Effect of Austenitizing Temperature and Prior Martensite on Ultra-Fine Bainite Transformation Kinetics
Abstract
1. Introduction
2. Material and Experimental Methods
2.1. Material Preparation
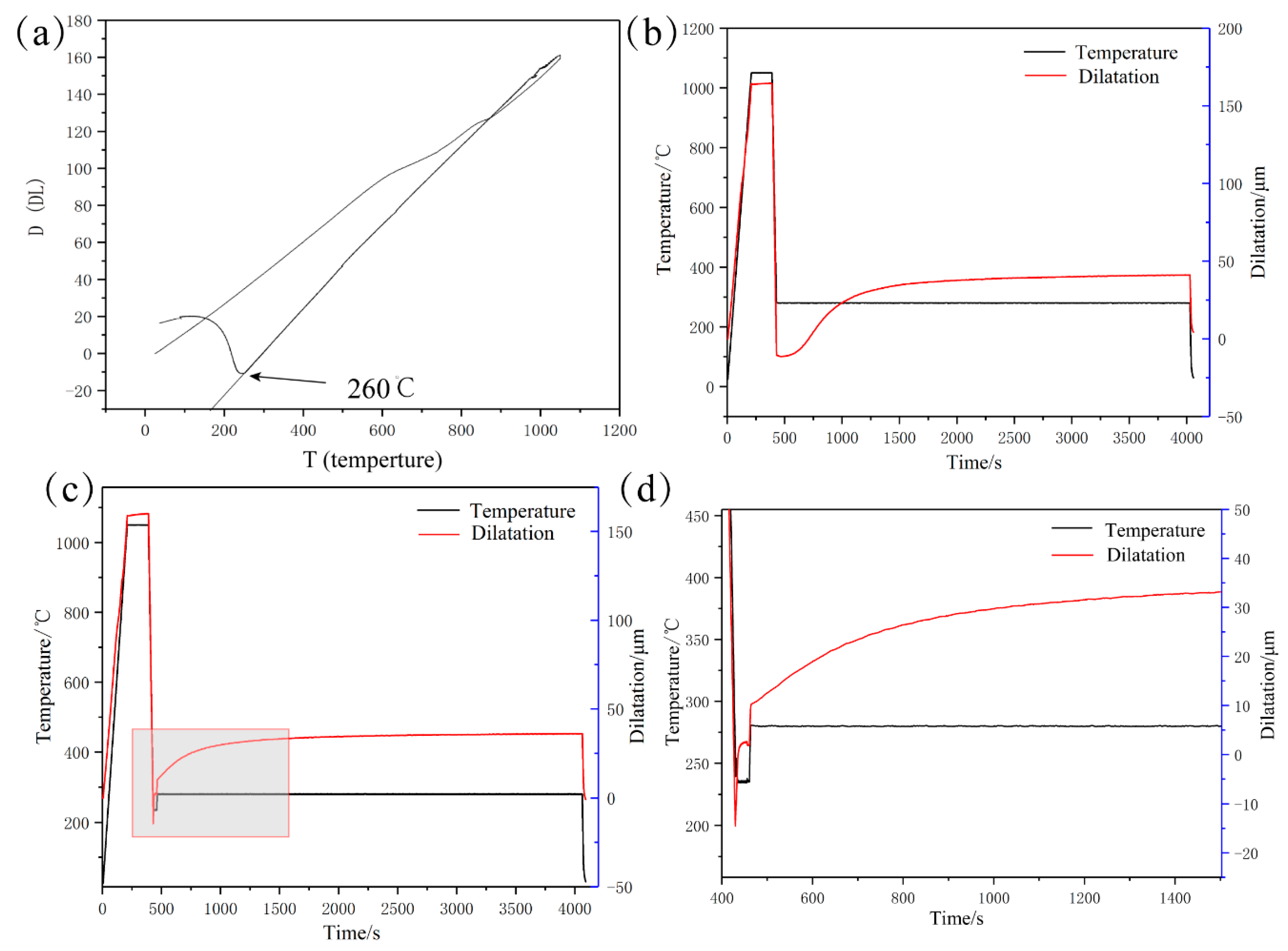
2.2. Determination of the Dilatometric Curve
2.3. Microstructure Observation
3. Results
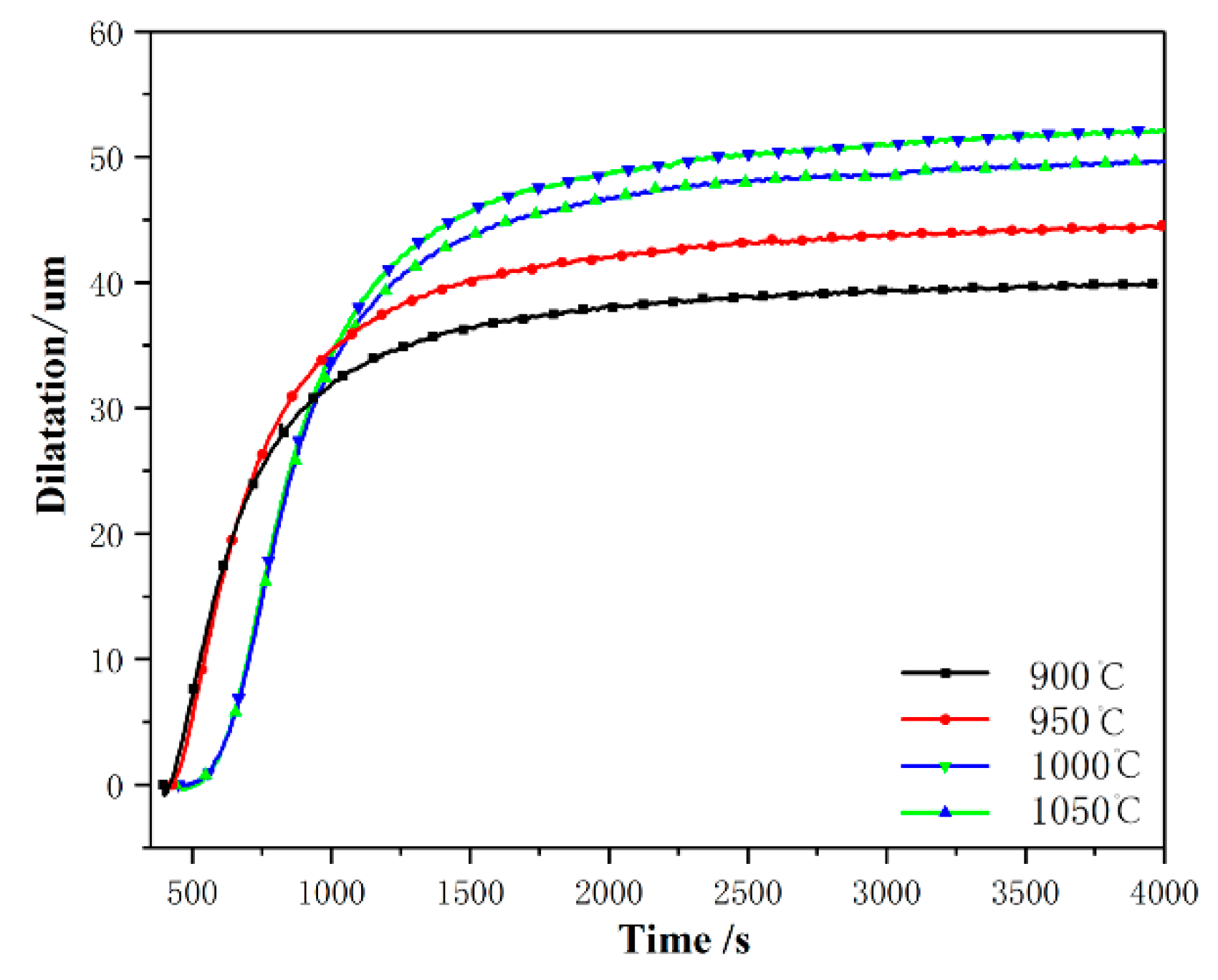
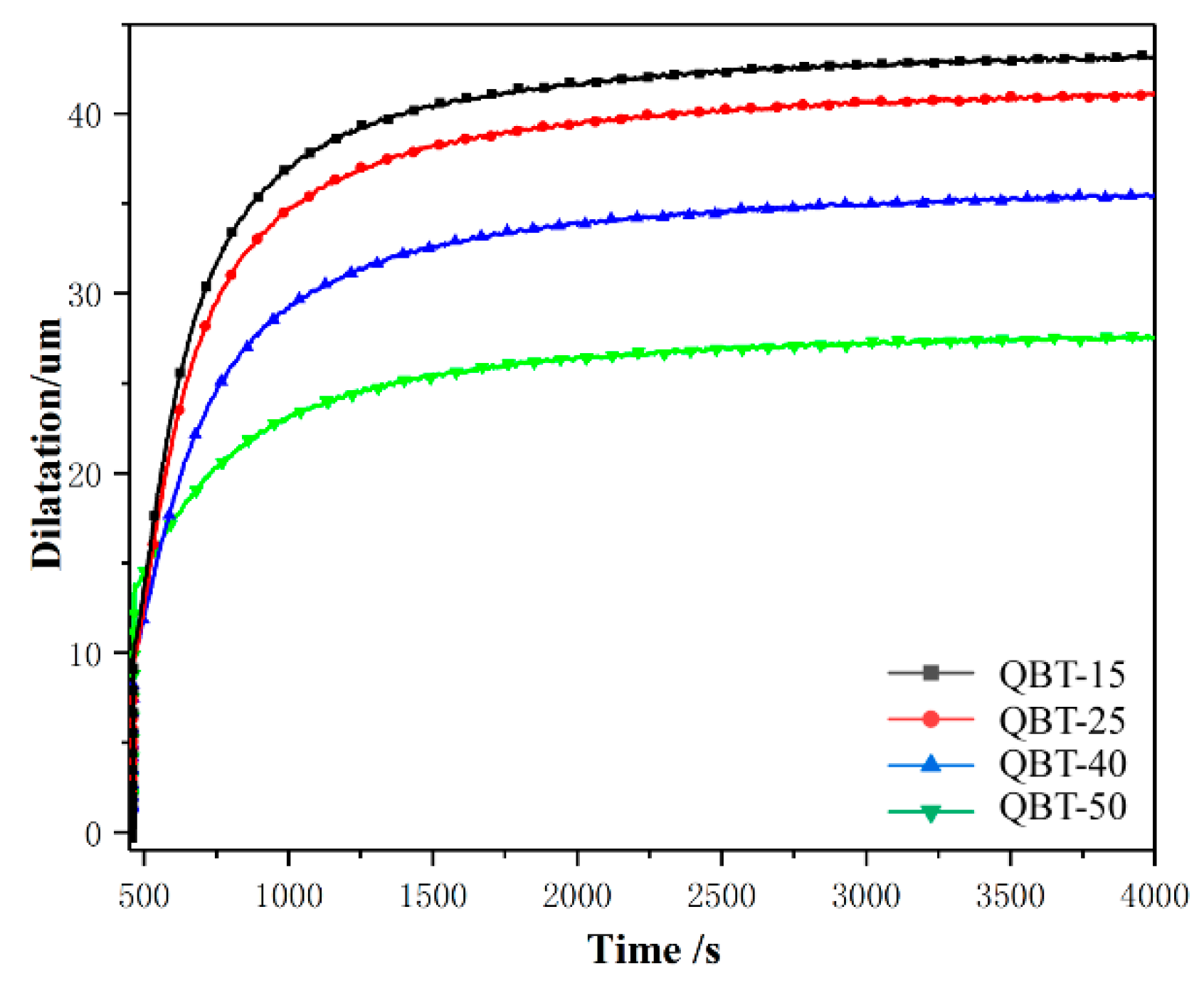
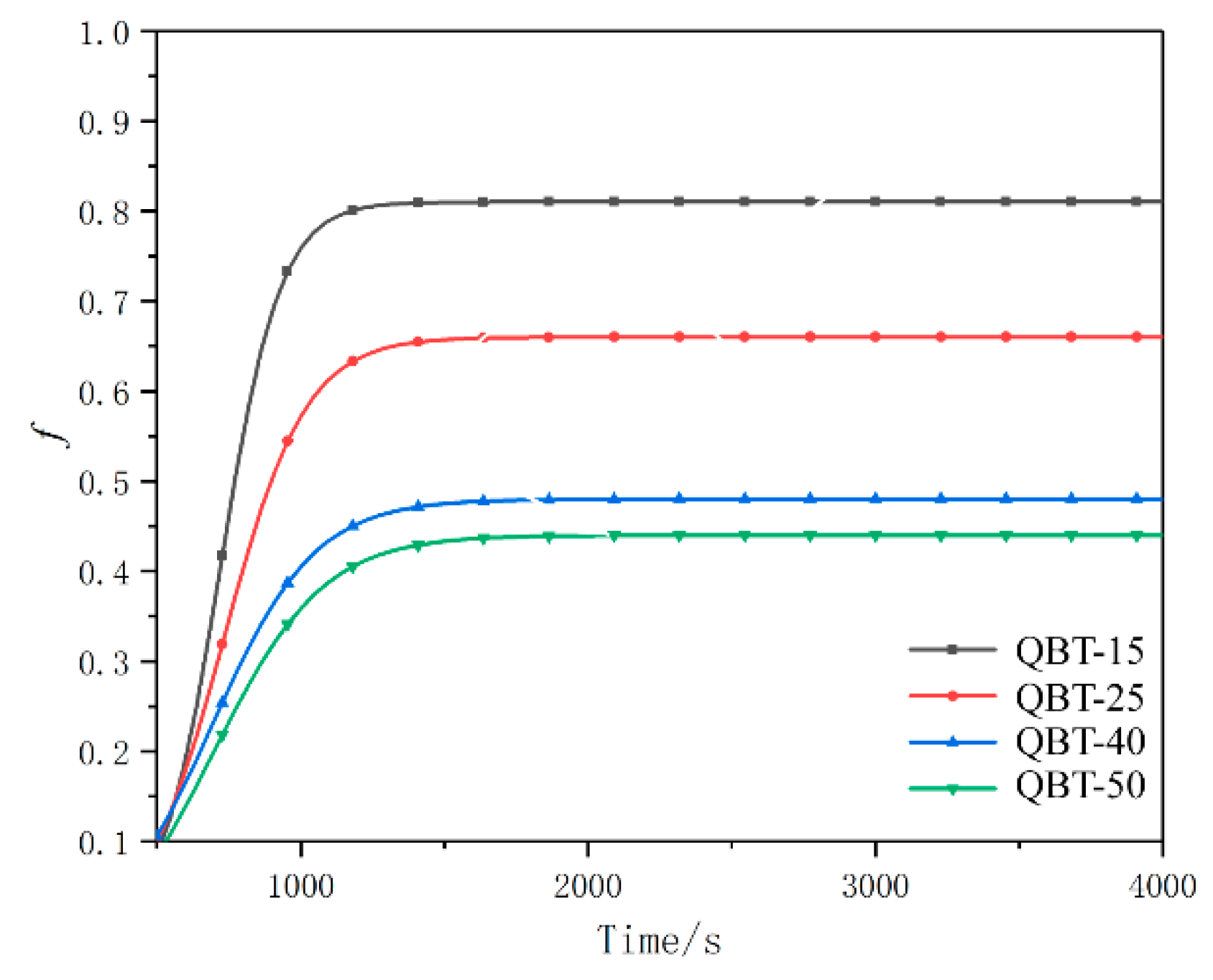
3.1. Phase Transformation
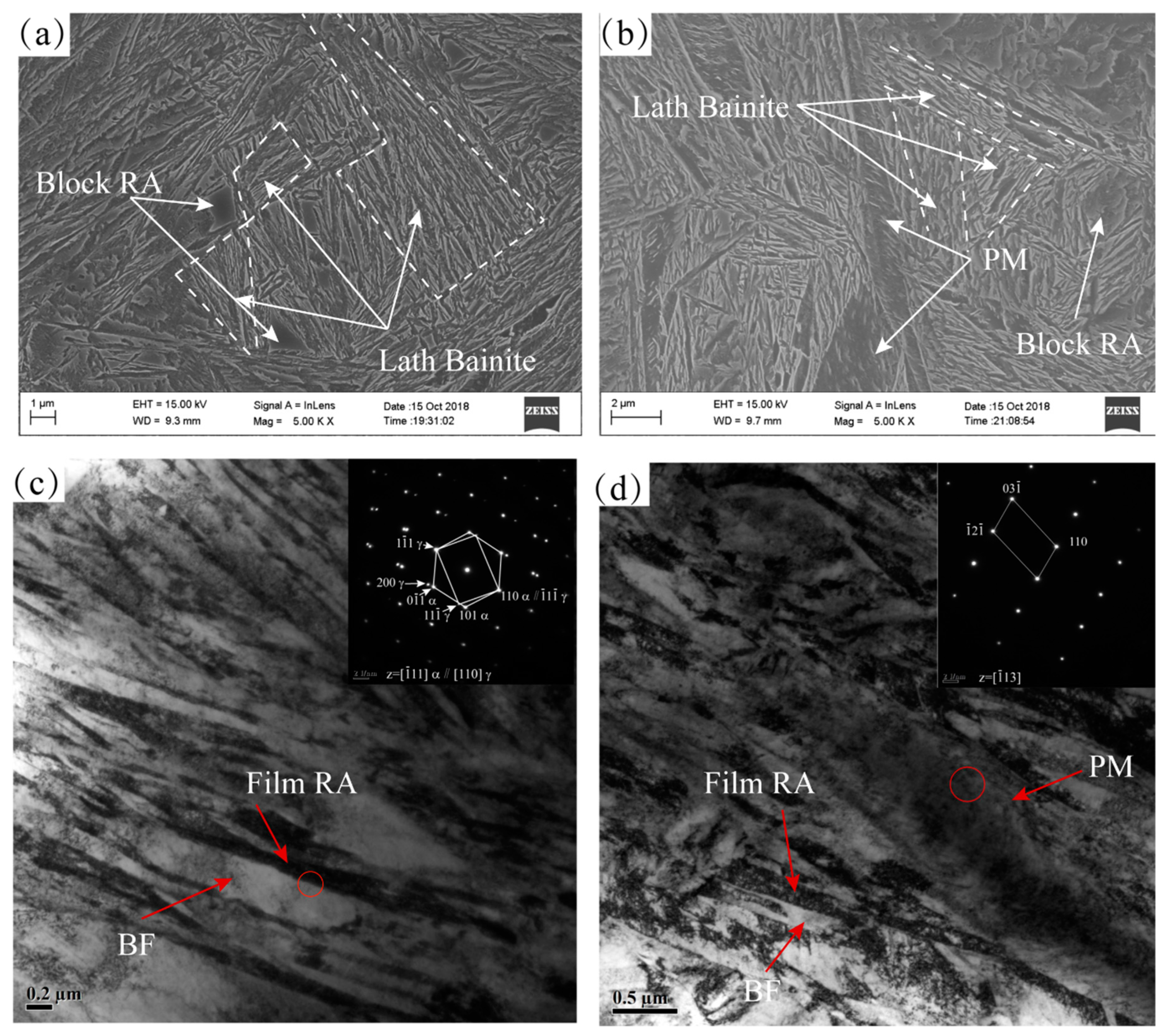
3.2. Microstructure Observation
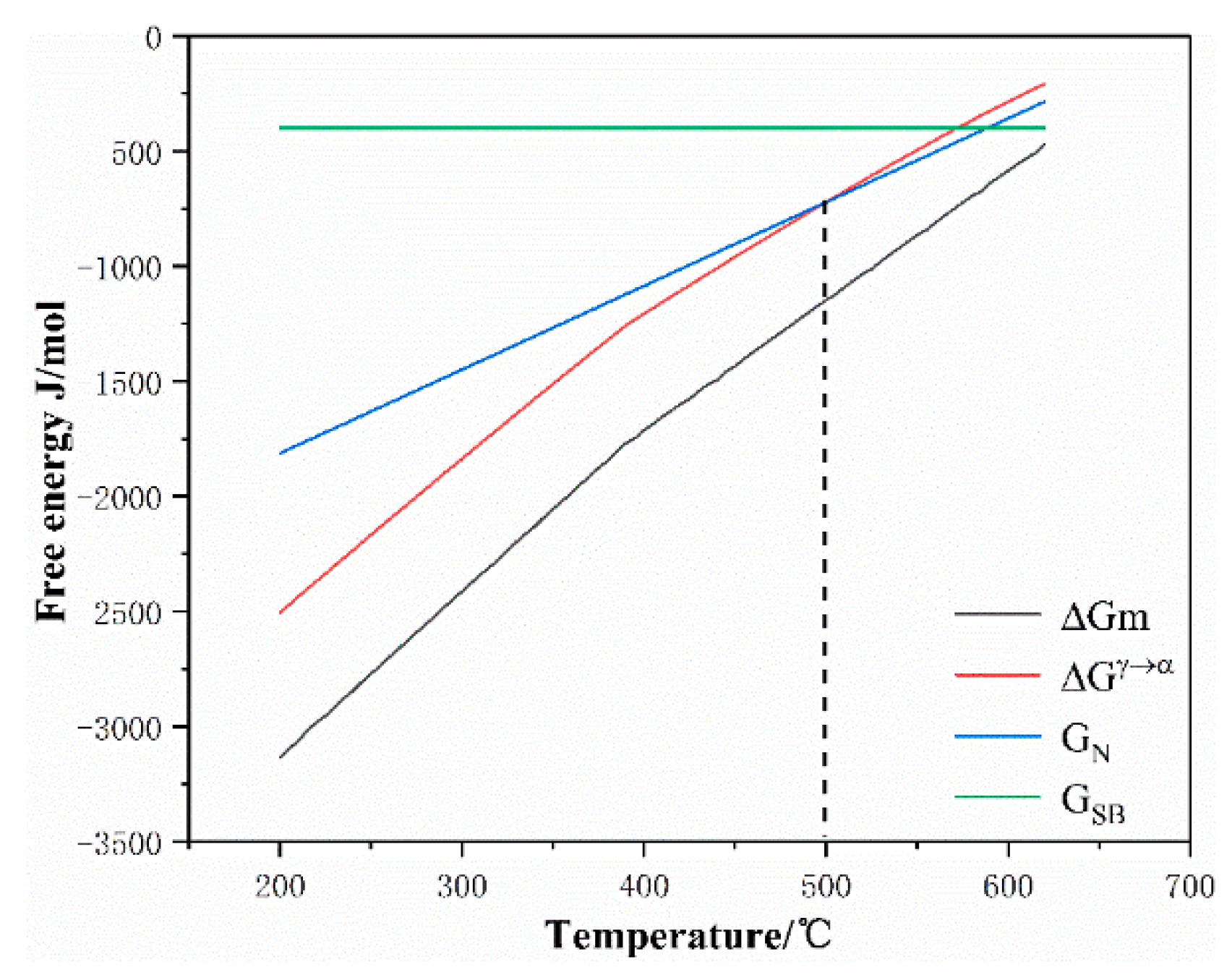
3.3. Model Establishment
4. Analysis and Discussion
4.1. Effect of the Prior Austenite Grain Size on the Transformation Kinetics of Ultra-Fine Bainite
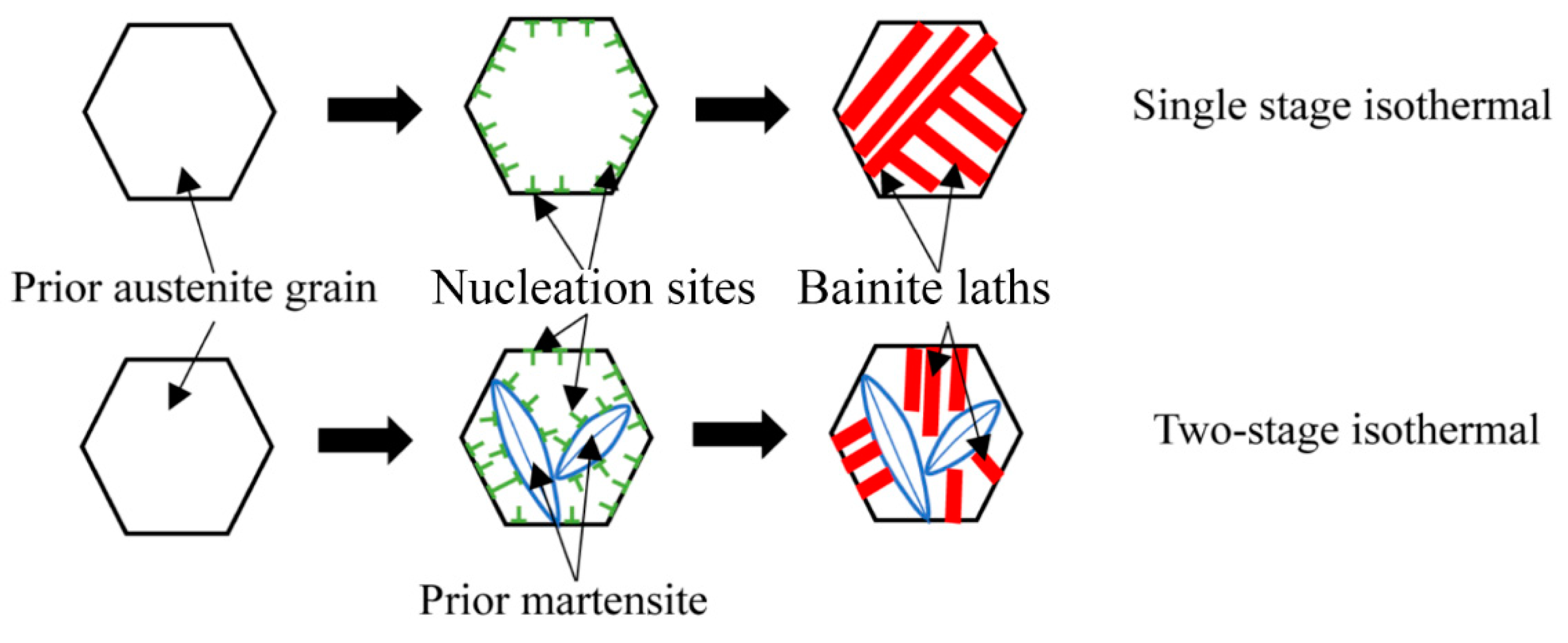
4.2. Effect of Martensite on the Transformation Kinetics of Ultra-Fine Bainite
5. Conclusions
- The decrease of austenite temperature will reduce the size of the prior austenite grains, reduce the activation of bainite transformation, and increase the nucleation rate and the number density of nucleation sites at the grain boundaries, thus accelerating the ultra-fine bainite phase transformation.
- Prior martensite can cause abnormal strain in the austenite, which reduces the activation energy of the ultra-fine bainite transformation, promotes the nucleation rate, increases the nucleation sites of the ultra-fine bainite, and increases the number density of nucleation sites Ni, thus accelerating the ultra-fine bainite phase transformation.
