On the Use of Functionally Graded Materials to Differentiate the Effects of Surface Severe Plastic Deformation, Roughness and Chemical Composition on Cell Proliferation
Abstract
1. Introduction
2. Experimental Procedure
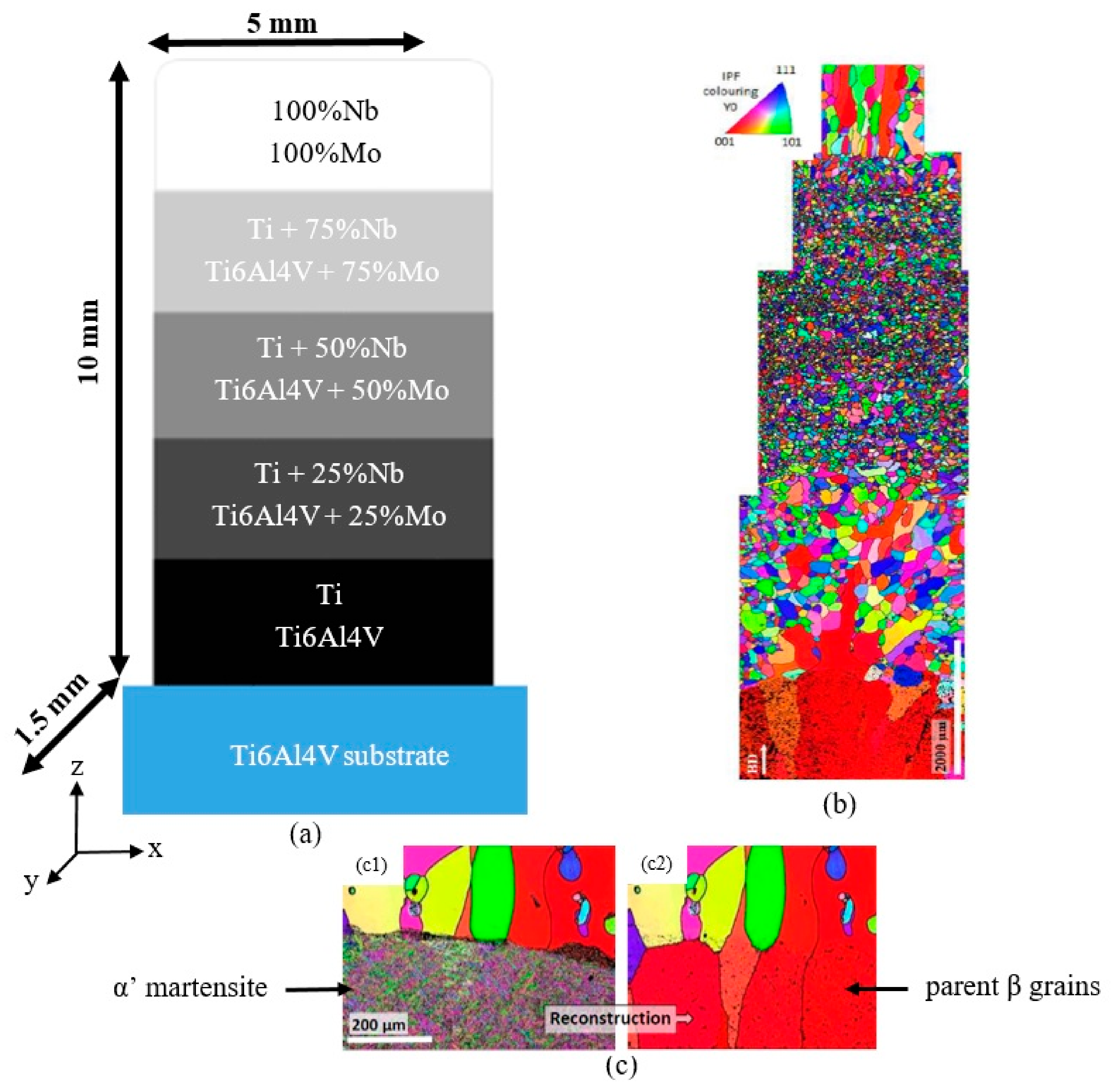
2.1. Sample Preparation
2.2. Roughness and Microscopy
