Direct Macroscopic Modeling of Grain Structure and Macrosegregation with a Cellular Automaton–Finite Element Model
Abstract
:1. Introduction
2. Model Descriptions
2.1. Macroscopic FE Model
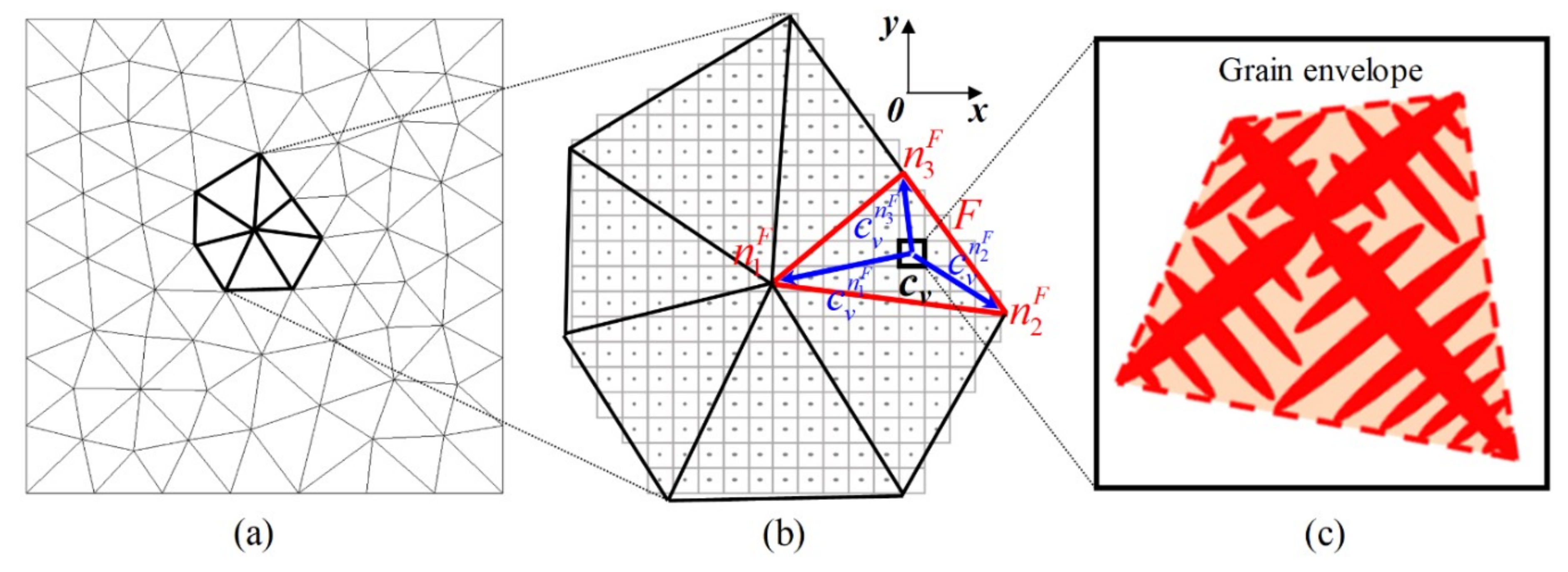
2.2. Microscopic CA Model
2.2.1. Nucleation
- , when cell v is liquid;
- , when cell v is not liquid, but at least one of its nearest neighboring cells, , is still liquid;
- , when cell v is not liquid, and all its nearest neighboring cells, , are not liquid; where is the number of the first and second nearest neighboring cells of cell v, thus .
2.2.2. Growth
2.2.3. Dendrite Tip Growth Kinetics
2.3. Coupling Strategy
3. Results and Discussion
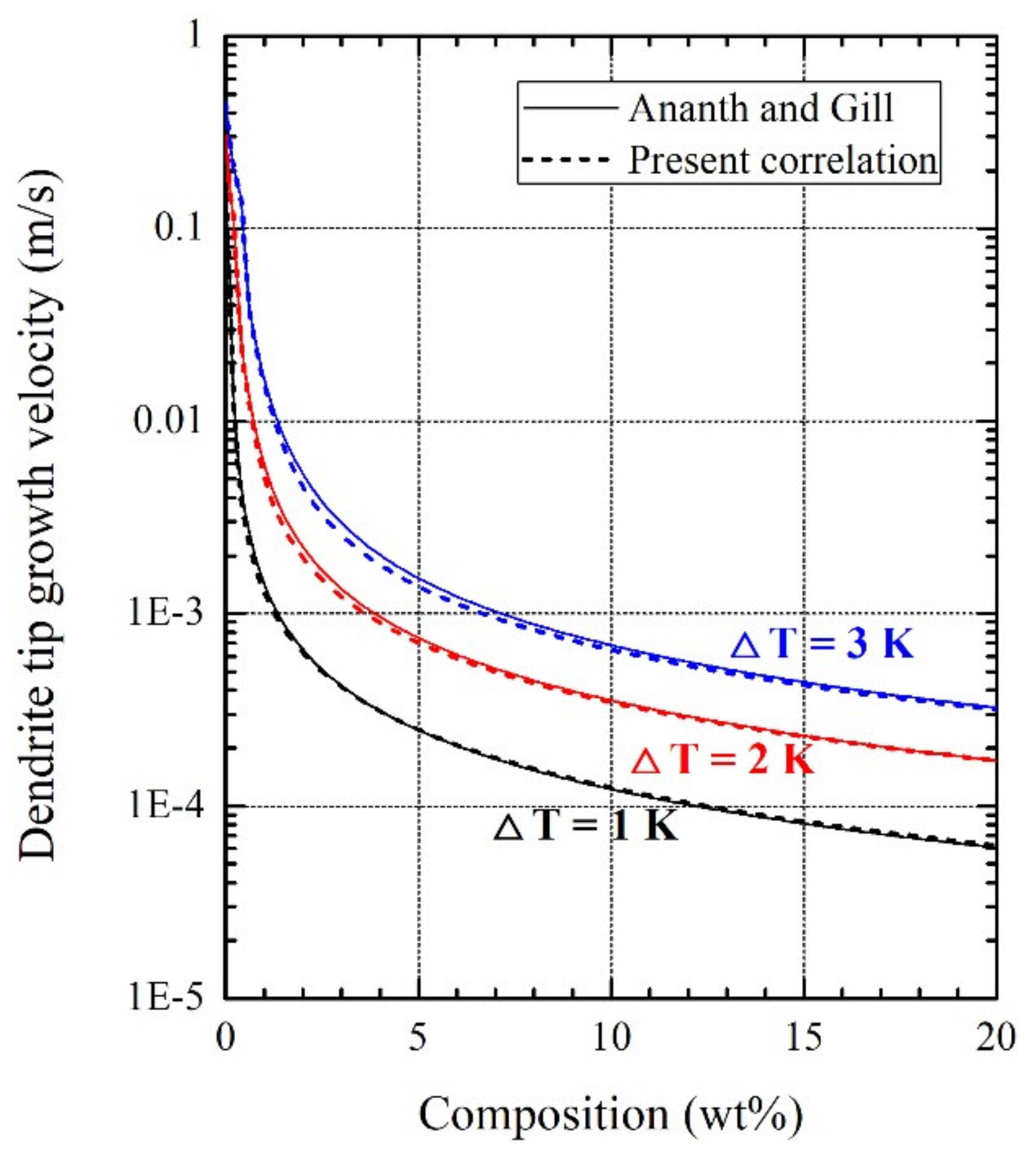
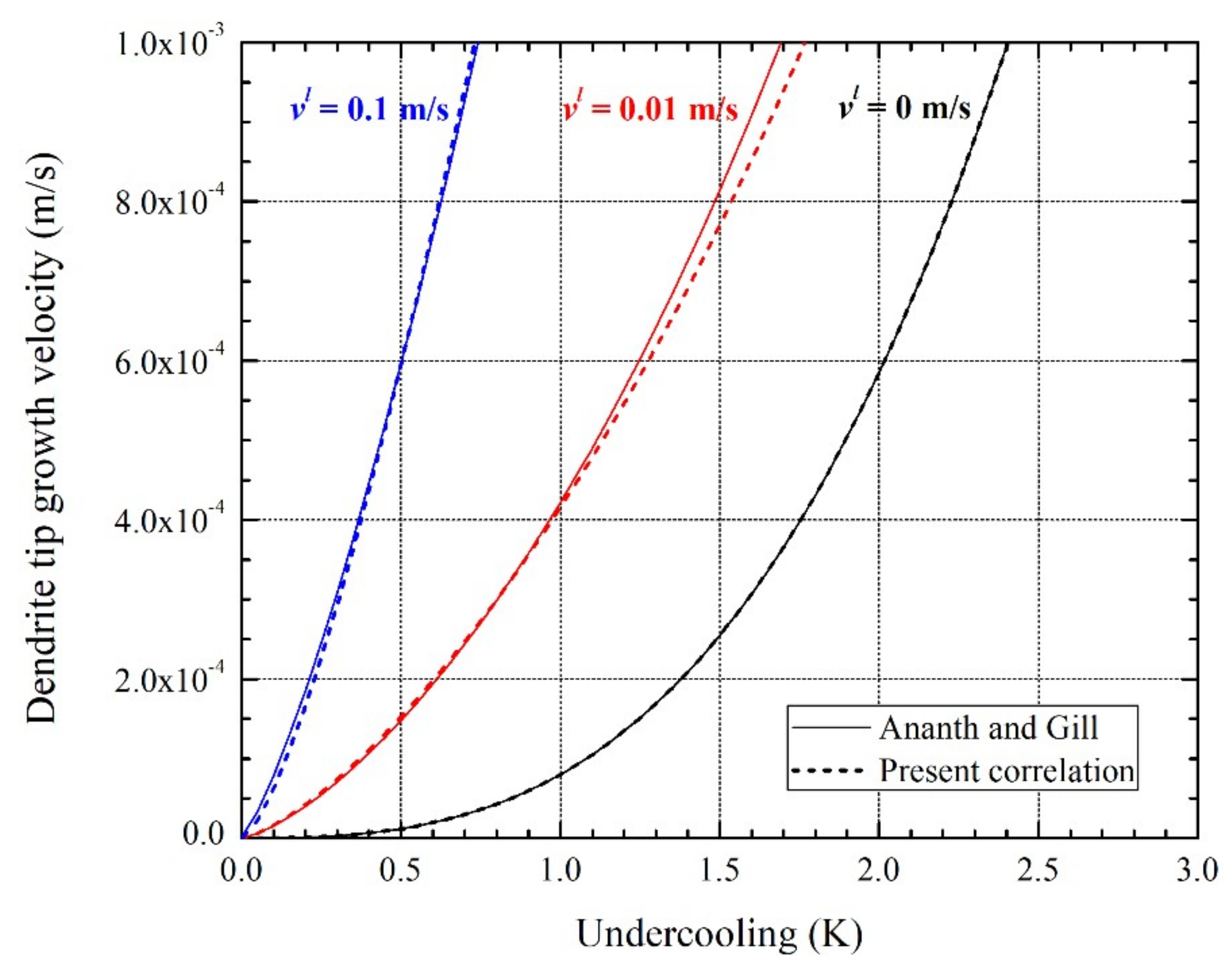
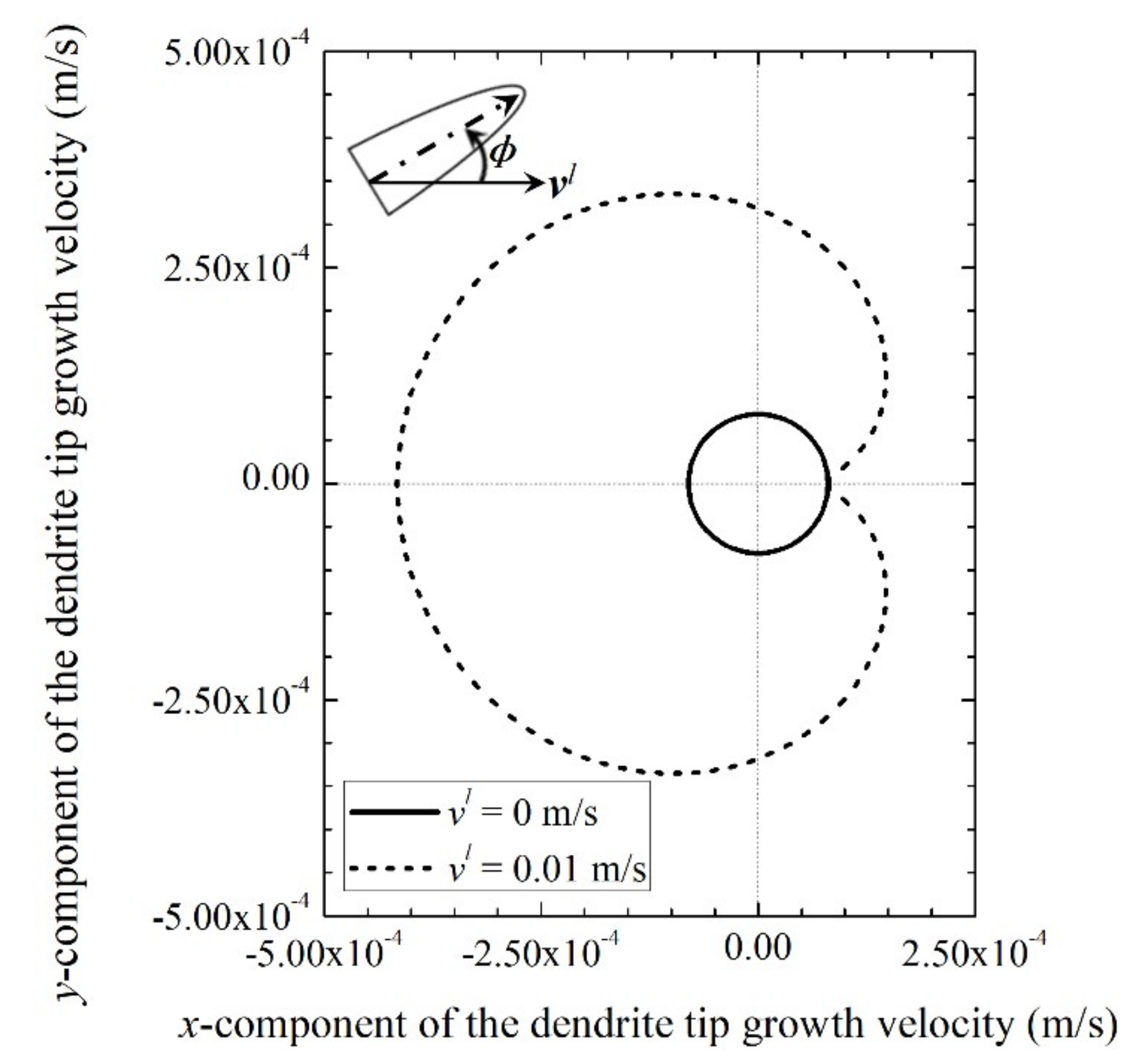
3.1. Dendrite Tip Growth Velocity in the Presence of Fluid Flow
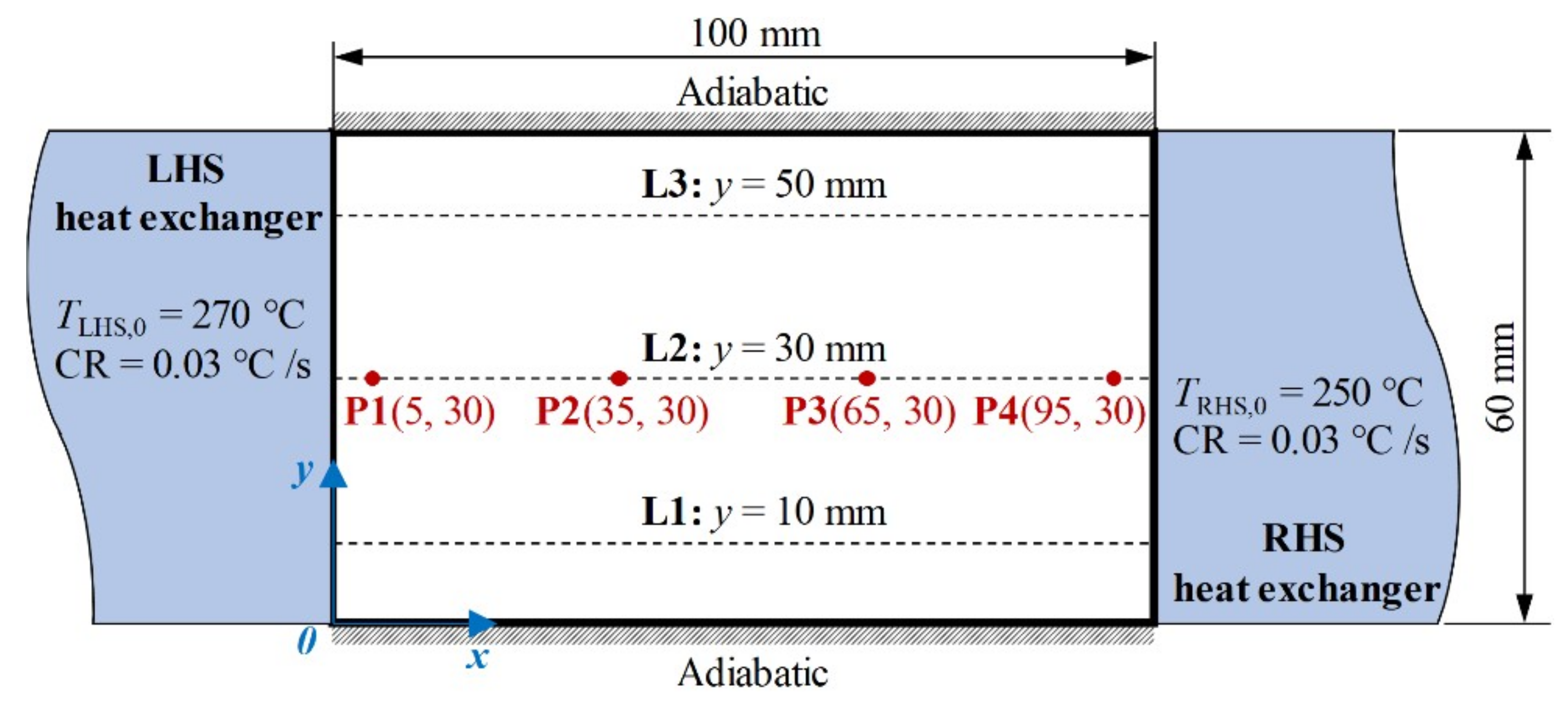
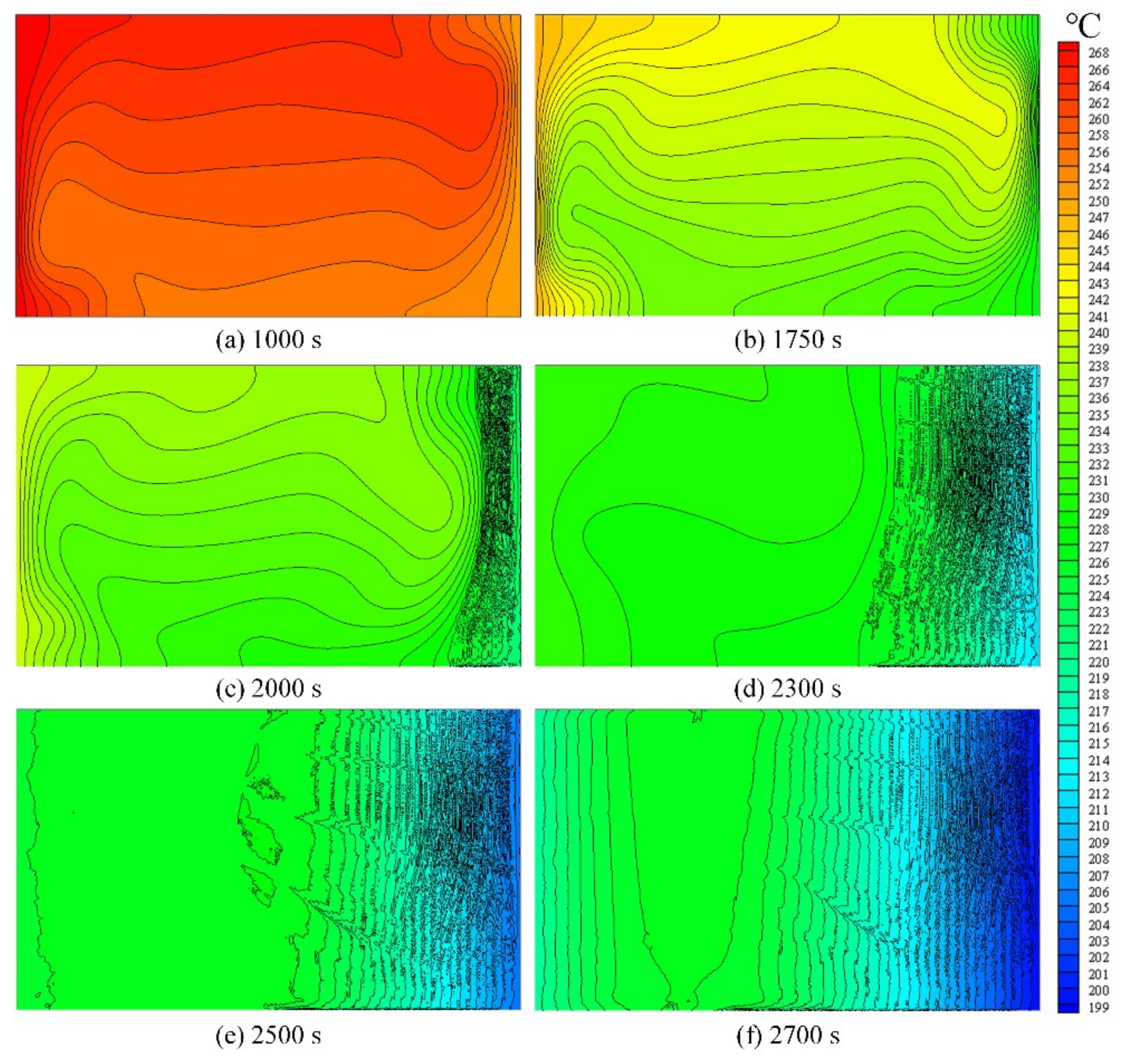
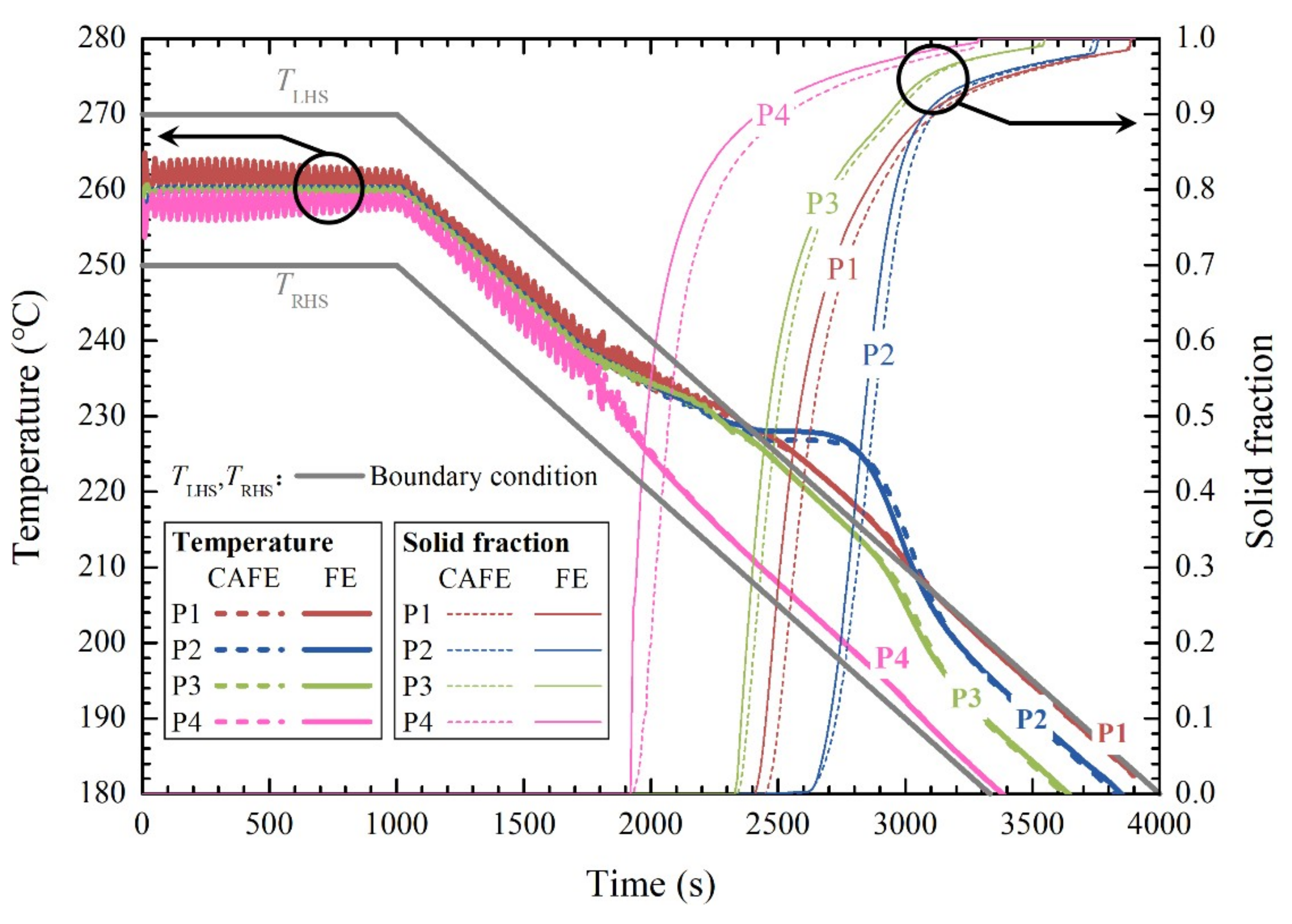
3.2. Evolution of the Temperature and Solid Fraction Fields
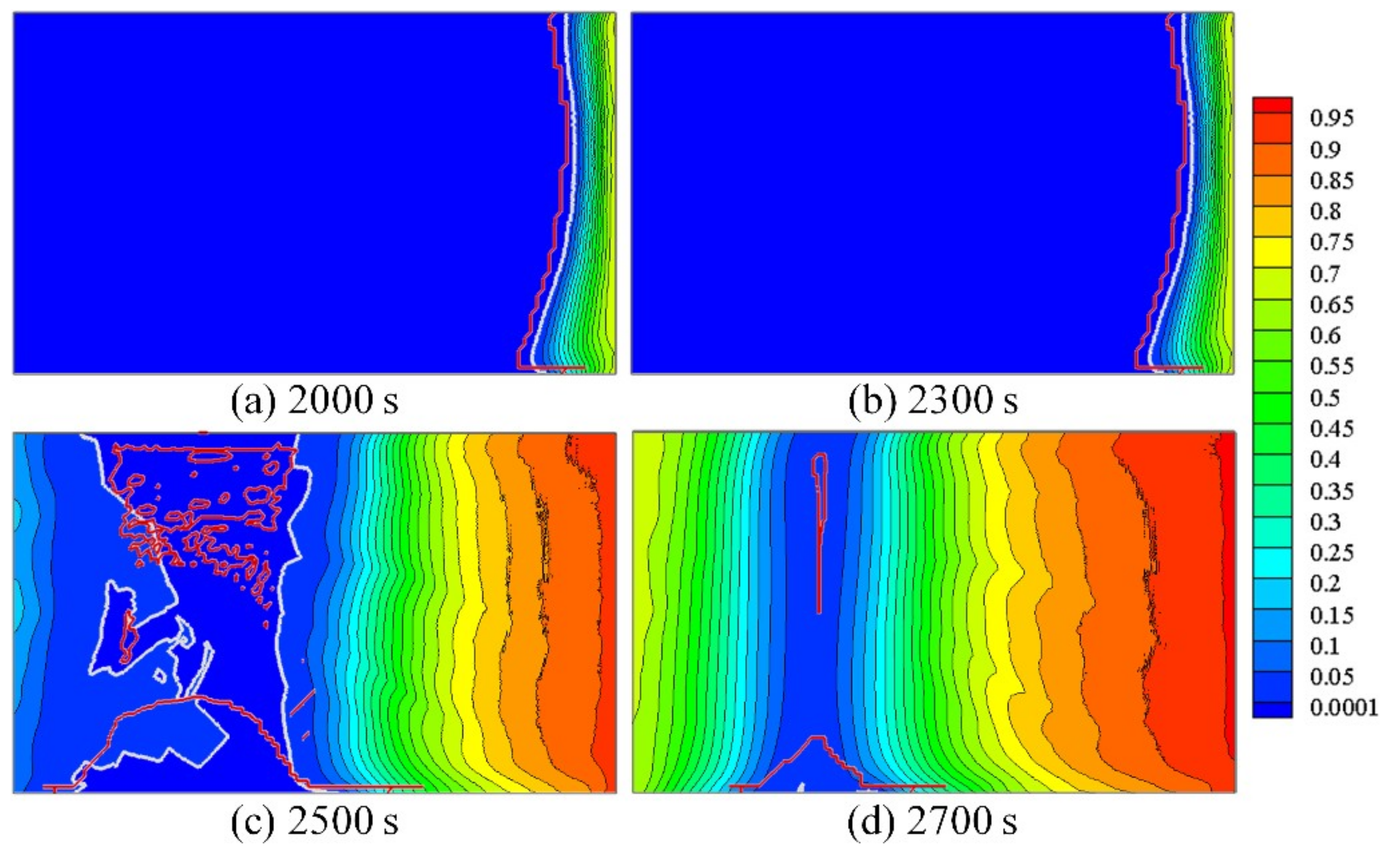
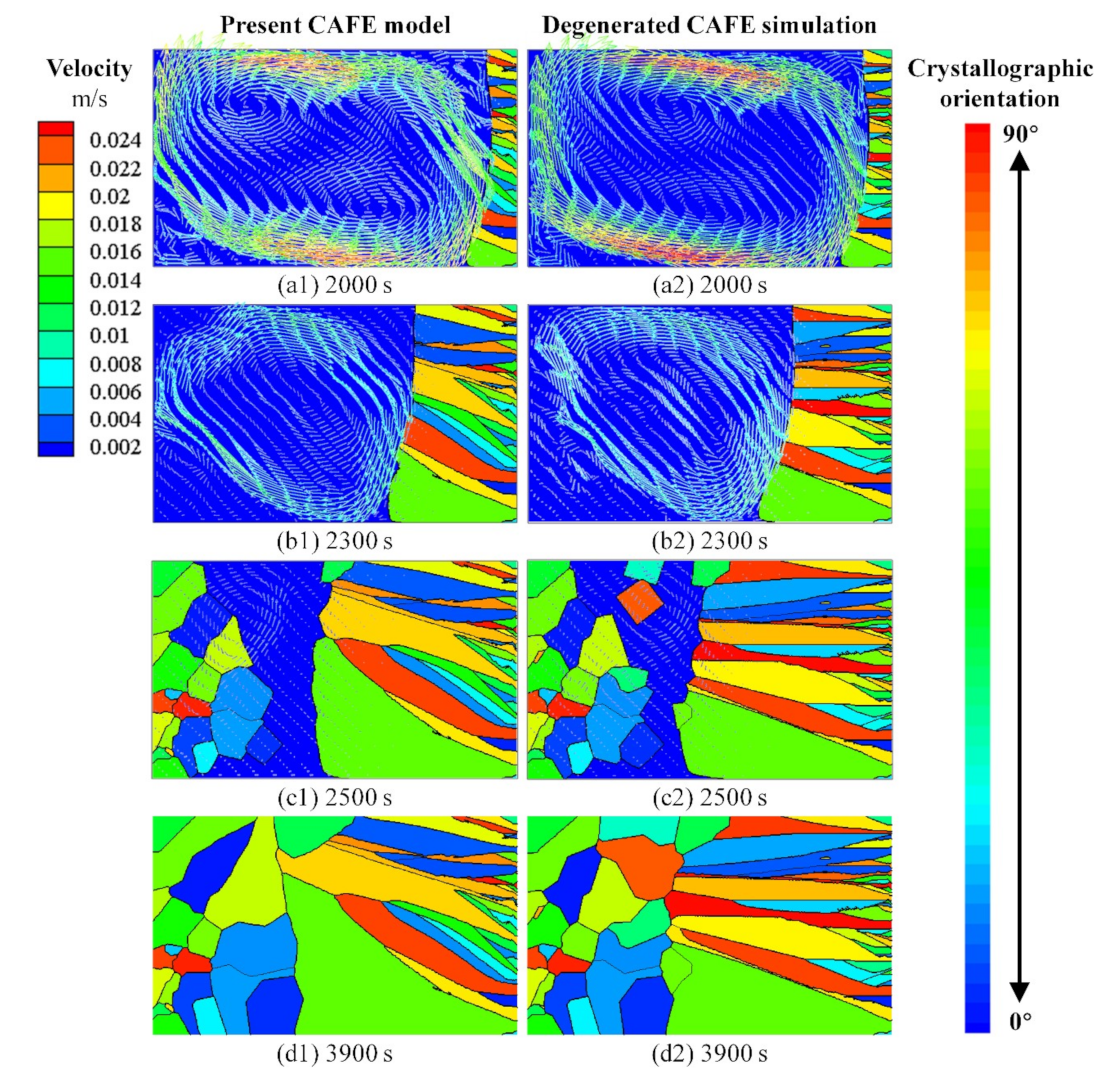
3.3. Interaction between the Fluid Flow and Grain Structure
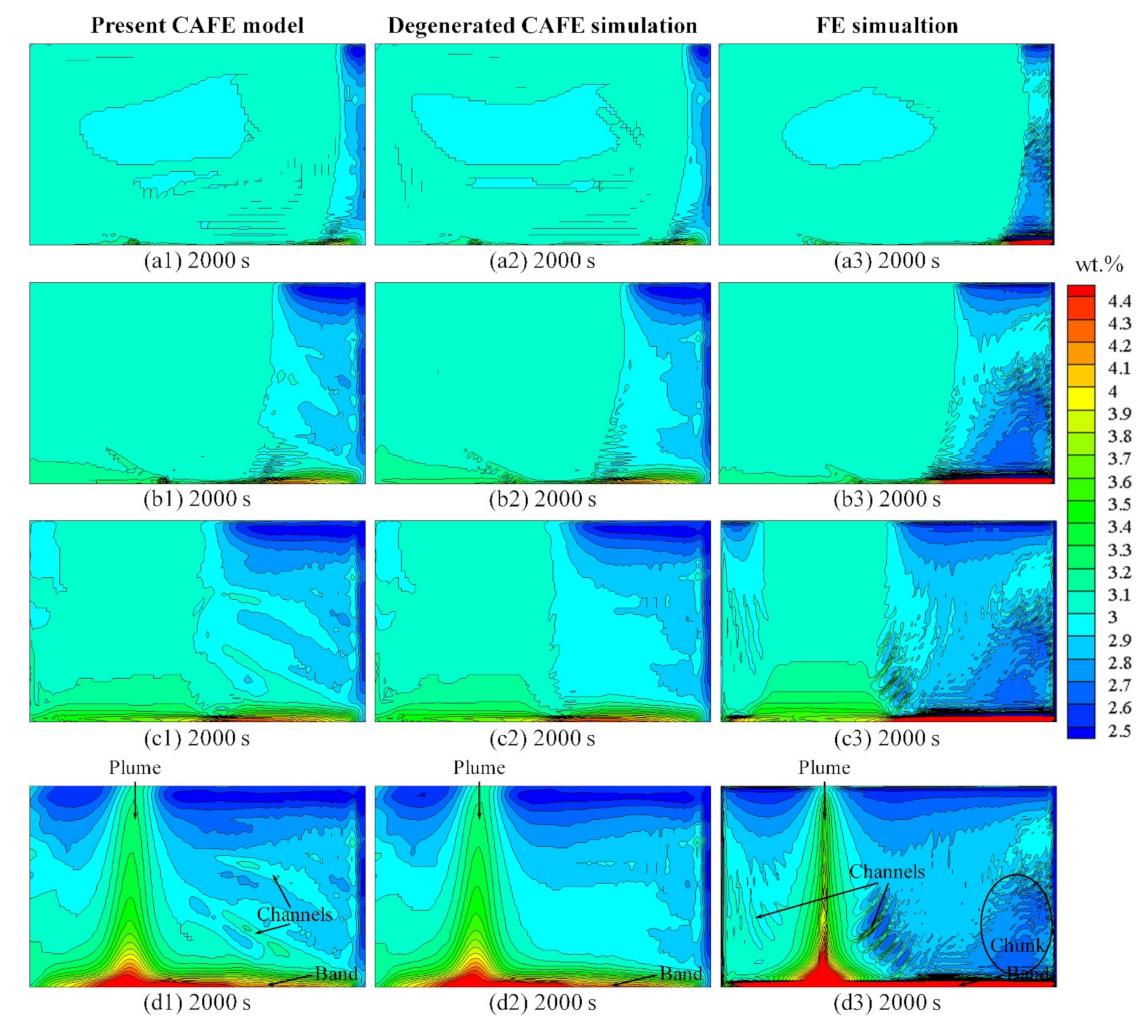
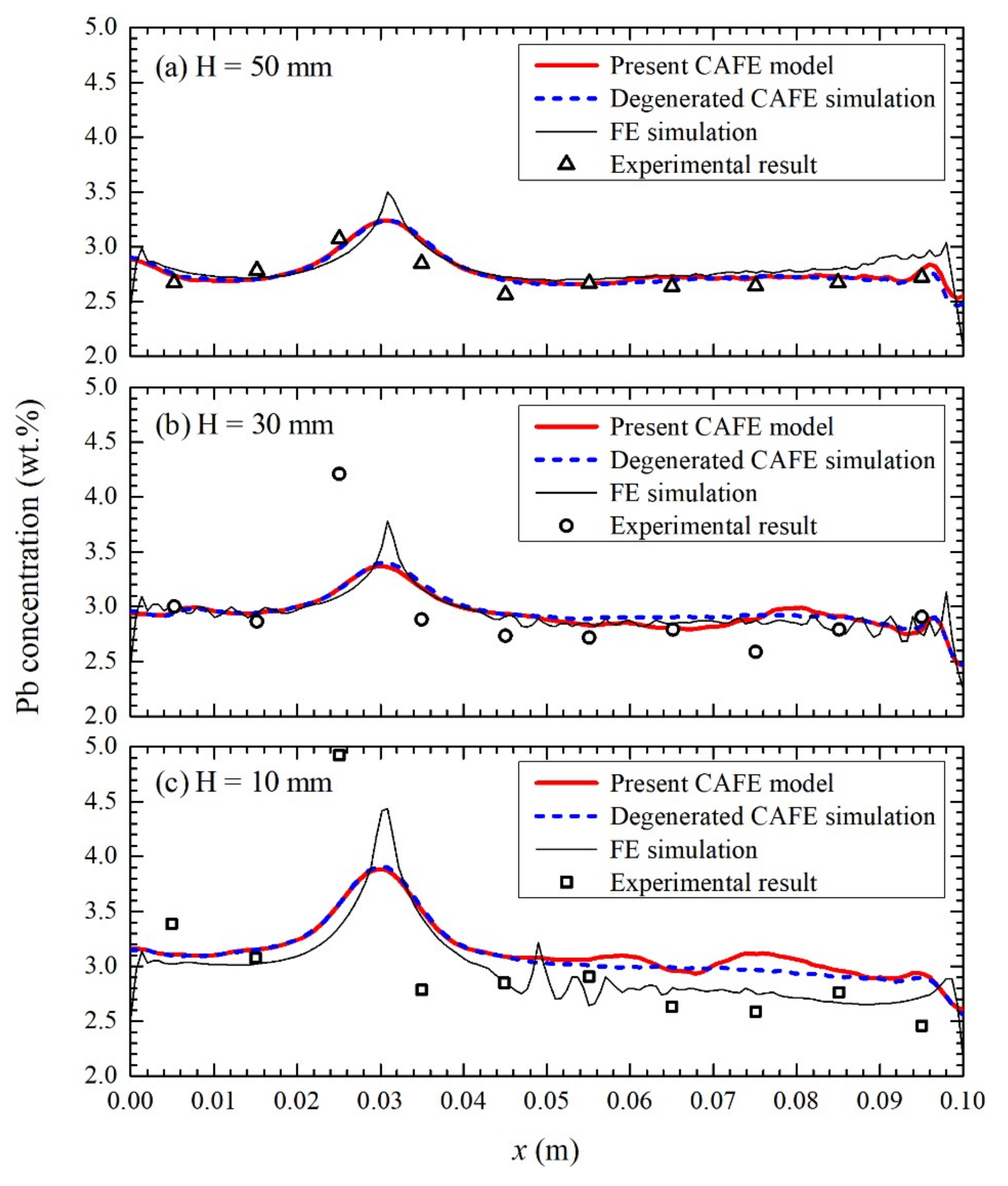
