Friction and Wear of Pd-Rich Amorphous Alloy (Pd43Cu27Ni10P20) with Ionic Liquid (IL) as Lubricant at High Temperatures
Abstract
:1. Introduction
2. Sample Preparation and Instrumentation
2.1. Ionic Liquid
2.2. Thermal Stability of IL [C9C1im][NTf2]
2.3. Palladium-Rich Amorphous Alloy and Pure Crystalline Palladium
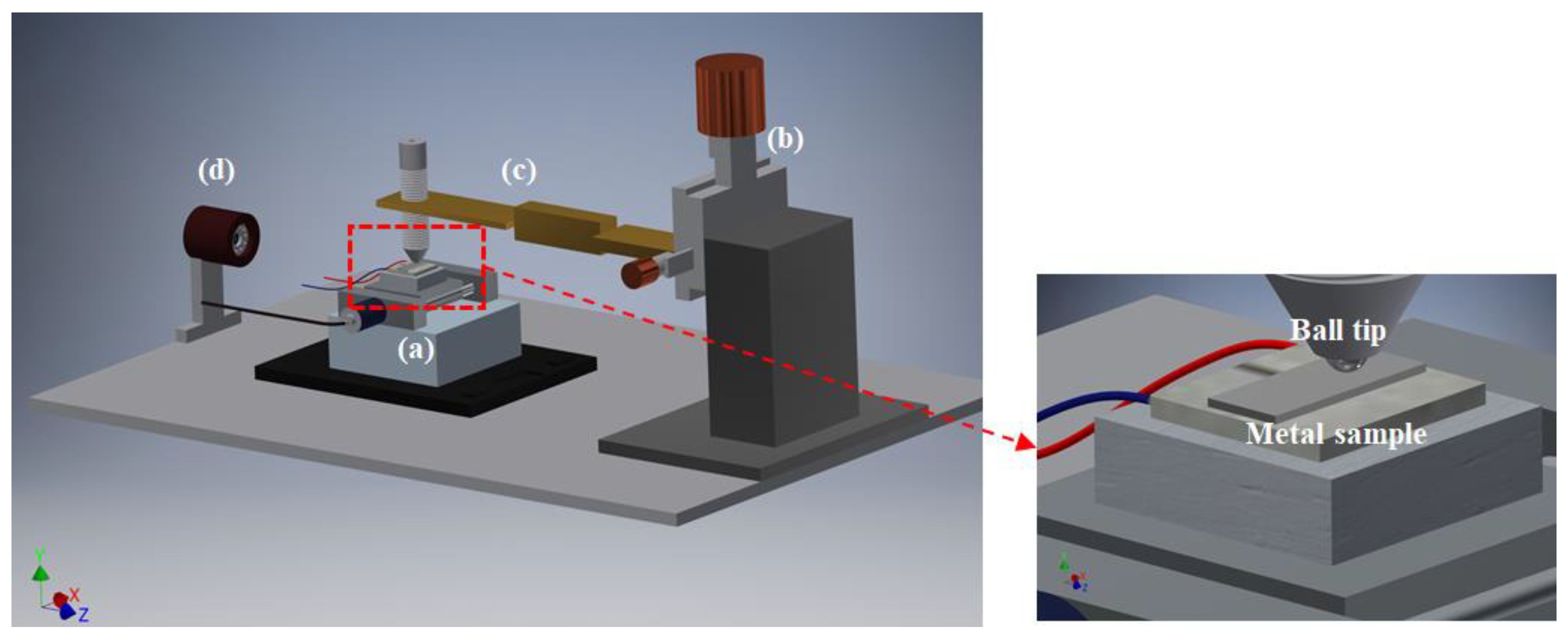
2.4. Experimental Procedure
2.4.1. Reciprocating Ball-on-Disc Test: In-Situ Friction Coefficient Measurement
2.4.2. Characterization of Wear Behavior: Wear Mechanism, Wear Coefficient, and Tribofilm Formation
3. Results and Discussion
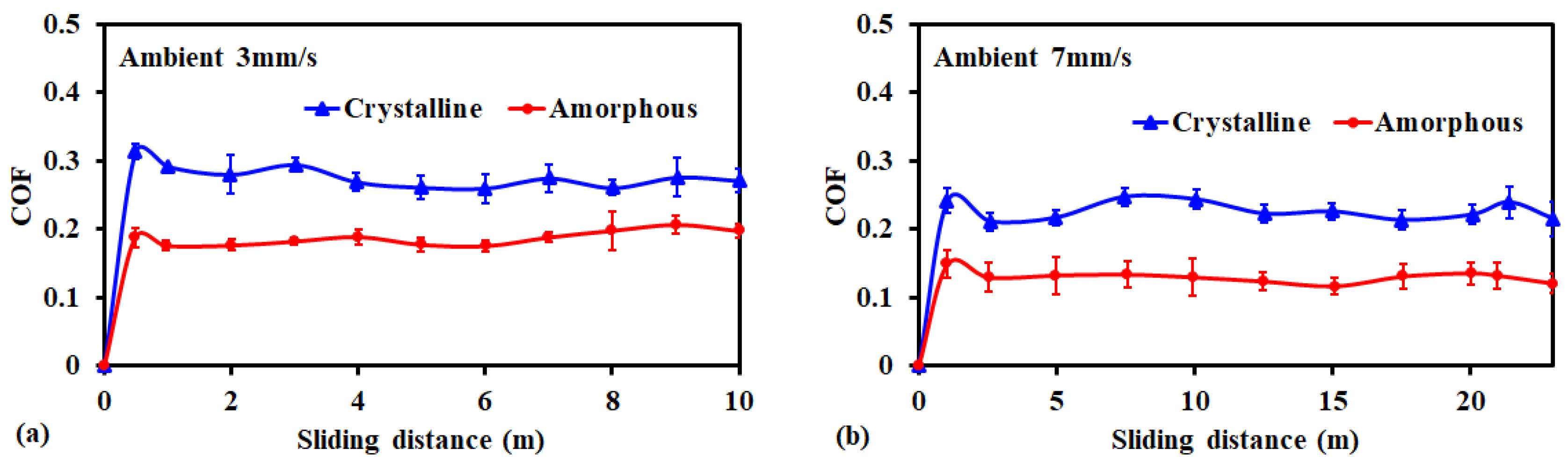
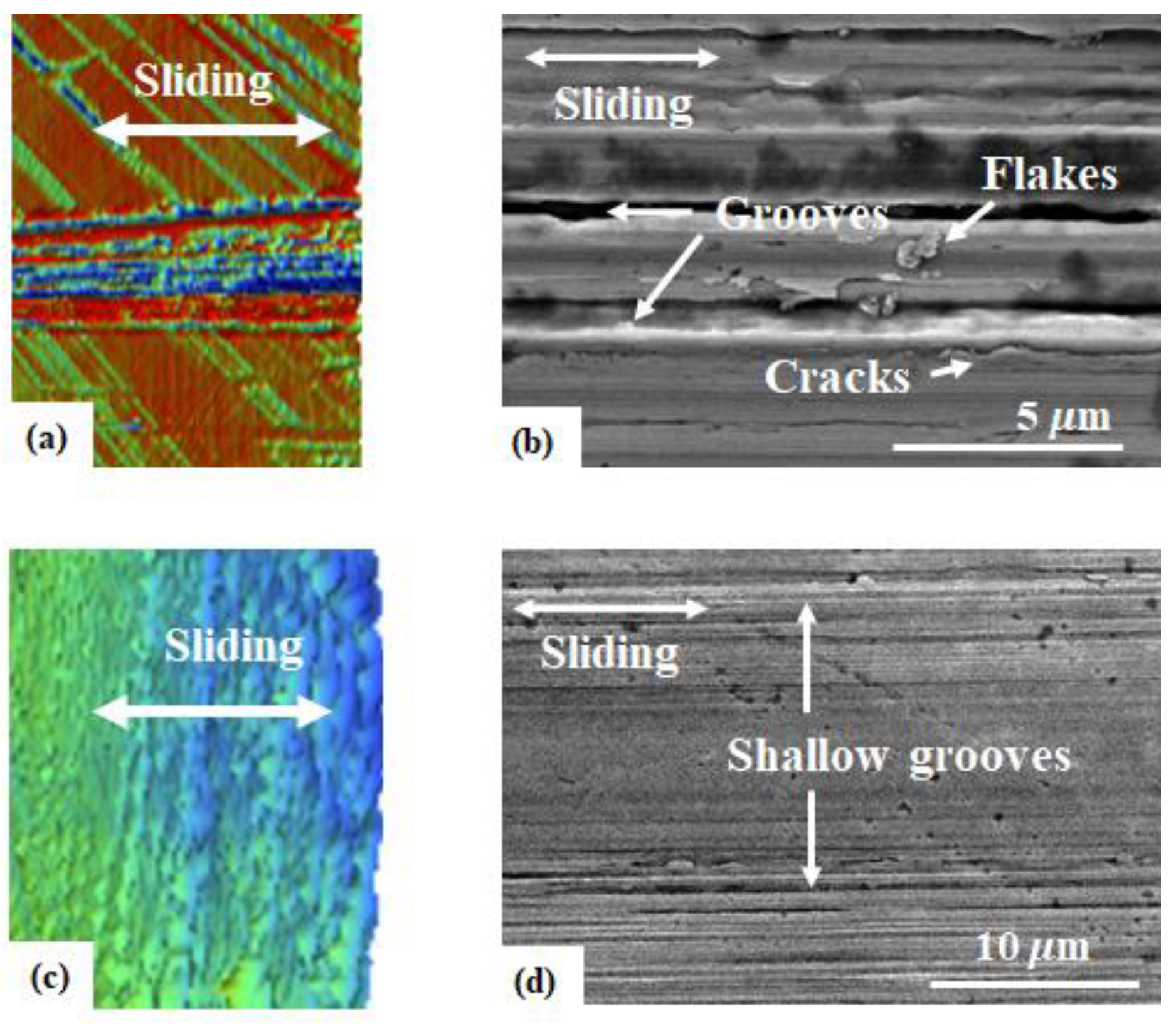
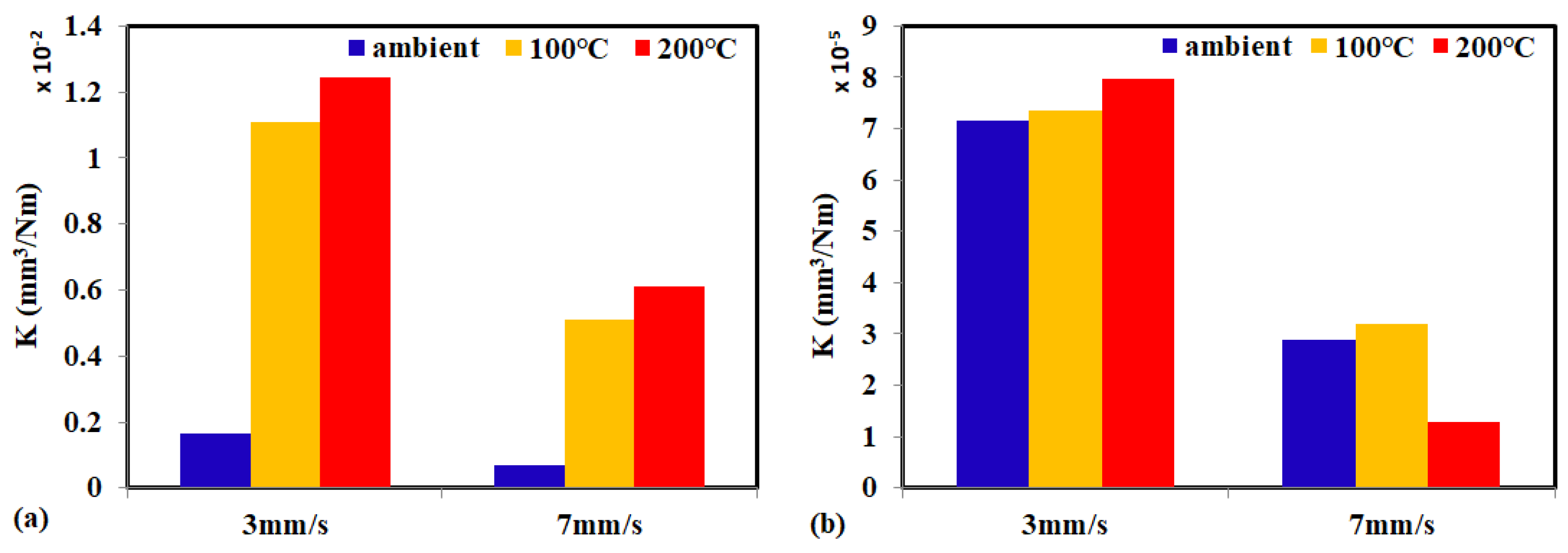
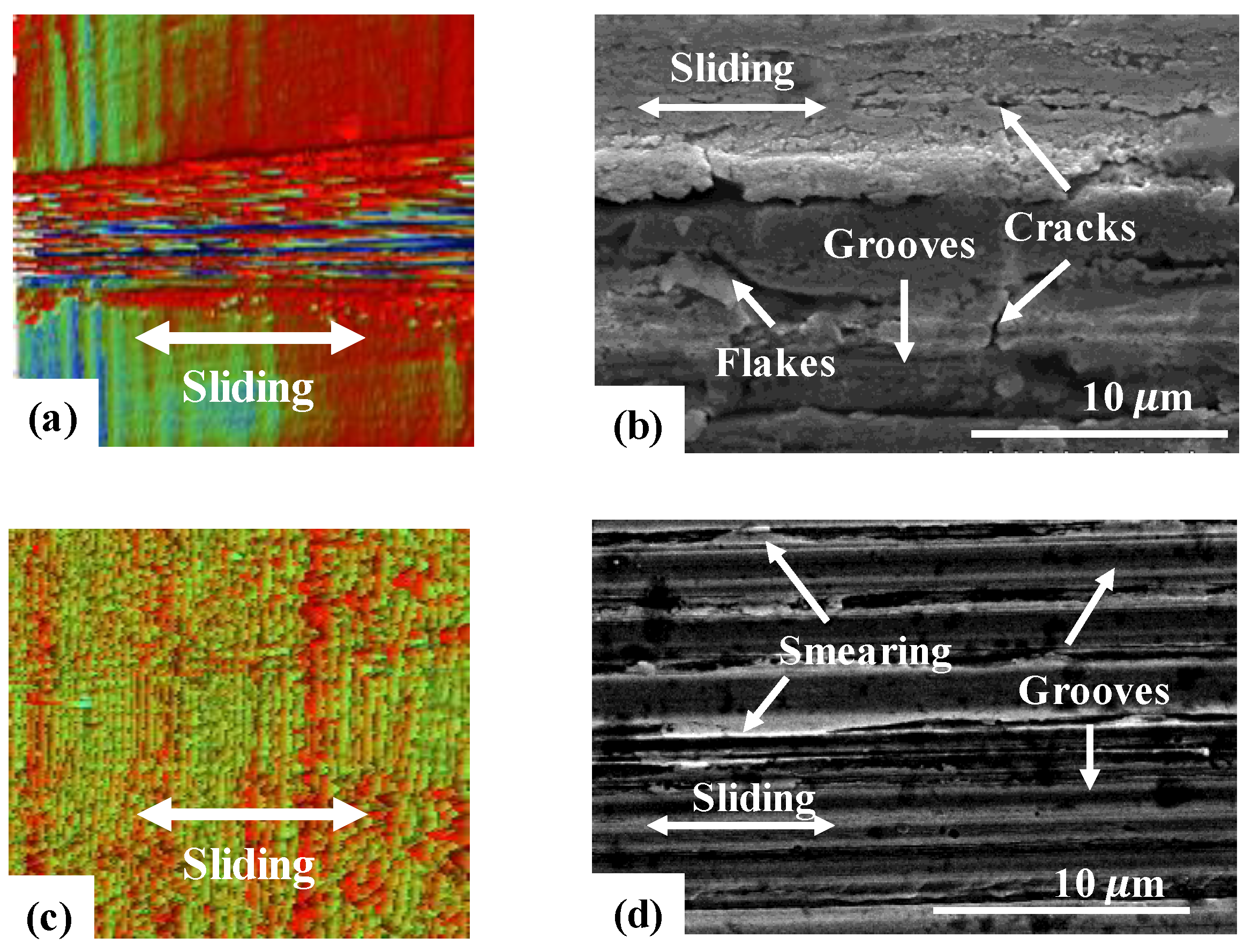
3.1. Friction and Wear Behavior at Ambient Temperature: Amorphous Pd Alloy vs. Crystalline Pd with IL Lubrication
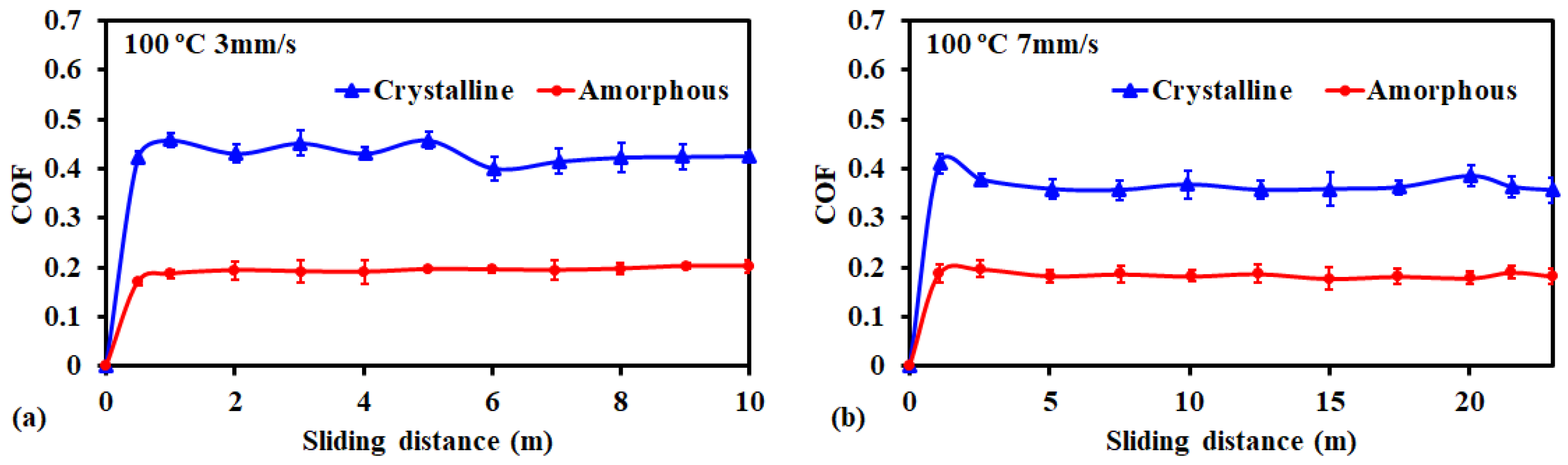
3.2. Friction and Wear Behavior at 100 °C: Amorphous Pd Alloy vs. Crystalline Pd under IL Lubrication
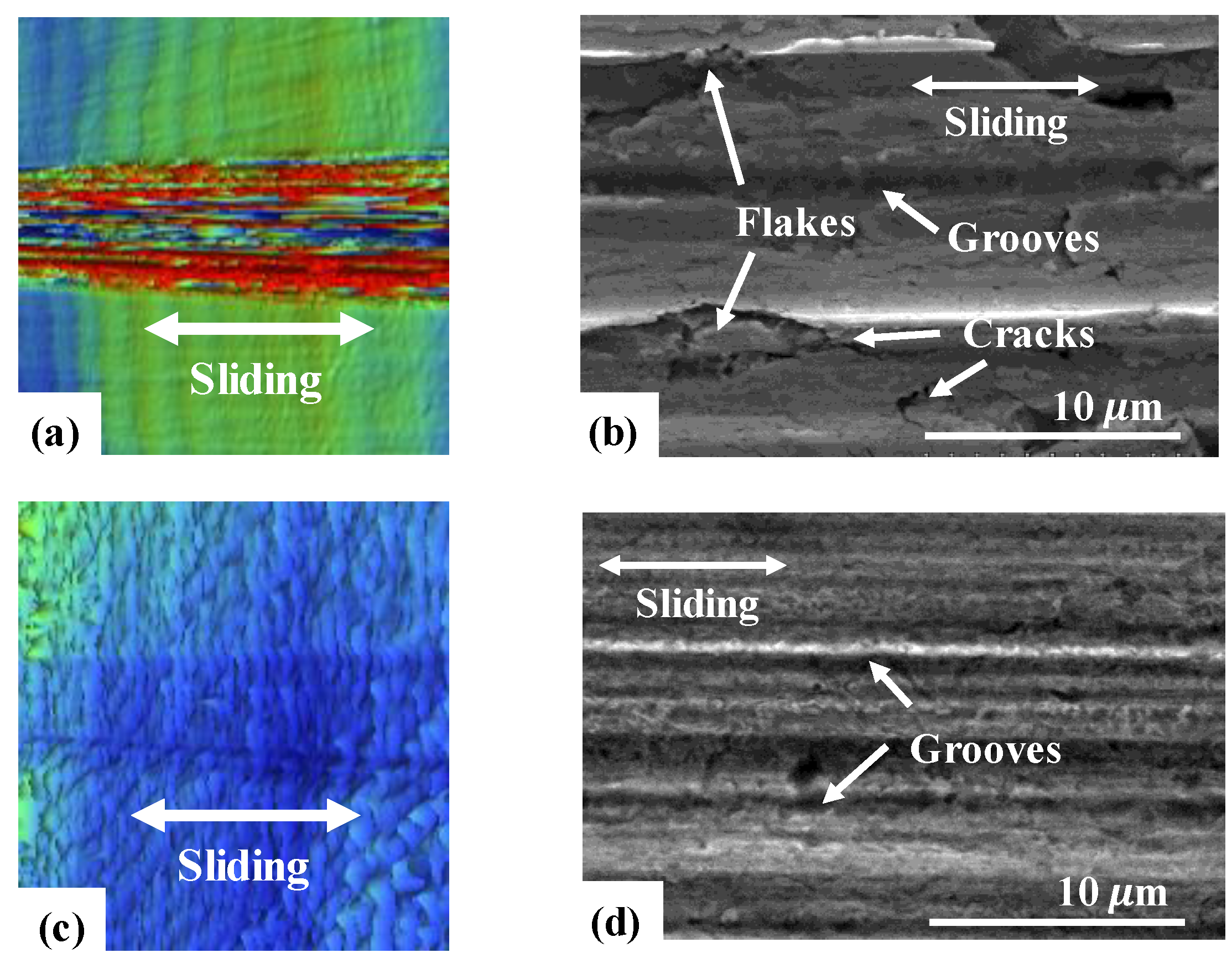
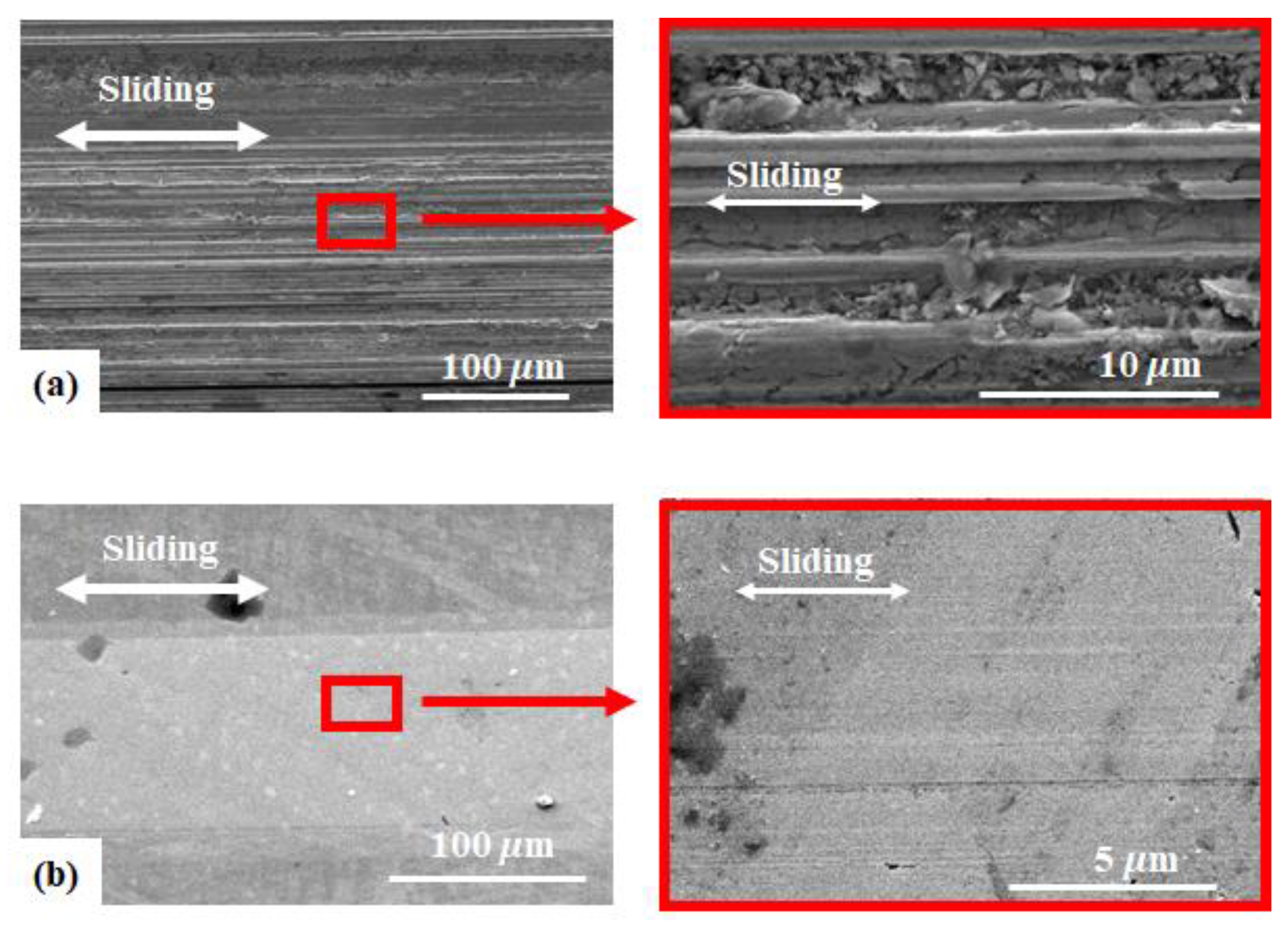
3.3. Friction and Wear Behavior at 200 °C: Amorphous Pd Alloy vs. Crystalline Pd with IL Lubrication
