A Novel Cyclic-Quenching-ART for Stabilizing Austenite in Nb–Mo Micro-Alloyed Medium-Mn Steel
Abstract
1. Introduction
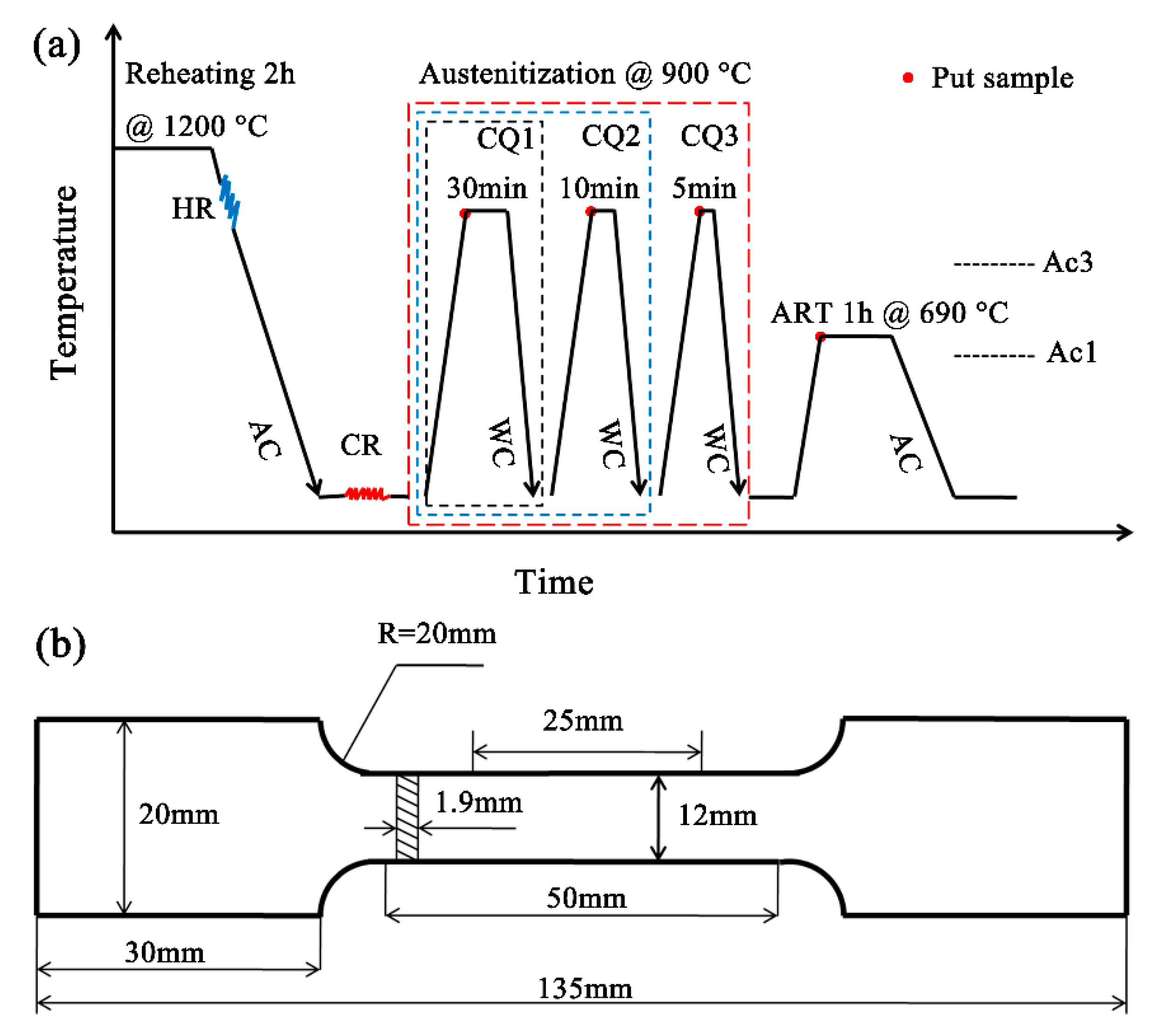
2. Experimental Procedure
3. Results and Discussion
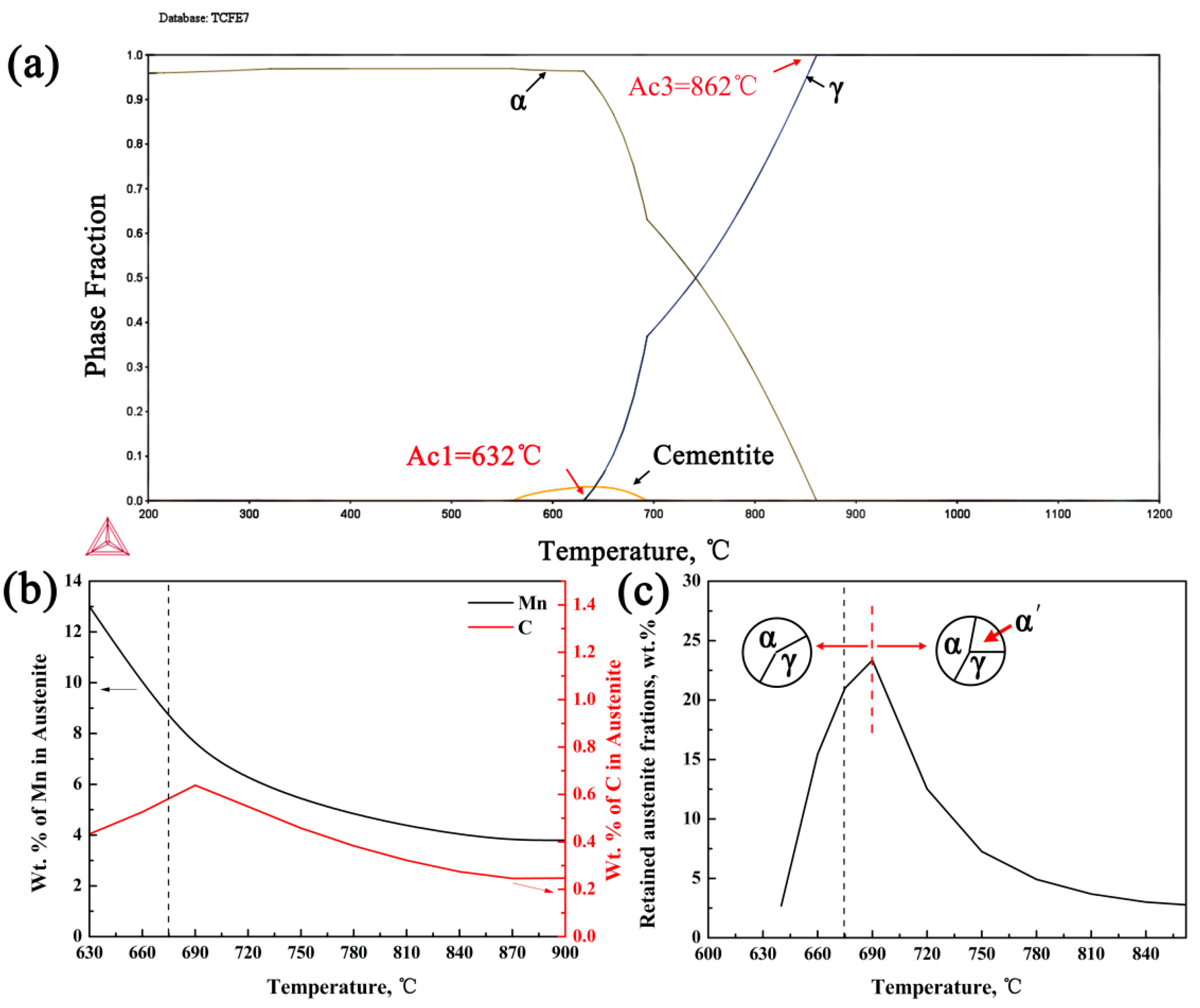
3.1. Microstructural Design Strategies
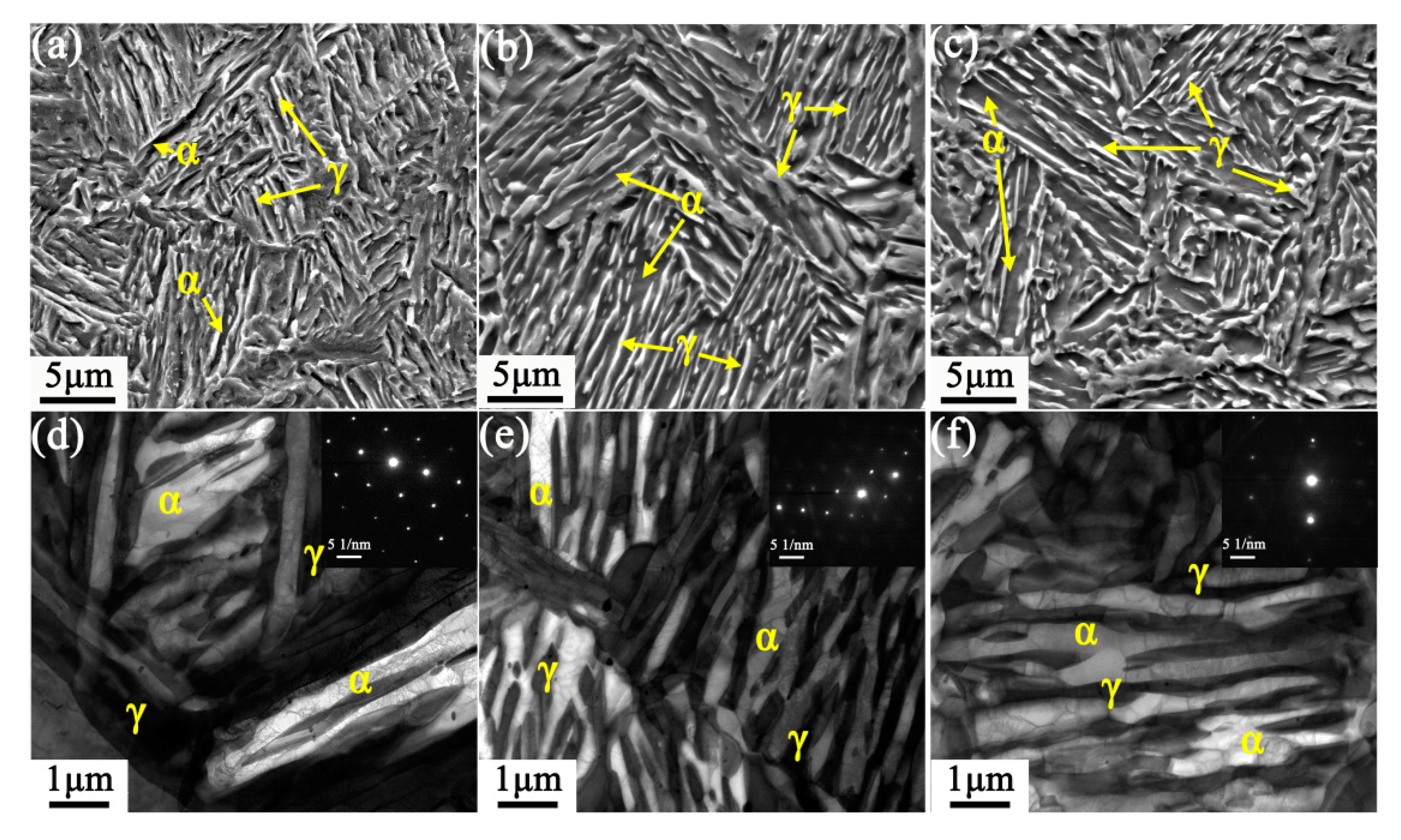
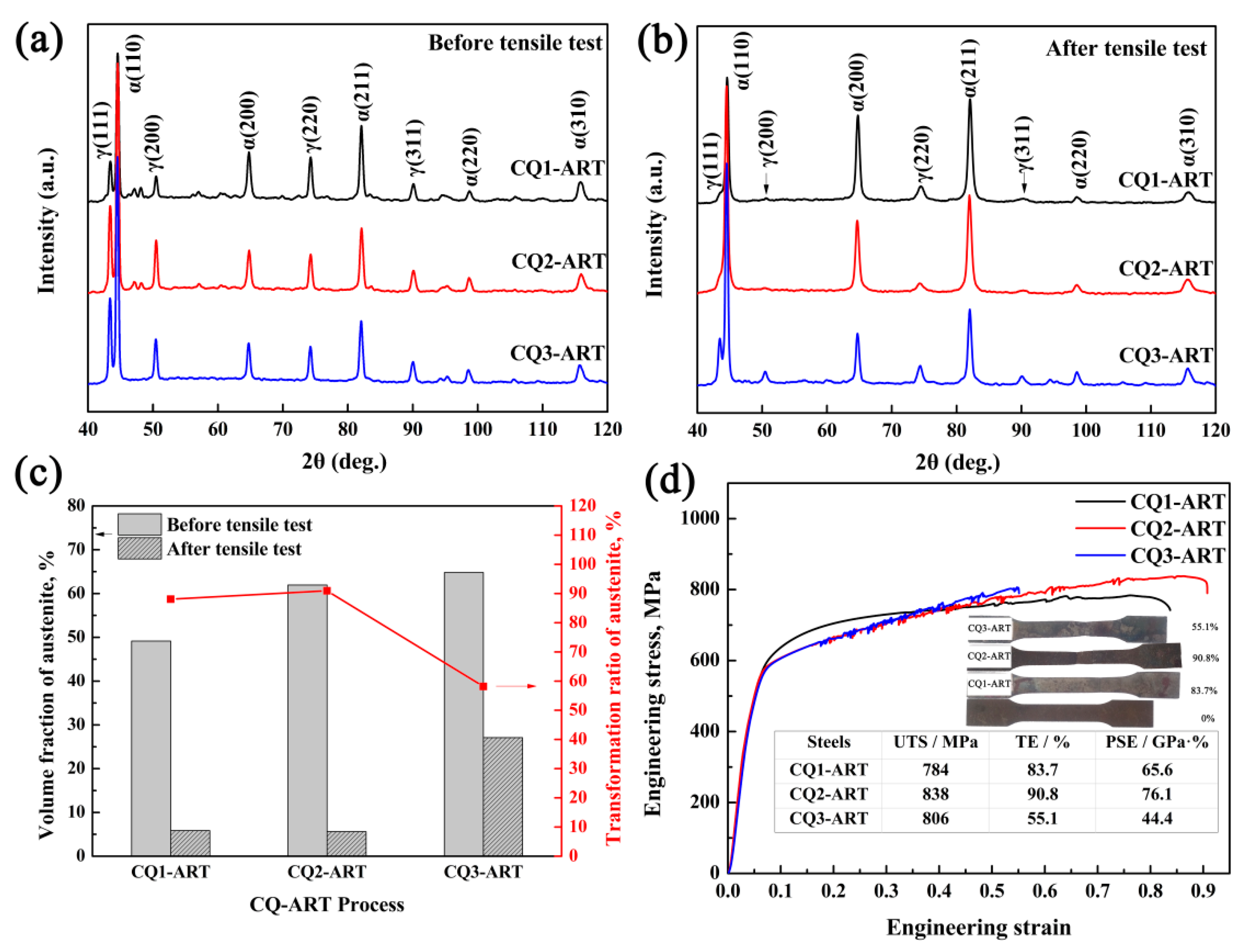
3.2. Microstructure and Mechanical Properties
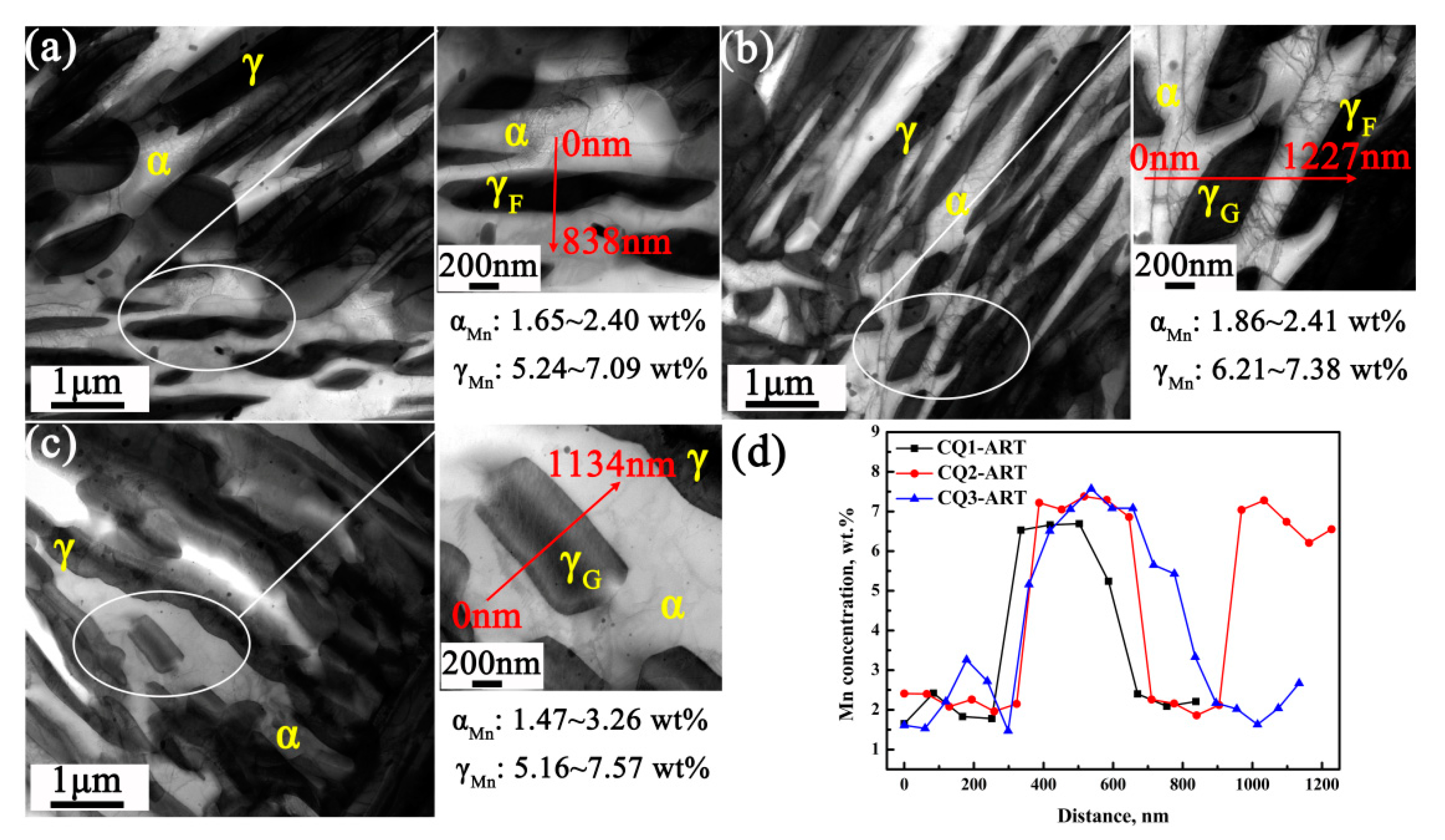
3.3. Mn and C Partitioning Behavior
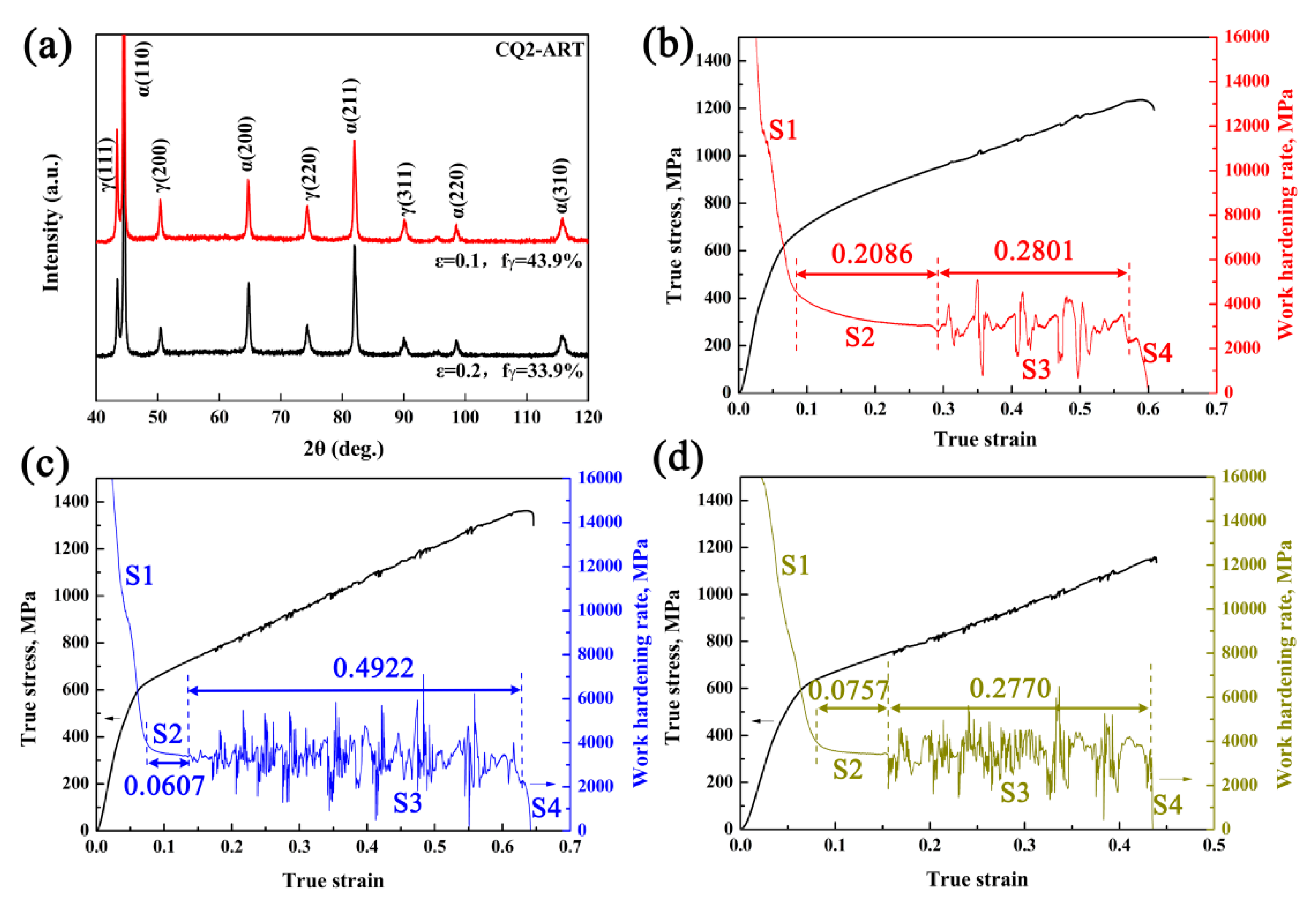
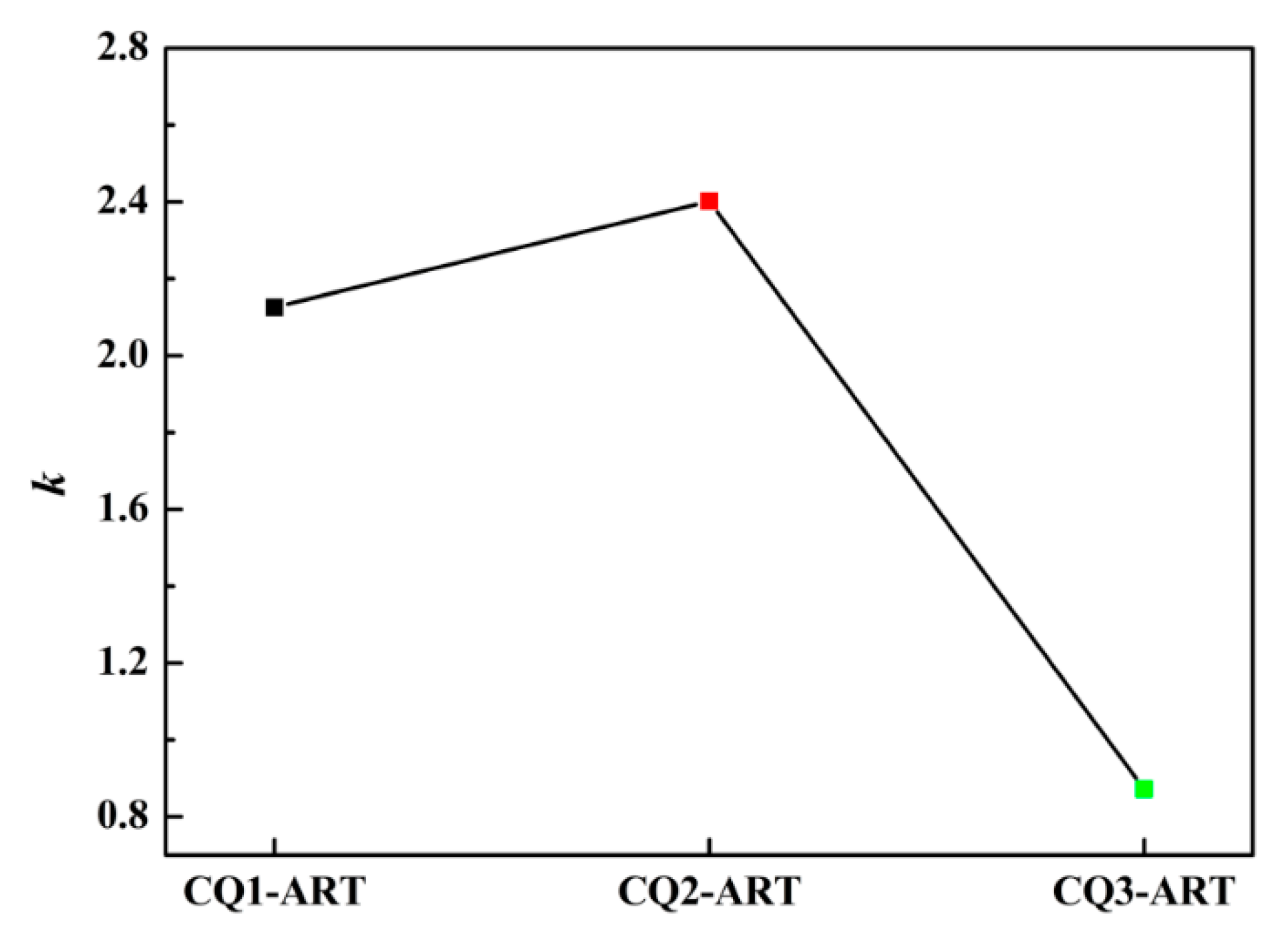
3.4. Deformation Behavior and Austenite Stability
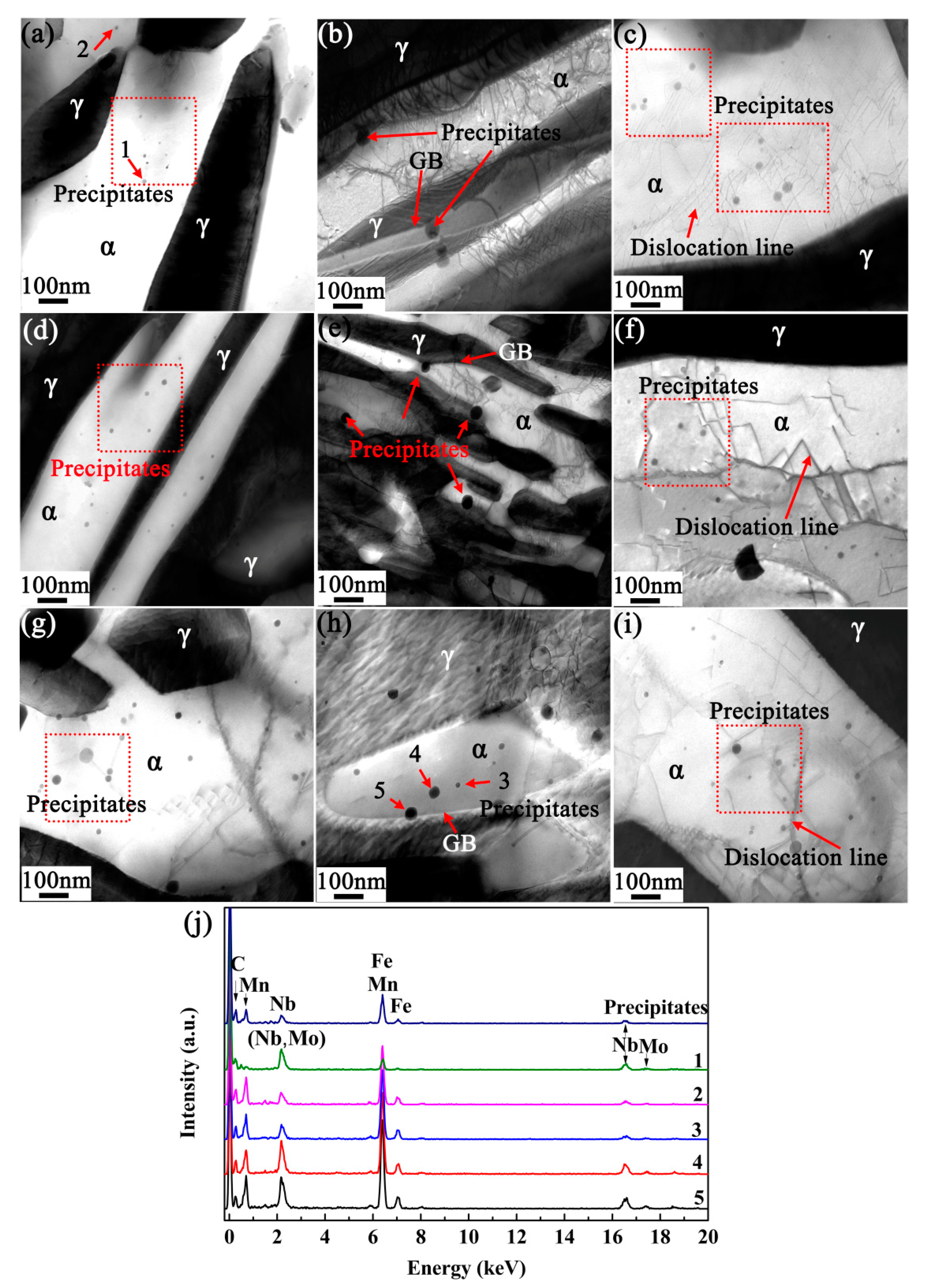
3.5. Strengthening Effect of Microalloying Elements Nb and Mo
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, H.J.; Cai, M.H.; Ding, H. Ultrahigh strength-ductile medium-Mn steel auto-parts combining warm stamping and quenching & partitioning. Mater. Sci. Technol. 2019, 35, 807–814. [Google Scholar]
- Cai, Z.H.; Ding, H.; Misra, R.D.K. Unique serrated flow dependence of critical stress in a hot-rolled Fe-Mn-Al-C steel. Scr. Mater. 2014, 71, 5–8. [Google Scholar] [CrossRef]
- He, B.B.; Liang, Z.Y.; Huang, M.X. Nanoindentation investigation on the initiation of yield point phenomenon in a medium Mn steel. Scr. Mater. 2018, 150, 134–138. [Google Scholar] [CrossRef]
