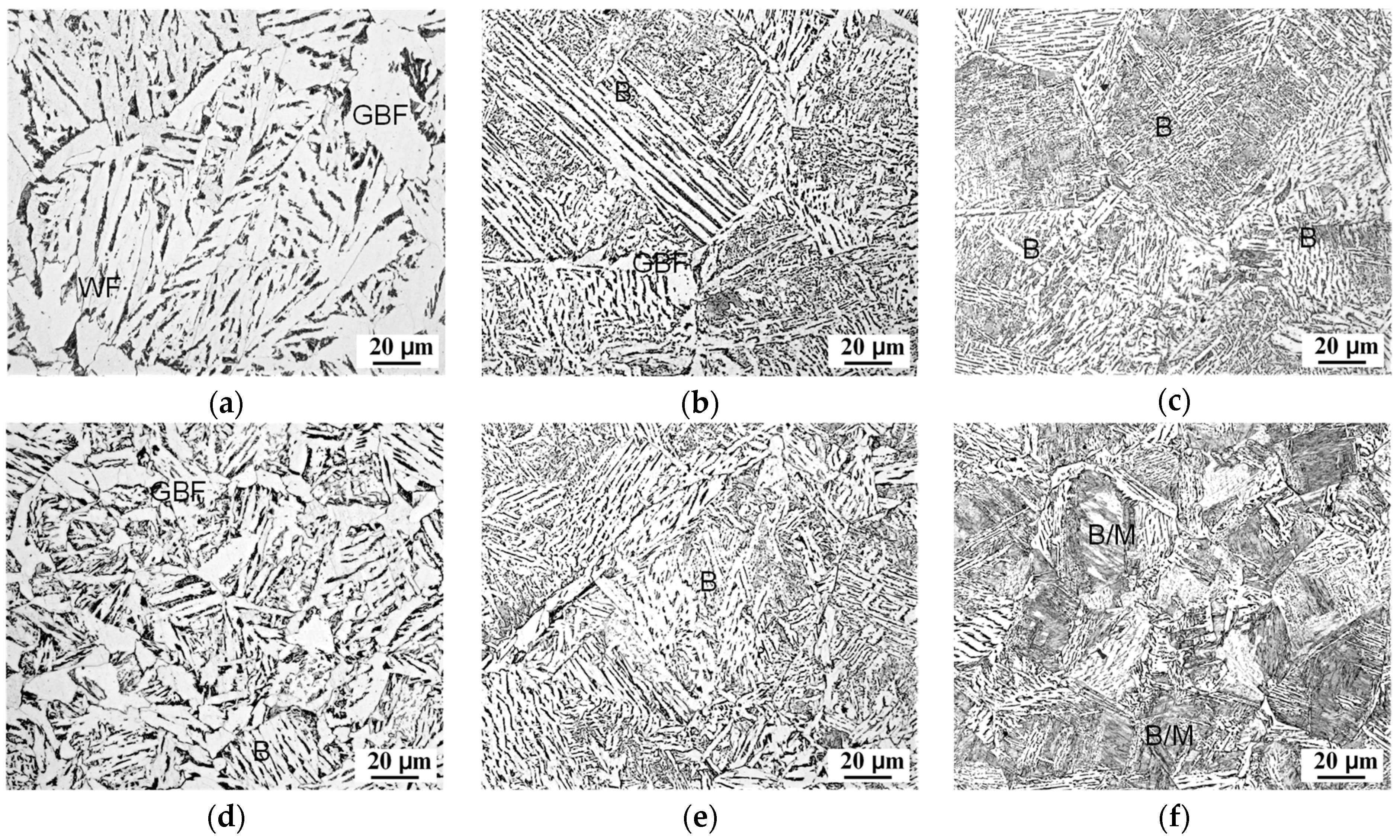
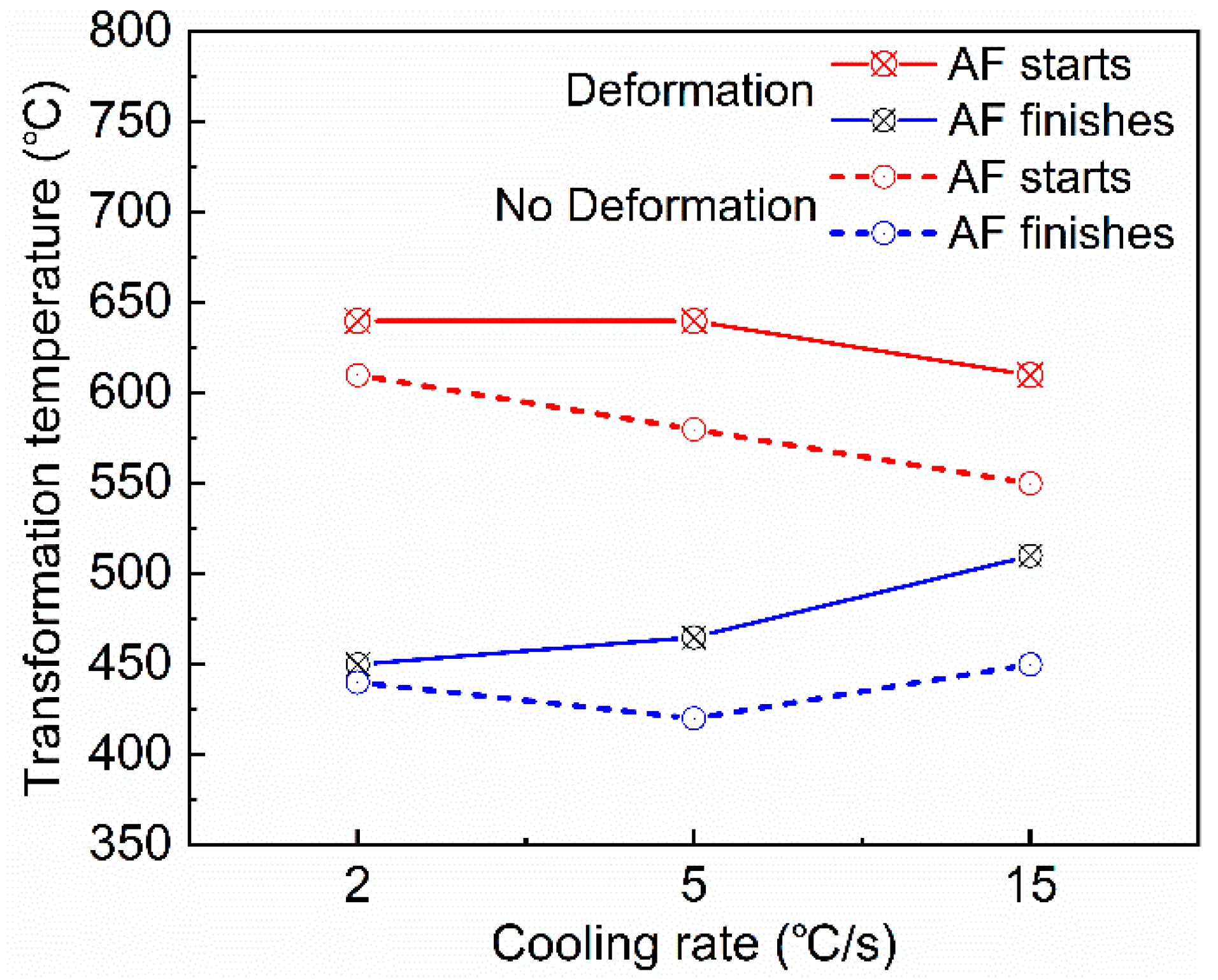
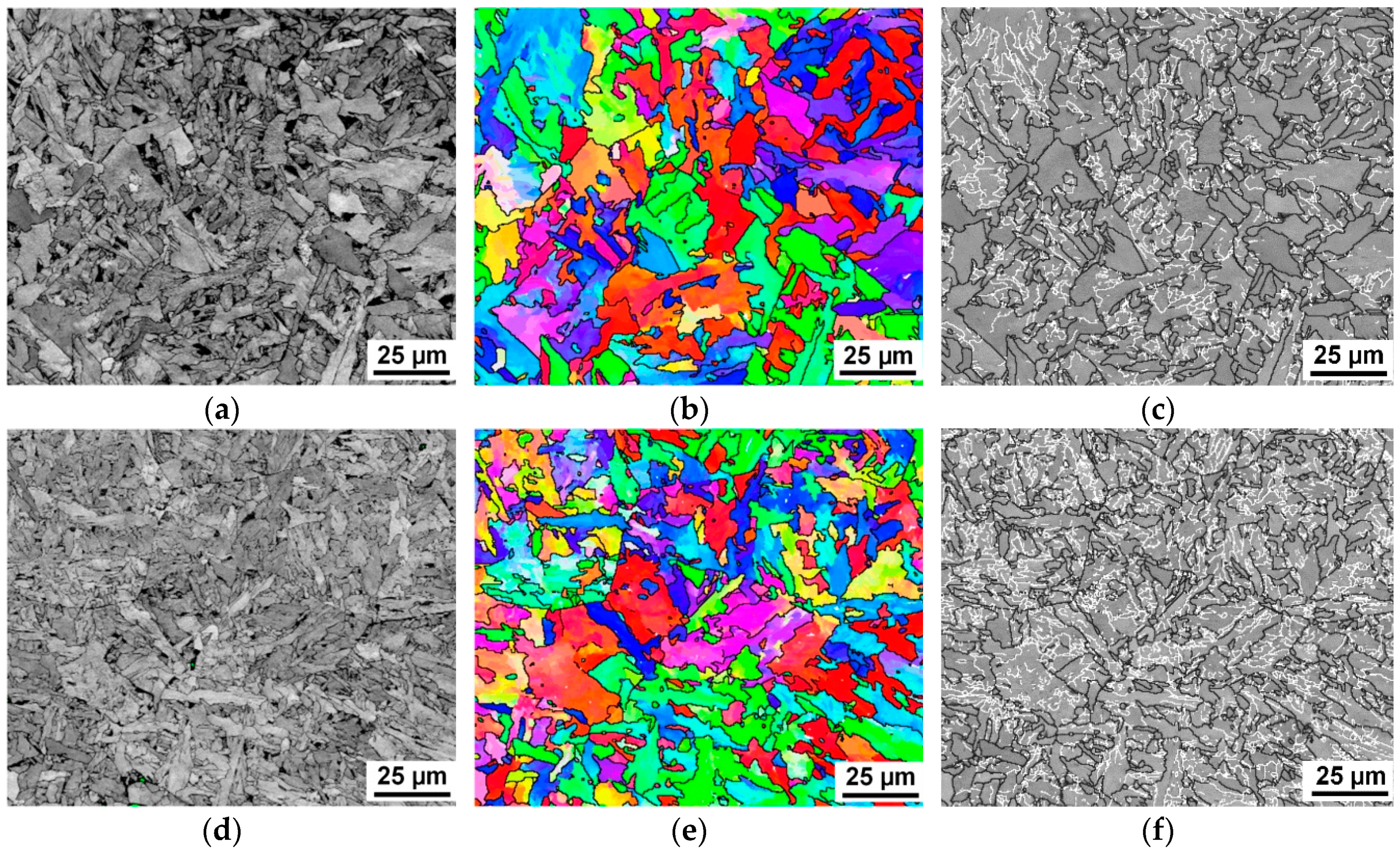
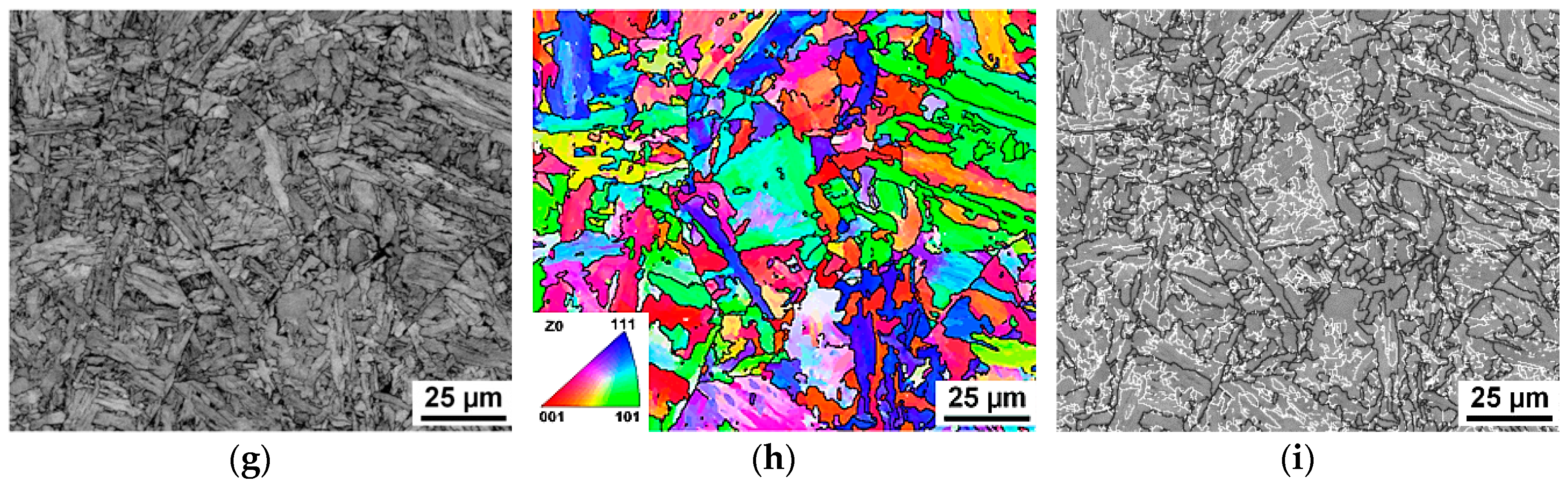
3.2. Microstructural Evolution Behaviors
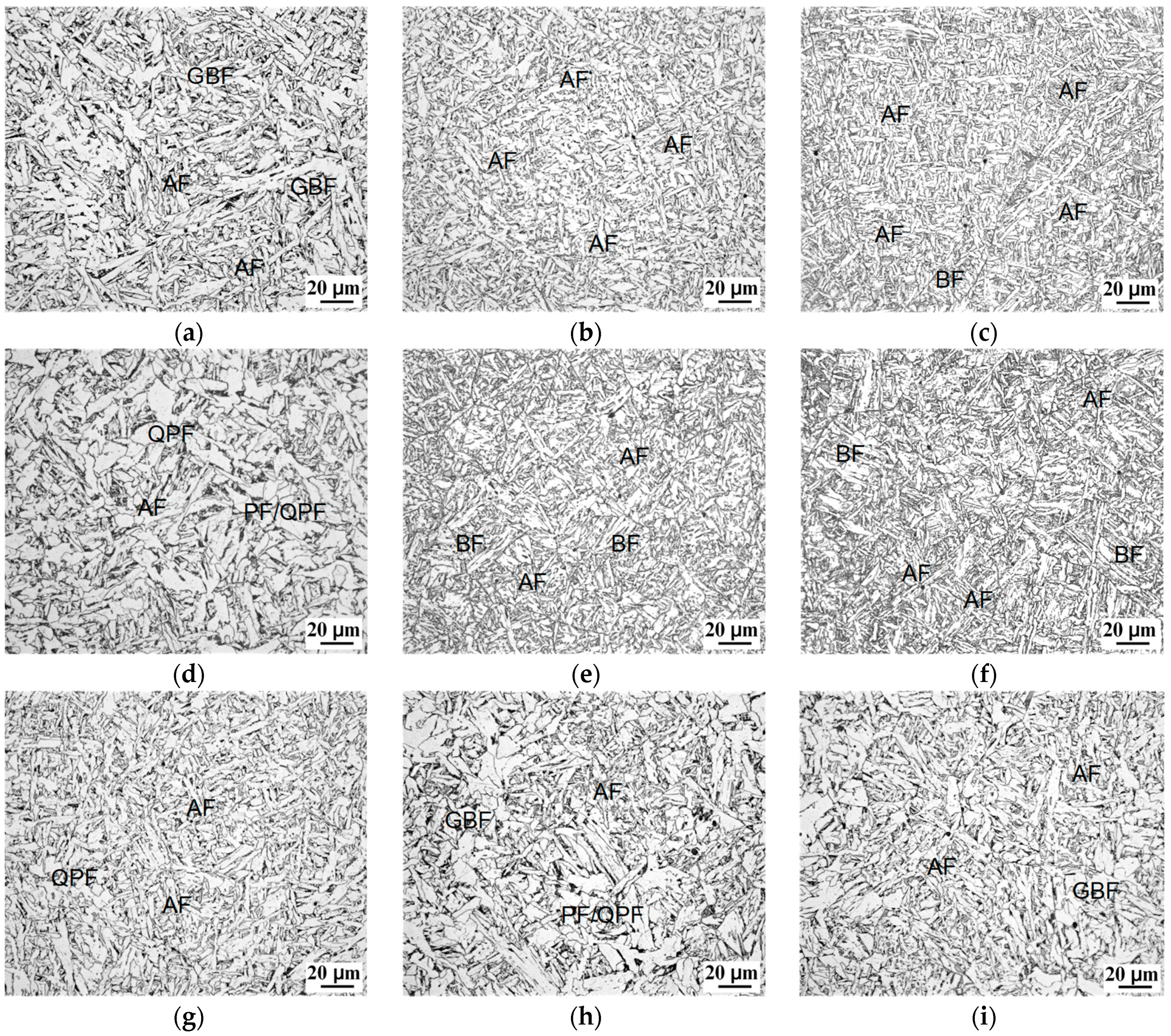
3.3. Hot Rolling Steels
4. Discussion
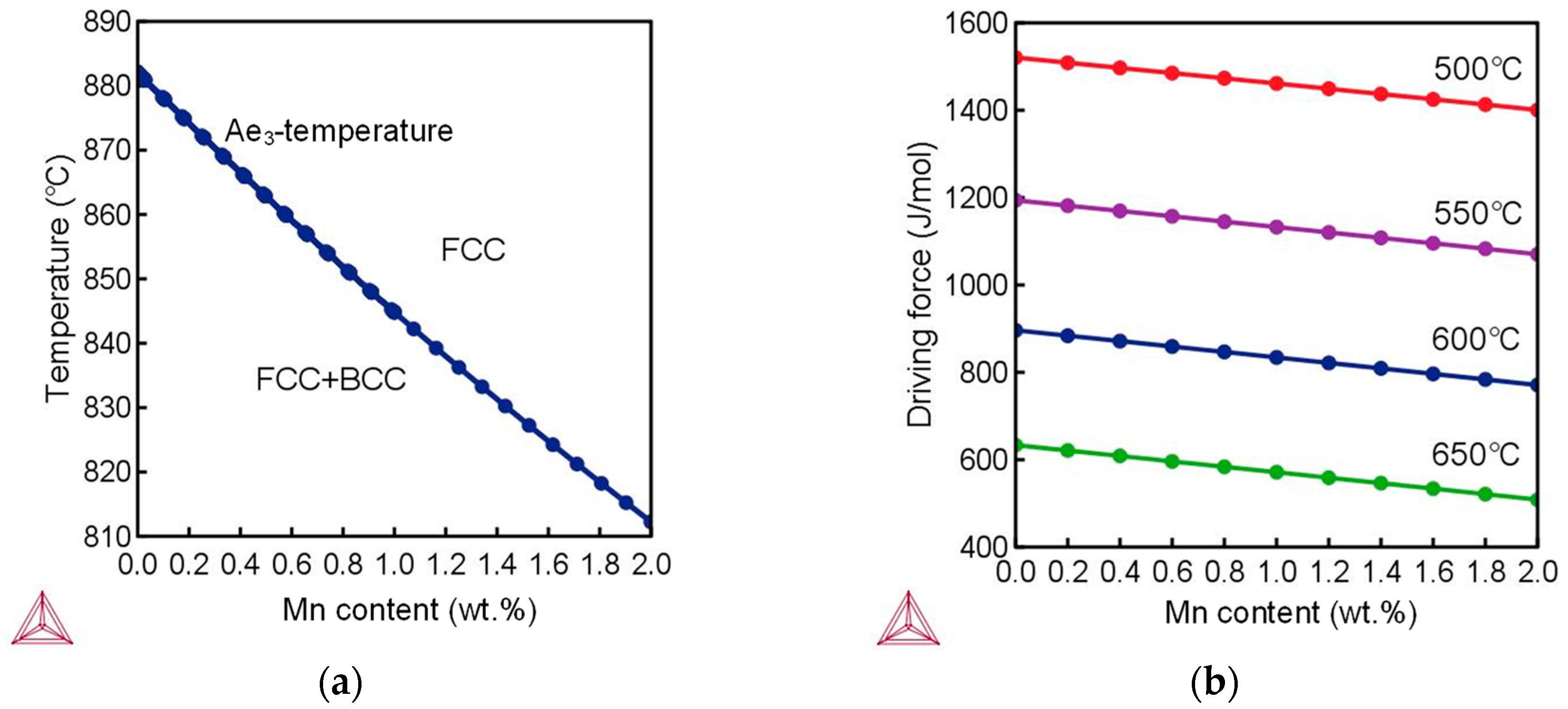
4.1. Mechanism of AF Nucleation Promoted by Ti–Ca Oxide Inclusions