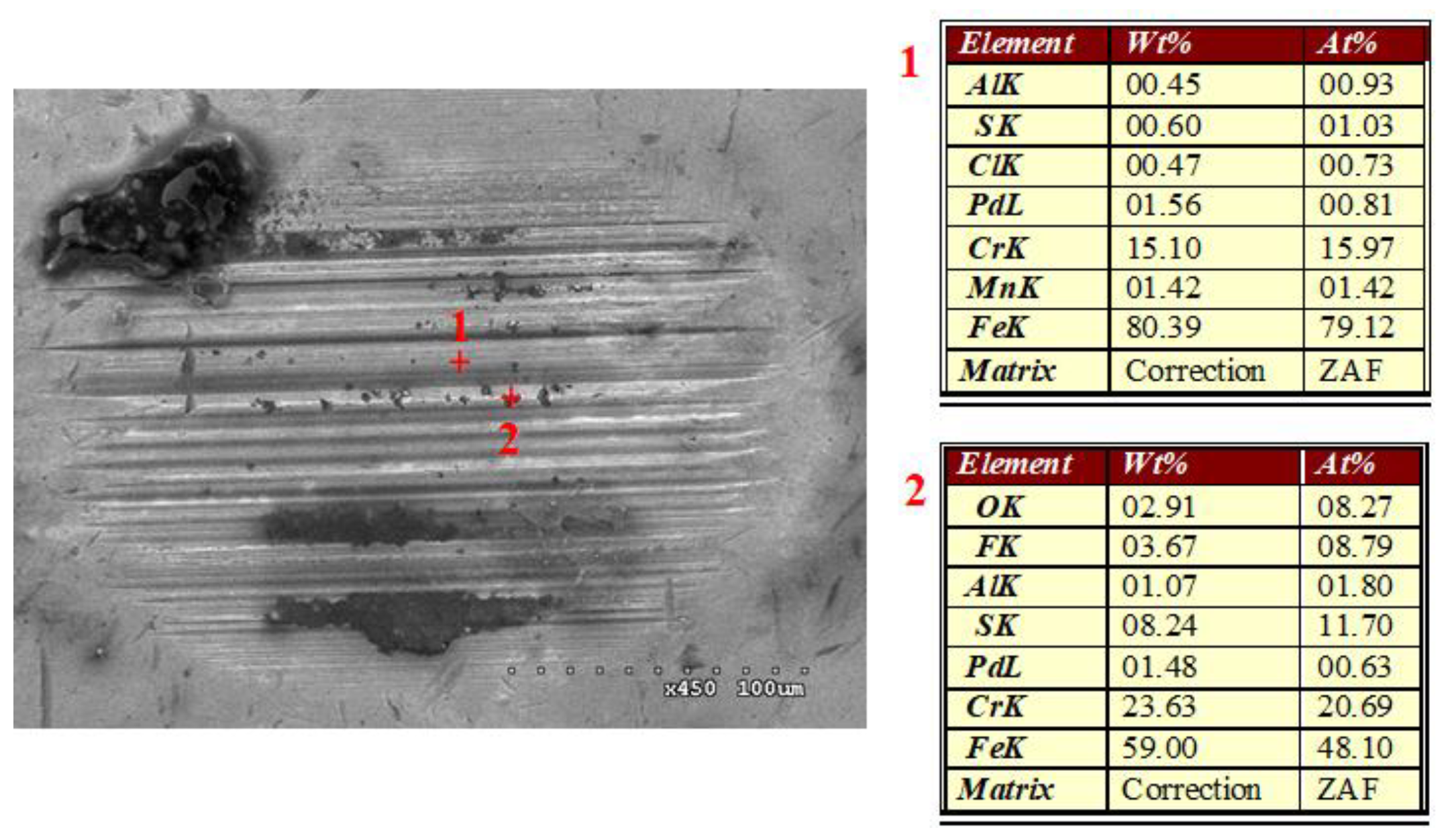
3.4. Formation of Tribofilm with Respect to Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, M.; Guo, R.; Zhou, F.; Liu, W. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Battez, A.H.; Blanco, D.; Fernández-González, A.; Mallada, M.T.; González, R.; Viesca, J.L. Friction, wear and tribofilm formation with a [NTf2] anion-based ionic liquid as neat lubricant. Tribol. Int. 2016, 103, 73–86. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L. Advancements in eco-friendly lubricants for tribological applications: Past, present, and future. Ecotribology 2016, 41–61. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium–steel contact. Part 2: friction, wear and surface interactions at high temperature. Tribol. Lett. 2010, 37, 431–443. [Google Scholar] [CrossRef]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-temperature ionic liquids: a novel versatile lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef]
- Wu, J.; Lu, X.; Feng, X.; Shi, Y. Halogen-free ionic liquids as excellent lubricants for PEEK-stainless steel contacts at elevated temperatures. Tribol. Int. 2016, 104, 1–9. [Google Scholar] [CrossRef]
