Abstract
Oxide Dispersion Strengthened (ODS) steel with a composition of Fe-14Cr-2W-0.2V-0.07Ta-1Al-0.3Y2O3 was fabricated by mechanical alloying (MA) and spark plasma sintering (SPS). All of the investigations were performed on the as-SPSed ODS sample without further heat treatment. The microstructure, particles, and tensile properties of the ODS sample were analyzed. According to the results, both submicron-sized and micron-sized grains existed in the microstructure, showing a bimodal structure. The nanoparticles were homogeneously distributed in the matrix, and the nanoparticles in the ODS sample were mainly Y-Al-O composite oxides. Two kinds of Y-Al-O nanoparticles were identified: Y3Al5O12 particles with body-centered cubic structures and Y4Al2O9 particles with monoclinic structures. The size of the nanoparticles ranged from 2 nm to 61.5 nm, and the average size of the nanoparticles was 11 ± 2.7 nm. Except for the nanoparticles, large M23C6 particles that were detrimental to the ductility of the ODS sample were also identified. These large M23C6 particles possessed a long strip or rod-like morphology, and were generally distributed along the grain boundaries. The generation of cracks generally occurred in the region where the large M23C6 particles were located during the tensile test. The as-SPSed ODS steel possessed a ductile/brittle mixed fracture mode according to the fracture surfaces analysis.
1. Introduction
ODS steels have been selected as the most promising candidates for advanced nuclear power plants due to their superior performance over traditional materials at high temperature and their excellent irradiation resistance [1,2,3,4,5,6]. ODS steels are usually fabricated via mechanical alloying (MA) and further consolidation such as hot pressing (HP), hot extrusion (HE), hot isostatic pressing (HIP), or spark plasma sintering (SPS) [7,8,9,10,11,12]. SPS is a relatively new consolidation method that possesses a very fast heating and cooling rate and is characterized by a very short holding time [9]. After sintering, ODS samples strengthened by homogeneous nanoparticles can be obtained. As a strong ferrite stabilizer, V has been commonly used in conventional ferritic steel to increase hardness and refine the microstructure [13]. The addition of V in reduced-activation ferritic/martensite (RAFM) steel also leads to high hardness and strength [14,15]. Based on the above analysis, some researchers have investigated the effect of V on the toughness of ODS steel and have found that an appropriate content of V would obviously reduce the ductile-to-brittle transition temperature (DBTT) [15]. Therefore, V was added to 14Cr ODS steel in order to improve solid solution strength and toughness. The main part of Ta is in a solution state in steels. It is known that Ta has a positive effect on the tensile and Charpy properties of RAFM steel [16,17]. Some researchers have also confirmed that the addition of Ta can effectively decrease the DBTT of 12YWT steel [18]. Ta is thus added to 14Cr ODS steel in order to improve mechanical performances. Alloying elements such as Al, Ti, and Zr are generally added to produce a high-density dispersion of (Y, Al, Ti, Zr)-rich oxides [19,20,21,22,23,24,25,26]. The existence of Al in ODS steel is beneficial to corrosion resistance via forming a protective alumina scale, and Y-Al-O nanoparticles act as strengthening phases, which can effectively improve the mechanical properties of ODS steel [19,21].
A large number of nanoparticles form during the manufacturing process, which is vital for the performance of ODS steels. Simultaneously, contamination is inevitable during the milling process, which leads to the formation of harmful particles with a large size. Ohsuka et al. [27] found serious contamination of oxygen after mechanical alloying, and controlled this phenomenon by improving the purity of argon. He et al. [28] identified elongated Cr carbides and the dissolution behavior of carbides after annealing. Hoelzer et al. [29] investigated the manufacture–mechanical properties relationship of several ODS samples and found that large particles have a detrimental effect on mechanical properties. Most of the research has been performed on as-heat-treated samples, and information about the characterization of as-consolidated ODS steels has been insufficient.
Therefore, the microstructure and mechanical properties of as-SPSed ODS steel without further heat treatment were investigated in this work. Particles with different sizes in the ODS sample were identified as well. A tensile test of the as-SPSed ODS sample was conducted at room temperature, and the fracture mode was analyzed.
2. Experiment
Powders of the ODS steel with a composition of Fe-14Cr-2W-0.2V-0.07Ta-1Al-0.3Y2O3 were obtained by mechanical alloying: 1 wt % Al (purity 99.99 wt %) and 0.3 wt % Y2O3 (purity 99.99 wt %) powders were added to the pre-alloyed powders (fabricated by atomization) with a nominal composition of Fe-14Cr-2W-0.2V-0.07Ta (wt %), and these mixed powders were mechanically alloyed in a planetary ball mill (QM-3SP4, Nanjing NanDa Instrument Plant, Nanjing, China) for 30 h at 400 rpm. The ball-to-powder ratio was 15:1, and the powder mass of each milling was 10 g. All of the handling of powders was conducted under a high-purity argon atmosphere in a glove box. The mechanically alloyed powders were then consolidated by spark plasma sintering (SPS). The powders were first heated to 800 °C for 5 min, and then continuously heated to 1100 °C for a soaking time of 10 min. The pressure applied during sintering was 40 MPa. No further heat treatment was performed.
Characterization of the as-SPSed ODS sample was carried out by optical microscope (OM, Leica DFC 450, Leica, Solms, Germany), scanning electron microscope (SEM, su1510, Hitachi, Tokyo, Japan), and transmission electron microscope (TEM, jem-2100f, JEOL, Tokyo, Japan). The samples for OM and SEM observation were prepared by grinding, polishing, and then etching in a solution composed of 5 g copper chloride, 100 mL hydrochloric acid, and 100 mL ethyl alcohol. Thin slices were sliced from the as-SPSed sample, mechanically thinned, and punched into 3-mm diameter discs to prepare TEM thin foils. The foils were then subjected to a double jet electropolishing device with a solution of 5% perchloric acid and 95% ethanol at −20 °C. The chemical extraction method was used to prepare XRD samples. The matrix was dissolved in Berzelius-type solution [30], except for precipitated particles. After multiple instances of centrifugation and washing, the obtained residues were dried and detected by X-ray diffraction (XRD, D8 Advanced, Bruker, Karlsruhe, Germany) using Cu Kα Radiation. The densities of the ODS samples were measured by electronic density balance based on the Archimedes principle. Ten measurements were performed on each sample. The average value of the tested results was considered to be the density of each sample. The relative density was the ratio of the measured density to the theoretical density. A tensile test was performed using sheet specimens at room temperature with a nominal strain rate of 10−3 s−1. The gauge length of the test sample was 4 mm, with a gauge section of 0.75 × 1.5 mm2. The sample size for the tensile test was the same as in Reference [31]. Three specimens were tested in order to obtain a reliable result. After the tensile test, fracture surfaces and the cross-section of fracture were investigated via SEM observation. The fracture mode was also analyzed.