- As the content of prior martensite increases, the average volume of ultra-fine bainite subunit decreases, the nucleation density and the nucleation rate of ultrafine bainite phase transformation will increases.
Author Contributions
Funding
Conflicts of Interest
References
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Very strong low temperature bainite. Mater. Sci. Technol. 2002, 18, 279–284. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainitic steels: Part 2. Mater. Sci. Technol. 2001, 17, 517–522. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainitic steels: Part 1. Mater. Sci. Technol. 2001, 17, 512–516. [Google Scholar] [CrossRef]
- Soliman, M.; Palkowski, H. Development of the low temperature bainite. Arch. Civ. Mech. Eng. 2016, 16, 403–412. [Google Scholar] [CrossRef]
- Hu, H.; Xu, G.; Wang, L.; Xue, Z.; Zhang, Y.; Liu, G. The effects of Nb and Mo addition on transformation and properties in low carbon bainitic steels. Mater. Des. 2015, 84, 95–99. [Google Scholar] [CrossRef]
- Huang, H.; Sherif, M.Y.; Rivera-Diaz-Del-Castillo, P.E.J. Combinatorial optimization of carbide-free bainitic nanostructures. Acta Mater. 2013, 61, 1639–1647. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.S.; Zhang, B.; Zhang, F.C. Microstructure and mechanical properties of high-carbon Si–Al-rich steel by low-temperature austempering. Mater. Des. 2012, 35, 170–174. [Google Scholar] [CrossRef]