3.4. Macrosegregation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saito, Y.; Goldbeckwood, G.; Mullerkrumbhaar, H. Numerical simulation of dendritic growth. Phys. Rev. A 1988, 38, 2148–2157. [Google Scholar] [CrossRef] [Green Version]
- Boettinger, W.J.; Warren, J.A.; Beckermann, C.; Karma, A. Phase-field simulation of solidification. Annu. Rev. Mater. Res. 2002, 32, 163–194. [Google Scholar] [CrossRef]
- Steinbach, I. Phase-field models in materials science. Model. Simul. Mater. Sc. 2009, 17, 073001. [Google Scholar] [CrossRef] [Green Version]
- Gandin, C.A.; Rappaz, M. A coupled finite-element cellular-automaton model for the prediction of dendritic grain structures in solidfication processes. Acta Mater. 1994, 42, 2233–2246. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Yang, C.; Zhang, H.; Yan, X.W.; Tang, N.; Liu, B.C. Multiscale Modeling and Simulation of Directional Solidification Process of Ni-Based Superalloy Turbine Blade Casting. Metals 2018, 8, 632. [Google Scholar] [CrossRef]
- Jacot, A.; Rappaz, M. A pseudo-front tracking technique for the modelling of solidification microstructures in multi-component alloys. Acta Mater. 2002, 50, 1909–1926. [Google Scholar] [CrossRef]
- Wang, W.; Lee, P.D.; McLean, M. A model of solidification microstructures in nickel-based superalloys: Predicting primary dendrite spacing selection. Acta Mater. 2003, 51, 2971–2987. [Google Scholar] [CrossRef]