3. Results and Discussion
3.1. Microstructure
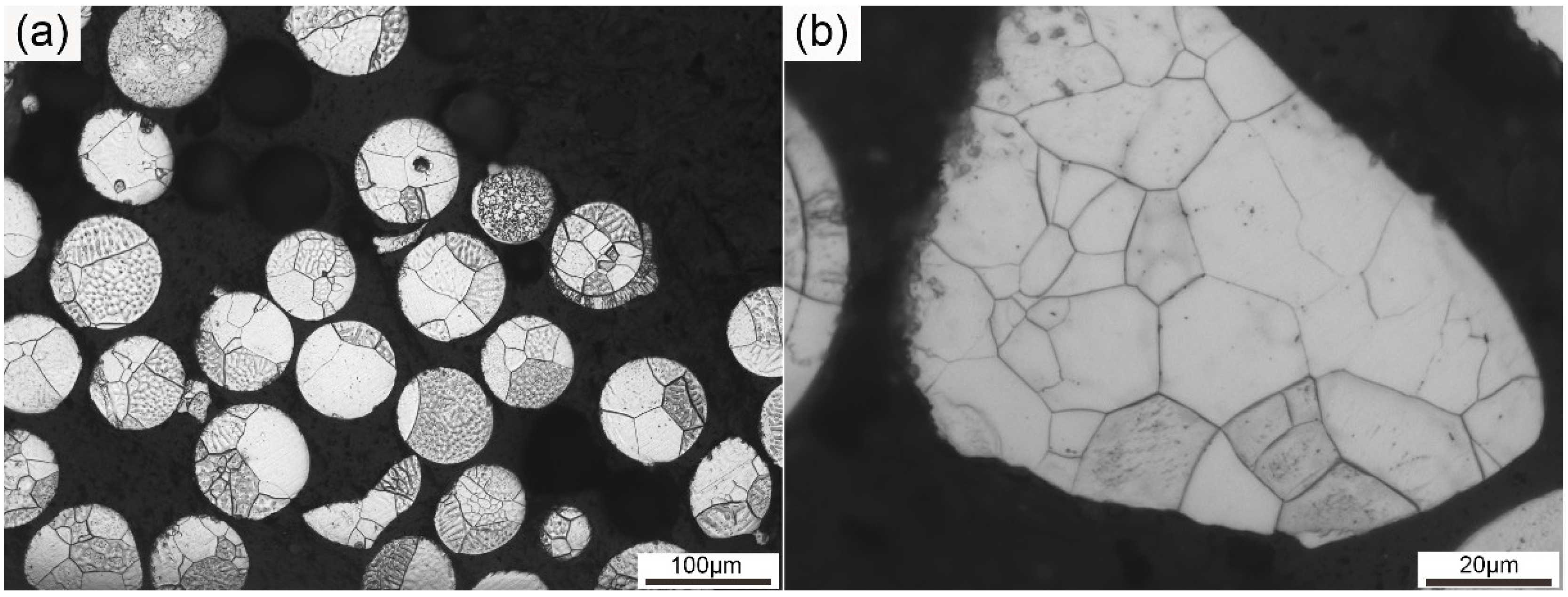
Figure 1 illustrates the OM graphs of the pre-alloyed powders. As can be seen from Figure 1a, the particle size of the pre-alloyed powders was approximately 100 μm. Most of the particles presented a spherical morphology, and particles in an irregular shape can also be seen at the same time. The size and morphology of the particles were basically uniform, though particles of a small size and irregular shape can also be seen. Each particle in Figure 1a possessed several equiaxed grains. The size of the equiaxed grains was heterogeneous. Both large and small grains existed in each particle. Figure 1b gives a magnified image of an irregular particle in the pre-alloyed powders. Equiaxed grains of different sizes can be seen in the particle. The size of the grains ranged from 2 to 20 μm. Grain size disparity leads to different deformation degrees during the mechanical alloying process, which influences recrystallization behavior during consolidation [32]. The grain size and particle size of the pre-alloyed powders played a key role in the final microstructure of the as-SPSed sample.
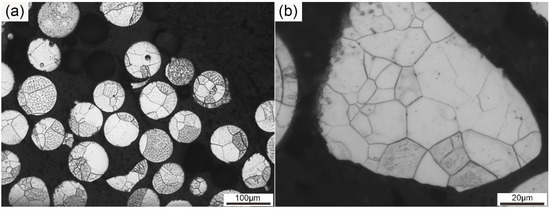
Figure 1.
Optical microscope (OM) graphs of the pre-alloyed powders at (a) low and (b) high magnification.
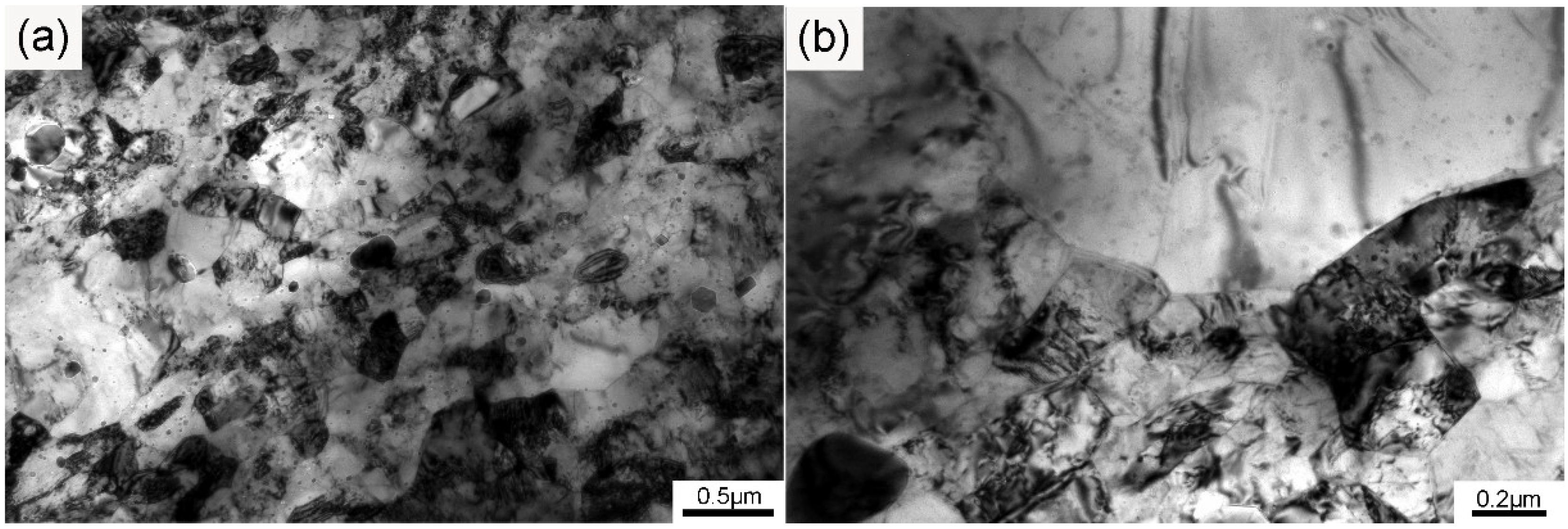
The TEM structure of the ODS sample is shown in Figure 2. As can be seen from the graph obtained at low magnification in Figure 2a, both submicron-sized and micron-sized grains existed in the sample. Besides, there were also some regions in which the grain morphology could not be clearly distinguished because of the large amounts of dislocations and precipitates. The integral grain morphology was not clear, so the typical area of the sample was magnified and is depicted in Figure 2b. Almost all of the grains possessed equiaxed morphology, and a large amount of dislocations existed in the submicron-sized grains. Dislocations could seldom be found in the micron-sized grains, which was different from the submicron-sized grains, but uniformly distributed nanoparticles were obviously present. The dislocations in the as-SPSed samples were introduced during the mechanical alloying and consolidation process. Movement of the dislocations in the submicron-sized grains could be hindered by the nanoparticles in the matrix. The grain boundaries were also regarded as dislocation barriers, thus increasing strain hardening. These phenomena were all beneficial to the high strength of the ODS sample. Grains of different sizes possess different deformation degrees after mechanical alloying, which influences the recrystallization process during the subsequent consolidation procedure [32]. The heterogeneous structure was introduced by pre-alloyed powders and mechanical alloying, and such heterogeneity was preserved by SPS. Therefore, a bimodal microstructure with both large and small grains was obtained. According to the Hall–Petch relationship [2,33], micron-sized grains are ductile because of the lower limit of the mean free path of the dislocations, which leads to more dislocation pile-up at the grain boundary, finally inducing slip. The combination of submicron-sized and micron-sized grains could effectively improve mechanical properties by balancing the strength and ductility of the ODS sample.
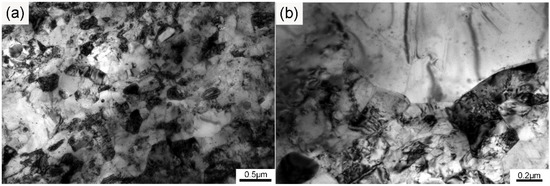
Figure 2.
TEM graphs of the sample at (a) low and (b) high magnification.