- Yoozbashi, M.N.; Yazdani, S.; Wang, T.S. Design of a new nanostructured, high-Si bainitic steel with lower cost production. Mater. Des. 2011, 32, 3248–3253. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef]
- Godet, S.; Harlet, P.; Delannay, F.; Jacques, P.J. Critical Assessment of the Bainite Transformation of Finely Grained and Deformed Austenite. Mater. Sci. Forum 2003, 6, 426–432. [Google Scholar] [CrossRef]
- García-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of Low-temperature Bainite. ISIJ Int. 2003, 43, 285–288. [Google Scholar] [CrossRef]
- Hu, F.; Hodgson, P.D.; Wu, K.M. Acceleration of the super bainite transformation through a coarse austenite grain size. Mater. Lett. 2014, 122, 240–243. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, K.M.; Hu, F.; Yu, L.; Wan, X.L. Multi-step isothermal bainitic transformation in medium-carbon steel. Scr. Mater. 2014, 74, 56–59. [Google Scholar] [CrossRef]
- Xu, G.; Feng, L.; Li, W.; Hu, H. A new approach to quantitative analysis of bainitic transformation in a superbainite steel. Scr. Mater. 2013, 68, 833–836. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Harjo, S.; Su, Y.H.; Aizawa, K. Effect of prior martensite on bainite transformation in nanobainite steel. Acta Mater. 2015, 85, 243–249. [Google Scholar] [CrossRef]