- Tan, L.J.; Zabaras, N. A level set simulation of dendritic solidification with combined features of front-tracking and fixed-domain methods. J. Comput. Phys. 2006, 211, 36–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Beckermann, C. A unified solute diffusion-model for columnar and equiaxed dendritic alloy solidification. Mater. Sci. Eng. A 1993, 171, 199–211. [Google Scholar] [CrossRef]
- Wang, C.Y.; Beckermann, C. A multiphase solute diffusion-model for dendritic alloy solidification. Metall. Trans. A 1993, 24, 2787–2802. [Google Scholar] [CrossRef]
- Wang, C.Y.; Beckermann, C. Equiaxed dendritic solidification with convection. 1. Multiscale/multiphase modeling. Metall. Mater. Trans. A 1996, 27, 2754–2764. [Google Scholar] [CrossRef]
- Ciobanas, A.I.; Fautrelle, Y. Ensemble averaged multiphase Eulerian model for columnar/equiaxed solidification of a binary alloy: I. The mathematical model. J. Phys. D 2007, 40, 3733–3762. [Google Scholar] [CrossRef]
- Appolaire, B.; Combeau, H.; Lesoult, G. Modeling of equiaxed growth in multicomponent alloys accounting for convection and for the globular/dendritic morphological transition. Mater. Sci. Eng. A 2008, 487, 33–45. [Google Scholar] [CrossRef]
