Combined Effects of Deformation and Undercooling on Isothermal Bainitic Transformation in an Fe-C-Mn-Si Alloy
Abstract
1. Introduction
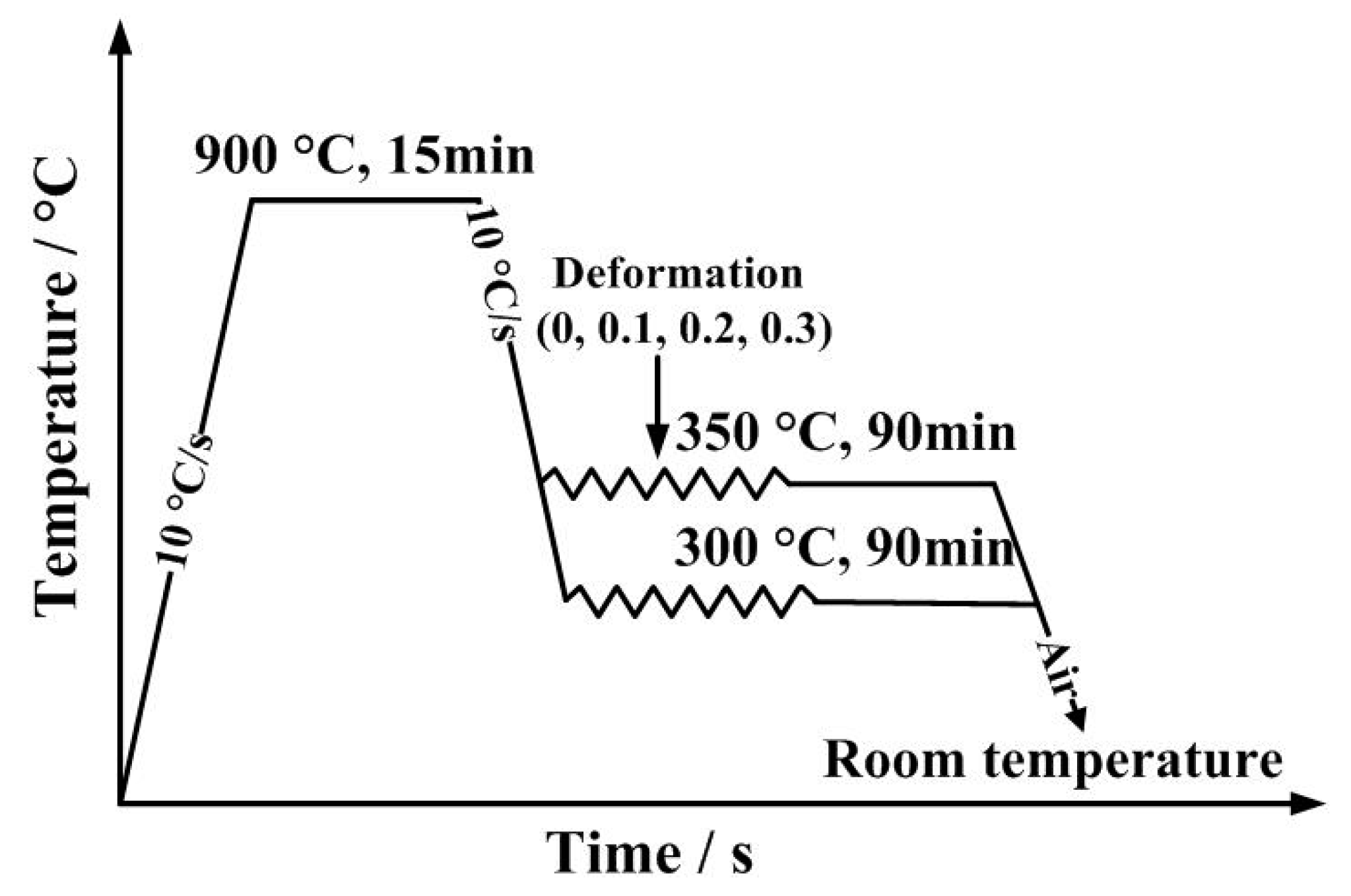
2. Materials and Methods
3. Results
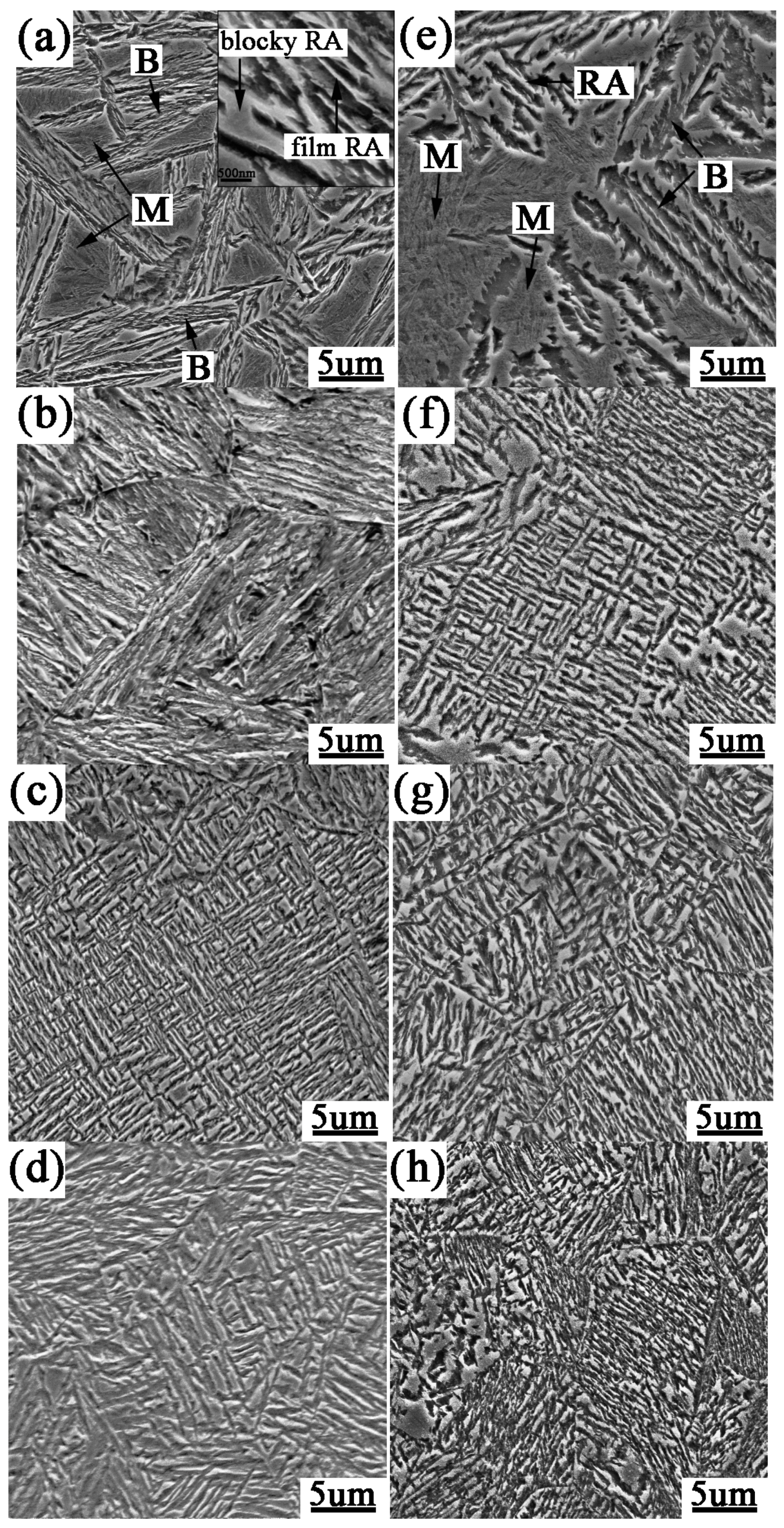
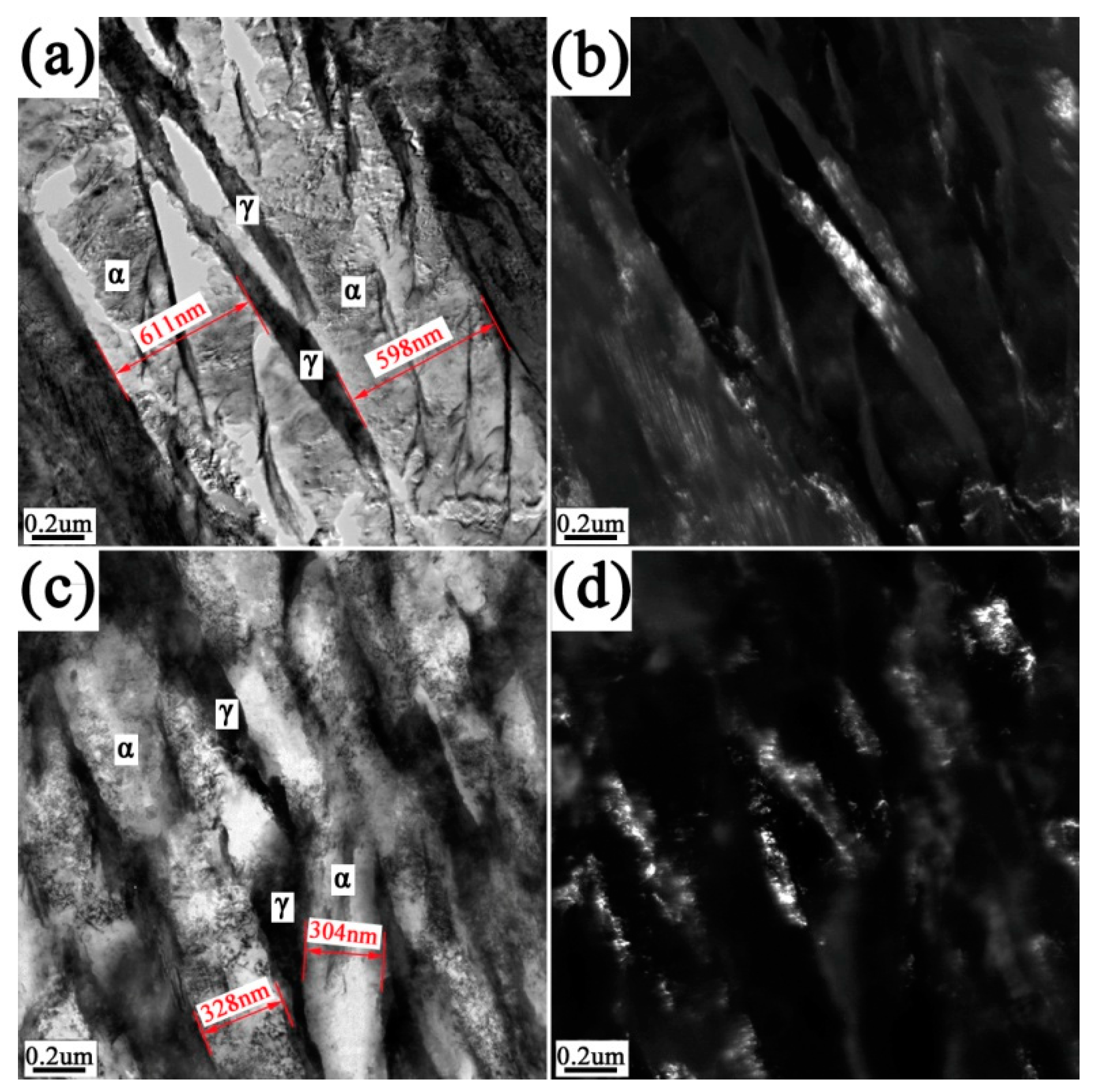
3.1. Microstructure
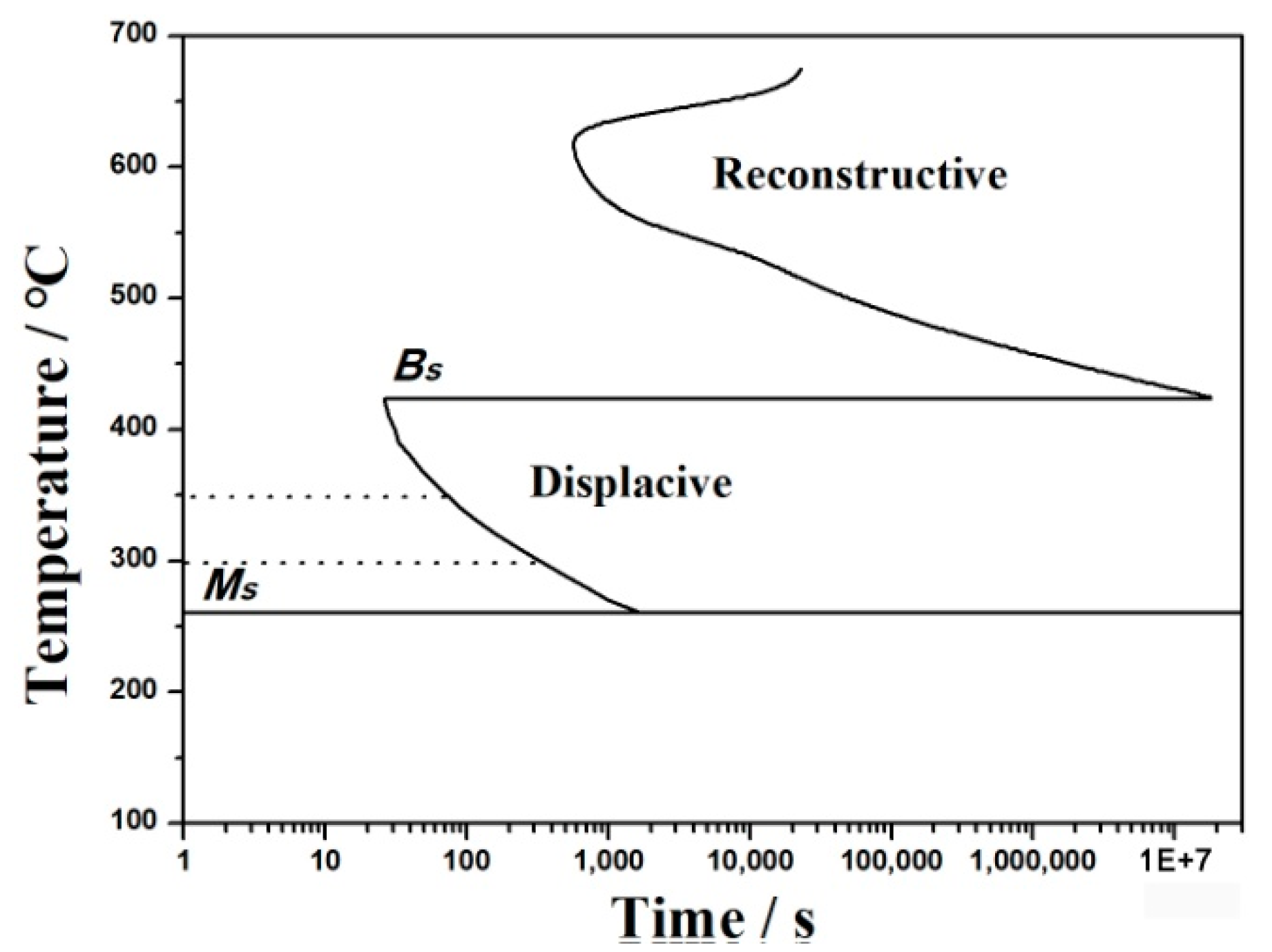
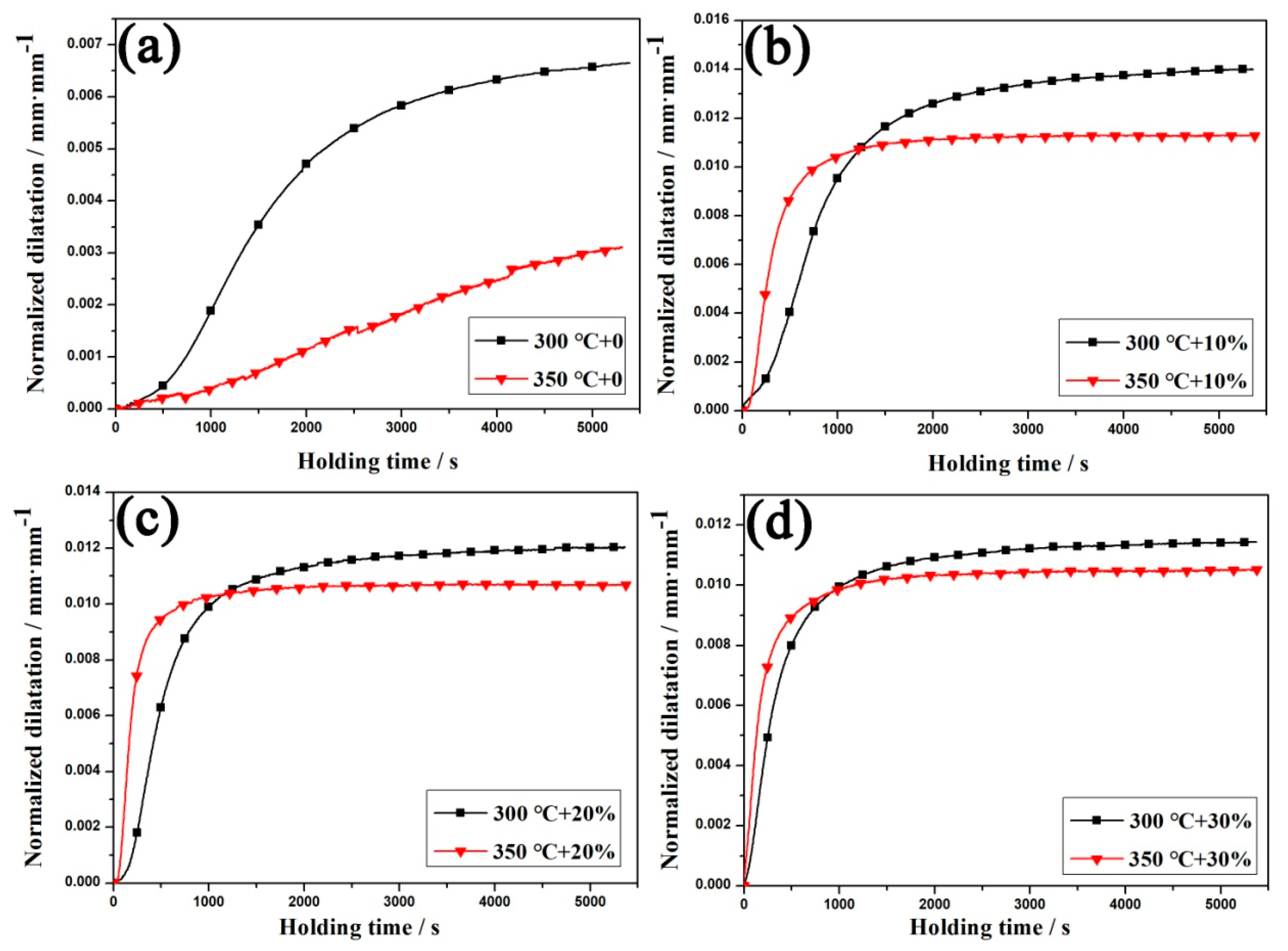
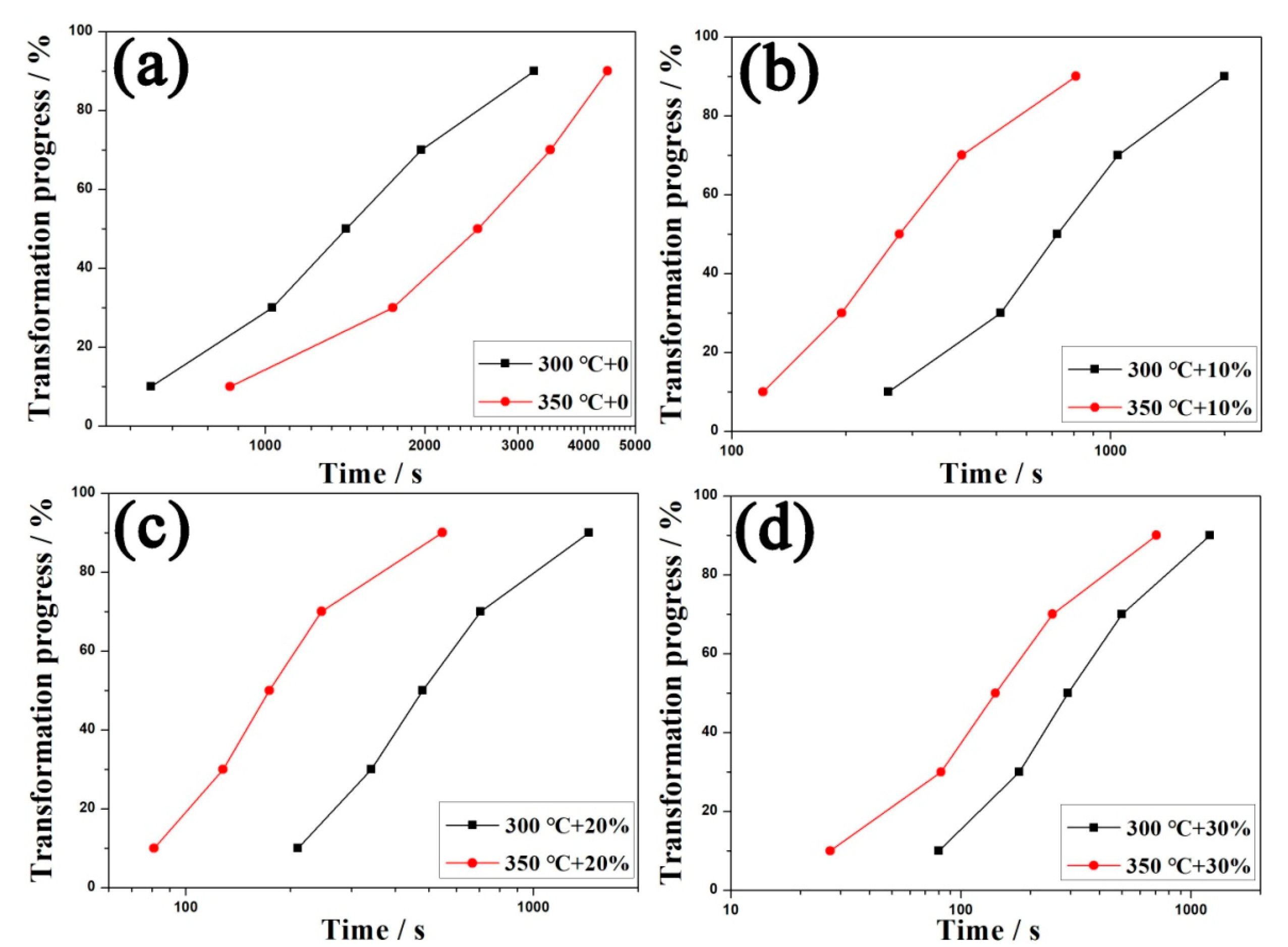
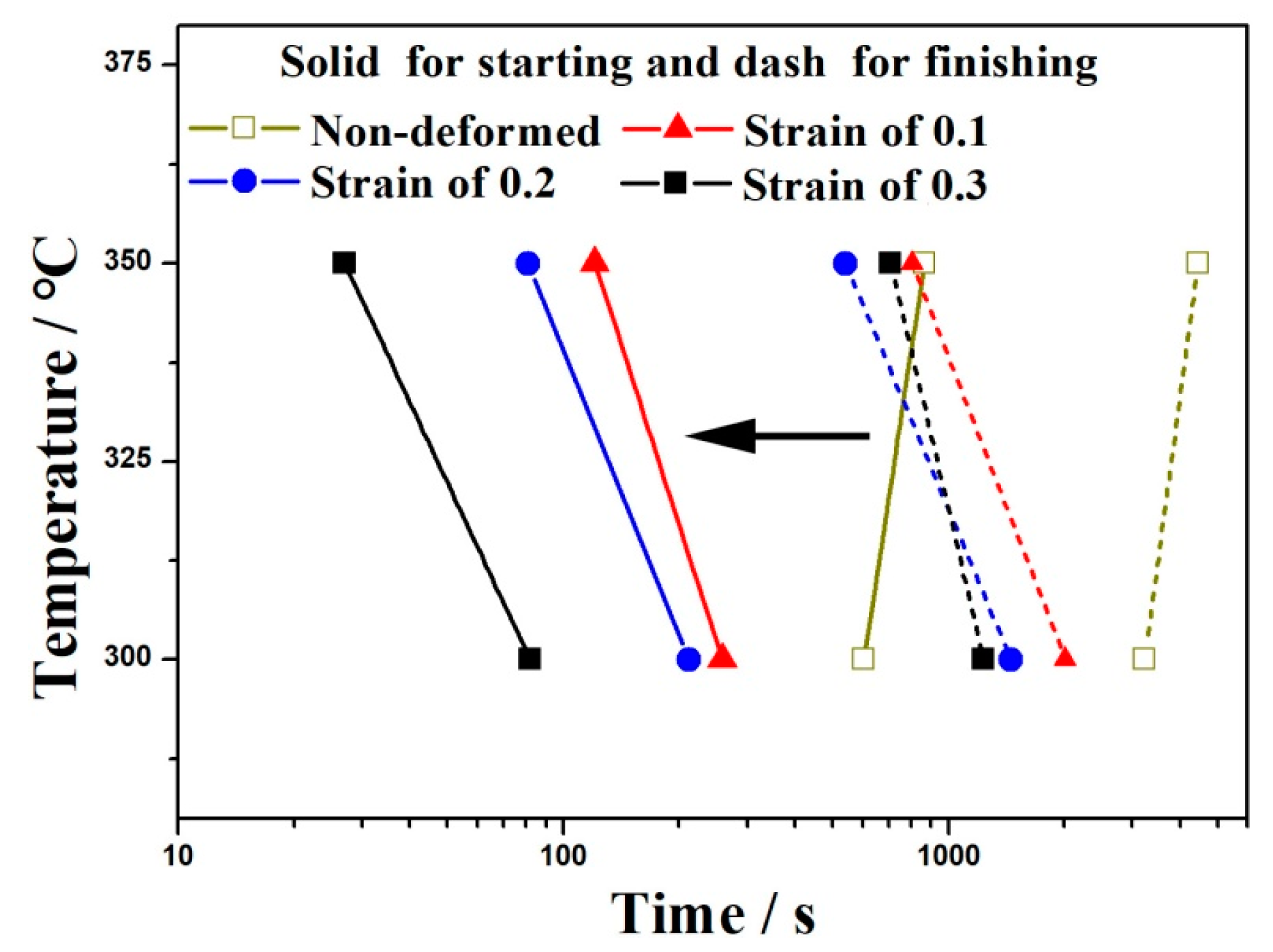
3.2. Kinetics of Bainitic Transformation
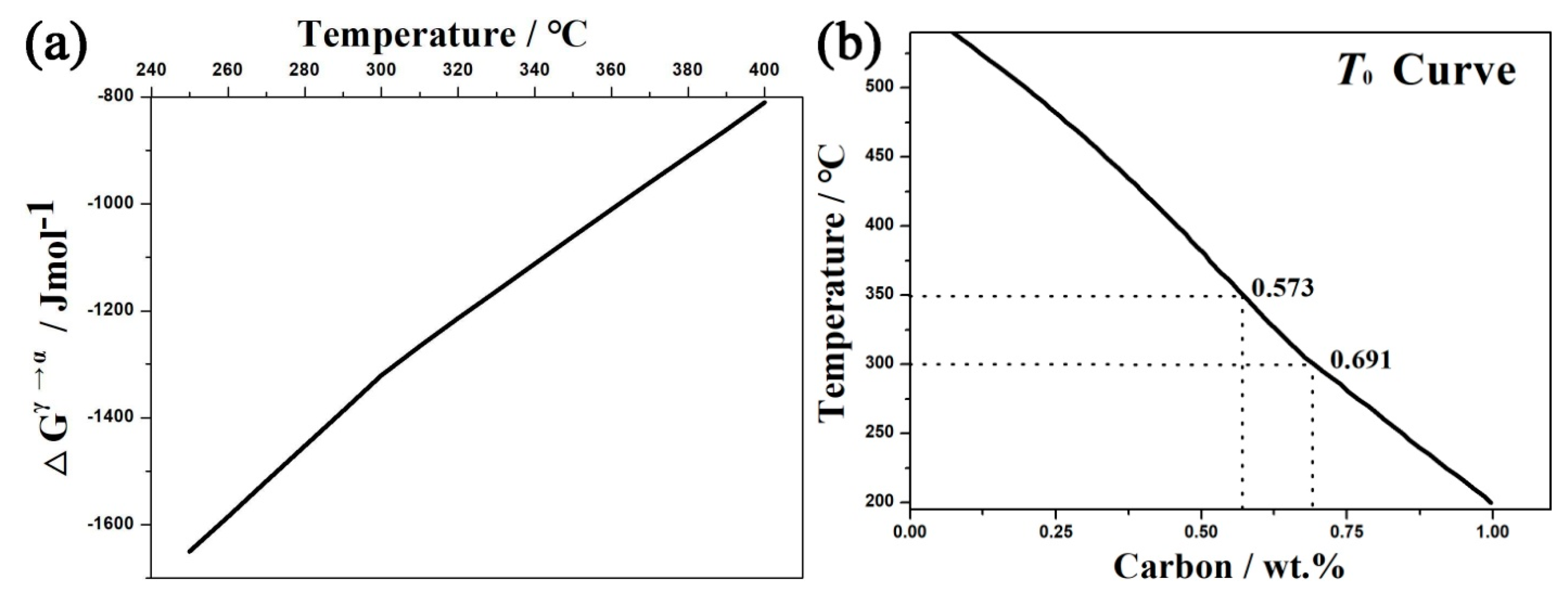
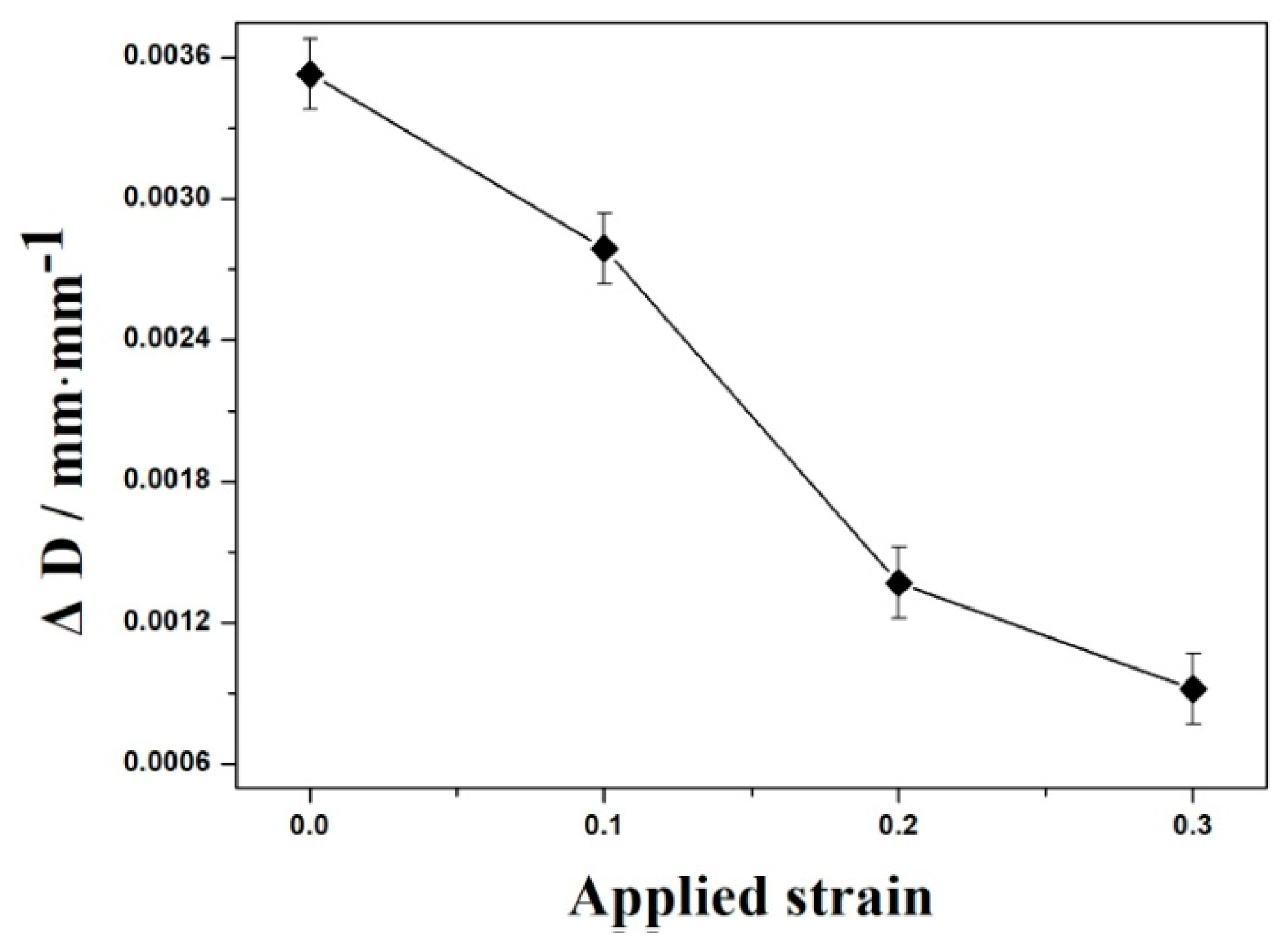
4. Discussions
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | retained austenite |
| MS | martensite starting temperature |
| MCBS | medium-carbon bainite steels |
| ICT | incomplete transformation |
| TTT | time-temperature-transformation |
| Bs | bainite starting temperature |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscope |
| B | bainite |
| M | martensite |
References