- Nancarrow, P.; Mohammed, H. Ionic liquids in space technology–current and future trends. ChemBioEng Rev. 2017, 4, 106–119. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A. Green Solvents II: Properties and Applications of Ionic Liquids; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 2, ISBN 978-94-007-2891-2. [Google Scholar]
- Somers, A.E.; Biddulph, S.M.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. A comparison of phosphorus and fluorine containing IL lubricants for steel on aluminium. Phys. Chem. Chem. Phys. 2012, 14, 8224–8231. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.; Howlett, P.; MacFarlane, D.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Lhermerout, R.; Diederichs, C.; Perkin, S. Are ionic liquids good boundary lubricants? A molecular perspective. Lubricants 2018, 6, 9. [Google Scholar] [CrossRef]
- Tao, P.; Yang, Y.; Xie, Z.; He, Y. Research on friction and wear behavior of a bulk metallic glass under different sliding velocity. Mater. Lett. 2015, 156, 177–179. [Google Scholar] [CrossRef]
- Watanabe, L.Y.; Roberts, S.N.; Baca, N.; Wiest, A.; Garrett, S.J.; Conner, R.D. Fatigue and corrosion of a Pd-based bulk metallic glass in various environments. Mater. Sci. Eng. C 2013, 33, 4021–4025. [Google Scholar] [CrossRef]