- Wu, M.; Ludwig, A. Modeling equiaxed solidification with melt convection and grain sedimentation-I: Model description. Acta Mater. 2009, 57, 5621–5631. [Google Scholar] [CrossRef]
- Schneider, M.C.; Beckermann, C. Formation of macrosegregation by multicomponent thermosolutal convection during the solidification of steel. Metall. Mater. Trans. A 1995, 26, 2373–2388. [Google Scholar] [CrossRef]
- Guillemot, G.; Gandin, C.A.; Combeau, H. Modeling of macrosegregation and solidification grain structures with a coupled Cellular Automaton-Finite Element model. ISIJ Int. 2006, 46, 880–895. [Google Scholar] [CrossRef]
- Gandin, C.A.; Guillemot, G.; Bellet, M. Interaction between single grain solidification and macrosegregation: Application of a cellular automaton-Finite element model. J. Cryst. Growth 2007, 303, 58–68. [Google Scholar]
- Carozzani, T.; Gandin, C.A.; Digonnet, H.; Bellet, M.; Zaidat, K.; Fautrelle, Y. Direct Simulation of a Solidification Benchmark Experiment. Metall. Mater. Trans. A 2013, 44, 873–887. [Google Scholar] [CrossRef]
- Quillet, G.; Ciobanas, A.; Lehmann, P.; Fautrelle, Y. A benchmark solidification experiment on an Sn-10 wt%Bi alloy. Int. J. Heat Mass Transf. 2007, 50, 654–666. [Google Scholar] [CrossRef]