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Very strong low temperature bainite. Mater. Sci. Technol. 2002, 18, 279–284. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Mechanical properties of low-temperature bainite. Mater. Sci. Forum 2005, 500–501, 495–502. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of low-temperature bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Yoozbashi, M.N.; Yazdani, S.; Wang, T.S. Design of a new nanostructured high-Si bainitic steel with lower cost production. Mater. Des. 2011, 32, 3248–3253. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.S.; Zhang, B.; Zhang, F.C. Microstructure and mechanical properties of high-carbon Si–Al-rich steel by low-temperature austempering. Mater. Des. 2012, 35, 170–174. [Google Scholar] [CrossRef]
- Yang, H.S.; Bhadeshia, H.K.D.H. Designing low carbon; low temperature bainite. Mater. Sci. Technol. 2008, 24, 335–342. [Google Scholar] [CrossRef]
- Hu, H.J.; Xu, G.; Liu, F.; Wang, L.; Zhou, L.X.; Xue, Z.L. Dynamic observation of twin evolution during austenite grain growth in an Fe–C–Mn–Si alloy. Int. J. Mater. Res. 2014, 105, 337–341. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Wang, L.; Hu, H.J. Combined effect of the prior deformation and applied stress on the bainite transformation. Met. Mater. Int. 2016, 22, 956–961. [Google Scholar] [CrossRef]
- Zhang, X.X.; Xu, G.; Wang, X.; Embury, D. Mechanical behavior of carbide-free medium carbon bainitic steels. Metall. Mater. Trans. A 2014, 45, 1352–1361. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.H.; Zheng, C.L.; Zhang, F.C.; Wang, T.S. Effects of ausforming on isothermal bainite transformation behavior and microstructural refinement in medium-carbon Si–Al-rich alloy steel. Mater. Des. 2014, 62, 168–174. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Hu, H.J.; Yuan, Q.; Tian, J.Y. Comprehensive analysis on the effects of different stress states on the bainitic transformation. Mater. Sci. Eng. A 2017, 704, 427–433. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Reynolds, W.T.; Purdy, G.R. The incomplete transformation phenomenon in steel. Metall. Mater. Trans. A 2006, 37, 1731–1745. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Waugh, A.R. Bainite: An atom-probe study of the incomplete reaction phenomenon. Acta Metall. 1982, 30, 775–784. [Google Scholar] [CrossRef]