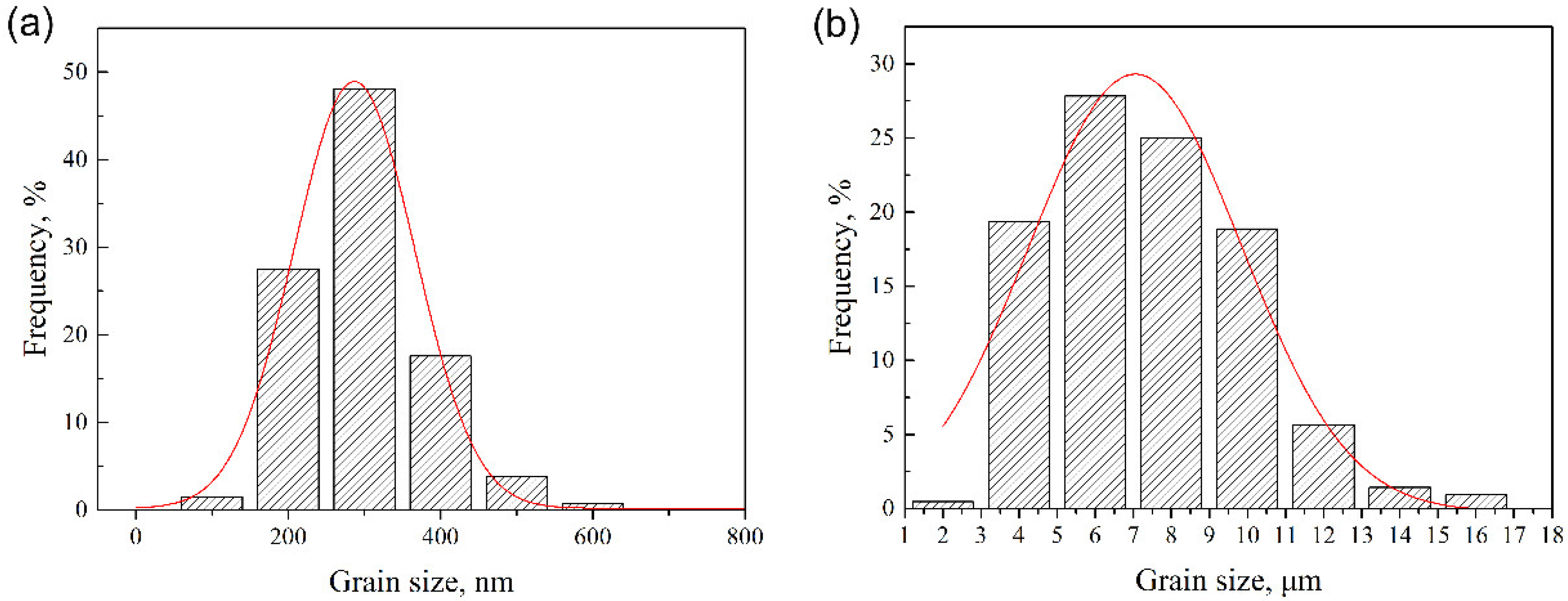
Grain size distribution of the as-SPSed sample was analyzed via TEM and SEM graphs, and the results are shown in Figure 3a,b, respectively. As depicted in Figure 2, both submicron-sized and micron-sized grains existed in the as-SPSed sample. Figure 3a illustrates the distribution result of the submicron-sized grains. The size of the submicron grains ranged from 100 to 600 nm, and mainly concentrated on approximately 300 ± 23 nm. A large number of micron-sized grains also existed in the as-SPSed sample apart from the submicron ones. The size distribution of the micron grains is shown in Figure 3b. The size of the micron grains ranged from 2 to 16 μm. Most of the micron grains concentrated around a size of 4–10 μm. The size distribution results depicted a bimodal character of the grains, which was consistent with the microstructure. A bimodal structure is primarily developed as a strategy for improving the ductility of ultrafine-grained and nanocrystalline metals [34,35]. In bimodal structures, submicron grains provide high strength, and micron grains are beneficial in improving ductility [35]. The combination of submicron and micron grains was an effective way to improve the mechanical performances of the as-SPSed sample.
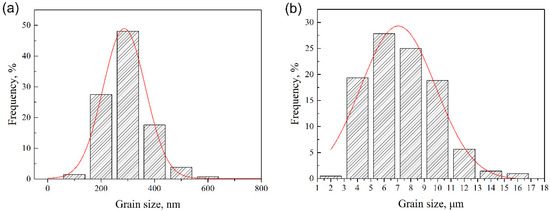
Figure 3.
Size distribution of (a) submicron and (b) micron grains in the as-spark plasma sintered (SPSed) sample.
3.2. Nanoparticles
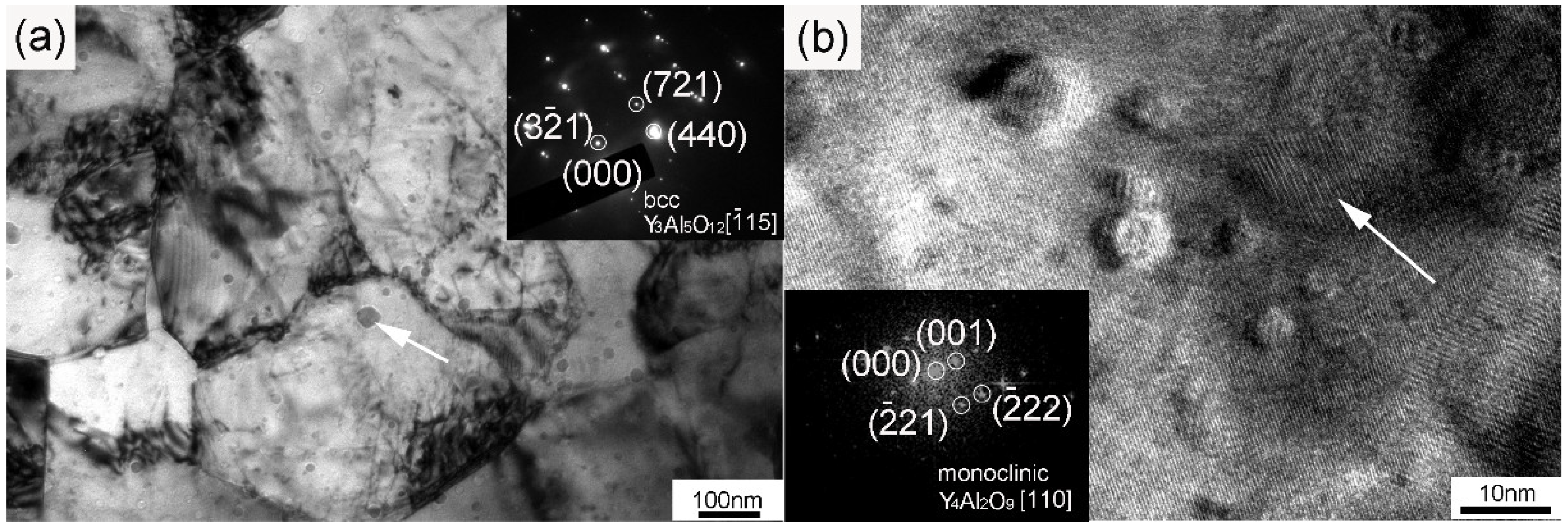
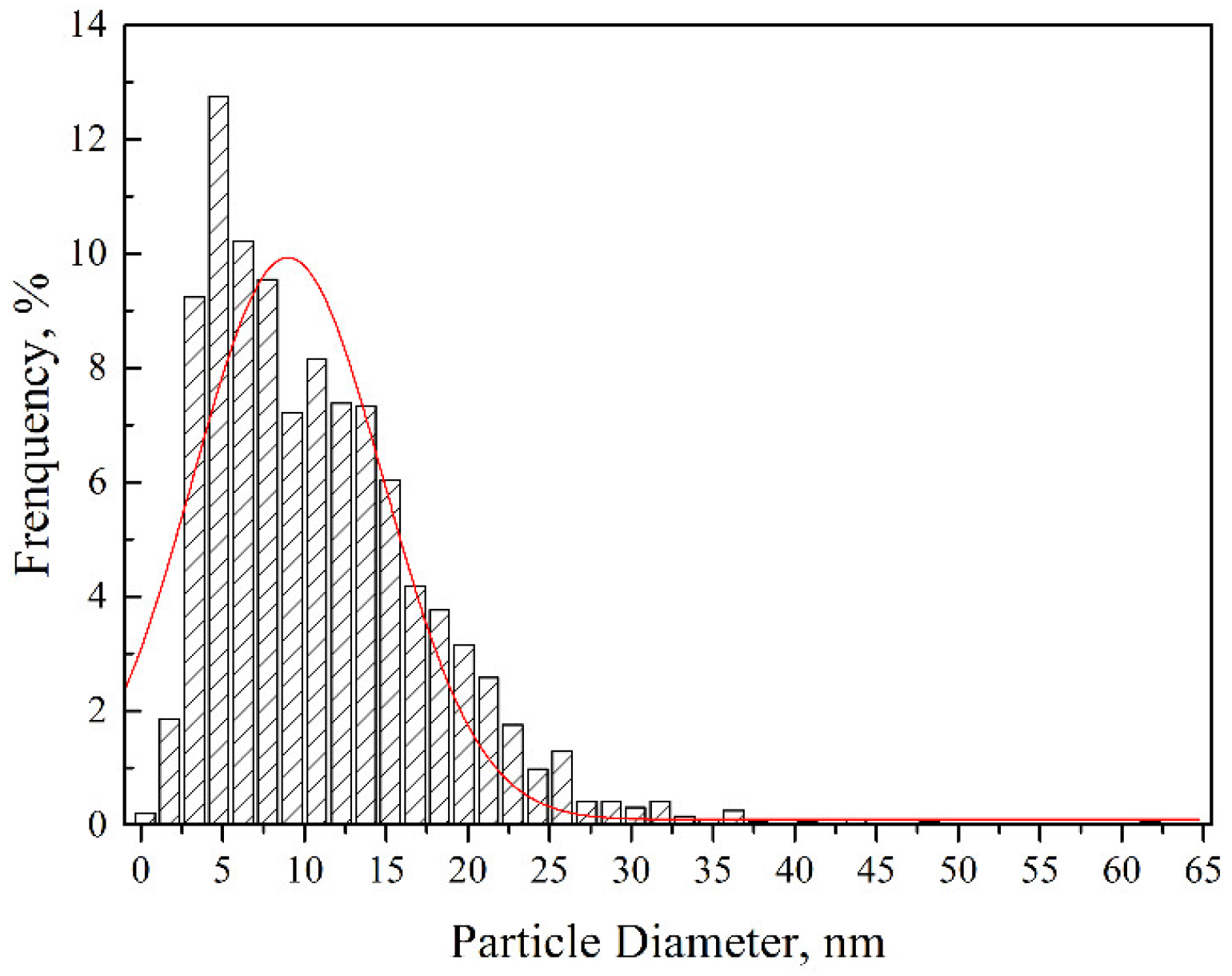
Figure 4 shows the TEM and High Resolution Transmission Electron Microscope (HRTEM) images of the dispersoids and the corresponding fast Fourier transformation (FFT) patterns. As depicted in Figure 4a, nanoparticles with sizes ranging from 10 to 50 nm were uniformly distributed inside the grains and along the grain boundaries. Besides, dislocation tangles distributed along grain boundaries can also be seen. According to the selected area diffraction (SAD) inserted into Figure 4a, the marked particle with a size of approximately 50 nm could be identified as Y3Al5O12 with a body-centered cubic structure. This Y3Al5O12 particle was oriented with a zone axis to the electron beam. Nanoparticles with sizes ranging from 4 to 10 nm could be distinctly seen when we further increased the