- Hachani, L.; Saadi, B.; Wang, X.D.; Nouri, A.; Zaidat, K.; Belgacem-Bouzida, A.; Ayouni-Derouiche, L.; Raimondi, G.; Fautrelle, Y. Experimental analysis of the solidification of Sn-3 wt.%Pb alloy under natural convection. Int. J. Heat Mass Transf. 2012, 55, 1986–1996. [Google Scholar] [CrossRef]
- Hachani, L.; Zaidat, K.; Saadi, B.; Wang, X.D.; Fautrelle, Y. Solidification of Sn-Pb alloys: Experiments on the influence of the initial concentration. Int. J. Therm. Sci. 2015, 91, 34–48. [Google Scholar] [CrossRef]
- Boussaa, R.; Hachani, L.; Budenkova, O.; Botton, V.; Henry, D.; Zaidat, K.; Ben Hadid, H.; Fautrelle, Y. Macrosegregations in Sn-3 wt%Pb alloy solidification: Experimental and 3D numerical simulation investigations. Int. J. Heat Mass Transf. 2016, 100, 680–690. [Google Scholar] [CrossRef]
- Chen, Q.P.; Shen, H.F. A finite element formulation for macrosegregation during alloy solidification using a fractional step method and equal-order elements. Comp. Mater. Sci. 2018, 154, 335–345. [Google Scholar] [CrossRef]
- Yeh, C.; Chang, L.; Straumal, B. Wetting transition of grain boundaries in tin-rich indium-based alloys and its influence on electrical properties. Mater. Trans. 2010, 51, 1677–1682. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kogtenkova, O.A.; Muktepavela, F.; Kolesnikova, K.I.; Bulatov, M.F.; Straumal, P.B.; Baretzky, B. Direct observation of strain-induced non-equilibrium grain boundaries. Mater. Lett. 2015, 159, 432–435. [Google Scholar] [CrossRef]