- Aydin, H.; Essadiqi, E.; Jung, I.H. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A 2013, 564, 501–508. [Google Scholar] [CrossRef]
- Li, X.; Song, R.B.; Zhou, N.P. An ultrahigh strength and enhanced ductility cold-rolled medium-Mn steel treated by intercritical annealing. Scr. Mater. 2018, 154, 30–33. [Google Scholar] [CrossRef]
- Sun, B.; Fazeli, F.; Scott, C. The influence of silicon additions on the deformation behavior of austenite-ferrite duplex medium manganese steels. Acta Mater. 2018, 148, 249–262. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M.; Szpunar, J.A. Effect of arisen dislocation density and texture components during cold rolling and annealing treatments on hydrogen induced cracking susceptibility in pipeline steel. J. Mater. Res. 2016, 31, 3390–3400. [Google Scholar] [CrossRef]
- Liu, C.Q.; Peng, Q.C.; Xue, Z.L. Microstructure-Tensile Properties Relationship and Austenite Stability of a Nb-Mo Micro-Alloyed Medium-Mn TRIP Steel. Materials 2018, 8, 615. [Google Scholar] [CrossRef]
- Liu, C.Q.; Peng, Q.C.; Xue, Z.L. Microstructure and Mechanical Properties of Hot-Rolled and Cold-Rolled Medium-Mn TRIP Steels. Materials 2018, 11, 2242. [Google Scholar] [CrossRef]