2.3. Sterilization and Cell Culture
2.4. Cell Adhesion and Cell Counting
3. Results and Discussion
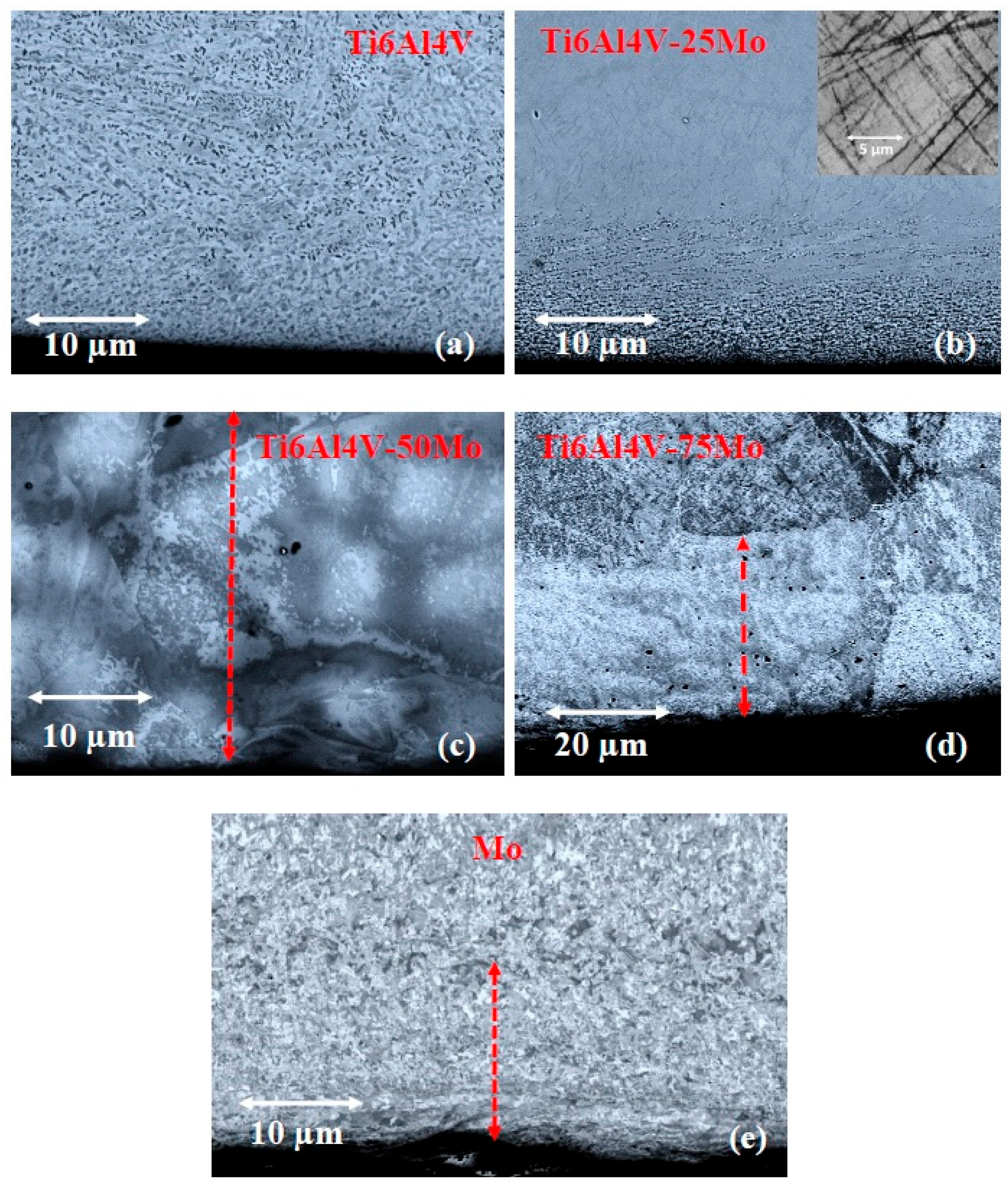
3.1. Evolution of the Microstructure Along the Gradient
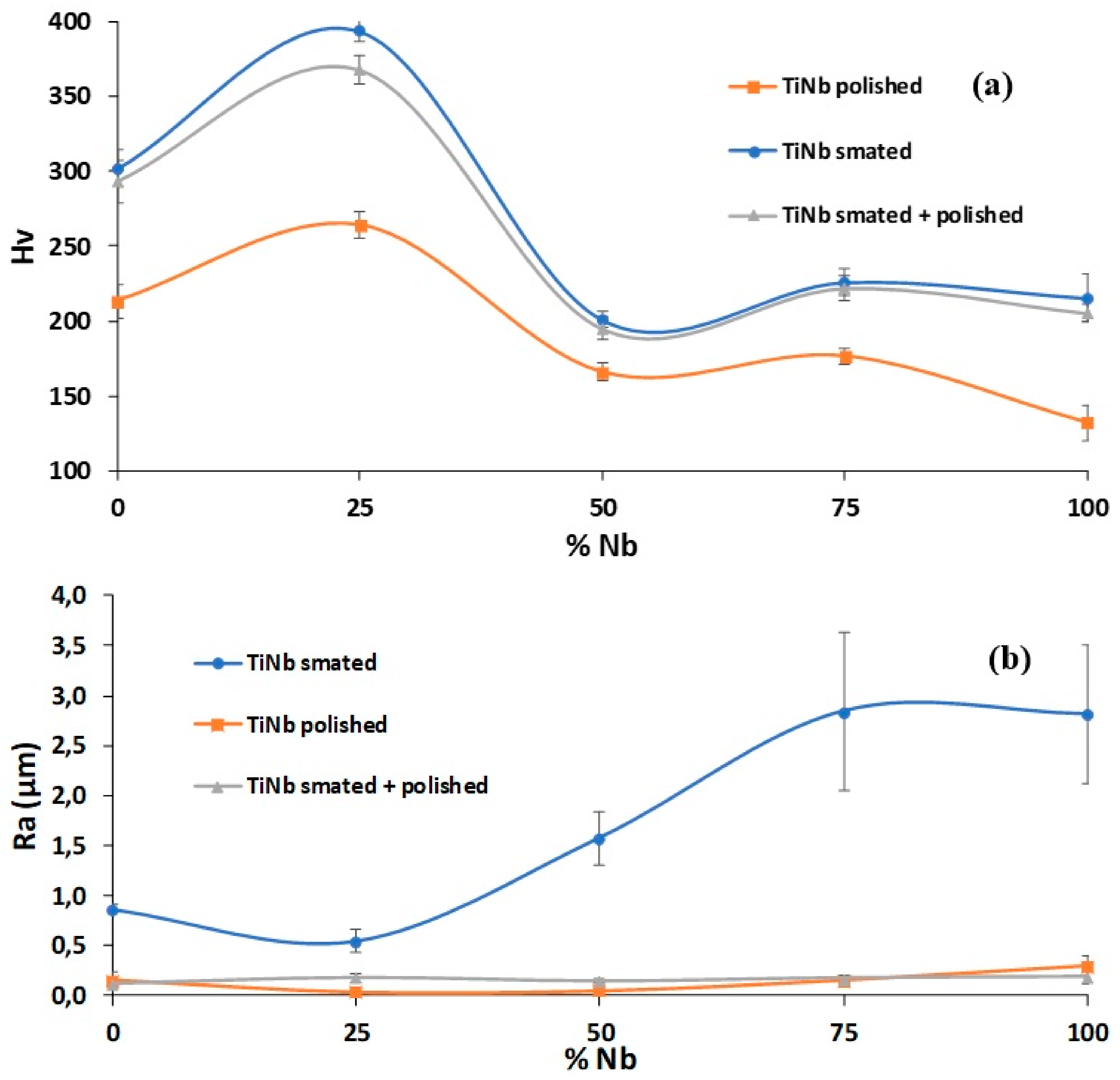
3.2. Hardness and Roughness Evolutions
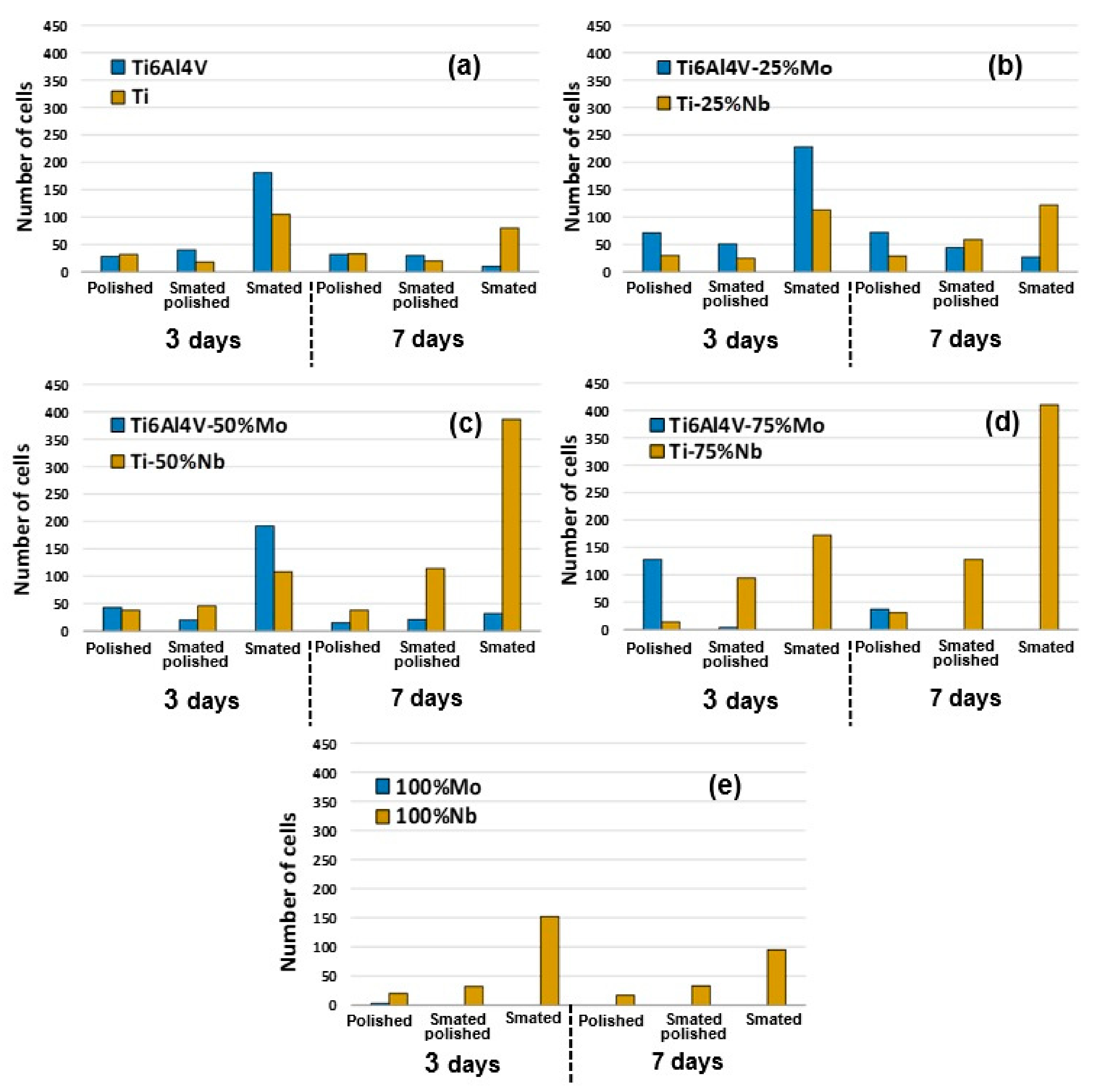
