Study of Nanometer-Sized Precipitation and Properties of Fire Resistant Hot-Rolled Steel
Abstract
1. Introduction
2. Experimental Material and Procedure
3. Results and Discussion
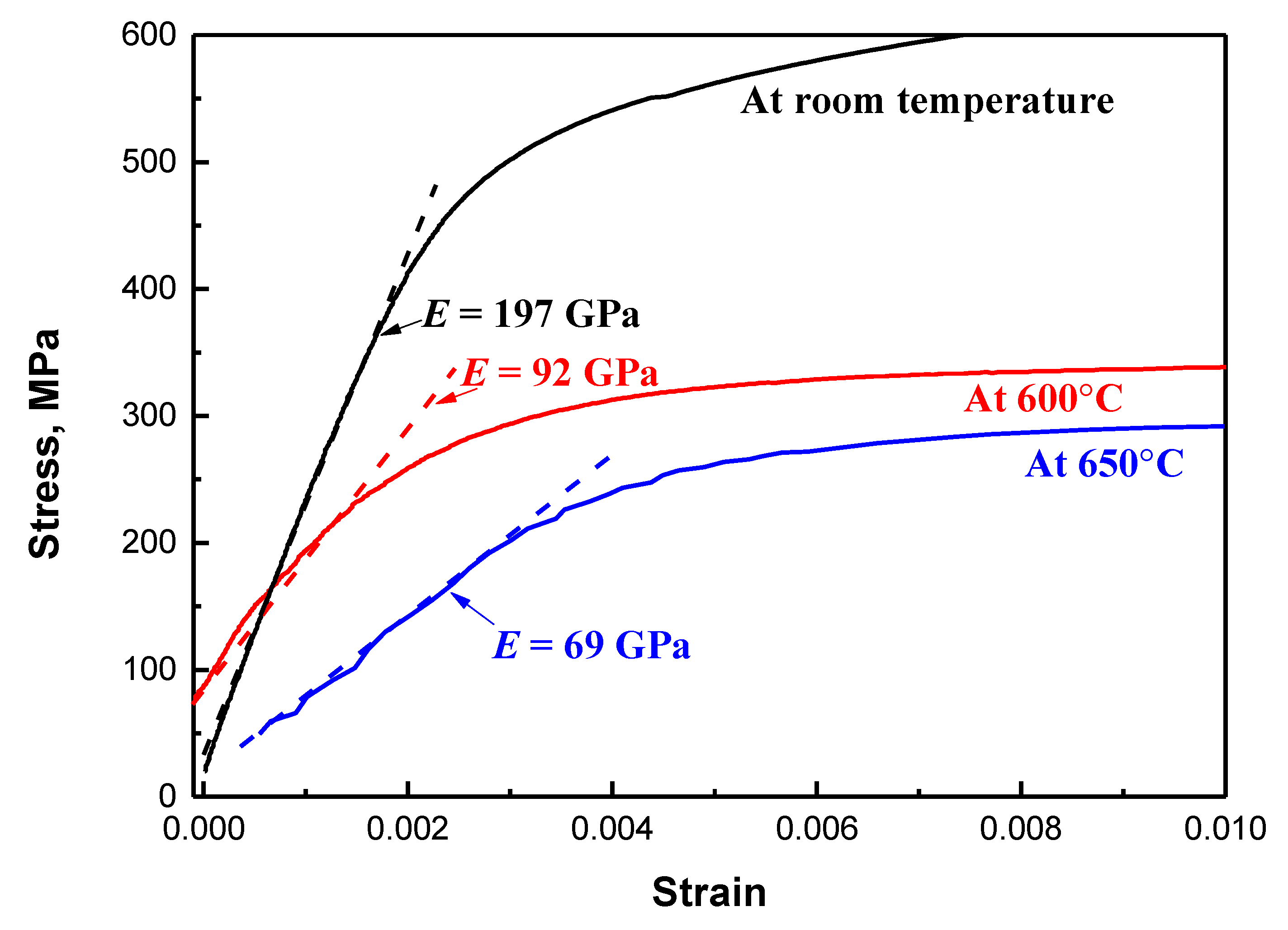