- Maddala, D.R.; Hebert, R.J. Sliding wear behavior of Fe50−xCr15Mo14C15B6Erx (x = 0, 1, 2 at%) bulk metallic glass. Wear 2012, 294, 246–256. [Google Scholar] [CrossRef]
- Thakurathi, M.; Gurung, E.; Cetin, M.M.; Thalangama-Arachchige, V.D.; Mayer, M.F.; Korzeniewski, C.; Quitevis, E.L. The Stokes-Einstein equation and the diffusion of ferrocene in imidazolium-based ionic liquids studied by cyclic voltammetry: Effects of cation ion symmetry and alkyl chain length. Electrochim. Acta 2018, 259, 245–252. [Google Scholar] [CrossRef]
- Dzyuba, S.V.; Bartsch, R.A. Efficient synthesis of 1-alkyl (aralkyl)-3-methyl (ethyl) imidazolium halides: precursors for room-temperature ionic liquids. J. Heterocycl. Chem. 2001, 38, 265–268. [Google Scholar] [CrossRef]
- Dzyuba, S.V.; Bartsch, R.A. Influence of structural variations in 1-alkyl (aralkyl)-3-methylimidazolium hexafluorophosphates and bis (trifluoromethylsulfonyl) imides on physical properties of the ionic liquids. ChemPhysChem 2002, 3, 161–166. [Google Scholar] [CrossRef]
