Application of Ion Exchange for Preparation of Selected Metal Perrhenates—Precursors for Superalloy Production
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. General Methodology
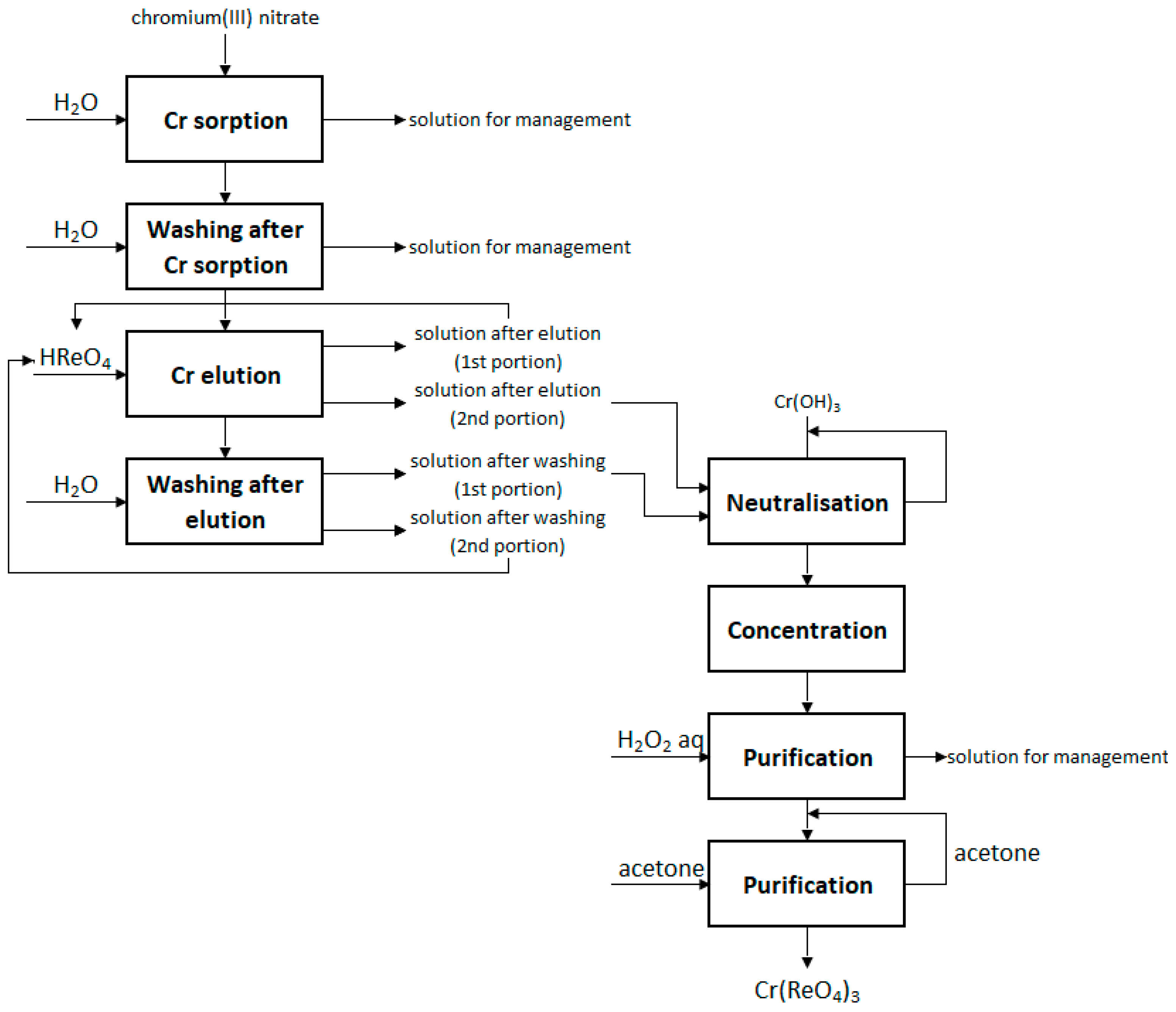
2.3. Preparation of Anhydrous Chromium(III) Perrhenate and Its Mixtures
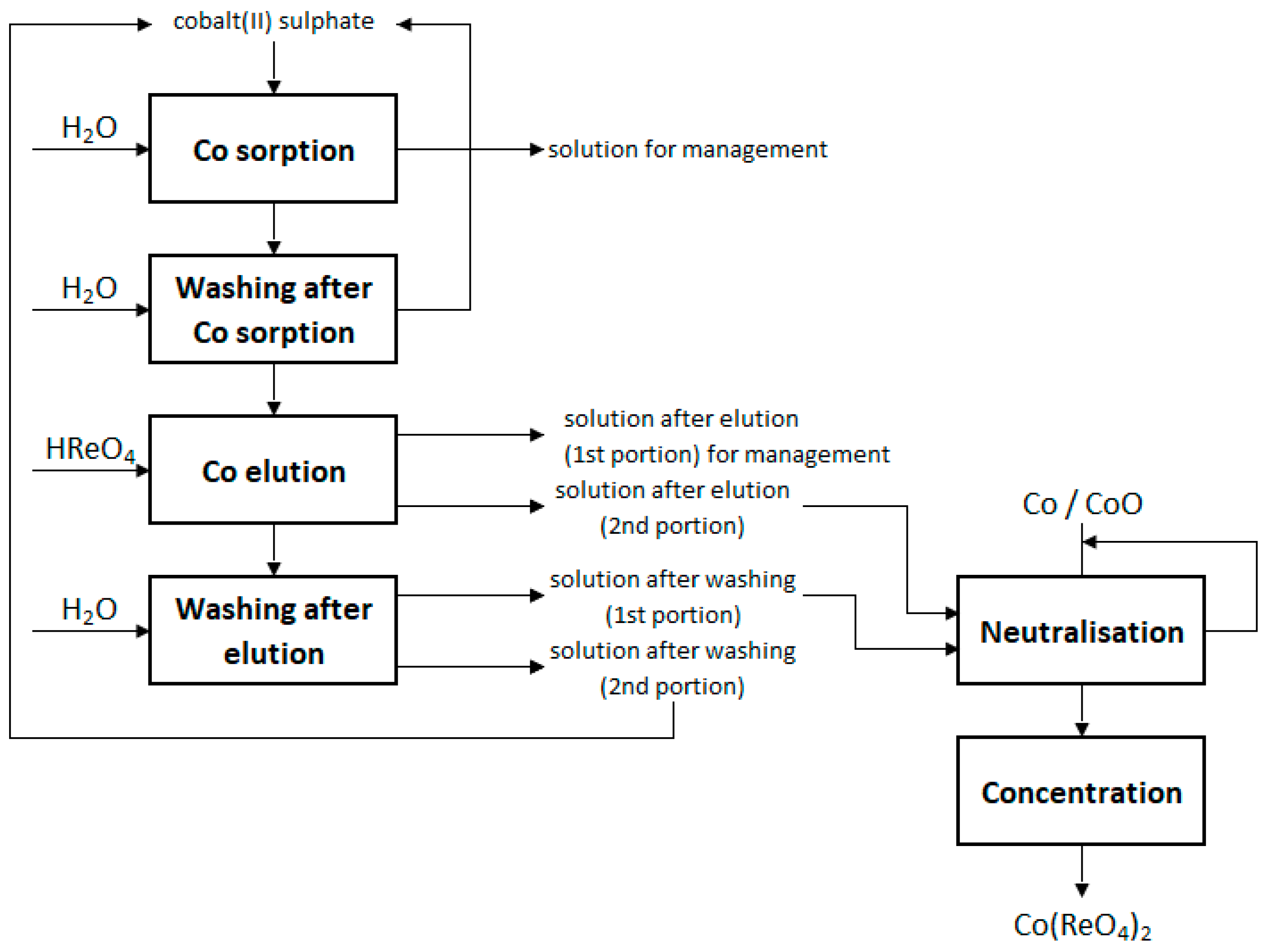
2.4. Preparation of Anhydrous Cobalt(II) Perrhenate and Its Mixtures
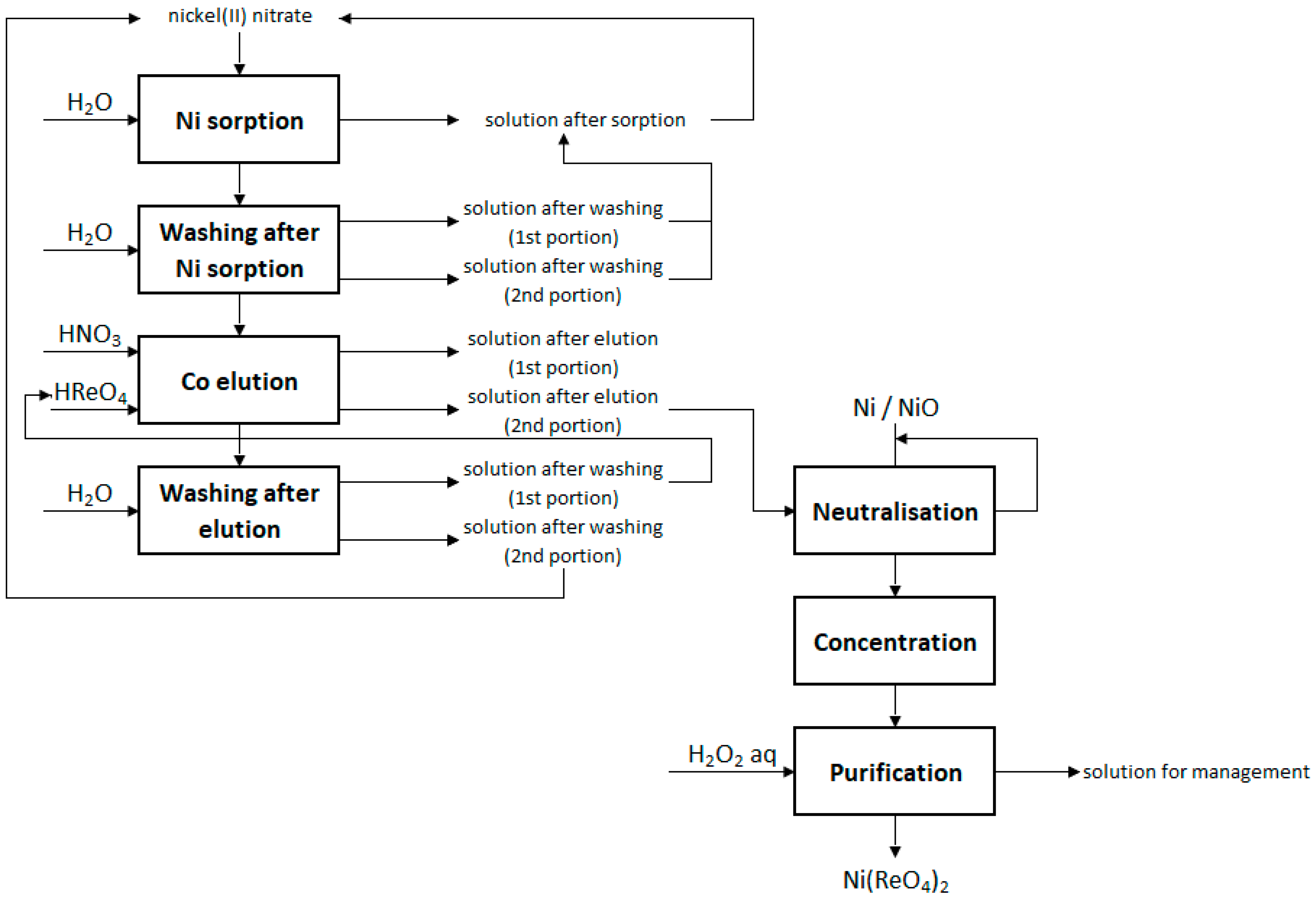
2.5. Preparation of Anhydrous Nickel(II) Perrhenate and Its Mixtures
2.6. Analytical Methods
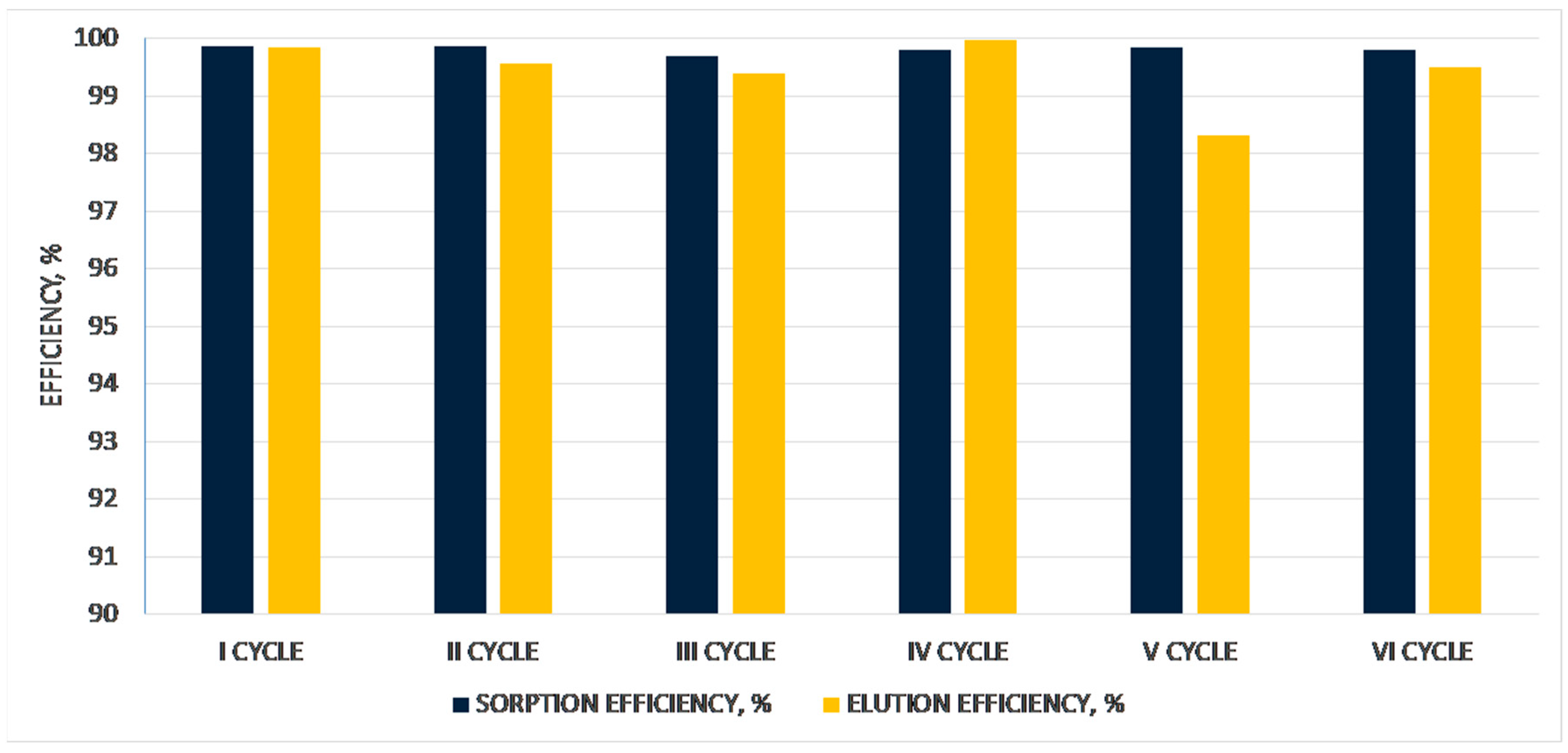
3. Results and Discussion
3.1. Preparation of Anhydrous Chromium(III) Perrhenate and Its Mixtures
3.2. Preparation of Anhydrous Cobalt(II) Perrhenate and Its Mixtures
3.3. Preparation of Anhydrous Nickel(II) Perrhenate and Its Mixtures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leddicotte, G.W. The Radiochemistry of Rhenium; Subcomitee on Radiochemistry National Academy of Sciences—Natinal Research Council: Washington, DC, USA, 1961. [Google Scholar]
- Colton, R. The Chemistry of Rhenium and Technetium; Interscience Publishers: London, UK, 1965; pp. 5–25. [Google Scholar]
- Roskil. Rhenium: Market Outlook to 2020; Roskill Information Services: London, UK, 2010; p. 51. [Google Scholar]
- Benke, G.; Anyszkiewicz, K.; Hac, D.; Litwinionek, K.; Leszczyńska-Sejda, K. Rozwój technologii odzysku renu z półproduktów przemysłu miedziowego. Przem. Chem. 2006, 85, 793–797. [Google Scholar]
- German, R.M.; Bose, A.; Jerman, G. Rhenium alloying of tungsten heavy alloys. Int. J. Powder Metall. 1989, 21, 9–12. [Google Scholar]
- Savickij, E.M.; Burhanov, G.S. Redkie metally i splavy: Fiziko-himicheskij analiz i metallovedenie; Nauka: Moscow, Russia, 1980; pp. 1–20. [Google Scholar]
- Hurd, L.C. The Determination of Rhenium. Ind. Eng. Chem. Anal. Ed. 1936, 8, 112–114. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Majewski, T.; Benke, G.; Piętaszewski, J.; Anyszkiewicz, K.; Michałowski, J.; Chmielarz, A. Production of high-purity ammonium perhenate for W-Re-Ni-Fe heavy alloys. J. Alloys Compd. 2012. [Google Scholar] [CrossRef]
- Majewski, T.; Leszczyńska-Sejda, K. Investigation of Application Possibilities of Re-Ni Alloy Powder to Tungsten Heavy Alloys Production. Solid State Phenom. 2016, 251, 14–19. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Krompiec, S.; Michalik, S.; Krompiec, M. Synthesis of perrhenic acid using ion exchange method. Hydrometallurgy 2007, 89, 289–296. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K. Zastosowanie jonitów do sorpcji jonów amonowych z wodnych roztworów renianu(VII) amonu. Przem. Chem. 2008, 87, 289–295. (In Polish) [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K. Opracowanie metod otrzymywania kwasu nadrenowego z nadrenianu amonu. Przem. Chem. 2006, 85, 847. (In Polish) [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Malarz, J.; Ciszewski, M.; Drzazga, M.; Malarz, J.; Machelska, G.; Witman, K.; Krompiec, S.; Zych, D.; et al. Sposób Wytwarzania Bezwodnego Renianu(VII) Chromu(III). Polish Patent PL228983, 16 December 2016. (In Polish). [Google Scholar]
- Satora, W.; Kozub, K.; Gambal, P.; Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K.; Chmielarz, A.; Machelska, G.; Witman, K.; Kurylak, W.; et al. Sposób Produkcji Bezwodnego Renianu(VII) Kobaltu(II). Polish Patent PL223067, 18 April 2013. (In Polish). [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Anyszkiewicz, K.; Satora, W.; Kozub, K. Hydrometallurgical Methods for Production of Ni(ReO4)2 and Co(ReO4)2. In Proceedings of the European Metallurgical Conference EMC 2013, Weimar, Germany, 23–26 June 2016; Eicke, S., Hahn, M., Eds.; GDMB: Clausthal-Zellerfeld, Germany, 2013; pp. 885–898. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A. Hydrometallurgical methods for production of nickel(II) and cobalt(II) perrhenates. World Metal. Erzmetal. 2013, 66, 5–11. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Kopyto, D.; Majewski, T.; Drzazga, M. Production of High-Purity Anhydrous Nickel(II) Perrhenate for Tungsten-Based Sintered Heavy Alloy. Materials 2017, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Satora, W.; Kozub, K.; Gambal, P.; Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K.; Chmielarz, A.; Machelska, G.; Witman, K.; Kurylak, W.; et al. Sposób Produkcji Bezwodnego Renianu(VII) Niklu(II). Polish Patent PL223066, 18 April 2013. (In Polish). [Google Scholar]
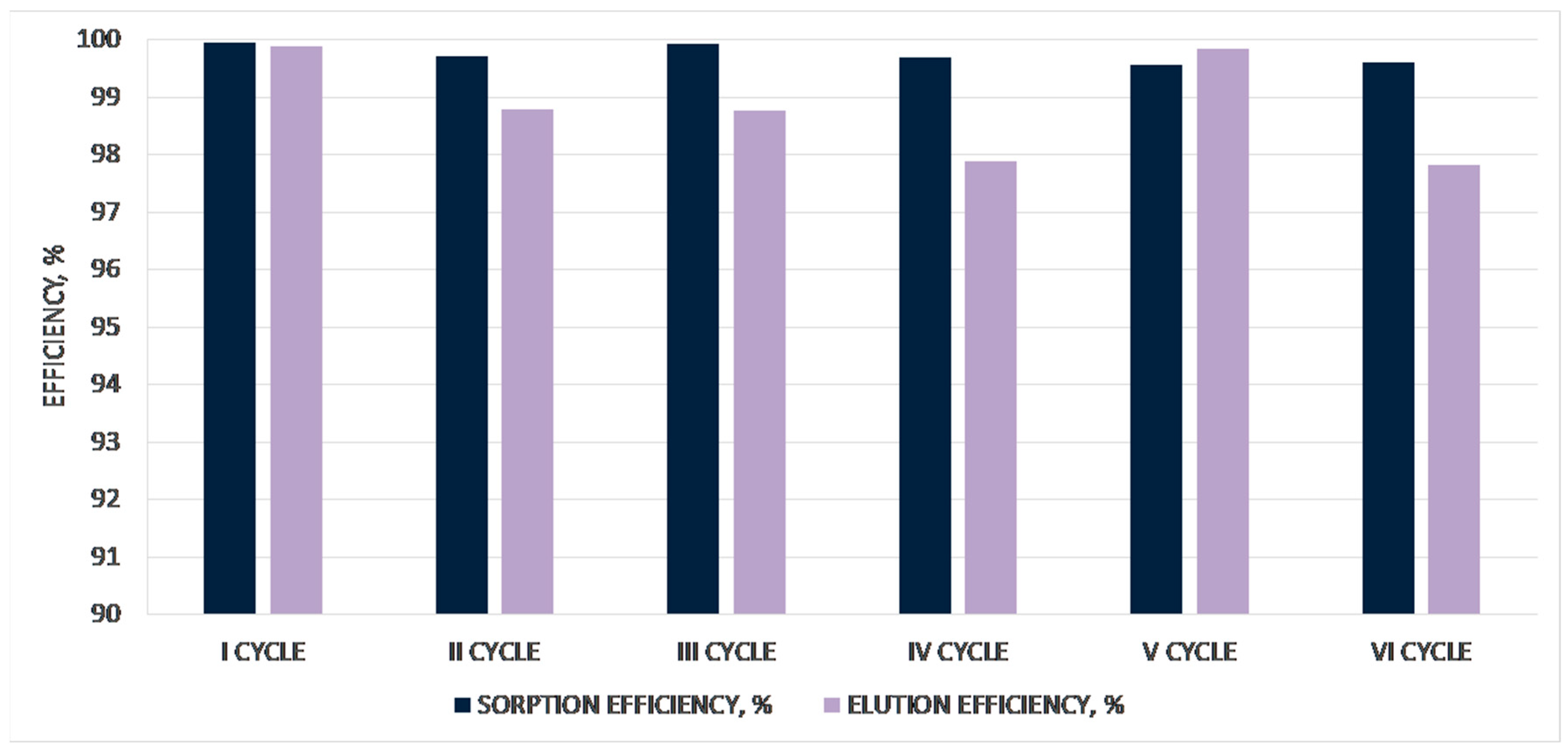
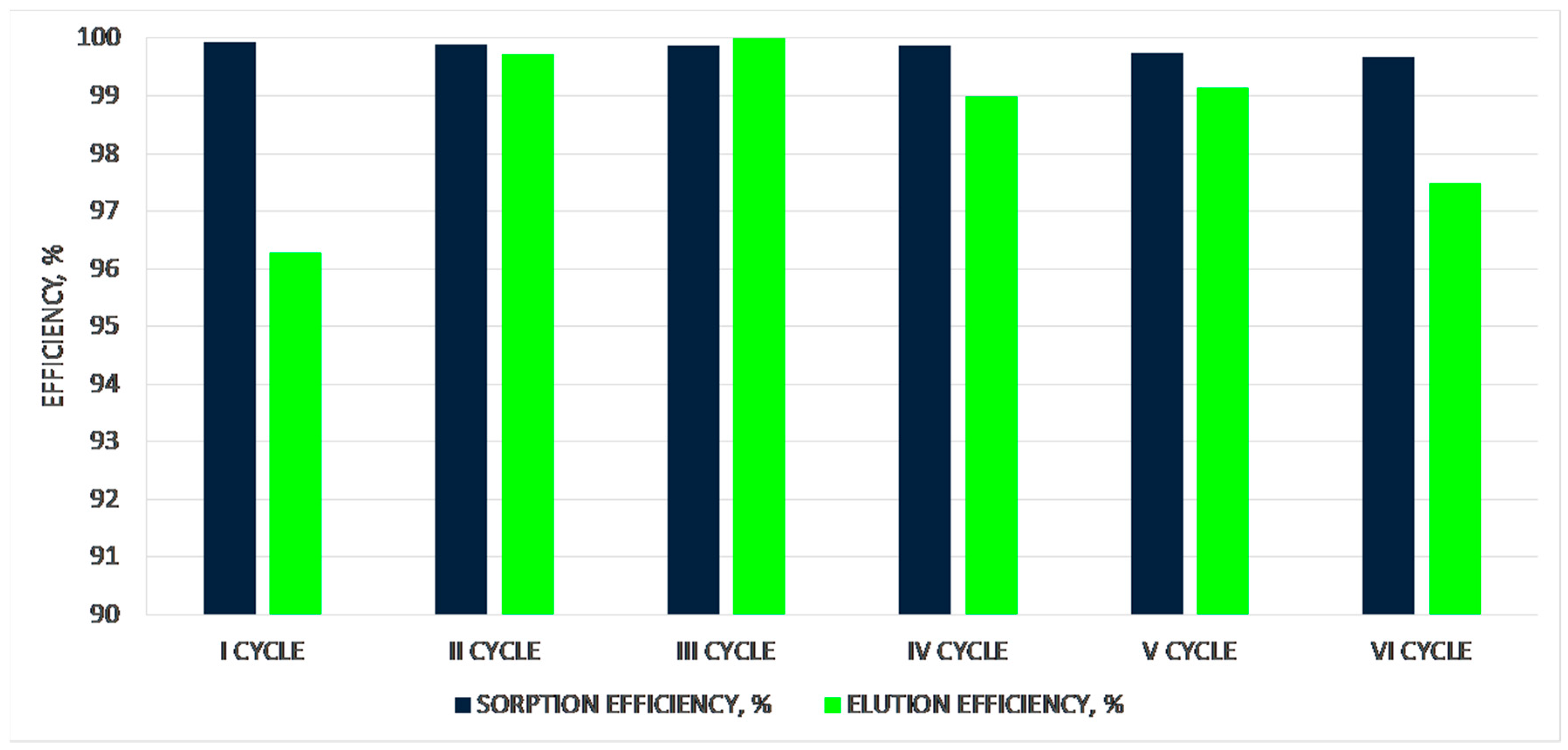
| 1st CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Cr Concentration, g/dm3 | Re Concentration, g/dm3 | Cr Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 15.0 | 2.000 | - | 30.000 | - | - |
| washing water | 2.50 | - | - | - | - | - | |
| solution after sorption | 15.70 | 0.002 | - | 0.031 | - | 99.87 | |
| washings | 1.80 | 0.005 | - | 0.009 | - | ||
| ELUTION | ionite | - | - | - | 29.960 | - | - |
| eluent | 2.00 | - | 600.00 | - | 1200.000 | - | |
| washing water | 3.00 | - | - | - | - | - | |
| elution (1st part) | 0.70 | 0.33 | 1.58 | 0.230 | 1.110 | - | |
| elution (2nd part) | 2.40 | 11.10 | 475.15 | 26.640 | 1140.360 | 99.84 | |
| washings (1st part) | 1.10 | 2.40 | 50.10 | 2.640 | 55.110 | ||
| washings (2nd part) | 0.800 | 0.50 | 4.16 | 0.400 | 3.330 | - | |