magnification, which is illustrated in Figure 4b. The nanoparticle marked by an arrow in Figure 4b was determined to be Y4Al2O9 with a zone axis [110] on the basis of the FFT diagram inserted into the bottom left corner. The Y4Al2O9 particles possessed a monoclinic structure with a diameter of around 10 nm. That is to say, the Y3Al5O12 particles with a body-centered cubic structure were larger than the monoclinic Y4Al2O9 particles after sufficient investigations. Both the nanoparticles with diameters of a few tens of nanometers and the ones with sizes less than 10 nm showed near-spherical morphologies. The formation of Y-Al-O nanoparticles was due to the reaction between Y2O3 and Al2O3 [22]. Most of the Al dissolved in the matrix during the milling process, and a part of the Al reacted with oxygen to form Al2O3. The generation of Al2O3 particles was further improved during the consolidation process, which facilitated the reaction between Y2O3 and Al2O3 particles, thus increasing the formation of Y-Al-O nanoparticles [19,21,22]. As strengthening phases, the size distribution of the nanoparticles also played a key role in the mechanical properties of the ODS sample. The histogram in Figure 5 gives the size distribution of the nanoparticles in the ODS sample. It should be mentioned that the statistical result shown in Figure 5 was obtained by counting the particles with sizes less than 100 nm. The number density of these nanoparticles was 7.64 × 1023 m−3. Particles with large sizes were excluded because of the limited pinning effect. The size of the nanoparticles ranged from 2 to 61.5 nm, and the average size of the nanoparticles was 11 ± 2.7 nm. Most of the nanoparticles concentrated around a size ranging from 3 to 20 nm. The uniform spatial and size distribution of the Y-Al-O nanoparticles may be ascribed to an effective milling process.
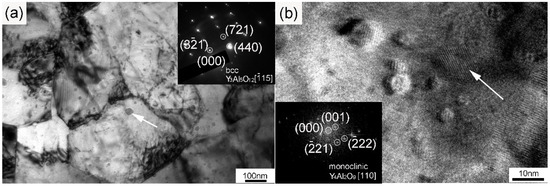
Figure 4.
(a) TEM image of dispersed nanoparticles in the sample with a selected area diffraction (SAD) pattern of a Y3Al5O12 nanoparticle (marked by the arrow); (b) HRTEM image of dispersed nanoparticles in the sample with a fast Fourier transformation (FFT) pattern of a Y4Al2O9 nanoparticle (marked by the arrow).
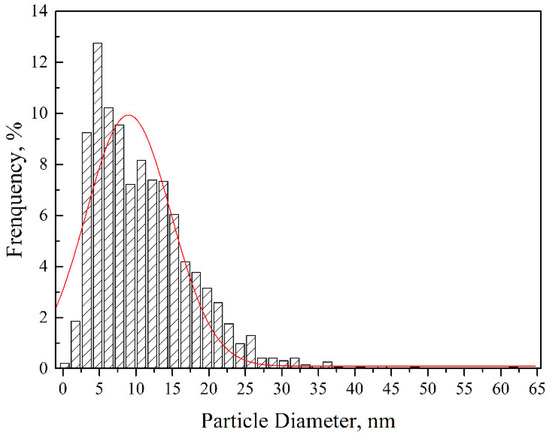
Figure 5.
Size distribution of nanoparticles in the ODS sample.