- Bellet, M.; Combeau, H.; Fautrelle, Y.; Gobin, D.; Rady, M.; Arquis, E.; Budenkova, O.; Dussoubs, B.; Duterrail, Y.; Kumar, A.; et al. Call for contributions to a numerical benchmark problem for 2D columnar solidification of binary alloys. Int. J. Therm. Sci. 2009, 48, 2013–2016. [Google Scholar] [CrossRef]
- Thevoz, P.; Desbiolles, J.L.; Rappaz, M. Modeling of equiaxed microstructure formation in casting. Metall. Trans. A 1989, 20, 311–322. [Google Scholar] [CrossRef]
- Rappaz, M.; Gandin, C.A. Probabilistic modeling of microstructure formation in solidification processes. Acta Mater. 1993, 41, 345–360. [Google Scholar] [CrossRef]
- Gandin, C.A.; Guillemot, G.; Appolaire, B.; Niane, N.T. Boundary layer correlation for dendrite tip growth with fluid flow. Mater. Sci. Eng. A 2003, 342, 44–50. [Google Scholar] [CrossRef]
- Lipton, J.; Glicksman, M.E.; Kurz, W. Dendritic growth into undercooled alloy melts. Mater. Sci. Eng. 1984, 65, 57–63. [Google Scholar] [CrossRef]
- Gandin, C.A.; Desbiolles, J.L.; Rappaz, M.; Thevoz, P. A three-dimensional cellular automaton-finite element model for the prediction of solidification grain structures. Metall. Mater. Trans. A 1999, 30, 3153–3165. [Google Scholar] [CrossRef]