- Xue, L.; Tamas, G.; Koh, Y.P.; Shadeck, M.; Gurung, E.; Simon, S.L.; Maroncelli, M.; Quitevis, E.L. Effect of Alkyl chain branching on physicochemical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2016, 61, 1078–1091. [Google Scholar] [CrossRef]
- Hasan, M.; Kahler, N.; Kumar, G. Shape-controlled metal–metal and metal–polymer Janus structures by thermoplastic embossing. ACS Appl. Mater. Interfaces 2016, 8, 11084–11090. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; He, M.; Yeo, C.D.; Kumar, G.; Hu, Z.; Quitevis, E.L.; Thalangama-Arachchige, V.D. Friction and wear of Pd-rich amorphous alloy (Pd43Cu27Ni10P20) under dry and ionic liquid (IL) lubricated conditions. Wear 2018, 408, 190–199. [Google Scholar] [CrossRef]
- Khonsari, M.M.; Booser, E.R. Applied Tribology: Bearing Design and Lubrication, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-0-470-05711-7. [Google Scholar]
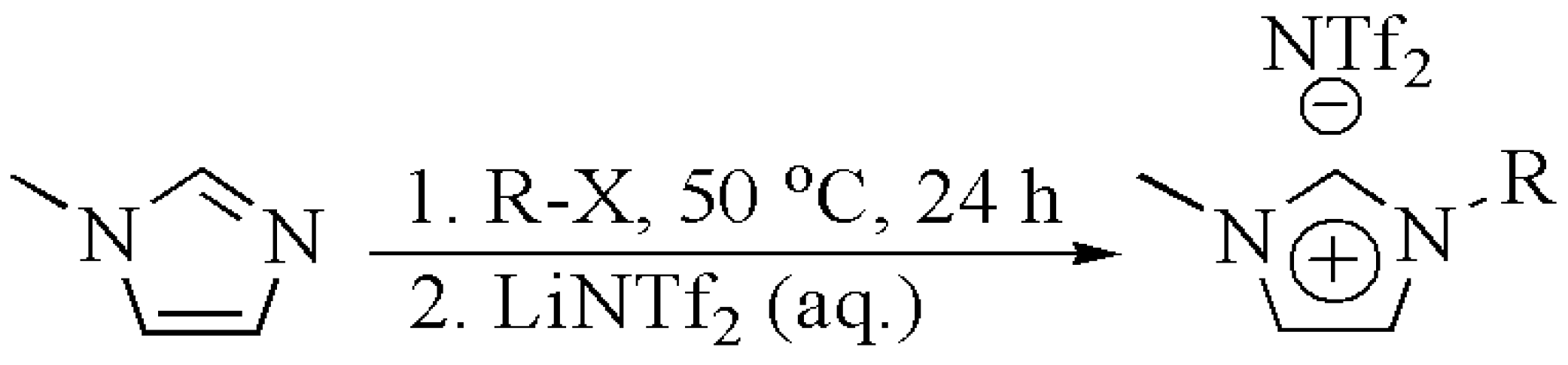
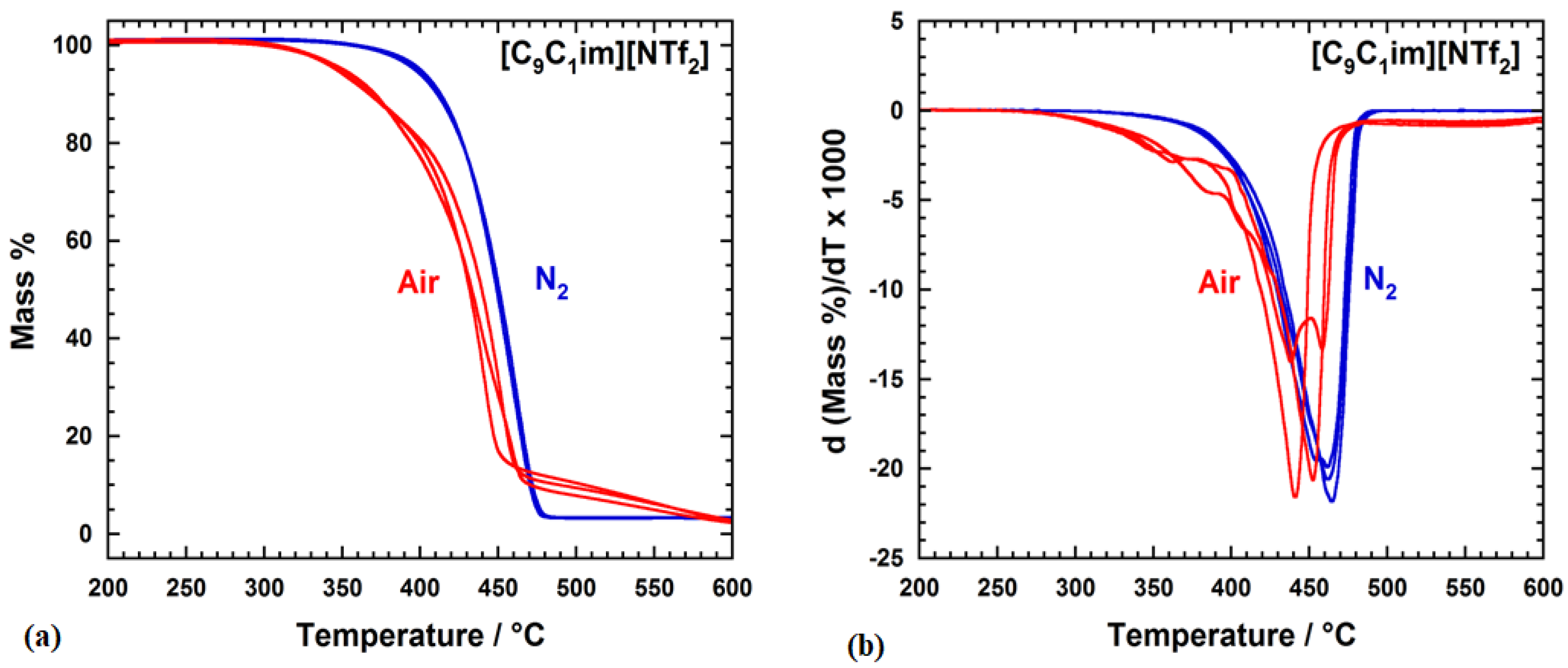


| Tonset (°C) | Tpeak (°C) | Tendset (°C) | ASTM D3850 | |||||
|---|---|---|---|---|---|---|---|---|
| T25% (°C) | T50% (°C) | T70% (°C) | T80% (°C) | T90% (°C) | ||||
| N2 | 428 ± 3 | 463 ± 2 | 474 ± 1 | 434 ± 2 | 451 ± 2 | 461 ± 2 | 466 ± 2 | 472 ± 2 |