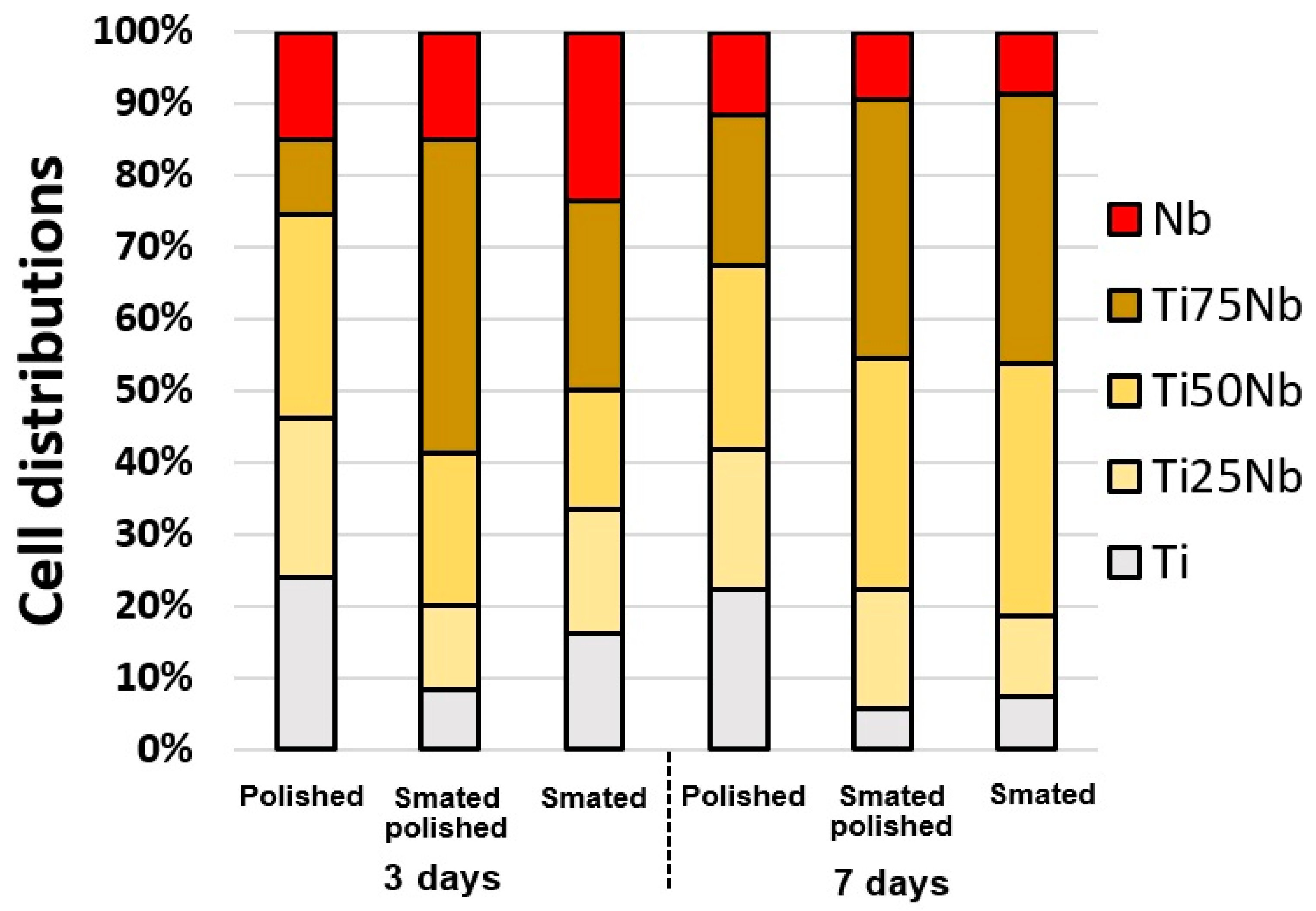
3.3. Cell Adhesion and Cell Proliferation
3.3.1. Comparison of the Effect of Nb and Mo on Titanium Biocompatibility
3.3.2. Cell Adhesion
3.3.3. Long Term Cell Proliferation on FGM
4. Conclusions
- The major findings can be summarized has follows:
- The use of FGMs can greatly reduce the number of samples needed in biocompatibility studies by ensuring at the same time that all tests are done under the same conditions.
- Increase in roughness was induced by SMAT trends to improve the cellular adhesion.
- Comparatively, this increase in roughness dud not modify the proliferation capability of the cells.
- The microstructure refinement and the presence of structural defects induced by severe plastic deformation have an effect on cell distribution during the first stages of proliferation.
- Chemistry modifications are the most important factor to ensure long-term cell proliferation.