- Kawata, H.; Hayashi, K.; Sugiura, N.; Yoshinaga, N.; Takahashi, M. Effect of martensite in initial structure on bainite transformation. Mater. Sci. Forum 2010, 638, 3307–3312. [Google Scholar] [CrossRef]
- Cui, X.; Northwood, D.O.; Liu, C. Role of Prior Martensite in a 2.0 GPa Multiple Phase Steel. Steel Res. Int. 2018, 89. [Google Scholar] [CrossRef]
- Miyamoto, G.; Iwata, N.; Takayama, N.; Furuhara, T. Variant selection of lath martensite and bainite transformation in low carbon steel by ausforming. J. Alloys Compd. 2013, 577, S528–S532. [Google Scholar] [CrossRef]
- Toji, Y.; Matsuda, H.; Raabe, D. Effect of Si on the acceleration of bainite transformation by pre-existing martensite. Acta Mater. 2016, 116, 250–262. [Google Scholar] [CrossRef]
- Quidort, D.; Brechet, Y.J.M. A model of isothermal and non isothermal transformation kinetics of bainite in 0.5% C steels. ISIJ Int. 2002, 42, 1010–1017. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, 2nd ed.; The Institute of Materials: London, UK, 2001. [Google Scholar]
- Van Bohemen, S.M.; Sietsma, J. Modeling of isothermal bainite formation based on the nucleation kinetics. Int. J. Mater. Res. 2008, 99, 739–747. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C. Autocatalytic nature of the bainitic transformation in steels: A new hypothesis. Philos. Mag. 2013, 93, 388–408. [Google Scholar] [CrossRef]