3.3. M23C6
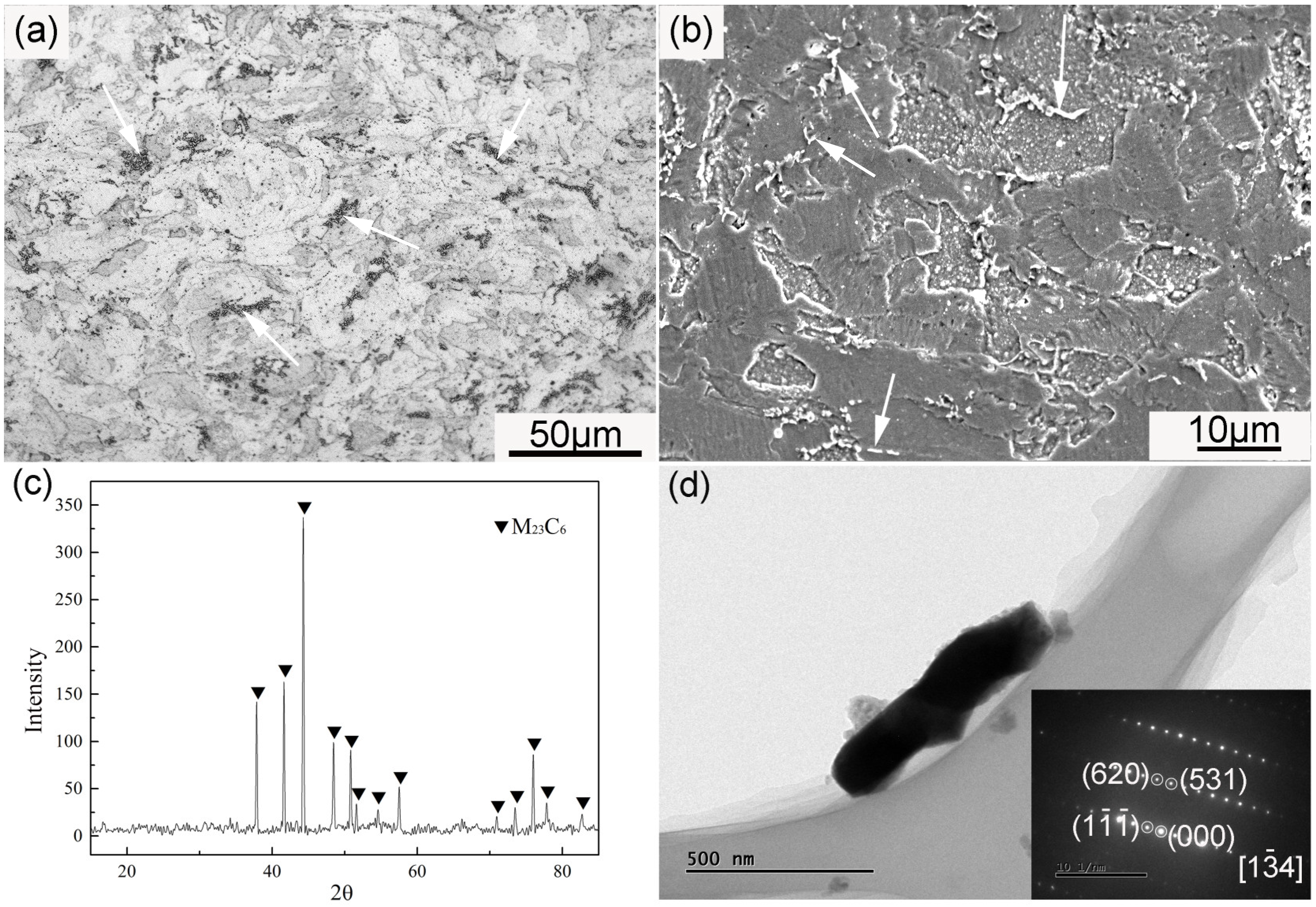
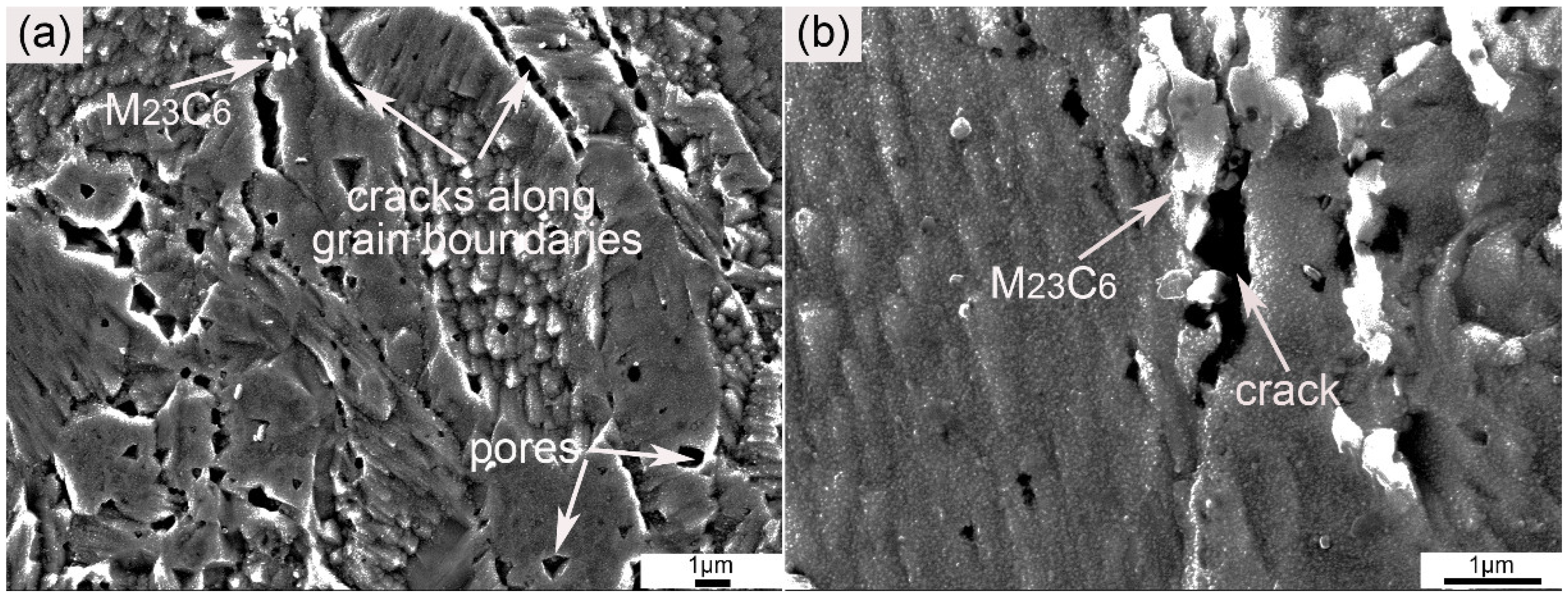
Figure 6 demonstrates the large particles in the ODS sample. The large precipitates arrowed in Figure 6a,b were rich in Cr and C according to EDS spectra (not given here). These large particles possessed a long strip or rod-like morphology, and were generally distributed along the grain boundaries. The average size of these particles was approximately 520 ± 25 nm. In order to characterize the particles in the ODS sample, the chemical extraction method was performed. The matrix dissolved in Berzelius-type solution, with undissolved particles remaining. The XRD pattern of the remained particles is shown in Figure 6c. All of the peaks shown in the XRD results were identified as M23C6, and no peaks of the Y-Al-O nanoparticles were found because of the low content and small size. The rod-like particle in Figure 6d was identified as M23C6 based on the SAD image inserted into Figure 6d. The formation of large amounts of M23C6 resulted from the contamination of carbon during the milling process. The content of carbon was determined by the burning method. The sample was heated and thoroughly burnt in oxygen fluid in a high-frequency induction furnace to obtain CO2, and the infrared absorption method was then used to measure the content of CO2. The carbon content was finally obtained by calculation. The content of carbon in the SPSed sample was 0.058 wt %. The introduction of impurities could not be completely avoided even when all of the handling of powders before mechanical alloying and the milling process were under an argon atmosphere. It has been proven that a longer milling time increases the contamination of impurities [20,36,37], which is ascribed to the wearing of milling balls. Deformation and fracture of powder grains can occur because of the high energy of milling. Such high energy is simultaneously capable of aggravating the abrasive action of milling balls. This phenomenon is consequently detrimental to the purity of the as-milled powders. According to some investigations, reducing the mechanical alloying time and improving the purity of the argon atmosphere are effective ways to reduce carbon contamination [27,28,29,36]. The existence of M23C6 particles could not effectively hinder dislocation motion and grain boundary movement because of their large size, and their presence concurrently decreased ductility. M23C6 particles were the crack source when stain was exerted on the sample.
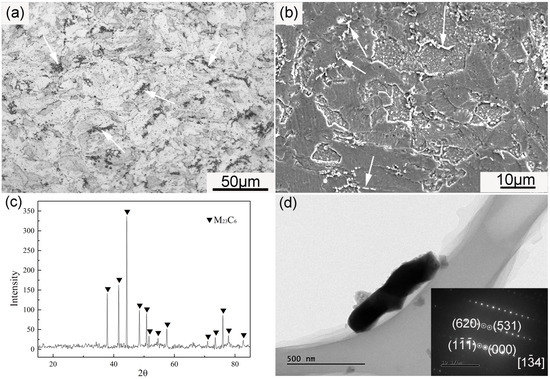
Figure 6.
(a) OM and (b) SEM images of the ODS sample, (c) XRD pattern of the residues extracted from the sample, (d) TEM image of a large extracted particle and the corresponding SAD pattern. Arrows in (a,b) show the distribution of M23C6 particles.