- Niobium has better long-term biocompatibility than molybdenum when it is pure or when it is alloyed with titanium.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Ghelichi, R.; Khademhosseini, A.; Guagliano, M. Cell Response to Nanocrystallized Metallic Substrates Obtained through Severe Plastic Deformation. ACS Appl. Mater. Interface 2014, 6, 7963–7985. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Balakrishnan, A.; Lee, B.C.; Kim, W.S.; Smetana, K.; Park, J.K.; Panigrahi, B.B. In vitro biocompatibility of equal channel angular processed (ECAP) titanium. Biomed. Mater. 2007, 2, S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Balakrishnan, A.; Lee, B.C.; Kim, W.S.; Dvorankova, B.; Smetana, K.; Park, J.K.; Panigrahi, B.B. In vitro fibroblast response to ultra fine grained titanium produced by a severe plastic deformation process. J. Mater. Sci. Mater. Med. 2008, 19, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.; Semenova, I.P.; Jakushina, E.; Latysh, V.V.; Rack, H.J.; Lowe, T.C.; Petruželka, J.; Dluhoš, L.; Hrušák, D.; Sochová, J. Nanostructured SPD Processed Titanium for Medical Implants. Mater. Sci. Forum 2008, 584, 49–54. [Google Scholar] [CrossRef]
- Estrin, Y.; Ivanova, E.P.; Michalska, A.; Truong, V.K.; Lapovok, R.; Boyd, R. Accelerated stem cell attachment to ultrafine grained titanium. Acta Biomater. 2011, 7, 900–906. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in vitro biocompatibility of ultrafine-grained titanium with hierarchical porous surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Park, C.H.; Lee, D.H.; Ko, Y.G.; Jang, J.H.; Lee, C.S. Enhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topography. Acta Biomater. 2009, 5, 3272–3280. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. In vitro corrosion and cytotoxicity on microcrystalline, nanocrystalline and amorphous NiTi alloy fabricated by high pressure torsion. Mater. Lett. 2010, 64, 983–986. [Google Scholar] [CrossRef]
- Faghihi, S.; Azari, F.; Zhilyaev, A.; Szpunar, J.; Vali, H.; Tabrizian, M. Cellular and molecular interactions between MC3T3-E1 pre-osteoblasts and nanostructured titanium produced by high-pressure torsion. Biomaterials 2007, 28, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Korotin, D.M.; Bartkowski, S.; Kurmaev, E.Z.; Neumann, M.; Yakushina, E.B.; Valiev, R.Z.; Cholakh, S.O. Surface Studies of Coarse-Grained and Nanostructured Titanium Implants. J. Nanosci. Nanotechnol. 2012, 12, 8567–8572. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Huo, W.; Zhang, W.; Zhao, Y.; Zhang, Y. Electrochemical corrosion characteristics and biocompatibility of nanostructured titanium for implants. Appl. Surf. Sci. 2018, 434, 63–72. [Google Scholar] [CrossRef]
- Bahl, S.; Aleti, B.T.; Suwas, S.; Chatterjee, K. Surface nanostructuring of titanium imparts multifunctional properties for orthopedic and cardiovascular applications. Mater. Des. 2018, 144, 169–181. [Google Scholar] [CrossRef]
- Nie, F.L.; Wang, S.G.; Wang, Y.B.; Wei, S.C.; Zheng, Y.F. Comparative study on corrosion resistance and in vitro biocompatibility of bulk nanocrystalline and microcrystalline biomedical 304 stainless steel. Dent. Mater. 2011, 27, 677–683. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Q.; Lai, W.; Zhao, X.; Shen, H.; Nie, F.; Zheng, Y.; Wei, S.; Ji, J. In vitro bioactivity and biocompatibility evaluation of bulk nanostructured titanium in osteoblast-like cells by quantitative proteomic analysis. J. Mater. Chem. B 2013, 1, 1926. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhang, L.C.; Niu, H.Z.; Bai, X.F.; Yu, S.; Ma, X.Q.; Yu, Z.T. Deformation twinning and localized amorphization in nanocrystalline tantalum induced by sliding friction. Mater. Lett. 2014, 127, 4–7. [Google Scholar] [CrossRef]
- Lu, K.; Lu, J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mater. Sci. Eng. A 2004, 375–377, 38–45. [Google Scholar] [CrossRef]
- Shot Peening for Medical Indusrty. Available online: https://sonats-et.com/en/our-markets/shot-peening-medical-industry/ (accessed on 12 December 2019).
