Surface Modification of Rusted Rebar and Enhanced Passivation/Anticorrosion Performance in Simulated Concrete Pore Solutions with Different Alkalinity
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials and Surface Modification Processes
2.2. Electrochemical Testing
2.3. Surface Characterization
3. Results and Discussion
3.1. Passivation Behavior of Rebar in the Cl−-Free SCPS of Different Alkalinity
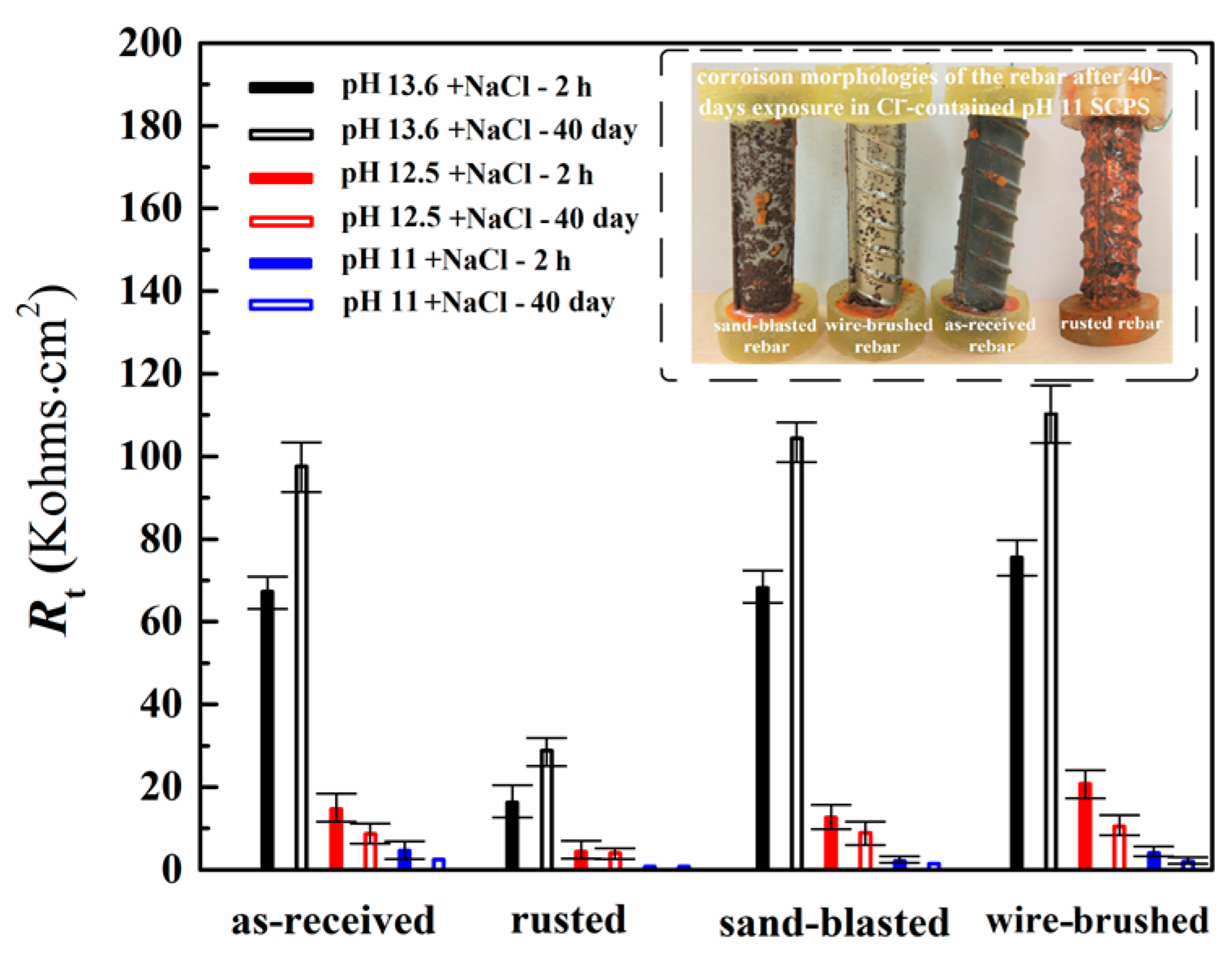
3.2. Corrosion Behavior of Rebar in the Cl−-containing SCPS of Different Alkalinity
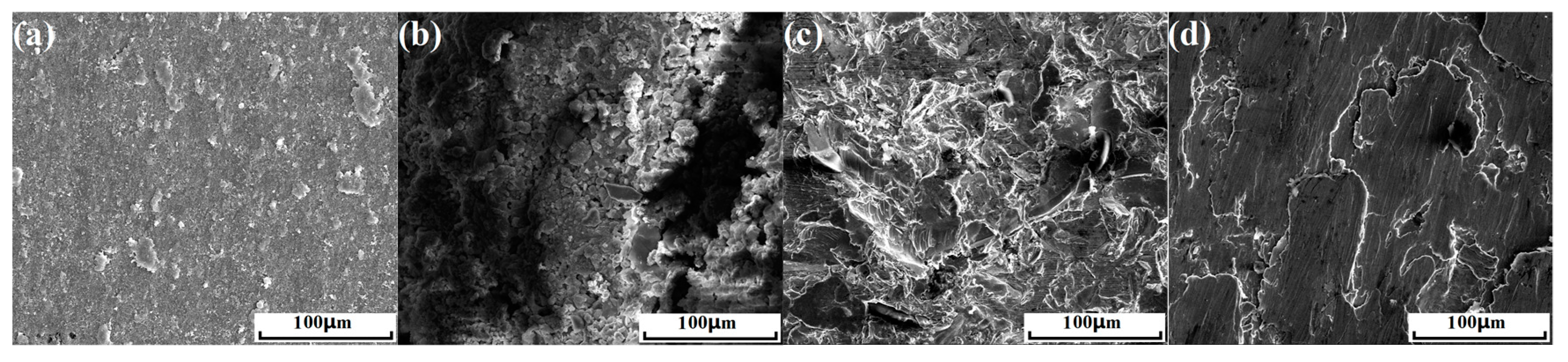
3.3. Surface Microstructure and its Effect on Corrosion Resistance of Rebar