- Ananth, R.; Gill, W.N. Self-consistent theory of dendritic growth with convection. J. Cryst. Growth 1991, 108, 173–189. [Google Scholar] [CrossRef]
| Parameter Description | Symbol | Value | Unit |
|---|---|---|---|
| Phase diagram | |||
| Melting temperature | 232.0 | °C | |
| Eutectic temperature | 183.0 | °C | |
| Eutectic composition | 38.1 | wt.% | |
| Partition coefficient | 0.0656 | - | |
| Physical properties | |||
| Reference density | 7130.0 | kg·m−3 | |
| Solutal expansion coefficient | −5.3 × 10−3 | wt.%−1 | |
| Thermal expansion coefficient | 9.5 × 10−5 | K−1 | |
| Specific heat of the liquid | 265.0 | J·kg−1·K−1 | |
| Specific heat of the solid | 226.0 | J·kg−1·K−1 | |
| Latent heat | 57,512.0 | J·kg−1 | |
| Thermal conductivity of the liquid | 55.0 | W·m−1·K−1 | |
| Thermal conductivity of the solid | 33.0 | W·m−1·K−1 | |
| Diffusion coefficient of Pb in liquid Sn | 3.0 × 10−9 | m2·s−1 | |
| Dynamic viscosity of the liquid | 2.0 × 10−3 | Pa·s | |
| Initial and boundary conditions | |||
| Nominal composition | 3.0 | wt.% | |
| Initial temperature | 258.6 | °C | |
| Initial velocity | 0 | m·s−1 | |
| Initial temperature of the LHS wall | 270.0 | °C | |
| Initial temperature of the RHS wall | 250.0 | °C | |