| 2nd CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Cr Concentration, g/dm3 | Re Concentration, g/dm3 | Cr Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 15.00 | 2.000 | - | 30.000 | - | - |
| washing water | 2.50 | - | - | - | - | - | |
| solution after sorption | 15.60 | 0.002 | - | 0.031 | - | 99.86 | |
| washings | 1.90 | 0.006 | - | 0.011 | - | ||
| ELUTION | ionite | - | - | - | 30.006 * | - | - |
| eluent | 3.00 | 0.21 | 451.48 | 0.631 | 1354.434 | - | |
| washing water | 3.00 | - | - | - | - | - | |
| elution (1st part) | 0.75 | 0.35 | 1.60 | 0.263 | 1.200 | - | |
| elution (2nd part) | 3.00 | 9.00 | 431.00 | 27.000 | 1293.000 | 99.56 | |
| washings (1st part) | 1.00 | 2.30 | 54.80 | 2.300 | 54.800 | ||
| washings (2nd part) | 1.25 | 0.25 | 4.16 | 0.313 | 5.205 | - | |
| 3rd CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Cr Concentration, g/dm3 | Re Concentration, g/dm3 | Cr Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 15.00 | 2.000 | - | 30.000 | - | - |
| washing water | 2.50 | - | - | - | - | - | |
| solution after sorption | 15.500 | 0.005 | - | 0.078 | - | 99.69 | |
| washings | 2.000 | 0.008 | - | 0.016 | - | ||
| ELUTION | ionite | - | - | - | 30.038 * | - | - |
| eluent | 3.20 | 0.18 | 362.13 | 0.575 | 1158.816 | - | |
| washing water | 2.50 | - | - | - | - | - | |
| elution (1st part) | 0.80 | 0.35 | 1.70 | 0.280 | 1.360 | ||
| elution (2nd part) | 3.10 | 8.71 | 355.00 | 27.001 | 1100.500 | 99.39 | |
| washings (1st part) | 1.10 | 2.20 | 48.10 | 2.420 | 52.910 | ||
| washings (2nd part) | 0.70 | 0.22 | 4.50 | 0.154 | 3.150 | - | |
| 4th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Cr Concentration, g/dm3 | Re Concentration, g/dm3 | Cr Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 15.00 | 3.000 | - | 45.000 | - | - |
| washing water | 2.50 | - | - | - | - | - | |
| solution after sorption | 15.80 | 0.005 | - | 0.079 | - | 99.79 | |
| washings | 1.70 | 0.010 | - | 0.017 | - | ||
| ELUTION | ionite | - | - | - | 45.086 * | - | - |
| eluent | 3.00 | 0.14 | 451.50 | 0.434 | 1354.510 | - | |
| washing water | 2.50 | - | - | - | - | - | |
| elution (1st part) | 0.80 | 0.320 | 1.60 | 0.256 | 1.280 | - | |
| elution (2nd part) | 2.90 | 14.500 | 451.00 | 42.050 | 1307.900 | 99.97 | |
| washings (1st part) | 1.00 | 2.500 | 40.00 | 2.500 | 40.000 | ||
| washings (2nd part) | 0.80 | 0.330 | 5.20 | 0.264 | 4.160 | - | |
| 5th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Cr Concentration, g/dm3 | Re Concentration, g/dm3 | Cr Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 15.00 | 3.000 | - | 45.000 | - | - |