3.4. Tensile Properties
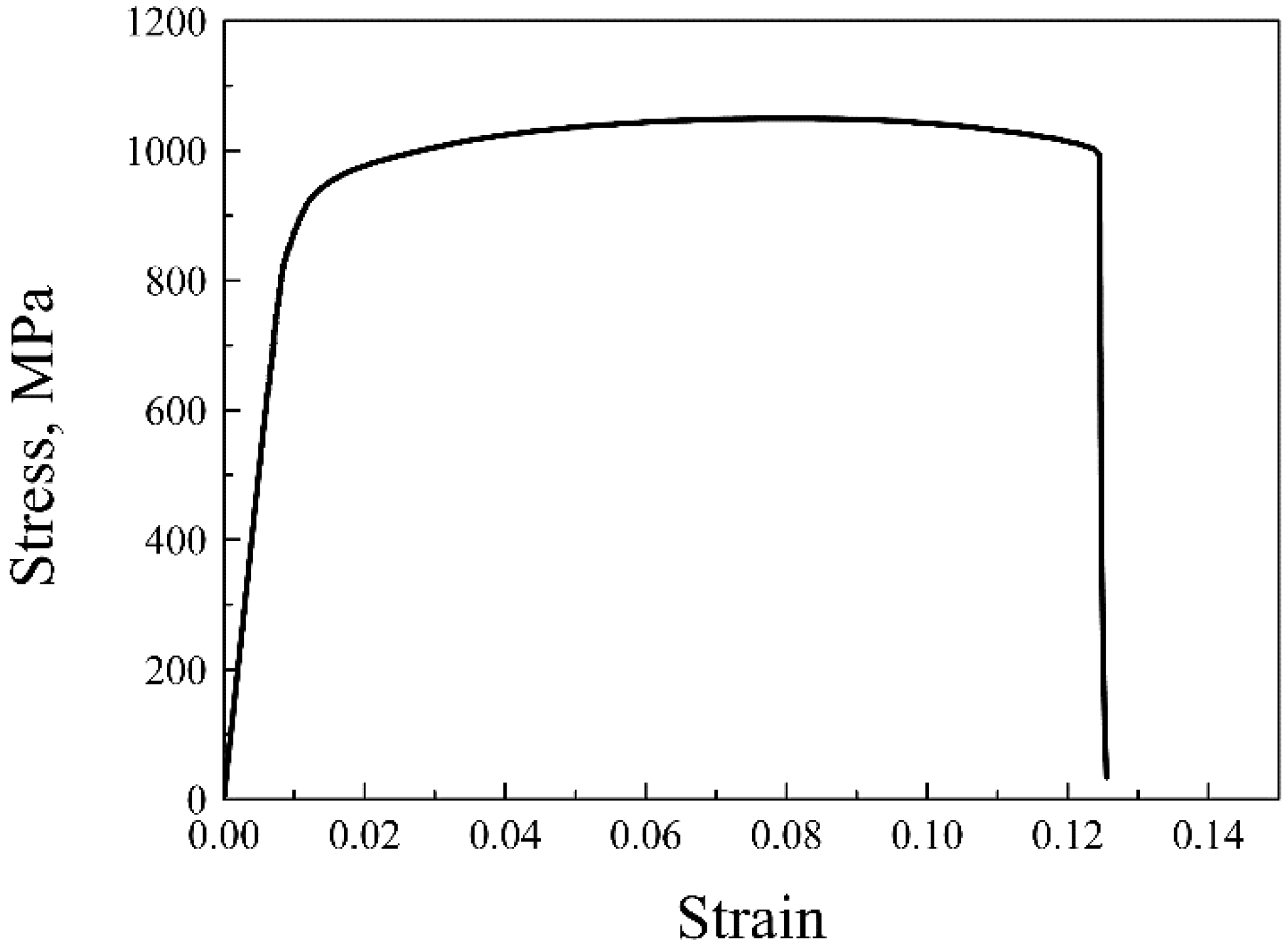
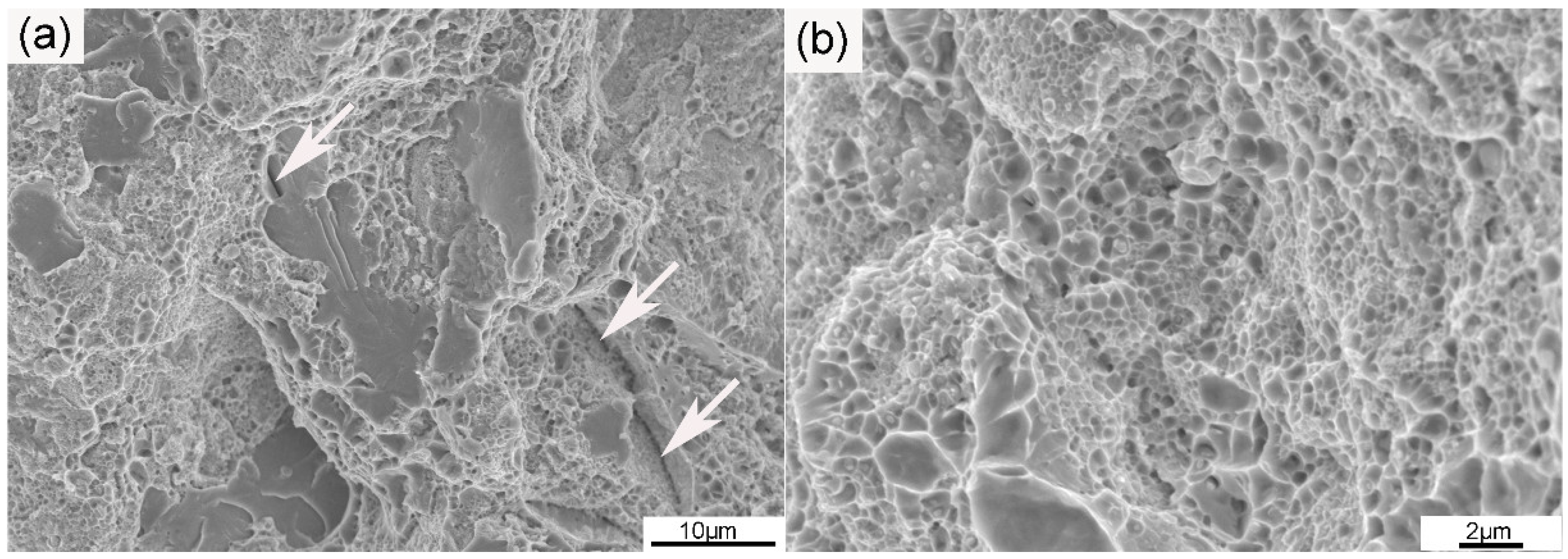
The tensile strain–stress curve of the ODS sample at room temperature is given in Figure 7. The yield and tensile strength of the ODS sample were 824 and 1052 MPa, respectively, and the ODS sample also had a high total elongation of 11.5%. ODS steel containing Al generally possesses lower strength than ODS steel without Al, but the ODS steel in this work was proven to be superior to ODS steels with similar compositions both in strength and ductility [8,9]. The total elongations of several 14Cr ODS steels were added and compared to the SPSed 14Cr ODS steel in this article. Liu et al. [38] fabricated a 14Cr ODS steel with a composition of Fe-14Cr-3Al-2W-0.1Ti-0.35Y2O3 by vacuum hot pressing (VHP) and hot isostatic pressing (HIP). The tensile test results showed that the VHP sample had a total strain of 5.8%, and the HIP sample had a total strain of 10.5%. The content of Al was higher than that of the SPSed sample, which was beneficial for ductility. However, the addition of Ti improved the strength and thus decreased ductility. Therefore, the ductility of the VHP and HIP samples were worse than in the SPSed sample in this work. Li et al. [39] fabricated 18Cr ODS steel with a composition of Fe-17.7Cr-3.84Al-2.13W-0.59Ti-0.05Ta-0.19Y-0.17O by HIP. A large number of Cr-rich phases were also identified in the 18Cr ODS steel. The total elongation of the 18 Cr ODS steel was 13%, which was a little higher than that of the SPSed sample. The higher elongation was due to the higher Al content. There was no obvious drop in ductility when compared to some other ODS steels with similar compositions. Figure 8 shows the fracture surfaces of the sample after tensile testing. As can be seen from the image at low magnification in Figure 8a, the fracture surfaces contained large area fractions of dimples and small area fractions of cleavage. The characterization of dimples is depicted in Figure 8b. The coexistence of cleavages and dimples indicated a ductile/brittle mixed fracture mode in the sample at room temperature. The generation of cracks generally occurred in the region where pores and large particles were located. The density of the SPSed sample was 7.6273 g/cm3 based on the density test results. The relative density of the SPSed sample was 99%, which was relatively high for the consolidated sample. The entrapment of air was unavoidable during the powder metallurgy process. Mechanical alloying was performed in an argon atmosphere, and the entrapment of argon was thus inevitable, which has been confirmed by many researchers [40]. The entrapped argon was then trapped by nanoparticles or accumulated to form pores during the consolidation procedure. The effect of densification was indeed one of the factors that could cause the formation of pores, though was not the major one. The entrapment of argon during mechanical milling leads to the formation of pores, and large M23C6 particles result from the contamination of the as-milled powders [36]. The existence of a large amount of brittle M23C6 particles was extremely detrimental to the ductility of the tensile sample.