4. Conclusions
- (1)
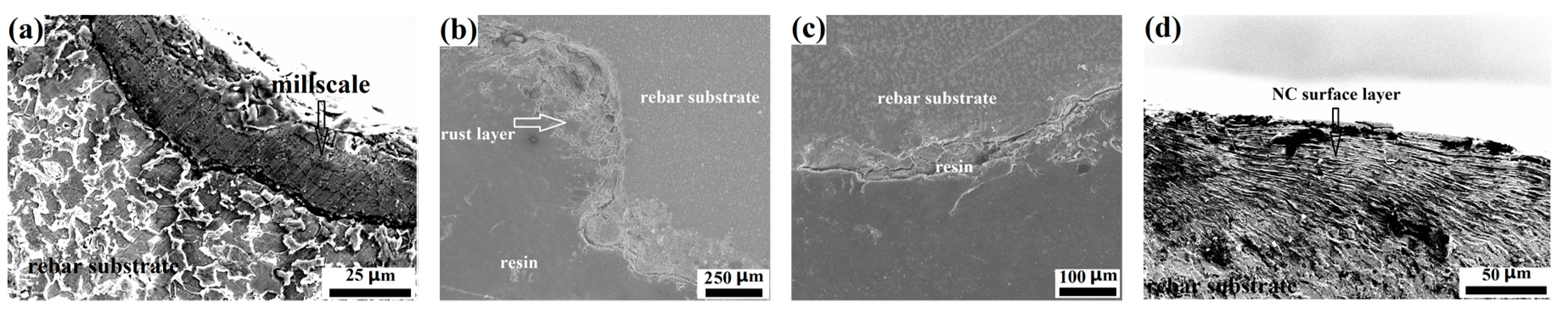
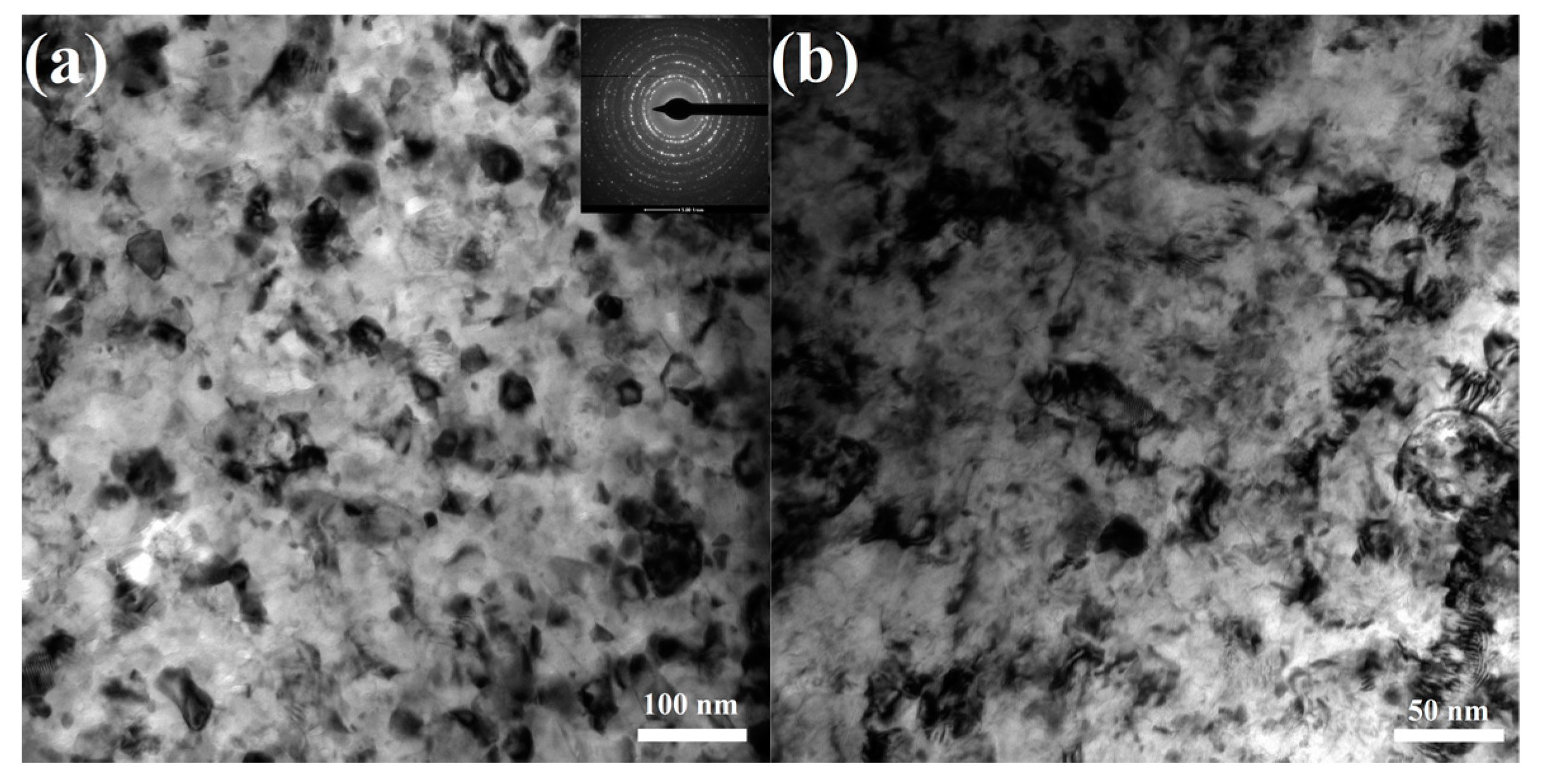
- The surfaces of the as-received rebar and the rusted rebar were covered with the mill scale and rust layers, respectively. Surface modification via sand blasting and wire brushing completely removed the rust layer, providing a fresh metallic surface to the treated rebars. The sand-blasted rebar had a modified surface with an abundance of impacted micro-fractures, presenting a higher roughness. The wire-brushed rebar had a modified surface layer with special nanograins, which restored a large amount of crystal defects and internal energies.
- (2)
- The surface modification greatly improved the rebar passivation. Complete removal of the rust layer and subsequent revealing of a fresh metallic surface dominated the enhanced passivation of the two surface-modified rebars. In addition, the wire-brushed rebar delivered the fast formation of the stable passive film and the best passivation of all the SCPSs due to its surface nanograins. However, due to a high surface roughness, the sand-blasted rebar presented slightly lower passivation in the pH-11 SCPS than the as-received rebar.