| Air | 408 ± 10 | 444 ± 7 | 457 ± 7 | 408 ± 4 | 435 ± 4 | 447 ± 5 | 454 ± 5 | 489 ± 17 |
| Temperature | Crystalline Pd | Amorphous Pd | ||
|---|---|---|---|---|
| 3 mm/s | 7 mm/s | 3 mm/s | 7 mm/s | |
| Ambient | 0.28 ± 0.01 | 0.24 ± 0.01 | 0.19 ± 0.01 | 0.12 ± 0.01 |
| 100 °C | 0.42 ± 0.02 | 0.36 ± 0.01 | 0.20 ± 0.01 | 0.18 ± 0.01 |
| 200 °C | 0.44 ± 0.03 | 0.33 ± 0.07 | 0.19 ± 0.02 | 0.17 ± 0.01 |
| (a) | Ambient | 100 °C | 200 °C | |||
| # of Sputtering | F (at%) | S (at%) | F (at%) | S (at%) | F (at%) | S (at%) |
| 0 | 0 | 4.1 | 0.1 | 0.1 | 2.7 | 1.8 |
| 1 | 0 | 0 | 0 | 0 | 2 | 1.4 |
| 2 | 0 | 0 | 0 | 0 | 0.9 | 1.4 |
| 3 | 0 | 0 | 0 | 0 | 2.6 | 1.5 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| (b) | Ambient | 100 °C | 200 °C | |||
| # of Sputtering | F (at%) | S (at%) | F (at%) | S (at%) | F (at%) | S (at%) |
| 0 | 4.6 | 4.7 | 11.2 | 3.9 | 0.6 | 2.7 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| (a) | Ambient | 100 °C | 200 °C | |||
| # of Sputtering | F (at%) | S (at%) | F (at%) | S (at%) | F (at%) | S (at%) |
| 0 | 0 | 4.9 | 0 | 1.3 | 6.5 | 3.7 |
| 1 | 0 | 3 | 0 | 1.7 | 1.7 | 3.4 |
| 2 | 0 | 0 | 0 | 1.3 | 0.1 | 2 |
| 3 | 0 | 0 | 0 | 1.5 | 0 | 3.2 |