- Xia, Y.; Miyamoto, G.; Yang, Z.G.; Zhang, C.; Furuhara, T. Direct measurement of carbon enrichment in the incomplete bainite transformation in Mo added low carbon steels. Acta Mater. 2015, 91, 10–18. [Google Scholar] [CrossRef]
- Hasan, H.S.; Peet, M.J.; Avettand-Fènoël, M.-N.; Bhadeshia, H.K.D.H. Effect of tempering upon the tensile properties of a nanostructured bainitic steel. Mater. Sci. Eng. A 2014, 615, 340–347. [Google Scholar] [CrossRef]
- Baradari, S.; Boutorabi, S.M. Effects of isothermal transformation conditions on the microstructure and hardness values of a high-carbon Al–Si alloyed steel. Mater. Des. 2015, 86, 603–609. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Wang, L.; Hu, H.J. Effect of undercooling and austenitic grain size on bainitic transformation in an Fe–C–Mn–Si superbainite steel. Trans. Indian Inst. Met. 2016, 69, 693–698. [Google Scholar] [CrossRef]
- Hu, H.J.; Zurob, H.S.; Xu, G.; Embury, D.; Purdy, G.R. New insights to the effects of ausforming on the bainitic transformation. Mater. Sci. Eng. A 2015, 626, 34–40. [Google Scholar] [CrossRef]
- Hu, H.J.; Xu, G.; Zhou, M.X.; Yuan, Q. New insights to the promoted bainitic transformation in prior deformed austenite in a Fe-C-Mn-Si alloy. Met. Mater. Int. 2017, 23, 233–238. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Koo, M.S.; Adachi, Y. Effect of ausforming on nanobainite steel. Scr. Mater. 2010, 63, 819–822. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Adachi, Y.; Paradowska, A.M.; Kelleher, J.F.; Zhang, S.Y. Effects of ausforming temperature on bainite transformation; microstructure and variant selection in nanobainite steel. Acta Mater. 2013, 61, 4142–4154. [Google Scholar] [CrossRef]
- Yang, Z.N.; Chu, C.H.; Jiang, F.; Qin, Y.M.; Long, X.Y.; Wang, S.L.; Chen, D.; Zhang, F.C. Accelerating nano-bainite transformation based on a new constructed microstructural predicting model. Mater. Sci. Eng. A 2019. [Google Scholar] [CrossRef]