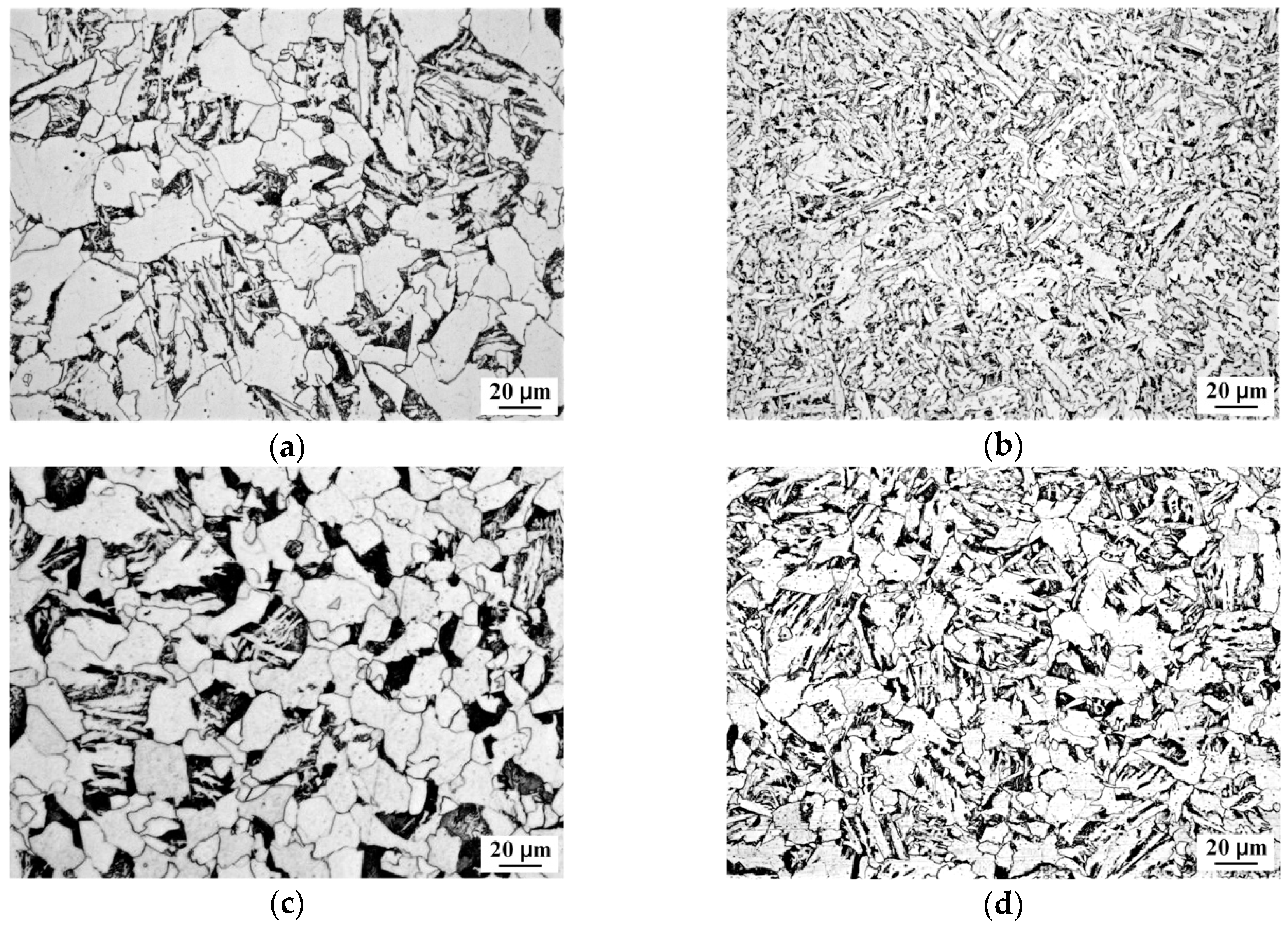
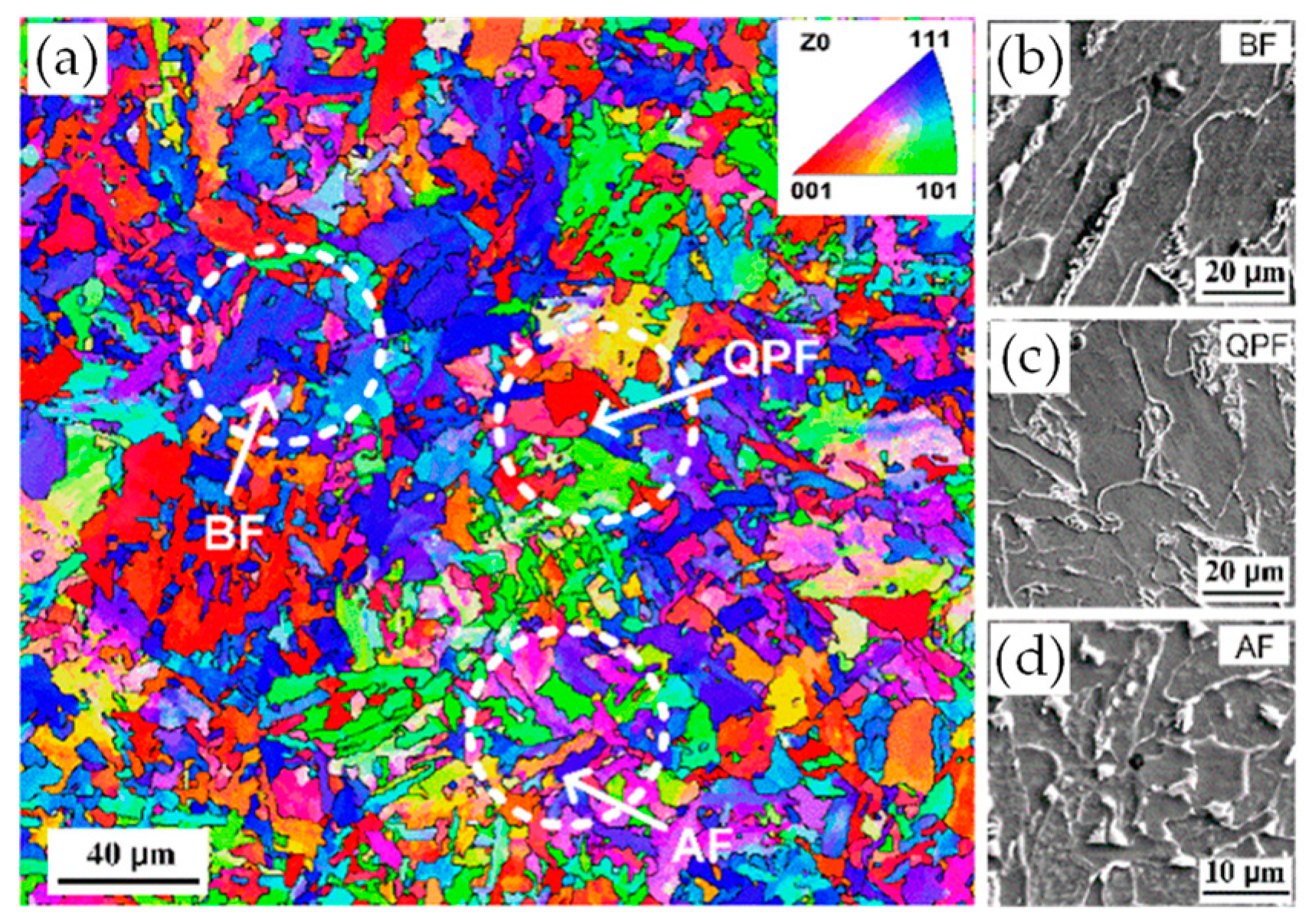
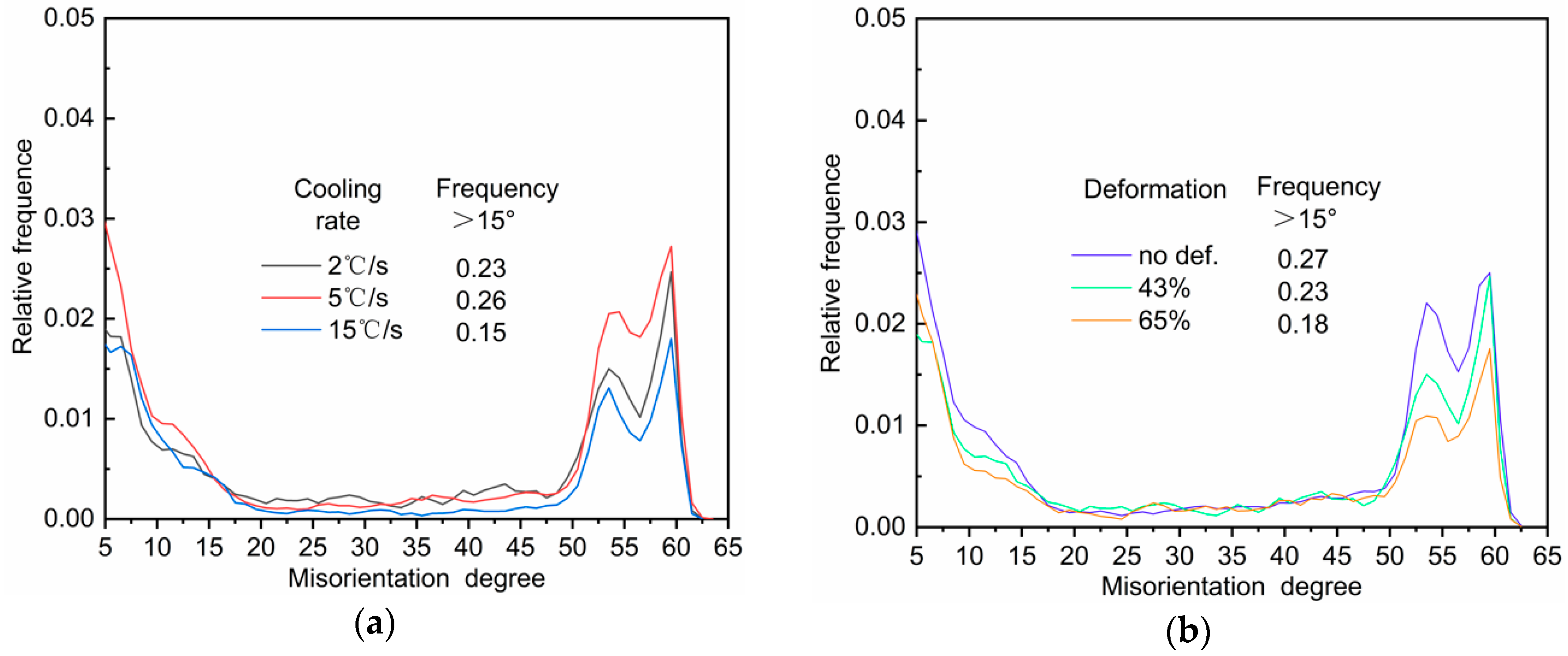
4.2. EBSD Interpretation of AF Microstructure
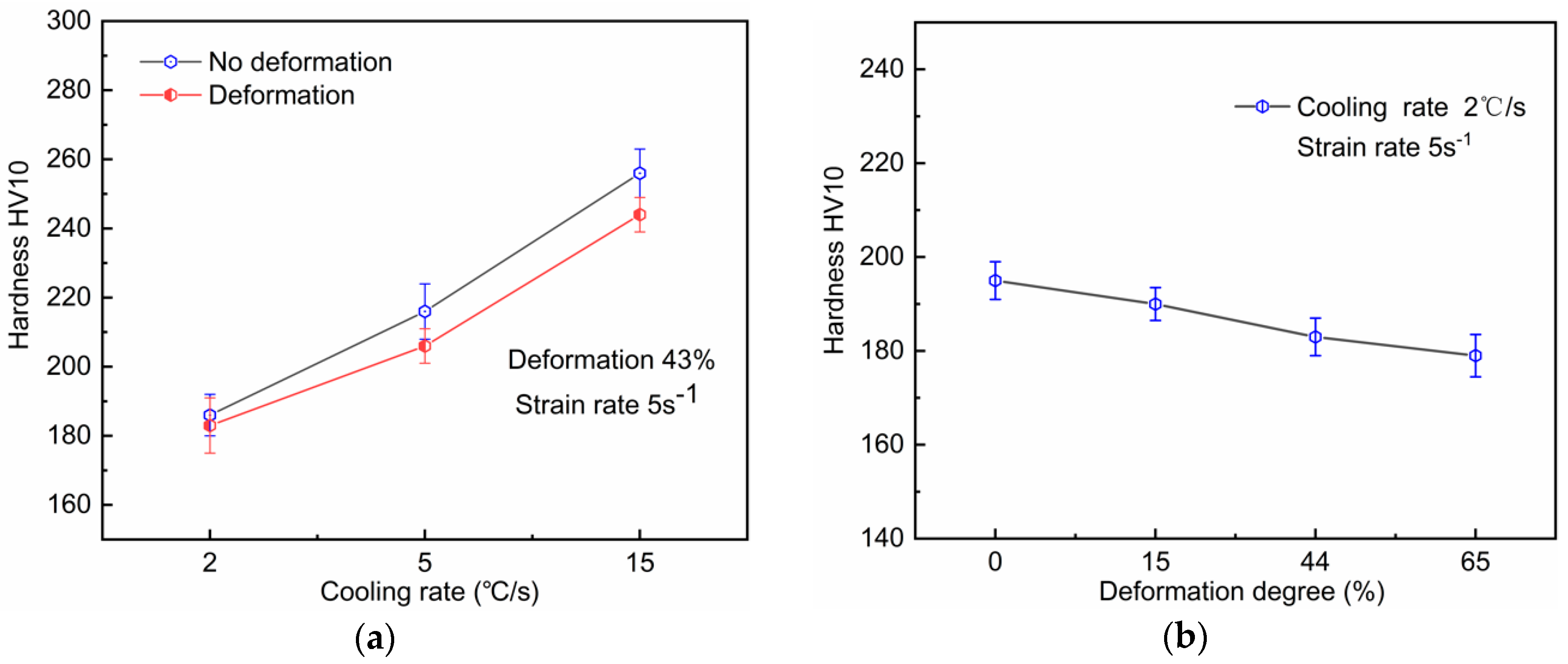
4.3. Process–Microstructure–Hardness Relationship
5. Conclusions
- (1)
- Inclusions in Ti–Ca deoxidized steel were mainly of a Ti-Al-Ca-O-Mn-S composite