| washing water | 2.50 | - | - | - | - | - | |
| solution after sorption | 15.90 | 0.004 | - | 0.064 | - | 99.83 | |
| washings | 1.60 | 0.008 | - | 0.013 | - | ||
| ELUTION | ionite | - | - | - | 44.940 * | - | - |
| eluent | 3.10 | 0.168 | 437.24 | 0.520 | 1355.440 | - | |
| washing water | 2.50 | - | - | - | - | - | |
| elution (1st part) | 0.90 | 0.320 | 1.70 | 0.288 | 1.530 | - | |
| elution (2nd part) | 2.80 | 14.600 | 465.50 | 40.880 | 1303.400 | 98.31 | |
| washings (1st part) | 1.10 | 2.500 | 41.00 | 2.750 | 45.100 | ||
| washings (2nd part) | 0.80 | 0.330 | 5.40 | 0.264 | 4.320 | - | |
| 6th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Cr Concentration, g/dm3 | Re Concentration, g/dm3 | Cr Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 15.00 | 3.000 | - | 45.000 | - | - |
| washing water | 2.50 | - | - | - | - | ||
| solution after sorption | 15.80 | 0.005 | - | 0.079 | - | 99.79 | |
| washings | 1.70 | 0.010 | - | 0.017 | - | ||
| ELUTION | ionite | 45.662 * | |||||
| eluent | 3.20 | 0.173 | 423.70 | 0.552 | 1355.850 | - | |
| washing water | 2.50 | - | - | - | - | - | |
| elution (1st part) | 0.75 | 0.330 | 1.70 | 0.248 | 1.275 | - | |
| elution (2nd part) | 2.90 | 14.400 | 445.00 | 41.760 | 1290.500 | 99.50 | |
| washings (1st part) | 1.25 | 2.500 | 47.00 | 3.125 | 58.750 | ||
| washings (2nd part) | 0.80 | 0.300 | 5.40 | 0.240 | 4.320 | - | |
| Material | Re % | Cr % | Ni | NH4+ ppm | NO3- ppm | Co ppm | Mg ppm | Ca ppm | Na ppm | Zn ppm | Mo ppm | K ppm | Pb ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr(ReO4)3 | 69.58 | 6.48 | <10ppm | <10 | <15 | <5 | <2 | <3 | <10 | <3 | <5 | <10 | <5 |
| mixture | 71.07 | 3.87 | 2,28% | <10 | <15 | <5 | <2 | <3 | <10 | <3 | <5 | <10 | <5 |
| 1st CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Co Concentration, g/dm3 | Re Concentration, g/dm3 | Co Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 12.00 | 5.000 | - | 60.000 | - | - |
| washing water | 3.00 | - | - | - | - | ||
| solution after sorption | 12.50 | 0.001 | - | 0.013 | - | 99.95 | |
| washings | 2.50 | 0.007 | - | 0.018 | - | ||
| ELUTION | ionite | - | - | - | 59.970 | - | - |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 3.50 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 0.200 | 0.03 | 0.100 | 0.015 | - | |
| elution (2nd part) | 2.00 | 26.400 | 475.15 | 52.800 | 950.300 | 99.88 | |
| washings (1st part) | 1.00 | 6.000 | 42.10 | 6.000 | 42.100 | ||
| washings (2nd part) | 2.00 | 0.500 | 0.20 | 1.000 | 0.400 | - | |