- Bagheri, S.; Guagliano, M. Review of shot peening processes to obtain nanocrystalline surfaces in metal alloys. Surf. Eng. 2009, 25, 3–14. [Google Scholar] [CrossRef]
- Grosdidier, T.; Novelli, M. Recent Developments in the Application of Surface Mechanical Attrition Treatments for Improved Gradient Structures: Processing Parameters and Surface Reactivity. Mater. Trans. 2019, 60, 1344–1355. [Google Scholar] [CrossRef]
- Arifvianto, B.; Suyitno; Mahardika, M.; Dewo, P.; Iswanto, P.T.; Salim, U.A. Effect of surface mechanical attrition treatment (SMAT) on microhardness, surface roughness and wettability of AISI 316L. Mater. Chem. Phys. 2011, 125, 418–426. [Google Scholar] [CrossRef]
- Lai, M.; Cai, K.; Hu, Y.; Yang, X.; Liu, Q. Regulation of the behaviors of mesenchymal stem cells by surface nanostructured titanium. Colloids Surf. B Biointerfaces 2012, 97, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ji, W.; Han, P.; Zhang, J.; Jiang, Y.; Zhang, X. In vitro and in vivo mineralization and osseointegration of nanostructured Ti6Al4V. J. Nanoparticle Res. 2011, 13, 645–654. [Google Scholar] [CrossRef]
- Koizumi, M. FGM activities in Japan. Compos. Part. B Eng. 1997, 28, 1–4. [Google Scholar] [CrossRef]
- Pei, E.; Loh, G.H.; Harrison, D.; de Amorim, H.A.; Monzón Verona, M.D.; Paz, R. A study of 4D printing and functionally graded additive manufacturing. Assem. Autom. 2017, 37, 147–153. [Google Scholar] [CrossRef]
- Roop Kumar, R.; Wang, M. Functionally graded bioactive coatings of hydroxyapatite/titanium oxide composite system. Mater. Lett. 2002, 55, 133–137. [Google Scholar] [CrossRef]
- Bogdanski, D.; Köller, M.; Müller, D.; Muhr, G.; Bram, M.; Buchkremer, H.P.; Stöver, D.; Choi, J.; Epple, M. Easy assessment of the biocompatibility of Ni–Ti alloys by in vitro cell culture experiments on a functionally graded Ni–NiTi–Ti material. Biomaterials 2002, 23, 4549–4555. [Google Scholar] [CrossRef]
- Qian, T.; Liu, D.; Tian, X.; Liu, C.; Wang, H. Microstructure of TA2/TA15 graded structural material by laser additive manufacturing process. Trans. Nonferrous Met. Soc. China 2014, 24, 2729–2736. [Google Scholar] [CrossRef]
- Ren, H.S.; Liu, D.; Tang, H.B.; Tian, X.J.; Zhu, Y.Y.; Wang, H.M. Microstructure and mechanical properties of a graded structural material. Mater. Sci. Eng. A 2014, 611, 362–369. [Google Scholar] [CrossRef]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Bartakova, S.; Prachar, P.; Kudrman, J.; Bresina, V.; Podhorna, B.; Cernochova, P.; Vanek, J.; Strecha, J. New titanuim β-alloys for dental implantology and their laboratory-based assays of biocompatibility. Scr. Med. 2009, 82, 76–82. [Google Scholar]
- Cremasco, A.; Messias, A.D.; Esposito, A.R.; de Rezende Duek, E.A.; Caram, R. Effects of alloying elements on the cytotoxic response of titanium alloys. Mater. Sci. Eng. C 2011, 31, 833–839. [Google Scholar] [CrossRef]
- Schneider-Maunoury, C.; Weiss, L.; Perroud, O.; Joguet, D.; Boisselier, D.; Laheurte, P. An application of differential injection to fabricate functionally graded Ti-Nb alloys using DED-CLAD® process. J. Mater. Process. Technol. 2019, 268, 171–180. [Google Scholar] [CrossRef]
- Schneider-Maunoury, C.; Weiss, L.; Acquier, P.; Boisselier, D.; Laheurte, P. Functionally graded Ti6Al4V-Mo alloy manufactured with DED-CLAD® process. Addit. Manuf. 2017, 17, 55–66. [Google Scholar] [CrossRef]
- Novelli, M.; Bocher, P.; Grosdidier, T. Effect of cryogenic temperatures and processing parameters on gradient-structure of a stainless steel treated by ultrasonic surface mechanical attrition treatment. Mater. Charact. 2018, 139, 197–207. [Google Scholar] [CrossRef]
- Samih, Y.; Novelli, M.; Thiriet, T.; Bolle, B.; Allain, N.; Fundenberger, J.J.; Marcos, G.; Czerwiec, T.; Grosdidier, T. Plastic deformation to enhance plasma-assisted nitriding: On surface contamination induced by Surface Mechanical Attrition Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2014, 63, 012020. [Google Scholar] [CrossRef]