| Cooling rate on the LHS and RHS walls | −0.03 | °C·s−1 | |
| Nucleation and grain growth | |||
| Gibbs–Thomson coefficient | 2.0 × 10−7 | m·K | |
| Gaussian distribution nucleation parameters on the RHS wall | |||
| Average undercooling | 1.0 | K | |
| Standard deviation | 0.5 | K | |
| Density of nucleation sites | 1.0 × 106 | m−2 | |
| Gaussian distribution nucleation parameters in the bulk melt | |||
| Average undercooling | 2.5 | K | |
| Standard deviation | 0.5 | K | |
| Density of nucleation sites | 1.0 × 108 | m−3 | |
| Additional parameters | |||
| FE mesh size | 1.0 × 10−3 | m | |
| FE time step | 0.05 | s | |
| CA cell size | 2.0 × 10−4 | m | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Shen, H. Direct Macroscopic Modeling of Grain Structure and Macrosegregation with a Cellular Automaton–Finite Element Model. Metals 2019, 9, 177. https://doi.org/10.3390/met9020177
Chen Q, Shen H. Direct Macroscopic Modeling of Grain Structure and Macrosegregation with a Cellular Automaton–Finite Element Model. Metals. 2019; 9(2):177. https://doi.org/10.3390/met9020177
Chicago/Turabian StyleChen, Qipeng, and Houfa Shen. 2019. "Direct Macroscopic Modeling of Grain Structure and Macrosegregation with a Cellular Automaton–Finite Element Model" Metals 9, no. 2: 177. https://doi.org/10.3390/met9020177