- Samanta, S.; Das, S. Development of Multiphase Microstructure with Bainite, Martensite, and Retained Austenite in a Co-Containing Steel Through Quenching and Partitioning (Q&P) Treatment. Metall. Mater. Trans. A 2013, 44, 5653–5664. [Google Scholar]
- Chester, N.A.; Bhadeshia, H.K.D.H. Mathematical Modelling of Bainite Transformation Kinetics. J. Phys. IV Fr. 1997, 07, C5-41–C5-46. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Caballero, F.G.; Capdevila, C.; Garcia-Mateo, C.; Garcia De Andres, C. Evaluation of displacive models for bainite transformation kinetics in steels. Mater. Trans. 2006, 47, 1492–1500. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, F.C.; Yang, X.W.; Lv, B.; Wu, K.M. Effect of tempering on the microstructure and mechanical properties of a medium carbon bainitic steel. Mater. Sci. Eng. A 2017, 686, 150–159. [Google Scholar] [CrossRef]
- Chang, L.C.; Bhadeshia, H.K.D.H. Austenite films in bainitic microstructures. Mater. Sci. Technol. 1995, 11, 874–882. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Zhao, A.; Li, Q.; Ma, J. Influence of prior martensite on bainite transformation, microstructures, and mechanical properties in ultra-fine bainitic steel. Materials 2019, 12, 527. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C. Modeling start curves of bainite formation. Metall. Mater. Trans. A 2010, 41, 285–296. [Google Scholar] [CrossRef]