- Eskandari, M.; Zarei-Hanzaki, A.; Szpunar, J.A.; Mohtadi-Bonab, M.A.; Kamali, A.R.; Nazarian-Samani, M. Microstructure evolution and mechanical behavior of a new microalloyed high Mn austenitic steel during compressive deformation. Mater. Sci. Eng. A 2014, 615, 424–435. [Google Scholar] [CrossRef]
- De Knijf, D.; Föjer, C.; Kestens, L.A.I. Factors influencing the austenite stability during tensile testing of Quenching and Partitioning steel determined via in-situ Electron Backscatter Diffraction. Mater. Sci. Eng. A 2015, 638, 219–227. [Google Scholar] [CrossRef]
- Cai, Z.H.; Ding, H.; Misra, R.D.K. Austenite stability and deformation behavior in a cold-rolled transformation-induced plasticity steel with medium manganese content. Acta Mater. 2015, 84, 229–236. [Google Scholar] [CrossRef]
- Grange, R.A. Strengthening Steel by Austenite Grain Refinement. ASM Trans. Quart 1966, 59, 26–48. [Google Scholar]
- Srivastava, A.K.; Bhattacharjee, D.; Jha, G. Microstructural and mechanical characterization of C-Mn-Al-Si cold-rolled TRIP-aided steel. Mater. Sci. Eng. A 2007, 445, 549–557. [Google Scholar] [CrossRef]
- Moor, E.D.; Matlock, D.K.; Speer, J.G. Austenite stabilization through manganese enrichment. Scr. Mater. 2011, 64, 185–188. [Google Scholar] [CrossRef]