type. The observed typical inclusions TiOx-MnS-Al2O3-CaO, TiOx-MnO-Al2O3-CaO, and TiOx-MnS were effective for intragranular acicular ferrite nucleation and responsible for microstructure refinement. The Mn-depletion zone mechanism was applicable to the present steel;
- (2)
- An increase in the cooling rate up to 15 °C/s could inhibit the grain boundary reaction product and reduce transformation temperature. The driving force for transformation from austenite to ferrite increased at lower temperatures, resulting in fine AF dominant microstructure formation. However, a high deformation of 43–65% discouraged the formation of full acicular ferrite microstructures because of the increase in austenite grain boundaries serving as nucleation sites.
- (3)
- High-angled grain boundaries acted as obstacles to cleavage crack propagation and improved toughness. The HAGB fraction of the sample cooled at 5 °C/s was the highest because of full AF structure formation. The grain boundary misorientation angle distribution of the AF structure exhibited a “double-peak” feature, which was similar to bainite transformation.
- (4)
- The hardness increased significantly as the cooling rate increased from 2 to 15 °C/s, while it decreased under the condition of deformation because of the formation of (quasi-)polygonal ferrite. By applying accelerated water cooling, the mechanical properties, particularly impact toughness, were significantly improved as a result of fine AF microstructure formation.