| 4 | 0 | 0 | 0 | 0.3 | 0 | 3.2 |
| 5 | 0 | 0 | 0 | 0 | 0 | 2.4 |
| 6 | 0 | 0 | 0 | 0 | 0 | 2.1 |
| 7 | 0 | 0 | 0 | 0 | 0 | 2.6 |
| 8 | 0 | 0 | 0 | 0 | 0 | 3 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| (b) | Ambient | 100 °C | 200 °C | |||
| # of Sputtering | F (at%) | S (at%) | F (at%) | S (at%) | F (at%) | S (at%) |
| 0 | 0 | 4.6 | 0.1 | 1.1 | 6.7 | 3.3 |
| 1 | 0 | 3.2 | 0 | 1.9 | 5.3 | 2.9 |
| 2 | 0 | 0 | 0 | 1.1 | 4.6 | 3 |
| 3 | 0 | 0 | 0 | 1 | 3.3 | 2.5 |
| 4 | 0 | 0 | 0 | 0.3 | 3.5 | 2 |
| 5 | 0 | 0 | 0 | 0.1 | 3.6 | 2.4 |
| 6 | 0 | 0 | 0 | 0 | 3.5 | 2.3 |
| 7 | 0 | 0 | 0 | 0 | 3 | 2.3 |
| 8 | 0 | 0 | 0 | 0 | 2.3 | 1.5 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| (a) | Ambient | 100 °C | 200 °C | |||
| # of Sputtering | F (at%) | S (at%) | F (at%) | S (at%) | F (at%) | S (at%) |
| 0 | 11.5 | 5.5 | 4.6 | 0.7 | 0 | 0.8 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| (b) | Ambient | 100 °C | 200 °C | |||
| # of Sputtering | F (at%) | S (at%) | F (at%) | S (at%) | F (at%) | S (at%) |
| 0 | 16.9 | 5.7 | 0 | 0 | 0 | 2.3 |