- He, B.B.; Xu, W.; Huang, M.X. Effect of ausforming temperature and strain on the bainitic transformation kinetics of a low carbon boron steel. Philos. Mag. 2015, 95, 1150–1163. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. A thermodynamic analysis of isothermal transformation diagrams. Met. Sci. 1982, 16, 159–165. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels; Institute of Materials: London, UK, 1992; pp. 1–450. [Google Scholar]
- Singh, S.B.; Bhadeshia, H.K.D.H. Estimation of bainite plate-thickness in low-alloy steels. Mater. Sci. Eng. A 1998, 245, 72–79. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Rationalisation of shear transformations in steels. Acta Metall. 1981, 29, 1117–1130. [Google Scholar] [CrossRef]
- Chen, H.; van der Zwaag, S. The effect of interfacial element partitioning on ferrite and bainite formation. JOM 2016, 68, 1320–1328. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Zhang, Y.L. The effects of external compressive stress on the kinetics of low temperature bainitic transformation and microstructure in a superbainite steel. Int. J. Mater. Res. 2015, 106, 1040–1045. [Google Scholar] [CrossRef]
- Hase, K.; Garcia-Mateo, C.; Bhadeshia, H.K.D.H. Bainite formation influenced by large stress. Mater. Sci. Technol. 2004, 20, 1499–1505. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, G.; Wang, L.; Yuan, Q. The varying effects of uniaxial compressive stress on the bainitic transformation under different austenitization temperatures. Metals 2016, 6, 119. [Google Scholar] [CrossRef]
- Eres-Castellanos, A.; Morales-Rivas, L.A.; Latz, A.; Caballero, F.G.; Garcia-Mateo, C. Effect of ausforming on the anisotropy of low temperature bainitic transformation. Mater. Charact. 2018, 145, 371–380. [Google Scholar] [CrossRef]
- Matsuda, H.; Bhadeshia, H.K.D.H. Kinetics of the bainite transformation. Proc. R. Soc. Lond. A 2004, 460, 1707–1722. [Google Scholar] [CrossRef]
- Quidort, D.; Brechet, Y.J.M. Isothermal kinetics of bainite in 0.5% C steels. Acta Mater. 2001, 49, 4161–4170. [Google Scholar] [CrossRef]