- Capdevila, C.; Caballero, F.G.; De Andres, C.G. Determination of Ms temperature in steels: A Bayesian neural network model. ISIJ Int. 2002, 42, 894–902. [Google Scholar] [CrossRef]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Morsdorf, L.; Jeannin, O.; Barbier, D. Multiple mechanisms of lath martensite plasticity. Acta Mater. 2016, 121, 202–214. [Google Scholar] [CrossRef]
- Li, Z.C.; Ding, H.; Misra, R.D.K. Microstructure-mechanical property relationship and austenite stability in medium-Mn TRIP steels: The effect of austenite-reverted transformation and quenching-tempering treatments. Mater. Sci. Eng. A 2017, 682, 211–219. [Google Scholar] [CrossRef]
- Mishra, G.; Chandan, A.K.; Kundu, S. Hot rolled and cold rolled medium manganese steel: Mechanical properties and microstructure. Mater. Sci. Eng. A 2017, 701, 319–327. [Google Scholar] [CrossRef]
- Choi, K.; Seo, C.; Lee, H. Effect of aging on the microstructure and deformation behavior of austenite base lightweight Fe-28Mn-9Al-0.8C steel. Scr. Mater. 2010, 63, 1028–1031. [Google Scholar] [CrossRef]
- Cai, Z.H.; Ding, H.; Xue, X.; Jiang, J. Significance of control of austenite stability and three-stage work-hardening behavior of an ultrahigh strength-high ductility combination transformation-induced plasticity steel. Scr. Mater. 2013, 68, 865–868. [Google Scholar] [CrossRef]
- Van Dijk, N.H.; Butt, A.M.; Zhao, L. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 53, 5439–5447. [Google Scholar] [CrossRef]
- Xu, H.F.; Zhao, J.; Cao, W.Q. Tempering Effects on the Stability of Retained Austenite and Mechanical Properties in a Medium Manganese Steel. ISIJ Int. 2012, 52, 868–873. [Google Scholar] [CrossRef]
- Hu, Z.P.; Xu, Y.B.; Zou, Y. Effect of intercritical rolling temperature on microstructure-mechanical property relationship in a medium Mn-TRIP steel containing δ ferrite. Mater. Sci. Eng. A 2018, 720, 1–10. [Google Scholar] [CrossRef]
- Lee, C.Y.; Jeong, J.; Han, J. Coupled strengthening in a medium manganese lightweight steel with an inhomogeneously grained structure of austenite. Acta Mater. 2015, 84, 1–8. [Google Scholar] [CrossRef]
- Godet, S.; Jacques, P.J. Beneficial influence of an intercritically rolled recovered ferritic matrix on the mechanical properties of TRIP-assisted multiphase steels. Mater. Sci. Eng. A 2015, 645, 20–27. [Google Scholar] [CrossRef]