- Liu, S.K.; Zhang, J. The influence of the Si and Mn concentrations on the kinetics of the bainite transformation in Fe-C-Si-Mn alloys. Metall. Trans. A 1990, 21, 1517–1525. [Google Scholar] [CrossRef]
- Ravi, A.M.; Sietsma, J.; Santofimia, M.J. Bainite formation kinetics in steels and the dynamic nature of the autocatalytic nucleation process. Scr. Mater. 2017, 140, 82–86. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. The mechanism of bainite formation in steels. Acta Metall. 1980, 28, 1265–1273. [Google Scholar] [CrossRef]
- Pickering, F.B. Bainite in Steels. Surf. Eng. 2014, 8, 264–265. [Google Scholar] [CrossRef]
- Singh, S.B. 10-Mechanisms of bainite transformation in steels. In Phase Transformations in Steels; Pereloma, E., Edmonds, D.V., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 385–416. [Google Scholar]
- Hillert, M.; Hoglund, L.; Ågren, J. Diffusion-controlled lengthening of Widmanstatten plates. Acta Mater. 2003, 51, 2089–2095. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Xie, F.; Lai, C.; Li, H.; Zhang, Q. Dynamic Recrystallization Behavior and Critical Strain of 51CrV4 High-Strength Spring Steel During Hot Deformation. JOM-US 2018, 70, 2385–2391. [Google Scholar] [CrossRef]