3.1. Tensile Properties
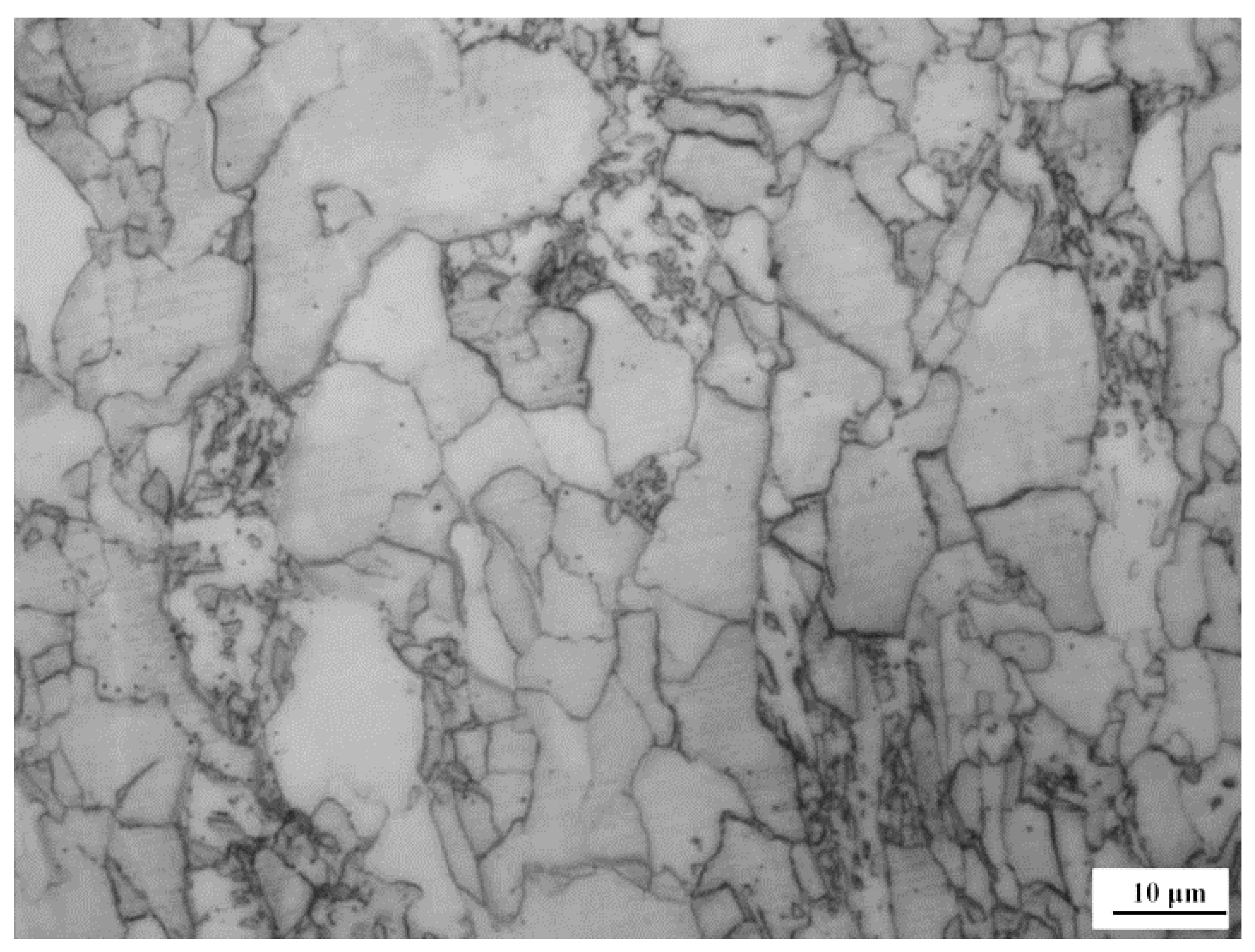
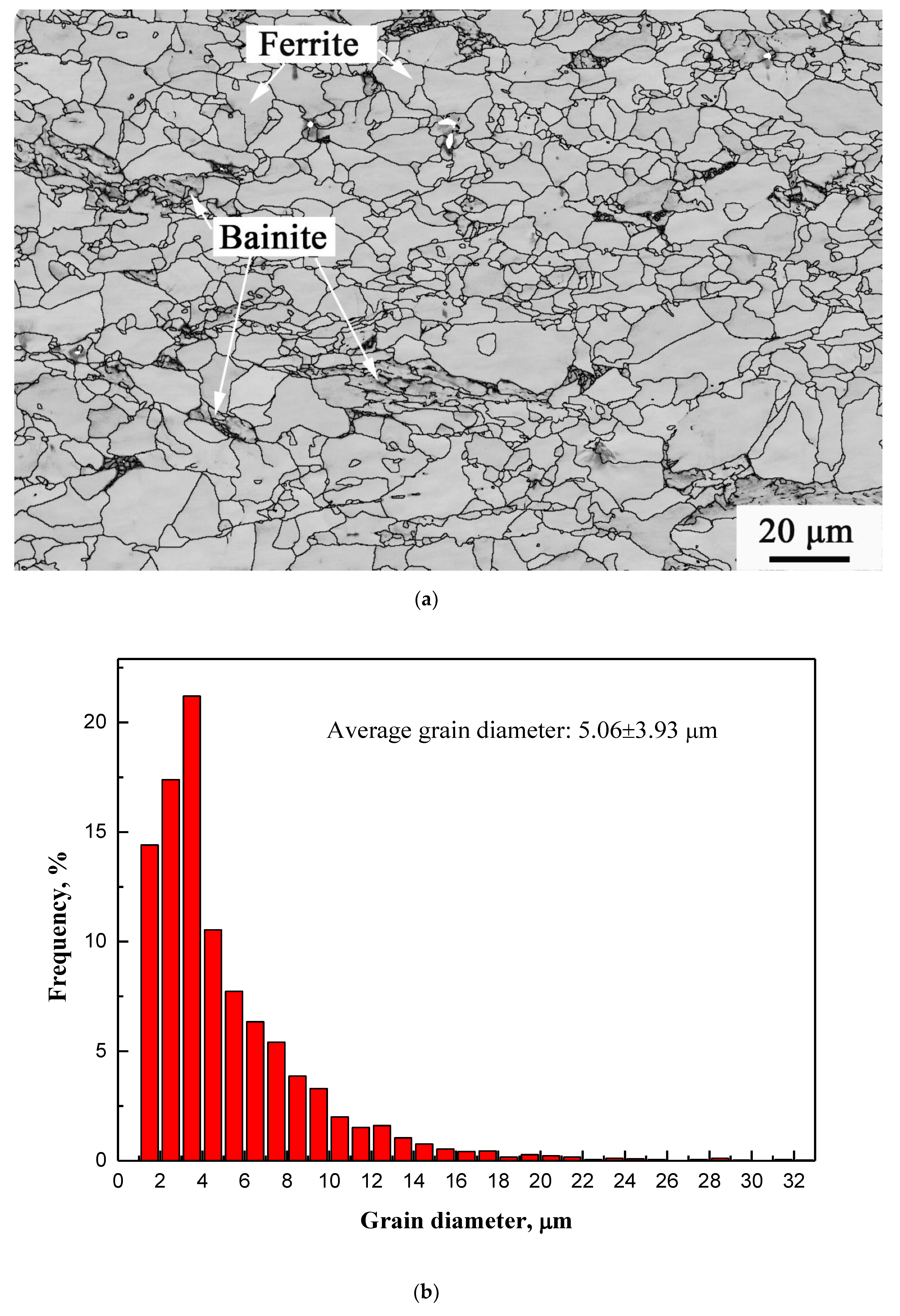
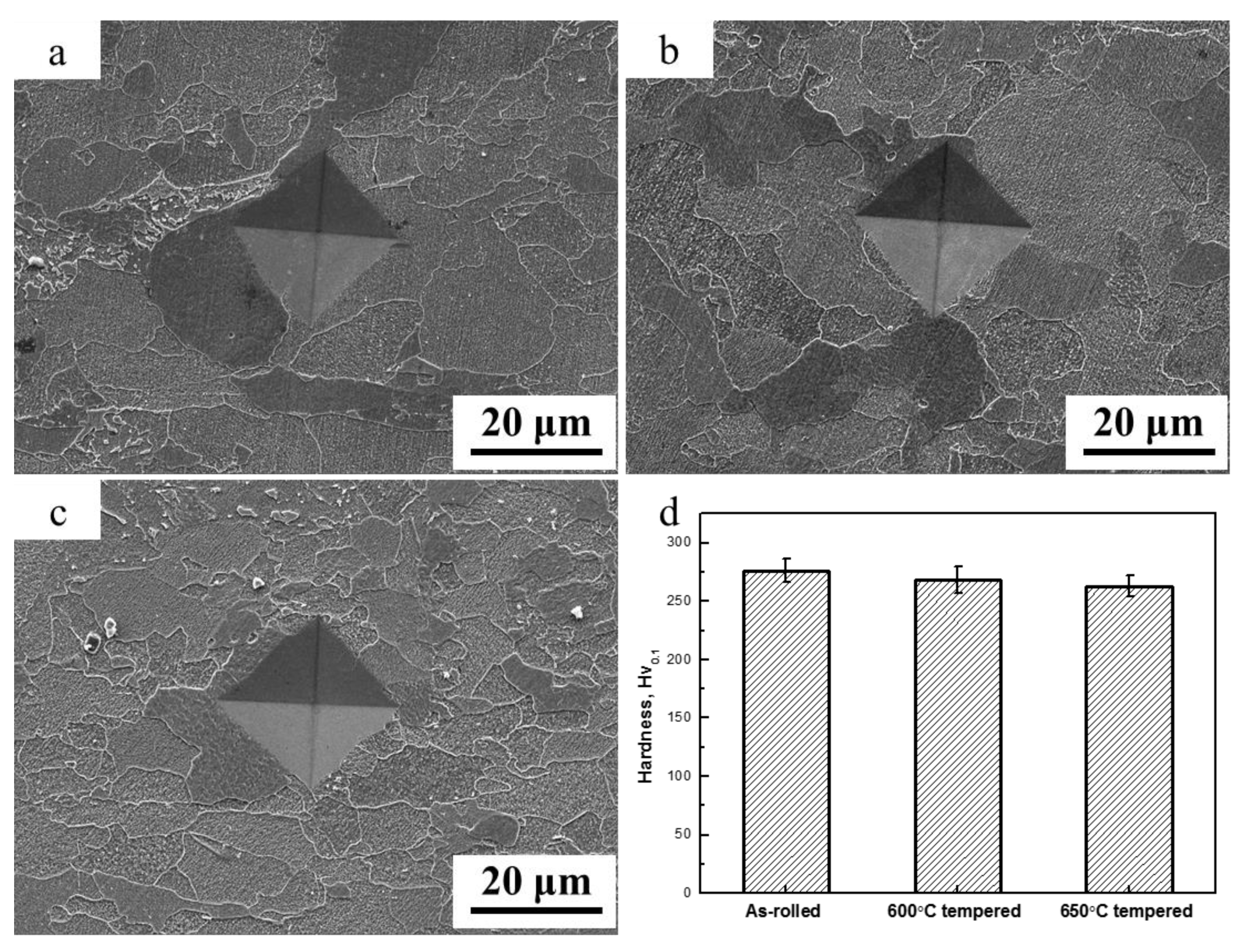
3.2. Microstructure and Microhardness