- Xu, Y.B.; Zou, Y.; Hu, Z.P. Correlation between deformation behavior and austenite characteristics in a Mn-Al type TRIP steel. Mater. Sci. Eng. A 2017, 698, 126–135. [Google Scholar] [CrossRef]
- Cai, Z.H.; Li, H.Y.; Jing, S.Y. Influence of annealing temperature on microstructure and tensile property of cold-rolled Fe-0.2C-11Mn-6Al steel. Mater. Charact. 2018, 137, 256–262. [Google Scholar] [CrossRef]
- Li, Z.C.; Ding, H.; Cai, Z.H. Mechanical properties and austenite stability in hot-rolled 0.2C-1.6/3.2 Al-6Mn-Fe TRIP steel. Mater. Sci. Eng. A 2015, 639, 559–566. [Google Scholar] [CrossRef]
- Li, Z.C.; Ding, H.; Misra, R.D.K. Microstructural evolution and deformation behavior in the Fe-(6,8.5) Mn-3Al-0.2 C TRIP steels. Mater. Sci. Eng. A 2016, 672, 161–169. [Google Scholar] [CrossRef]
- Li, Z.C.; Misra, R.D.K.; Cai, Z.H. Mechanical properties and deformation behavior in hot-rolled 0.2C-1.5/3Al-8.5 Mn-Fe TRIP steel: The discontinuous TRIP effect. Mater. Sci. Eng. A 2016, 673, 63–72. [Google Scholar] [CrossRef]
- Embury, D.; Bouaziz, O. Steel-based composites: Driving forces and classifications. Annu. Rev. Mater. Res. 2010, 40, 243–270. [Google Scholar] [CrossRef]
- Speer, J.G.; Streicher, A.M.; Matlock, D.K. Austenite Formation and Decomposition; Damm, E.B., Merwin, M., Eds.; TMS/ISS: Warrendale, PA, USA, 2003; pp. 505–522. [Google Scholar]