| 2nd CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Co Concentration, g/dm3 | Re Concentration, g/dm3 | Co Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 12.00 | 5.000 | 0.033 | 60.000 | 0.400 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 12.40 | 0.003 | 0.032 | 0.037 | 0.400 | 99.72 | |
| washings | 2.60 | 0.050 | <0.001 | 0.130 | - | ||
| ELUTION | ionite | - | - | - | 59.903 * | - | - |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 3.20 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 0.190 | 0.03 | 0.095 | 0.015 | ||
| elution (2nd part) | 2.10 | 25.200 | 456.0 | 52.920 | 957.600 | 98.79 | |
| washings (1st part) | 0.90 | 6.000 | 42.50 | 5.400 | 38.250 | ||
| washings (2nd part) | 1.70 | 0.450 | 0.18 | 0.765 | 0.306 | ||
| 3rd CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Co Concentration, g/dm3 | Re Concentration, g/dm3 | Co Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 12.00 | 5.000 | 0.026 | 60.000 | 0.306 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 12.40 | 0.002 | 0.025 | 0.025 | 0.306 | 99.93 | |
| washings | 2.60 | 0.006 | <0.001 | 0.016 | - | ||
| ELUTION | ionite | - | - | - | 60.682 * | - | - |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 3.10 | - | - | - | - | - | |
| elution (1st part) | 0.40 | 0.180 | 0.03 | 0.072 | 0.012 | ||
| elution (2nd part) | 2.20 | 24.200 | 431.50 | 53.240 | 949.300 | 98.77 | |
| washings (1st part) | 1.00 | 5.800 | 45.00 | 5.800 | 45.000 | ||
| washings (2nd part) | 1.50 | 0.550 | 0.18 | 0.825 | 0.270 | ||
| 4th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Co Concentration, g/dm3 | Re Concentration, g/dm3 | Co Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 12.00 | 5.000 | 0.015 | 60.000 | 0.180 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 12.10 | 0.004 | - | 0.048 | - | 99.68 | |
| washings | 2.90 | 0.050 | <0.001 | 0.145 | - | ||
| ELUTION | ionite | - | - | - | 60.552 * | - | - |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 3.10 | - | - | - | - | - | |
| elution (1st part) | 0.60 | 0.18 | 0.005 | 0.108 | 0.003 | - | |
| elution (2nd part) | 2.10 | 25.40 | 456.00 | 53.340 | 957.600 | 97.98 | |
| washings (1st part) | 0.90 | 5.60 | 45.00 | 5.040 | 40.500 | ||
| washings (2nd part) | 1.50 | 0.56 | 0.200 | 0.840 | 0.300 | - | |
| 5th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Co Concentration, g/dm3 | Re Concentration, g/dm3 | Co Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 12.00 | 5.000 | 0.025 | 60.000 | 0.300 | - |
| washing water | 3.00 | - | - | 0.000 | - | - | |
| solution after sorption | 12.20 | 0.005 | - | 0.061 | - | 99.57 | |
| washings | 2.80 | 0.070 | <0.001 | 0.196 | - | ||
| ELUTION | ionite | - | - | - | 60.967 * | - | |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 3.10 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 0.20 | 0.52 | 0.100 | 0.260 | - | |