- Shi, Z.M.; Li, J.; Chi, B.; Pu, J.; Zhang, Y.S.; Wang, M.Q.; Shi, J.; Dong, H. Martensitic phase transformation from non-isothermally deformed austenite in high strength steel 22SiMn2TiB. Metall. Mater. Trans. A 2013, 44, 4136–4142. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Hu, H.; Xu, G.; Xiong, Z.; Dai, F. Combined Effects of Deformation and Undercooling on Isothermal Bainitic Transformation in an Fe-C-Mn-Si Alloy. Metals 2019, 9, 138. https://doi.org/10.3390/met9020138
Zou H, Hu H, Xu G, Xiong Z, Dai F. Combined Effects of Deformation and Undercooling on Isothermal Bainitic Transformation in an Fe-C-Mn-Si Alloy. Metals. 2019; 9(2):138. https://doi.org/10.3390/met9020138
Chicago/Turabian StyleZou, Hang, Haijiang Hu, Guang Xu, Ziliu Xiong, and Fangqin Dai. 2019. "Combined Effects of Deformation and Undercooling on Isothermal Bainitic Transformation in an Fe-C-Mn-Si Alloy" Metals 9, no. 2: 138. https://doi.org/10.3390/met9020138
APA StyleZou, H., Hu, H., Xu, G., Xiong, Z., & Dai, F. (2019). Combined Effects of Deformation and Undercooling on Isothermal Bainitic Transformation in an Fe-C-Mn-Si Alloy. Metals, 9(2), 138. https://doi.org/10.3390/met9020138