| 1 | 0 | 1.5 | 0 | 0 | 0 | 1.5 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yeo, C.-D.; Hu, Z.; Thalangama-Arachchige, V.D.; Kaur, J.; Quitevis, E.L.; Kumar, G.; Koh, Y.P.; Simon, S. Friction and Wear of Pd-Rich Amorphous Alloy (Pd43Cu27Ni10P20) with Ionic Liquid (IL) as Lubricant at High Temperatures. Metals 2019, 9, 1180. https://doi.org/10.3390/met9111180
Lee J, Yeo C-D, Hu Z, Thalangama-Arachchige VD, Kaur J, Quitevis EL, Kumar G, Koh YP, Simon S. Friction and Wear of Pd-Rich Amorphous Alloy (Pd43Cu27Ni10P20) with Ionic Liquid (IL) as Lubricant at High Temperatures. Metals. 2019; 9(11):1180. https://doi.org/10.3390/met9111180
Chicago/Turabian StyleLee, Jaeho, Chang-Dong Yeo, Zhonglue Hu, Vidura D. Thalangama-Arachchige, Jagdeep Kaur, Edward L. Quitevis, Golden Kumar, Yung P. Koh, and Sindee Simon. 2019. "Friction and Wear of Pd-Rich Amorphous Alloy (Pd43Cu27Ni10P20) with Ionic Liquid (IL) as Lubricant at High Temperatures" Metals 9, no. 11: 1180. https://doi.org/10.3390/met9111180
APA StyleLee, J., Yeo, C.-D., Hu, Z., Thalangama-Arachchige, V. D., Kaur, J., Quitevis, E. L., Kumar, G., Koh, Y. P., & Simon, S. (2019). Friction and Wear of Pd-Rich Amorphous Alloy (Pd43Cu27Ni10P20) with Ionic Liquid (IL) as Lubricant at High Temperatures. Metals, 9(11), 1180. https://doi.org/10.3390/met9111180