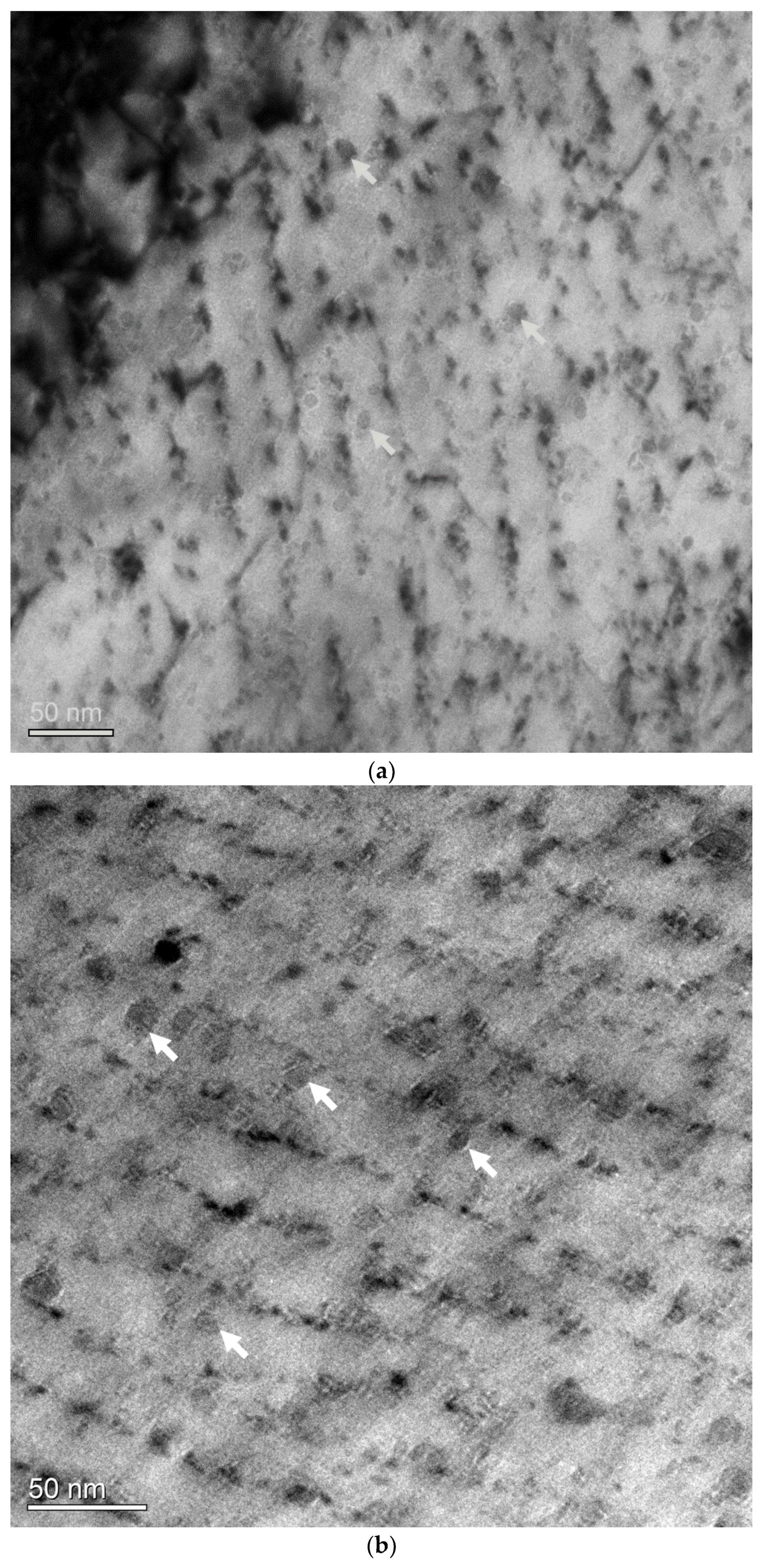
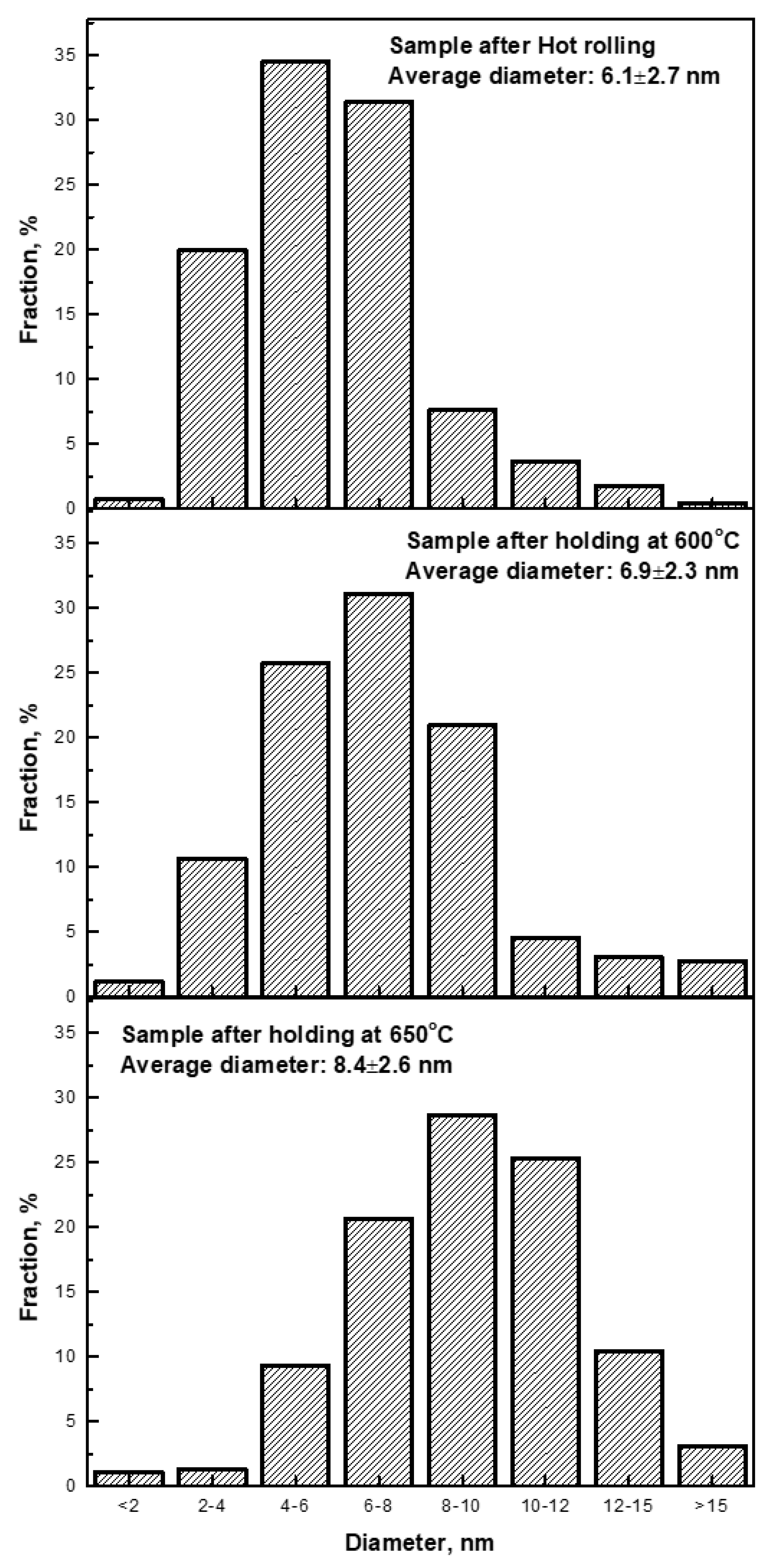
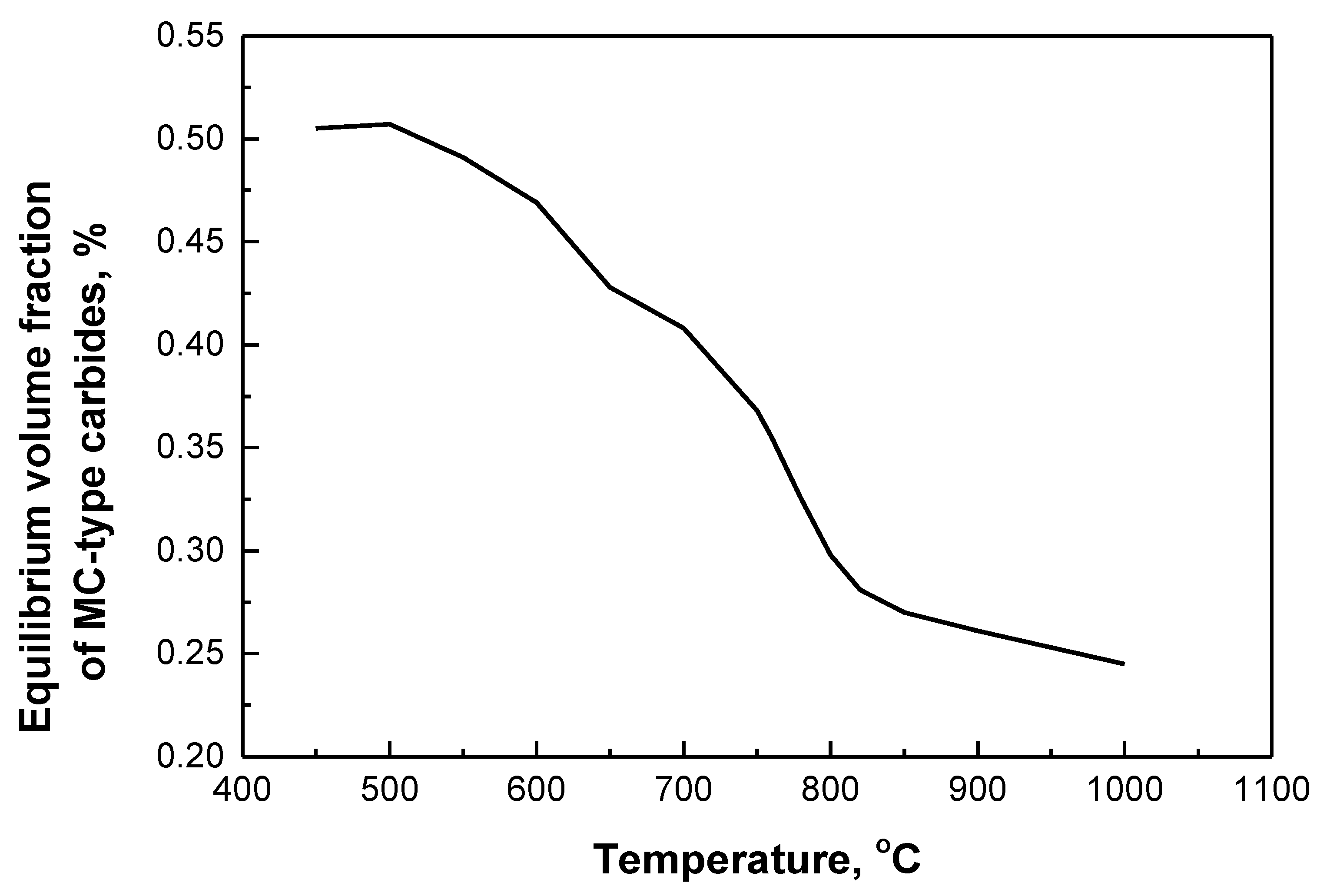
3.3. Nanometer-Sized Precipitation
3.4. Strengthening Mechanism
4. Conclusions
- (1)
- Nanometer-sized interphase precipitates were obtained in ferritic matrix. The interphase precipitated carbides have been found to exhibit an average diameter of 6.1 ± 2.7 nm, with an average distance of ~24–34 nm by TEM observation. EDX results indicated that the precipitates were (Ti, V)C complex carbides.
- (2)
- The nanometer-sized precipitation exhibited high stability against tempering at high temperatures of 600 °C and 650 °C for 3 h. Average diameters of carbides were measured to be equal to ~6.9 ± 2.3 nm and 8.4 ± 2.6 nm after annealing at high temperatures of 600 °C and 650 °C for 3 h, respectively.