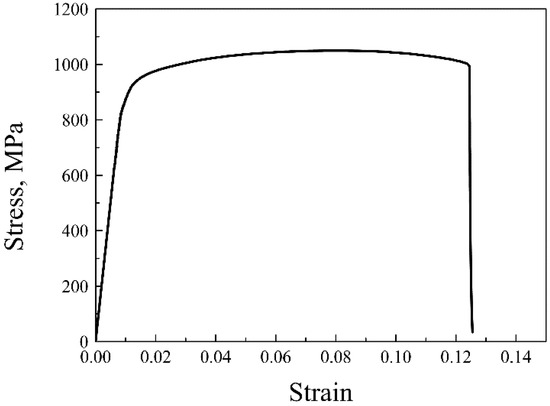
Figure 7.
Tensile strain–stress curve of the 14Cr ODS steel at room temperature.
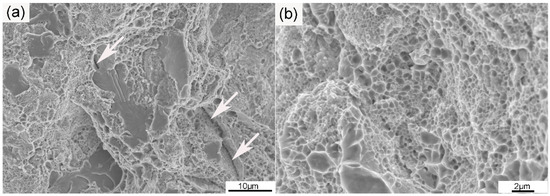
Figure 8.
SEM images of (a) the typical fracture surface at low magnification and (b) the dimple fracture region at high magnification of the ODS sample. Arrows in (a) show the distribution of cracks.
In order to investigate the effect of M23C6 on the tensile properties, the cross-section morphology of the fracture was characterized by SEM, and the results are shown in Figure 9. As shown in Figure 9a, a large amount of cracks originated from the grain boundaries. Some pores in the interior of the grains can also be seen. The generation of pores was discussed above. Pores in the as-SPSed sample were mainly due to the entrapment of argon. The entrapped argon moved to the grain boundaries or was trapped by the nanoparticles during the consolidation process [40]. The rest free air accumulated and formed pores with large sizes during tensile testing. Besides the cracks generated along grain boundaries, cracks originated by M23C6 can also be seen. Such a phenomenon can be clearly distinguished in Figure 9b. The marked crack originated from the region where the M23C6 particles were located. The existence of the M23C6 particles obviously weakened the grain boundaries. Therefore, the cracks tended to originate from the locations where the M23C6 particles were precipitated. Characterization of the cross-section of the fracture was consistent with the discussion above.
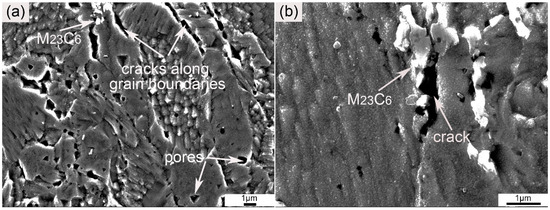
Figure 9.
SEM graphs of cross-section morphology of the fracture at (a) low and (b) high magnification.
Generally speaking, size and size distribution of particles in the matrix were vital to mechanical properties. Y-Al-O nanoparticles were beneficial to mechanical properties, while large particles such as M23C6 were harmful to the ductility of the sample. In order to improve the mechanical properties of the as-SPSed ODS sample, the issue of carbon contamination should be solved.
4. Conclusions
Microstructure, particles, and tensile properties of the as-SPSed ODS sample were investigated in this paper, and the main results can be concluded as follows:
- (1)
- Both submicron-sized and micron-sized grains existed in the microstructure of the as-SPSed ODS sample;
- (2)
- Body-centered cubic Y3Al5O12 particles and monoclinic Y4Al2O9 particles were identified, and these nanoparticles were found homogeneously distributed in the ODS sample;
- (3)
- The existence of large M23C6 particles in the ODS sample was confirmed;
- (4)
- Tensile properties of the as-SPSed ODS sample were analyzed, and the fracture mode was predominantly ductile with regions of brittle fracture.
Author Contributions
Q.Z. and Z.Q. performed the experiments and drafted the paper. Y.L. and H.L. provided the conceptualization. L.Y. collected the experimental data. Y.H. performed the formal analysis. Q.G. provided the methodology and analyzed the experimental results.
Funding
This research was funded by Key Technologies R & D Program of Tianjin (Grant No. 18YFZCGX00050), the National Natural Science Foundation of China (Grant Nos. 51474156 and U1660201), and the National Magnetic Confinement Fusion Energy Research Project (Grant No. 2015GB119000).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klueh, R.L. Elevated temperature ferritic and martensitic steels and their application to future nuclear reactors. Int. Mater. Rev. 2005, 50, 287–310. [Google Scholar] [CrossRef]
- Mao, C.; Liu, C.; Yu, L.; Li, H.; Liu, Y. The correlation among microstructural parameter and dynamic strain aging (DSA) in influencing the mechanical properties of a reduced activated ferritic-martensitic (RAFM) steel. Mater. Sci. Eng. A 2019, 739, 90–98. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhou, X.; Liu, C.; Yu, J.; Huang, Y.; Li, H.; Li, W. Precipitation and hot deformation behavior of austenitic heat-resistant steels: A review. J. Mater. Sci. Technol. 2017, 33, 1448–1456. [Google Scholar] [CrossRef]
- Wharry, J.P.; Swenson, M.J.; Yano, K.H. A review of the irradiation evolution of dispersed oxide nanoparticles in the bcc Fe-Cr system: Current understanding and future directions. J. Nucl. Mater. 2017, 486, 11–20. [Google Scholar] [CrossRef]
- Odette, G.R. On the status and prospects for nanostructured ferritic alloys for nuclear fission and fusion application with emphasis on the underlying science. Scr. Mater. 2018, 143, 142–148. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, L.; Wang, D.; Liu, Y.; Liu, C.; Li, H. The heterogeneous microstructure of heat affect zone and its effect on creep resistance for friction stir joints on 9Cr–1.5W heat resistant steel. Scr. Mater. 2019, 158, 6–10. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nakayama, T.; Suematsu, H.; Suzuki, T.; Nanko, M.; Cho, H.B.; Huynh, M.T.T.; Jiang, W.H.; Niihara, K. Synthesis of molten-metal corrosion resistant yttria-based refractory by hot-pressing and densification. J. Eur. Ceram. Soc. 2015, 35, 2651–2662. [Google Scholar] [CrossRef]
- Hilger, I.; Boulnat, X.; Hoffmann, J.; Testani, C.; Bergner, F.; De Carlan, Y.; Ferraro, F.; Ulbricht, A. Fabrication and characterization of oxide dispersion strengthened (ODS) 14Cr steels consolidated by means of hot isostatic pressing, hot extrusion and spark plasma sintering. J. Nucl. Mater. 2016, 472, 206–214. [Google Scholar] [CrossRef]
- Auger, M.A.; De Castro, V.; Leguey, T.; Muñoz, A.; Pareja, R. Microstructure and mechanical behavior of ODS and non-ODS Fe–14Cr model alloys produced by spark plasma sintering. J. Nucl. Mater. 2013, 436, 68–75. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Yu, L.; Ma, Z.; Guo, Q.; Huang, Y.; Li, H. Microstructure characteristic and mechanical property of transformable 9Cr-ODS steel fabricated by spark plasma sintering. Mater. Des. 2017, 132, 158–169. [Google Scholar] [CrossRef]
- Chauhan, A.; Bergner, F.; Etienne, A.; Aktaa, J.; De Carlan, Y.; Heintze, C.; Litvinov, D.; Hernandez-Mayoral, M.; Oñorbe, E.; Radiguet, B.; et al. Microstructure characterization and strengthening mechanisms of oxide dispersion strengthened (ODS) Fe-9% Cr and Fe-14% Cr extruded bars. J. Nucl. Mater. 2017, 495, 6–19. [Google Scholar] [CrossRef]