- Sugimoto, K.I.; Kobayashi, M.; Hashimoto, S.I. Ductility and strain-induced transformation in a high-strength transformation-induced plasticity-aided dual-phase steel. Metall. Trans. A 1992, 23, 3085–3091. [Google Scholar] [CrossRef]
- Hong, S.C.; Lim, S.H.; Hong, H.S. Effects of Nb on strain induced ferrite transformation in C-Mn steel. Mater. Sci. Eng. A 2003, 355, 241–248. [Google Scholar] [CrossRef]
- Bleck, W.; Frehn, A.; Kechagias, E. Control of Microstructure in TRIP Steels by Niobium. Mater. Sci. Forum 2003, 426, 43–48. [Google Scholar] [CrossRef]
- Cao, J.C.; Yong, Q.L.; Liu, Q.Y. Precipitation of MC phase and precipitation strengthening in hot rolled Nb–Mo and Nb–Ti steels. J. Mater. Sci. 2007, 42, 10080–10084. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.D.; Yong, Q.L. Precipitation behavior of carbide during heating process in Nb and Nb-Mo micro-alloyed steels. Acta Metall. Sin. 2015, 51, 315–324. [Google Scholar]
- Zhang, Z.Y.; Sun, X.J.; Yong, Q.L.; Li, Z.D. Precipitation behavior of nano-sized carbides in Nb-Mo microalloyed high strength steel and its strengthening mechanism. Acta Metall. Sin. 2016, 52, 410–418. [Google Scholar]
| C | Mn | Al | Si | Mo | Nb |
|---|---|---|---|---|---|
| 0.25 | 3.98 | 1.22 | 0.20 | 0.19 | 0.03 |