- (3)
- Yield strength of 578 ± 20 MPa and tensile strength of 813 ± 25 MPa were achieved with high elongation of 25.0 ± 0.5% at room temperature. In addition, yield strength of 325 ± 13 MPa and 278 ± 4 MPa was achieved at elevated temperatures of 600 °C and 650 °C, respectively. Nanometer-sized precipitation contributed ~318 MPa to yield strength at room temperature, and the yield strength contributions decreased to ~133 MPa and ~84 MPa at 600 °C and 650 °C, respectively. The significant decrease of yield strength at 650 °C was attributed to the large decrease in the shear modulus.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.Y.; Yong, Q.L.; Sun, X.J.; Li, Z.D.; Kang, J.Y.; Wang, G.D. Microstructure and mechanical properties of precipitation strengthened fire resistant steel containing high Nb and low Mo. J. Iron Steel Res. Int. 2015, 22, 337–343. [Google Scholar] [CrossRef]
- Chijiiwa, R.; Yoshida, Y.; Uemori, R.; Tamehiro, H.; Funato, K.; Horii, Y. Development and practical application of fire-resistant steel for buildings. Nippon Steel Tech. Rep. 1993, 58, 47–55. [Google Scholar]
- Gross, C.T.; Isheim, D.; Vaynman, S.; Fine, M.E.; Chung, Y. Design and development of lightly alloyed ferritic fire-resistant structural steels. Metall. Mater. Trans. A 2019, 50, 209–219. [Google Scholar] [CrossRef]
- Miyata, K.; Sawaragi, Y. Effect of Mo and W on the phase stability of precipitates in low Cr heat resistant steels. ISIJ Int. 2001, 41, 281–289. [Google Scholar] [CrossRef]
- Mizutani, Y.; Ishibashi, K.; Yoshii, K.; Watanabe, Y.; Chijhwa, R.; Yoshida, Y. 590MPa class of fire-resistant steel for building structural use. Nippon Steel Tech. Rep. 2004, 90, 38–44. [Google Scholar]
- Wan, R.C.; Sun, F.; Zhang, L.T.; Shan, A.D. Effects of Mo on high-temperature strength of fire-resistant steel. Mater. Des. 2012, 35, 335–341. [Google Scholar] [CrossRef]
- Wan, R.C.; Sun, F.; Zhang, L.T.; Shan, A.D. Effect of Mo addition on strength of fire-resistant steel at elevated temperature. J. Mater. Eng. Perform. 2014, 23, 2780–2786. [Google Scholar] [CrossRef]
- Lee, W.B.; Hong, S.G.; Park, C.G.; Park, S.H. Carbide precipitation and high-temperature strength of hot-rolled high-strength, low-alloy steels containing Nb and Mo. Metall. Mater. Trans. A 2002, 33, 1689–1698. [Google Scholar] [CrossRef]
- Wan, R.C.; Sun, F.; Zhang, L.T.; Shan, A.D. Development and study of high-strength low-Mo fire-resistant steel. Mater. Des. 2012, 36, 227–232. [Google Scholar] [CrossRef]
- Assefpour-Dezfuly, M.; Hugaas, B.A.; Brownrigg, A. Fire resistant high strength low alloy steels. Mater. Sci. Technol. 1990, 6, 1210–1214. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of high strength hot-rolled sheet steel consisting of ferrite and nanometer-sized carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Khalid, F.A.; Edmonds, D.V. Interphase precipitation in microalloyed engineering steels and model alloy. Mater. Sci. Technol. 1993, 9, 384–396. [Google Scholar] [CrossRef]
- Jang, J.H.; Heo, Y.U.; Lee, C.H.; Bhadeshia, H.K.D.H.; Suh, D.W. Interphase precipitation in Ti–Nb and Ti–Nb–Mo bearing steel. Mater. Sci. Technol. 2013, 29, 309–313. [Google Scholar] [CrossRef]
- Miyamoto, G.; Hori, R.; Poorganji, B.; Furuhara, T. Interphase precipitation of VC and resultant hardening in V-added medium carbon steels. ISIJ Int. 2011, 51, 1733–1739. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, P.Y.; Huang, C.Y.; Yang, J.R. Interphase precipitation of nanometer-sized carbides in a titanium–molybdenum-bearing low-carbon steel. Acta Mater. 2011, 59, 6264–6274. [Google Scholar] [CrossRef]
- Bu, F.Z.; Wang, X.M.; Yang, S.W.; Shang, C.J.; Misra, R.D.K. Contribution of interphase precipitation on yield strength in thermomechanically simulated Ti–Nb and Ti–Nb–Mo microalloyed steels. Mater. Sci. Eng. A 2015, 620, 22–29. [Google Scholar] [CrossRef]