- Rees, G.I.; Bhadeshia, H.K.D.H. Bainite transformation kinetics. Part 1. Modified model. Mater. Sci. Technol. 1992, 8, 985–993. [Google Scholar] [CrossRef]
- Garbarz, B.; Niżnik Harańczyk, B. Modification of microstructure to increase impact toughness of nanostructured bainite–austenite steel. Mater. Sci. Technol. 2015, 31, 773–780. [Google Scholar] [CrossRef]
- Smanio, V.; Sourmail, T. Effect of Partial Martensite Transformation on Bainite Reaction Kinetics in Different 1%C Steels. Solid State Phenom. 2011, 172–174, 821–826. [Google Scholar] [CrossRef]





| Elements | C | Si | Mn | Co | Cr | Mo | Ni | Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Composition | 0.51 | 1.72 | 0.83 | 0.56 | 0.98 | 0.25 | 0.60 | 0.04 | Bal. |
| Taus/ °C | Grain Size d/μm | λ | Vb m3 | κ | Q* KJ/mol | Ni m−3 | dN/dt m−3s−1 |
|---|---|---|---|---|---|---|---|
| 1050 | 92.82 ± 2.99 | 78 | 1.36 × 10−18 | 5.04 × 10−5 | 146.14 | 1.67 × 1014 | 7.22 × 1013 |
| 1000 | 72.21 ± 1.99 | 57 | 1.36 × 10−18 | 7.19 × 10−5 | 145.66 | 2.15 × 1014 | 8.37 × 1013 |
| 950 | 49.8 ± 2.34 | 24 | 1.36 × 10−18 | 1.94 × 10−4 | 142.81 | 3.11 × 1014 | 1.75 × 1014 |
| 900 | 32.74 ± 3.82 | 17 | 1.36 × 10−18 | 4.60 × 10−4 | 140.77 | 4.74 × 1014 | 3.91 × 1014 |
| Taus °C | Heat Treatment Parameters | fM % | λ | Vb m3 | κ | Q* KJ/mol | Ni m−3 | dN/dt m−3s−1 |
|---|---|---|---|---|---|---|---|---|
| 1050 | DIT | 0 | 78 | 1.36 × 1018 | 5.40 × 105 | 146.14 | 1.67 × 1014 | 7.22 × 1013 |