Author Contributions
Funding
Conflicts of Interest
References
- Takamura, J.; Mizoguchi, S. Roles of oxides in steels performance–metallurgy of oxides in steels. In Proceedings of the 6th International Iron and Steel Congress, Nagoya, Japan, 21–26 October 1990. [Google Scholar]
- Min, Y.; Li, X.B.; Yu, Z.; Liu, C.L.; Jiang, M.F. Characterization of the acicular ferrite in Al-deoxidized low-carbon steel combined with Zr and Mg additions. Steel Res. Int. 2016, 87, 1503–1510. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K. Systematic investigation of acicular ferrite formation on laboratory scale. Mater. Sci. Technol. 2017, 33, 162–171. [Google Scholar] [CrossRef]
- Ilman, M.N.; Cochrane, R.C.; Evans, G.M. The development of acicular ferrite in reheated Ti–B–Al–N-type steel weld metals containing various levels of aluminium and nitrogen. Weld. World 2015, 59, 565–575. [Google Scholar] [CrossRef]
- Mu, W.; Mao, H.; Jönsson, P.G.; Nakajima, K. Effect of carbon content on the potency of the intragranular ferrite formation. Steel Res. Int. 2016, 87, 311–319. [Google Scholar] [CrossRef]
- Capdevila, C.; García-Mateo, C.; Chao, J.; Caballero, F.G. Effect of V and N precipitation on acicular ferrite formation in sulfur-lean vanadium steels. Metall. Mater. Trans. A 2009, 40A, 522–538. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Mu, W.; Jönsson, P.G.; Nakajima, K. Recent aspects on the effect of inclusion characteristics on the intragranular ferrite formation in low alloy steels: a review. High Temp. Mater. Processes 2017, 36, 1–17. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Bernhard, C. Acicular ferrite formation and its influencing factors-a review. J. Mater. Sci. Res. 2017, 6, 24–43. [Google Scholar] [CrossRef]
- Li, X.; Min, Y.; Liu, C.; Jiang, M.F. Study on the formation of intragranular acicular ferrite in a Zr-Mg-Al deoxidized low carbon steel. Steel Res. Int. 2016, 87, 622–632. [Google Scholar] [CrossRef]
- Hui, K.; Shao, S.; Shen, Y.F.; Yan, Y.H.; Qiang, Y.; Zang, J.F.; Cai, Z.Y. Effect of aluminum on the nucleation of intragranular ferrite in Ti-added carbon structural steel. High Temp. Mater. Processes 2013, 32, 323–329. [Google Scholar] [CrossRef]
- Mu, W.; Jönsson, P.G.; Shibata, H.; Nakajima, K. Inclusion and microstructure characteristics in steels with TiN additions. Steel Res. Int. 2016, 87, 339–348. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Wang, G. Effect of hot deformation and controlled cooling process on microstructures of Ti-Zr deoxidized low carbon steel. ISIJ Int. 2016, 56, 1800–1807. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.; Jiang, M.; Hu, Z.Y.; Yang, C.W. Effects of Ti-Al complex deoxidization inclusions on nucleation of intragranular acicular ferrite in C-Mn steel. Steel Res. Int. 2016, 87, 445–455. [Google Scholar] [CrossRef]
- Kang, Y.; Jeong, S.; Kang, J.H.; Lee, C. Factors affecting the inclusion potency for acicular ferrite nucleation in high-strength steel welds. Metall. Mater. Trans. A 2016, 47A, 2842–2854. [Google Scholar] [CrossRef]
- Boratto, F.; Barbosa, R.; Yue, S.; Jonas, J.J. Effect of chemical composition on the critical temperature of microalloyed steels. In Proceedings of the International Conference on Physical Metallurgy of Thermomechanical Processing of Steels and Other Metals (THERMEC-88), Tokyo, Japan, 6–10 June 1988; Tamura, I., Ed.; ISIJ: Tokyo, Japan, 1988. [Google Scholar]
- Bhadeshia, H.K.D.H. Bainite in Steels, 2nd ed.; IOM Communication Ltd.: London, UK, 2001; pp. 101–106. [Google Scholar]
- Krauss, G.; Thompson, S.W. Ferritic microstructures in continuously cooled low-and ultralow-carbon steels. ISIJ Int. 1995, 35, 937–945. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Developments in martensitic and bainitic steels: role of the shape deformation. Mater. Sci. Eng. A 2004, 378, 34–39. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.V.; Sietsma, J. The kinetics of bainite and martensite formation in steels during cooling. Mater. Sci. Eng. A 2010, 527, 6672–6676. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, H.; Masse, J.P.; Bouzaiz, O.; Gacheta, G. The effect of prior ferrite formation on bainite and martensite transformation kinetics in advanced high-strength steels. Acta Mater. 2013, 61, 6025–6036. [Google Scholar] [CrossRef]