- Alikhani Chamgordani, S.; Miresmaeili, R.; Aliofkhazraei, M. Improvement in tribological behavior of commercial pure titanium (CP-Ti) by surface mechanical attrition treatment (SMAT). Tribol. Int. 2018, 119, 744–752. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Guo, L.X.; Ouyang, J.H. Iron-rich layer introduced by SMAT and its effect on corrosion resistance and wear behavior of 2024 Al alloy. Mater. Chem. Phys. 2011, 126, 301–309. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Jin, Y.; Ren, X. Comparison of corrosion behaviour of nanocrystalline 2024-T4 Al alloy processed by surface mechanical attrition treatment with two different mediums. Corros. Eng. Sci. Technol. 2015, 50, 425–432. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, W.; Wang, Y.; Zhang, S. Kink deformation in a beta titanium alloy at high strain rate. Mater. Sci. Eng. A 2017, 702, 218–224. [Google Scholar] [CrossRef]
- Lee, C.M.; Ju, C.P.; Chern Lin, J.H. Structure-property relationship of cast Ti-Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Thoemmes, A.; Bataev, I.A.; Belousova, N.S.; Lazurenko, D.V. Microstructure and mechanical properties of binary Ti-Nb alloys for application in medicine. In Proceedings of the 2016 11th International Forum on Strategic Technology (IFOST), Novosibirsk, Russia, 1–3 June 2016; pp. 26–29. [Google Scholar]
- Ho, W.F. Effect of omega phase on mechanical properties of Ti-Mo alloys for biomediacal applications. J. Med. Biol. Eng. 2007, 28, 47–51. [Google Scholar]
- Sadeghpour, S.; Abbasi, S.M.; Morakabati, M.; Karjalainen, L.P. Effect of dislocation channeling and kink band formation on enhanced tensile properties of a new beta Ti alloy. J. Alloys Compd. 2019, 808, 151741. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti}6Al}4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Huang, H.H.; Ho, C.T.; Lee, T.H.; Lee, T.L.; Liao, K.K.; Chen, F.L. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol. Eng. 2004, 21, 93–97. [Google Scholar] [CrossRef]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramón-Torregrosa, P.J.; Díaz-Rodríguez, L.; García-Martínez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vílchez, M.A. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf. Physicochem. Eng. Asp. 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Johansson, C.B.; Albrektsson, T.; Ericson, L.E.; Thomsen, P. A quantitative comparison of the cell response to commercially pure titanium and Ti-6Al-4V implants in the abdominal wall of rats. J. Mater. Sci. Mater. Med. 1992, 3, 126–136. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Ju, C.P.; Lee, C.M.; Chern Lin, J.H. Medical Implant Made of Biocompatible Low Modulus High Strength Titanium-Niobium Alloy and Method of Using the Same. Patent US20020162608A1, 7 November 2002. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, L.; Nessler, Y.; Novelli, M.; Laheurte, P.; Grosdidier, T. On the Use of Functionally Graded Materials to Differentiate the Effects of Surface Severe Plastic Deformation, Roughness and Chemical Composition on Cell Proliferation. Metals 2019, 9, 1344. https://doi.org/10.3390/met9121344
Weiss L, Nessler Y, Novelli M, Laheurte P, Grosdidier T. On the Use of Functionally Graded Materials to Differentiate the Effects of Surface Severe Plastic Deformation, Roughness and Chemical Composition on Cell Proliferation. Metals. 2019; 9(12):1344. https://doi.org/10.3390/met9121344
Chicago/Turabian StyleWeiss, Laurent, Yaël Nessler, Marc Novelli, Pascal Laheurte, and Thierry Grosdidier. 2019. "On the Use of Functionally Graded Materials to Differentiate the Effects of Surface Severe Plastic Deformation, Roughness and Chemical Composition on Cell Proliferation" Metals 9, no. 12: 1344. https://doi.org/10.3390/met9121344
APA StyleWeiss, L., Nessler, Y., Novelli, M., Laheurte, P., & Grosdidier, T. (2019). On the Use of Functionally Graded Materials to Differentiate the Effects of Surface Severe Plastic Deformation, Roughness and Chemical Composition on Cell Proliferation. Metals, 9(12), 1344. https://doi.org/10.3390/met9121344