- Nagini, M.; Vijay, R.; Rajulapati, K.V.; Reddy, A.V.; Sundararajan, G. Microstructure–mechanical property correlation in oxide dispersion strengthened 18Cr ferritic steel. Mater. Sci. Eng. A 2017, 708, 451–459. [Google Scholar] [CrossRef]
- Abbasi, S.M.; Shokuhfar, A. Improvement of Mechanical Properties of Cr-Ni-Mo-Cu-Ti Stainless Steel with Addition of Vanadium. J. Iron Steel Res. Int. 2007, 14, 74–78. [Google Scholar] [CrossRef]
- Rogozhkin, S.V.; Aleev, A.A.; Zaluzhnyi, A.G.; Nikitin, A.A.; Iskandarov, N.A.; Vladimirov, P.; Lindau, R.; Möslang, A. Atom probe characterization of nano-scaled features in irradiated ODS Eurofer steel. J. Nucl. Mater. 2011, 409, 94–99. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Lewandowska, M.; Kurzydlowski, K.J.; Baluc, N. Effect of vanadium addition on the microstructure and mechanical properties of the ODS ferritic steels. J. Nucl. Mater. 2013, 442, S84–S88. [Google Scholar] [CrossRef]
- Klueh, R.L.; Alexander, D.J.; Sokolov, M.A. Effect of chromium, tungsten, tantalum, and boron on mechanical properties of 5–9Cr–WVTaB steels. J. Nucl. Mater. 2002, 304, 139–152. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Liu, C.; Yan, B.; Li, H. Effects of tantalum on austenitic transformation kinetics of RAFM steel. J. Iron Steel Res. Int. 2017, 24, 705–710. [Google Scholar] [CrossRef]
- Rahmanifard, R.; Farhangi, H.; Novinrooz, A.J. Development of mechanical performance of 12YWT steel nanocomposite by addition of zirconium and tantalum. J. Alloys Compd. 2016, 657, 646–654. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Li, S.F.; Zhou, Z.J.; Wang, P.H.; Sun, H.Y.; Wang, M.; Zhang, G.M. Long-term thermal-aging stability of a 16Cr-oxide dispersion strengthened ferritic steel at 973 K. Mater. Des. 2016, 90, 318–329. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. TEM and HRTEM study of oxide particles in an Al-alloyed high-Cr oxide dispersion strengthened steel with Zr addition. J. Nucl. Mater. 2014, 444, 441–453. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.M.; Liu, Y.C.; Huang, Y.; Guo, Q.Y.; Li, H.J.; Wu, J.F. Evolution of Al-containing phases in ODS steel by hot pressing and annealing. Powder Technol. 2017, 311, 449–455. [Google Scholar] [CrossRef]
- Li, W.; Hao, T.; Gao, R.; Wang, X.; Zhang, T.; Fang, Q.; Liu, C. The effect of Zr, Ti addition on the particle size and microstructure evolution of yttria nanoparticle in ODS steel. Powder Technol. 2017, 319, 172–182. [Google Scholar] [CrossRef]
- Xie, R.; Lu, Z.; Lu, C.; Li, Z.; Ding, X.; Liu, C. Microstructures and mechanical properties of 9Cr oxide dispersion strengthened steel produced by spark plasma sintering. Fusion Eng. Des. 2017, 115, 67–73. [Google Scholar] [CrossRef]
- Mao, X.; Oh, K.H.; Jang, J. Evolution of ultrafine grained microstructure and nano-sized semi-coherent oxide particles in austenitic oxide dispersion strengthened steel. Mater. Charact. 2016, 117, 91–98. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F.; Jiang, S.; Yang, Z. TEM and HRTEM study of oxide particles in an Al-alloyed high-Cr oxide dispersion strengthened ferritic steel with Hf addition. J. Nucl. Mater. 2017, 485, 189–201. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Ukai, S.; Fujiwara, M.; Kaito, T.; Narita, T. Nano-structure control in ODS martensitic steels by means of selecting titanium and oxygen contents. J. Phys. Chem. Solids 2005, 66, 571–575. [Google Scholar] [CrossRef]
- He, P.; Klimenkov, M.; Lindau, R.; Möslang, A. Characterization of precipitates in nano structured 14% Cr ODS alloys for fusion application. J. Nucl. Mater. 2012, 428, 131–138. [Google Scholar] [CrossRef]
- Hoelzer, D.T.; Unocic, K.A.; Sokolov, M.A.; Byun, T.S. Influence of processing on the microstructure and mechanical properties of 14YWT. J. Nucl. Mater. 2016, 471, 251–265. [Google Scholar] [CrossRef]
- Burke, K.E. Chemical extraction of refractory inclusions from iron-and nickel-base alloys. Metallography 1975, 8, 473–488. [Google Scholar] [CrossRef]
- Gao, R.; Xia, L.L.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S. Oxidation resistance in LBE and air and tensile properties of ODS ferritic steels containing Al/Zr elements. J. Nucl. Mater. 2014, 455, 407–411. [Google Scholar] [CrossRef]
- Zhang, Z.; Orlov, D.; Vajpai, S.K.; Tong, B.; Ameyama, K. Importance of bimodal structure topology in the control of mechanical properties of a stainless steel. Adv. Eng. Mater. 2015, 17, 791–795. [Google Scholar] [CrossRef]
- Pande, C.S.; Cooper, K.P. Nanomechanics of Hall–Petch relationship in nanocrystalline materials. Prog. Mater. Sci. 2009, 54, 689–706. [Google Scholar] [CrossRef]
- Ramtani, S.; Dirras, G.; Bui, H.Q. A bimodal bulk ultra-fine-grained nickel: Experimental and micromechanical investigations. Mech. Mater. 2010, 42, 522–536. [Google Scholar] [CrossRef]
- Magee, A.; Ladani, L.; Topping, T.D.; Lavernia, E.J. Effects of tensile test parameters on the mechanical properties of a bimodal Al–Mg alloy. Acta Mater. 2012, 60, 5838–5849. [Google Scholar] [CrossRef]
- Oliera, P.; Couvrat, M.; Cayrond, C.; Lochet, N.; Chaffron, L. Incidence of mechanical alloying contamination on oxides and carbides formation in ODS ferritic steels. J. Nucl. Mater. 2013, 442, s106–s111. [Google Scholar] [CrossRef]
- Nagini, M.; Vijay, R.; Ramakrishna, M.; Reddy, A.V.; Sundararajan, G. Influence of the duration of high energy ball milling on the microstructure and mechanical properties of a 9Cr oxide dispersion strengthened ferritic–martensitic steel. Mater. Sci. Eng. A 2017, 620, 490–499. [Google Scholar] [CrossRef]
- Liu, T.; Shen, H.; Wang, C.; Chou, W. Microstructure and mechanical properties of Al containing ODS ferritic alloys by VHP and HIP. Mater. Res. Innov. 2015, 18, 410–413. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Z.; Li, M.; Wang, M.; Zhang, G. Microstructure characterization and tensile properties of 18Cr–4Al-oxide dispersion strengthened ferritic steel. J. Alloys Compd. 2015, 648, 39–45. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Z.; He, P.; Xu, Y.; Liao, L. Annealing behavior of 12-Cr based mechanical alloyed oxide dispersion strengthened ferritic alloys. J. Nucl. Mater. 2011, 417, 189–192. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).