| Retained Austenite Width | CQ1-ART | CQ2-ART | CQ3-ART |
|---|---|---|---|
| RAave (μm) | 0.62 | 0.40 | 0.42 |
| RAmin (μm) | 0.16 | 0.13 | 0.12 |
| RAmax (μm) | 1.85 | 1.25 | 1.60 |
| Mn Concentration | CQ1-ART | CQ2-ART | CQ3-ART |
|---|---|---|---|
| RAave (wt.%) | 6.16 | 7.31 | 7.19 |
| RAmin(wt.%) | 5.24 | 6.07 | 5.01 |
| RAmax (wt.%) | 7.69 | 7.93 | 8.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Peng, Q.; Xue, Z.; Yang, C. A Novel Cyclic-Quenching-ART for Stabilizing Austenite in Nb–Mo Micro-Alloyed Medium-Mn Steel. Metals 2019, 9, 1090. https://doi.org/10.3390/met9101090
Liu C, Peng Q, Xue Z, Yang C. A Novel Cyclic-Quenching-ART for Stabilizing Austenite in Nb–Mo Micro-Alloyed Medium-Mn Steel. Metals. 2019; 9(10):1090. https://doi.org/10.3390/met9101090
Chicago/Turabian StyleLiu, Chunquan, Qichun Peng, Zhengliang Xue, and Chengwei Yang. 2019. "A Novel Cyclic-Quenching-ART for Stabilizing Austenite in Nb–Mo Micro-Alloyed Medium-Mn Steel" Metals 9, no. 10: 1090. https://doi.org/10.3390/met9101090
APA StyleLiu, C., Peng, Q., Xue, Z., & Yang, C. (2019). A Novel Cyclic-Quenching-ART for Stabilizing Austenite in Nb–Mo Micro-Alloyed Medium-Mn Steel. Metals, 9(10), 1090. https://doi.org/10.3390/met9101090