| 1050 | QBT-15 | 21 | 670 | 6.50 × 1019 | 1.43 × 105 | 145.86 | 3.50 × 1014 | 1.67 × 1014 |
| 1050 | QBT-25 | 35 | 130 | 5.20 × 1019 | 5.36 × 105 | 142.60 | 4.37 × 1014 | 2.3 × 1014 |
| 1050 | QBT-40 | 49 | 50 | 3.90 × 1019 | 1.09 × 104 | 139.65 | 5.83 × 1014 | 4.15 × 1014 |
| 1050 | QBT-50 | 56 | 24 | 2.60 × 1019 | 2.07 × 104 | 136.84 | 8.75 × 1014 | 9.77 × 1014 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, P.; Luo, Y.; Zhou, X.; Qi, L.; Li, S.; Wang, Z. Effect of Austenitizing Temperature and Prior Martensite on Ultra-Fine Bainite Transformation Kinetics. Metals 2019, 9, 1309. https://doi.org/10.3390/met9121309
Li Z, Li P, Luo Y, Zhou X, Qi L, Li S, Wang Z. Effect of Austenitizing Temperature and Prior Martensite on Ultra-Fine Bainite Transformation Kinetics. Metals. 2019; 9(12):1309. https://doi.org/10.3390/met9121309
Chicago/Turabian StyleLi, Zhiyong, Pengfei Li, Yang Luo, Xiyue Zhou, Liang Qi, Shengci Li, and Zhigang Wang. 2019. "Effect of Austenitizing Temperature and Prior Martensite on Ultra-Fine Bainite Transformation Kinetics" Metals 9, no. 12: 1309. https://doi.org/10.3390/met9121309
APA StyleLi, Z., Li, P., Luo, Y., Zhou, X., Qi, L., Li, S., & Wang, Z. (2019). Effect of Austenitizing Temperature and Prior Martensite on Ultra-Fine Bainite Transformation Kinetics. Metals, 9(12), 1309. https://doi.org/10.3390/met9121309