| elution (2nd part) | 2.20 | 25.40 | 436.00 | 55.880 | 959.200 | 99.85 | |
| washings (1st part) | 0.80 | 5.00 | 44.00 | 4.000 | 35.200 | ||
| washings (2nd part) | 1.60 | 0.56 | 0.23 | 0.896 | 0.368 | - | |
| 6th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Co Concentration, g/dm3 | Re Concentration, g/dm3 | Co Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 12.00 | 5.000 | 0.031 | 60.000 | 0.368 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 12.00 | 0.005 | - | 0.060 | - | 99.60 | |
| washings | 3.00 | 0.060 | <0.001 | 0.180 | - | ||
| ELUTION | ionite | - | - | - | 59.851 * | - | - |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 3.10 | - | - | - | - | - | |
| elution (1st part) | 0.40 | 0.11 | 0.60 | 0.044 | 0.240 | - | |
| elution (2nd part) | 2.10 | 25.40 | 456.00 | 53.340 | 957.600 | 97.82 | |
| washings (1st part) | 0.90 | 4.60 | 44.00 | 4.140 | 39.600 | ||
| washings (2nd part) | 1.70 | 0.60 | 0.22 | 1.020 | 0.374 | - | |
| Material/Neutralizing Agent | Re % | Co % | Ni | NH4+ ppm | Cr ppm | Mg ppm | Ca ppm | Na ppm | Zn ppm | Mo ppm | K ppm | Pb ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co(ReO4)2/CoO | 66.55 | 10.55 | 20ppm | <10 | <5 | 5 | 4 | 11 | <3 | <5 | 11 | <5 |
| Co(ReO4)2/Comet | 66.55 | 10.55 | <10ppm | <10 | <5 | <2 | <3 | <10 | <3 | <5 | <10 | <5 |
| mixture/NiO | 66.57 | 3.92 | 6.60% | <10 | <5 | <2 | <3 | <10 | <3 | <5 | <10 | <5 |
| 1st CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Ni Concentration, g/dm3 | Re Concentration, g/dm3 | Ni Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 14.00 | 5.000 | - | 70.000 | - | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 14.10 | 0.002 | - | 0.028 | - | 99.93 | |
| washings | 2.90 | 0.008 | - | 0.023 | - | ||
| ELUTION | ionite | - | - | - | 69.949 | - | - |
| eluent | 2.00 | - | 500.00 | - | 1000.000 | - | |
| washing water | 2.00 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 1.200 | 6.00 | 0.600 | 3.000 | ||
| elution (2nd part) | 2.00 | 32.000 | 482.00 | 64.000 | 964.000 | 96.29 | |
| washings (1st part) | 0.50 | 4.000 | 47.00 | 2.000 | 23.500 | ||
| washings (2nd part) | 1.50 | 0.500 | 0.20 | 0.750 | 0.300 | ||
| 2nd CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Opertation | Materials | Solution Volume, dm3 | Ni Concentration, g/dm3 | Re Concentration, g/dm3 | Ni Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 14.00 | 5.000 | 0.021 | 70.000 | 0.300 | - |
| washing water | 3.00 | - | - | 0.000 | - | - | |
| solution after sorption | 14.20 | 0.002 | - | 0.028 | - | 99.89 | |