- Ferreira, D.; Alves, A.; Cruz Neto, R.; Martins, T.; Brandi, S. A new approach to simulate HSLA steel multipass welding through distributed point heat sources model. Metals 2018, 8, 951. [Google Scholar] [CrossRef]
- Wang, S.T.; Yang, S.W.; Gao, K.W.; He, X.L. Corrosion resistance of low alloying weathering steels in environment containing chloride ion. Trans. Mater. Heat Treat. 2008, 4, 170–175. [Google Scholar]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar]
- Chen, C.Y.; Chen, S.F.; Chen, C.C.; Yang, J.R. Control of precipitation morphology in the novel HSLA steel. Mater. Sci. Eng. A 2015, 634, 123–133. [Google Scholar]
- Tsai, S.P.; Tsai, Y.T.; Chen, Y.W.; Yang, J.R.; Chen, C.Y.; Wang, Y.T.; Huang, C.Y. Precipitation behavior in bimodal ferrite grains in a low carbon Ti-V-bearing steel. Scr. Mater. 2018, 143, 103–107. [Google Scholar] [CrossRef]
- Honeycombe, R.W.K.; Mehl, R.F. Transformation from austenite in alloy steels. Metall. Trans. A 1976, 7, 915–936. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Diffusional transformations: A theory for the formation of superledges. Phys. Stat. Sol. (a) 1982, 69, 745–750. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Miyamoto, G.; Yang, Z.G.; Furuhara, T. Direct measurement of carbon enrichment during austenite to ferrite transformation in hypoeutectoid Fe–2Mn–C alloys. Acta Mater. 2013, 61, 3120–3129. [Google Scholar] [CrossRef]
- Chen, M.Y.; Yen, H.W.; Yang, J.R. The transition from interphase-precipitated carbides to fibrous carbides in a vanadium-containing medium-carbon steel. Scr. Mater. 2013, 68, 829–832. [Google Scholar] [CrossRef]
- Dunlop, G.; Honeycombe, R. Ageing characteristics of VC, TiC, and (V, Ti) C dispersions in ferrite. Metal Sci. 1978, 12, 367–371. [Google Scholar] [CrossRef]
- Ashby, M.F. Physics of Strength and Plasticity; The MIT Press: Cambridge, MA, USA, 1969. [Google Scholar]
- Kelly, A.; Nicholson, R.B. Precipitation hardening. Prog. Mater. Sci. 1963, 10, 151–391. [Google Scholar]
- Chin, G.Y.; Mammel, W.L. Computer solutions of the Taylor analysis for axisymmetric flow. Trans. Metall. Soc. AIME 1967, 239, 1400–1405. [Google Scholar]
- Kaye, G.W.C.; Laby, T.H. Tables of Physical and Chemical Constants and Some Mathematical Functions, 14th ed.; Longman: London, UK, 1973. [Google Scholar]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzaki, K.; Furuhara, T. Stress–strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta Mater. 2015, 83, 383–396. [Google Scholar] [CrossRef]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.F.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Thermo-Calc Software. Available online: http://www.thermocalc.com/ (accessed on 1 October 2019).

| Testing Temperature | Yield Strength, MPa | Tensile Strength, MPa | Elongation, % |
|---|---|---|---|
| Room temperature | 578 ± 20 | 812 ± 25 | 25.0 ± 0.5 |
| 600 °C | 325 ± 13 | - | - |
| 650 °C | 278 ± 4 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Song, Z.; Chen, K.; Jiang, M.; Tao, Y.; Wang, X.; Shang, C. Study of Nanometer-Sized Precipitation and Properties of Fire Resistant Hot-Rolled Steel. Metals 2019, 9, 1230. https://doi.org/10.3390/met9111230
Xie Z, Song Z, Chen K, Jiang M, Tao Y, Wang X, Shang C. Study of Nanometer-Sized Precipitation and Properties of Fire Resistant Hot-Rolled Steel. Metals. 2019; 9(11):1230. https://doi.org/10.3390/met9111230
Chicago/Turabian StyleXie, Zhenjia, Zhendong Song, Kun Chen, Minghong Jiang, Ya Tao, Xuemin Wang, and Chengjia Shang. 2019. "Study of Nanometer-Sized Precipitation and Properties of Fire Resistant Hot-Rolled Steel" Metals 9, no. 11: 1230. https://doi.org/10.3390/met9111230
APA StyleXie, Z., Song, Z., Chen, K., Jiang, M., Tao, Y., Wang, X., & Shang, C. (2019). Study of Nanometer-Sized Precipitation and Properties of Fire Resistant Hot-Rolled Steel. Metals, 9(11), 1230. https://doi.org/10.3390/met9111230