- Kang, H.C.; Park, B.J.; Jang, J.H.; Jang, K.S.; Lee, K.J. Determination of the continuous cooling transformation diagram of a high strength low alloyed steel. Met. Mater. Int. 2016, 22, 949–955. [Google Scholar] [CrossRef]
- Seo, K.; Kim, Y.M.; Evans, G.M.; Kim, H.J.; Lee, C. Formation of Mn-depleted zone in Ti-containing weld metals. Weld. World 2015, 59, 373–380. [Google Scholar] [CrossRef]
- Byun, J.S.; Shim, J.H.; Cho, Y.W.; Lee, D.N. Non-metallic inclusion and intragranular nucleation of ferrite in Ti-killed C–Mn steel. Acta Mater. 2003, 51, 1593–1606. [Google Scholar] [CrossRef]
- Cabus, C.; Réglé, H.; Bacroix, B. Orientation relationship between austenite and bainite in a multiphased steel. Mater. Charact. 2007, 58, 332–338. [Google Scholar] [CrossRef]
- Caballero, F.G.; Yen, H.W.; Miller, M.K.; Yang, J.R.; Cornidea, J.; Garcia-Mateoa, C. Complementary use of transmission electron microscopy and atom probe tomography for the examination of plastic accommodation in nanocrystalline bainitic steels. Acta Mater. 2011, 59, 6117–6123. [Google Scholar] [CrossRef]
- Yang, J.R.; Huang, C.Y.; Huang, C.F.; Aoh, J.N. Influence of acicular ferrite and bainite microstructures on toughness for an ultra-low-carbon alloy steel weld metal. J. Mater. Sci. Lett. 1993, 12, 1290–1293. [Google Scholar] [CrossRef]
- Wan, X.L.; Wu, K.M.; Huang, G.; Wei, R. In situ observations of the formation of fine-grained mixed microstructures of acicular ferrite and bainite in the simulated coarse-grained heated-affected zone. Steel Res. Int. 2014, 85, 243–250. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in steels. Metal. Mater. Trans. 1990, 21A, 767–797. [Google Scholar] [CrossRef]
- Gourgues, A.F.; Flower, H.M.; Lindley, T.C. Electron backscattering diffraction study of acicular ferrite, bainite, and martensite steel microstructures. J. Mater. Sci. Technol. 2000, 16, 26–40. [Google Scholar] [CrossRef]
- Shibata, K.; Asakura, K. Transformation behavior and microstructures in ultra-low carbon steels. ISIJ Int. 1995, 35, 982–991. [Google Scholar] [CrossRef]
- Zhao, H.; Palmiere, E.J. Effect of austenite deformation on the microstructure evolution and grain refinement under accelerated cooling conditions. Metall. Mater. Trans. A 2017, 48, 3389–3399. [Google Scholar] [CrossRef]
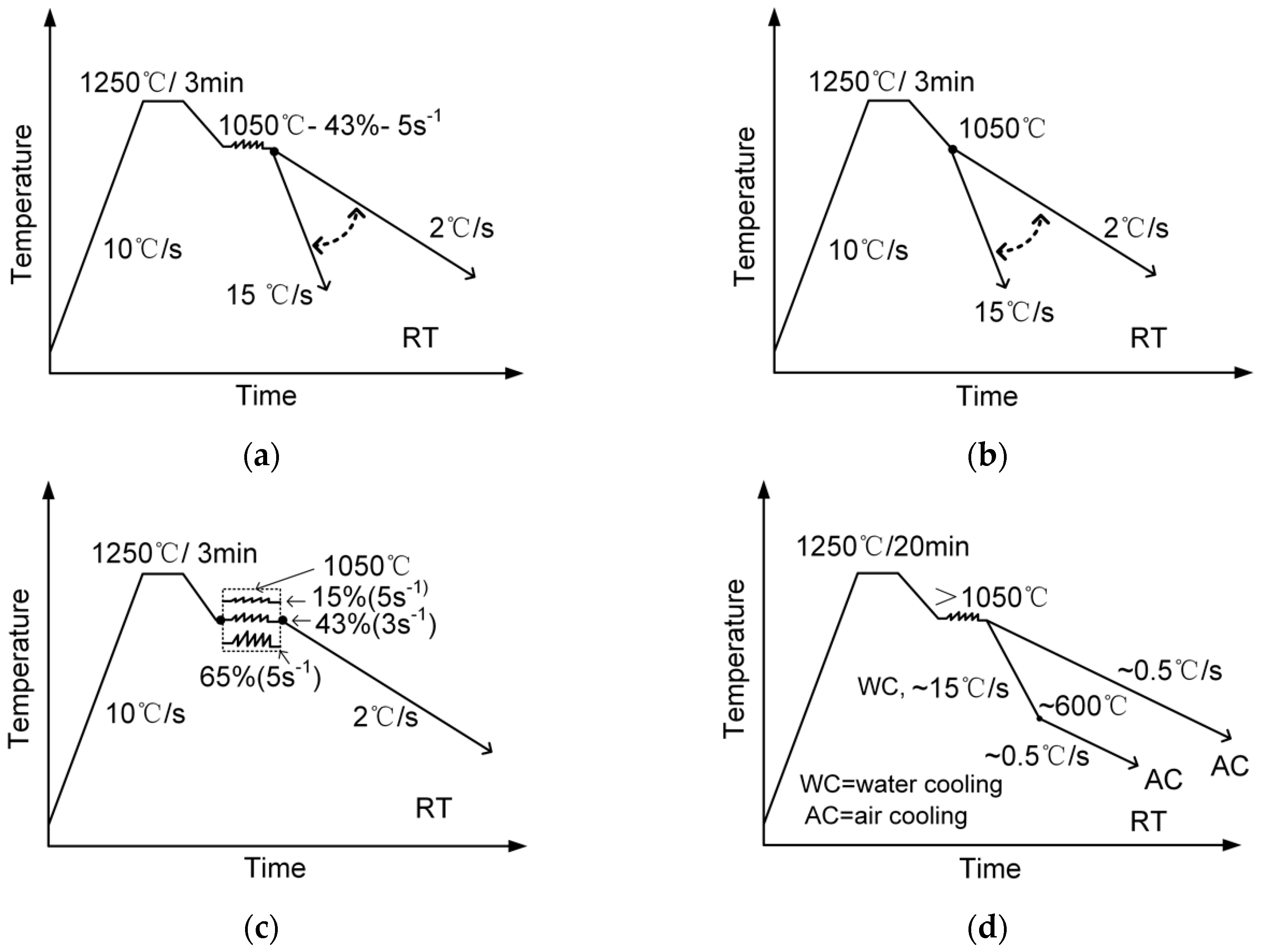

| Steel | C | Si | Mn | Al | Ti | Ca | Nb | N | S | Fe | Ae3 (°C) | Tnr (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC# | 0.07 | 0.06 | 1.5 | 0.01 | 0.01 | 0.001 | - | 0.003 | 0.006 | Bal. | 831 | 911 |
| AC# | 0.07 | 0.05 | 1.4 | 0.035 | - | 0.001 | 0.038 | 0.002 | 0.005 | Bal. | 839 | 1034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, X.; Kang, J.; Yuan, G.; Wang, G. Effect of Thermomechanical Treatment on Acicular Ferrite Formation in Ti–Ca Deoxidized Low Carbon Steel. Metals 2019, 9, 296. https://doi.org/10.3390/met9030296
Wang C, Wang X, Kang J, Yuan G, Wang G. Effect of Thermomechanical Treatment on Acicular Ferrite Formation in Ti–Ca Deoxidized Low Carbon Steel. Metals. 2019; 9(3):296. https://doi.org/10.3390/met9030296
Chicago/Turabian StyleWang, Chao, Xin Wang, Jian Kang, Guo Yuan, and Guodong Wang. 2019. "Effect of Thermomechanical Treatment on Acicular Ferrite Formation in Ti–Ca Deoxidized Low Carbon Steel" Metals 9, no. 3: 296. https://doi.org/10.3390/met9030296
APA StyleWang, C., Wang, X., Kang, J., Yuan, G., & Wang, G. (2019). Effect of Thermomechanical Treatment on Acicular Ferrite Formation in Ti–Ca Deoxidized Low Carbon Steel. Metals, 9(3), 296. https://doi.org/10.3390/met9030296