| washings | 2.80 | 0.009 | - | 0.007 | - | ||
| ELUTION | ionite | - | - | - | 72.563 * | - | - |
| eluent | 2.00 | 1.300 | 463.25 | 2.600 | 926.500 | - | |
| washing water | 3.20 | - | - | - | - | - | |
| elution (1st part) | 0.30 | 0.26 | 1.20 | 0.078 | 0.360 | ||
| elution (2nd part) | 2.10 | 30.10 | 450.00 | 63.210 | 945.000 | 99.73 | |
| washings (1st part) | 0.90 | 6.20 | 162.00 | 5.580 | 145.800 | ||
| washings (2nd part) | 2.25 | 0.55 | 5.00 | 1.238 | 11.250 | ||
| 3rd CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Ni Concentration, g/dm3 | Re Concentration, g/dm3 | Ni Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 14.00 | 5.000 | 0.023 | 70.000 | 0.324 | - |
| washing water | 3.00 | - | - | - | 0.000 | - | |
| solution after sorption | 12.40 | 0.004 | 0.026 | 0.050 | 0.324 | 99.88 | |
| washings | 4.60 | 0.008 | <0.001 | 0.037 | - | ||
| ELUTION | ionite | - | - | - | 70.111 * | - | - |
| eluent | 2.45 | 1.76 | 452.25 | 4.312 | 1108.013 | - | |
| washing water | 3.10 | - | - | - | - | - | |
| elution (1st part) | 0.30 | 0.26 | 0.10 | 0.078 | 0.030 | ||
| elution (2nd part) | 2.10 | 30.10 | 501.00 | 63.210 | 1052.100 | 99.99 | |
| washings (1st part) | 0.90 | 6.20 | 54.00 | 5.580 | 48.600 | ||
| washings (2nd part) | 2.25 | 0.55 | 0.80 | 1.238 | 1.800 | ||
| 4th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Ni Concentration, g/dm3 | Re Concentration, g/dm3 | Ni Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 14.00 | 5.000 | 0.057 | 70.000 | 0.800 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 14.50 | 0.004 | - | 0.062 | - | 99.88 | |
| washings | 2.50 | 0.009 | <0.001 | 0.023 | - | ||
| ELUTION | ionite | - | - | - | 69.920 * | - | - |
| eluent | 2.00 | 2.572 | 431.20 | 5.144 | 862.391 | - | |
| washing water | 2.50 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 0.20 | 0.60 | 0.100 | 0.300 | - | |
| elution (2nd part) | 2.00 | 32.0 | 452.00 | 64.000 | 904.000 | 99.00 | |
| washings (1st part) | 1.00 | 4.600 | 40.00 | 4.600 | 40.000 | ||
| washings (2nd part) | 1.00 | 0.52 | 0.20 | 0.520 | 0.200 | - | |
| 5th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Ni Concentration, g/dm3 | Re Concentration, g/dm3 | Ni Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 14.00 | 5.000 | 0.014 | 70.000 | 0.200 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 13.90 | 0.010 | - | 0.139 | - | 99.74 | |
| washings | 3.10 | 0.015 | <0.001 | 0.047 | - | ||
| ELUTION | ionite | - | - | - | 70.515 * | - | - |
| eluent | 3.00 | 1.567 | 463.43 | 4.700 | 1390.300 | - | |
| washing water | 2.00 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 0.23 | 1.2 | 0.115 | 0.600 | ||
| elution (2nd part) | 2.75 | 24.00 | 463.000 | 66.000 | 1273.250 | 99.15 | |
| washings (1st part) | 0.80 | 4.0 | 136.00 | 3.200 | 108.800 | ||
| washings (2nd part) | 0.95 | 0.630 | 1.10 | 0.599 | 1.045 | ||
| 6th CYCLE | |||||||
|---|---|---|---|---|---|---|---|
| Operation | Materials | Solution Volume, dm3 | Ni Concentration, g/dm3 | Re Concentration, g/dm3 | Ni Mass, g | Re Mass, g | Process Efficiency, % |
| SORPTION | solution for sorption | 14.00 | 5.000 | 0.075 | 70.000 | 1.045 | - |
| washing water | 3.00 | - | - | - | - | - | |
| solution after sorption | 15.00 | 0.006 | - | 0.090 | - | 99.67 | |
| washings | 2.00 | 0.070 | <0.001 | 0.140 | - | ||
| ELUTION | ionite | - | - | - | 70.371 * | - | - |
| eluent | 2.30 | 1.441 | 438.87 | 3.315 | 1009.400 | - | |
| washing water | 3.10 | - | - | - | - | - | |
| elution (1st part) | 0.50 | 0.11 | 0.60 | 0.055 | 0.300 | ||
| elution (2nd part) | 2.50 | 25.40 | 395.00 | 63.500 | 987.500 | 97.48 | |
| washings (1st part) | 0.90 | 4.60 | 21.00 | 4.140 | 18.900 | ||
| washings (2nd part) | 1.50 | 0.60 | 0.22 | 0.900 | 0.330 | ||
| Material/Neutralizing Agent | Re % | Ni % | Co | NH4+ ppm | NO3- ppm | Cr ppm | Mg ppm | Ca ppm | Na ppm | Zn ppm | Mo ppm | K ppm | Pb ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni(ReO4)2/NiO | 66.58 | 10.51 | <10 ppm | <10 | <15 | <5 | <2 | 3 | 11 | <3 | <5 | <10 | <5 |
| NiReO4)2/Nimet | 66.57 | 10.51 | <10 ppm | <10 | <15 | <5 | <2 | <3 | <10 | <3 | <5 | <10 | <5 |
| mixture/CoO | 66.55 | 3.78 | 6.78% | <10 | <15 | <5 | <2 | <3 | <10 | <3 | <5 | <10 | <5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leszczyńska-Sejda, K.; Benke, G.; Kopyto, D.; Drzazga, M.; Ciszewski, M. Application of Ion Exchange for Preparation of Selected Metal Perrhenates—Precursors for Superalloy Production. Metals 2019, 9, 201. https://doi.org/10.3390/met9020201
Leszczyńska-Sejda K, Benke G, Kopyto D, Drzazga M, Ciszewski M. Application of Ion Exchange for Preparation of Selected Metal Perrhenates—Precursors for Superalloy Production. Metals. 2019; 9(2):201. https://doi.org/10.3390/met9020201
Chicago/Turabian StyleLeszczyńska-Sejda, Katarzyna, Grzegorz Benke, Dorota Kopyto, Michał Drzazga, and Mateusz Ciszewski. 2019. "Application of Ion Exchange for Preparation of Selected Metal Perrhenates—Precursors for Superalloy Production" Metals 9, no. 2: 201. https://doi.org/10.3390/met9020201
APA StyleLeszczyńska-Sejda, K., Benke, G., Kopyto, D., Drzazga, M., & Ciszewski, M. (2019). Application of Ion Exchange for Preparation of Selected Metal Perrhenates—Precursors for Superalloy Production. Metals, 9(2), 201. https://doi.org/10.3390/met9020201