- (3)
- The surface modification of the rusted rebar greatly improved the anticorrosion resistance of the rusted rebar against Cl− due to the elimination of the pre-corroded surface and improvement of the passivation. However, due to the direct exposure of the metallic surface to the Cl− without a barrier of mill scale and the higher surface roughness, the wire-brushed rebar presented slightly lower corrosion resistance than the as-received rebar in the Cl−-containing pH–11 SCPS, whereas the sand-blasted rebar presented a lower corrosion resistance in all three Cl−-containing SCPSs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete–A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Moser, R.D.; Shingh, P.M.; Kahn, L.F.; Kurtis, K.E. Chloride-induced corrosion resistance of high-strength stainless steels in simulated alkaline and carbonated concrete pore solutions. Corros. Sci. 2012, 57, 241–253. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction–a review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Shi, X.M.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. The presentation of the chloride threshold level for corrosion of steel in concrete. Corros. Sci. 1997, 39, 1001–1013. [Google Scholar] [CrossRef]
- Cao, Y.; Gehlen, C.; Angstc, U.; Wang, L.; Wang, Z.D.; Yao, Y. Critical chloride content in reinforced concrete—An updated review considering Chinese experience. Cem. Concr. Res. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete–A critical review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.Q.; Li, X.G.; Zhou, C.; Tan, H.L. Effect of carbonation on the electrochemical behavior of corrosion resistance low alloy steel rebars in cement extract solution. Constr. Build. Mater. 2017, 130, 193–201. [Google Scholar] [CrossRef]
- Alonso, M.C.; Luna, F.J.; Criado, M. Corrosion behavior of duplex stainless steel reinforcement in ternary binder concrete exposed to natural chloride penetration. Constr. Build. Mater. 2019, 199, 385–395. [Google Scholar] [CrossRef]
- Williamson, J.; Isgor, O.B. The effect of simulated concrete pore solution composition and chlorides on the electronic properties of passive films on carbon steel rebar. Corros. Sci. 2016, 106, 82–95. [Google Scholar] [CrossRef]
- Ai, Z.Y.; Jiang, J.Y.; Sun, W.; Jiang, X.L.; Yu, B.; Wang, K.; Zhang, Z.F.; Song, D.; Ma, H.; Zhang, J.C. Enhanced passivation of alloy corrosion-resistant steel Cr10Mo1 under carbonation–Passive film formation, the kinetics and mechanism analysis. Cem. Concr. Compos. 2018, 92, 178–187. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.Q.; Li, X.G.; Jin, Z.; Liu, H.X. Corrosion behavior of Cr modified HRB400 steel rebar in simulated concrete pore solution. Constr. Build. Mater. 2015, 93, 884–890. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Carpenter, G.J.C.; Li, J.; McRae, G.A.; Gu, G.P. Nano-scale study of passive films and chloride-induced depassivation of carbon steel rebar in simulated concrete pore solutions using FIB/TEM. Cem. Concr. Res. 2013, 47, 55–68. [Google Scholar] [CrossRef]
- Imperatore, S.; Rinaldi, Z.; Drago, C. Degradation relationships for the mechanical properties of corroded steel rebars. Constr. Build. Mater. 2017, 148, 219–230. [Google Scholar] [CrossRef]
- Martinez-Echevarria, M.J.; Lopez-Alonso, M.; Romero, D.C.; Montero, J.R. Influence of the previous state of corrosion of rebars in predicting the service life of reinforced concrete structures. Constr. Build. Mater. 2018, 188, 915–923. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Ramírez, E.; Bautista, A.; Feliu, S. The behaviour of pre-rusted steel in concrete. Cem. Concr. Res. 1996, 26, 501–511. [Google Scholar] [CrossRef]
- Sun, X.Y.; Kong, H.T.; Wang, H.L.; Zhang, Z.C. Evaluation of corrosion characteristics and corrosion effects on the mechanical properties of reinforcing steel bars based on three-dimensional scanning. Corros. Sci. 2018, 142, 284–294. [Google Scholar] [CrossRef]
- González, J.A.; Miranda, J.M.; Otero, E.; Feliu, S. Effect of electrochemically reactive rust layers on the corrosion of steel in a Ca(OH)2 solution. Corros. Sci. 2007, 49, 438–448. [Google Scholar] [CrossRef]
- Bensabra, H.; Azzouz, N. Study of rust effect on the corrosion behavior of reinforcement steel using impedance spectroscopy. Metall. Mater. Trans. A 2013, 44, 5703–5710. [Google Scholar] [CrossRef]
- Miranda, J.M.; González, J.A.; Cobo, A.; Otero, E. Several questions about electrochemical rehabilitation methods for reinforced concrete structures. Corros. Sci. 2006, 48, 2172–2188. [Google Scholar] [CrossRef]
- Novak, P.; Mala, R.; Joska, L. Influence of pre-rusting on steel corrosion in concrete. Cem. Concr. Compos. 2001, 31, 589–593. [Google Scholar]
- Papadopoulos, M.P.; Apostolopoulos, C.A.; Zervaki, A.D.; Haidemenopoulos, G.N. Corrosion of exposed rebars, associated mechanical degradation and correlation with accelerated corrosion tests. Constr. Build. Mater. 2011, 25, 3367–3374. [Google Scholar] [CrossRef]
- AI-Tayyib, A.J.; Shamim Khan, M.; Allam, I.M.; AI-Mana, A.I. Corrosion behavior of pre-rusted rebars after placement in concrete. Cem. Concr. Res. 1990, 20, 955–960. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Al-Zahrani, M.; Al-Dulaijan, M.; Abdulquddus, S.U.; Rehman, S.; Ahsan, S.N. Effect of steel manufacturing process and atmospheric corrosion on the corrosion-resistance of steel bars in concrete. Cem. Concr. Compos. 2002, 24, 151–158. [Google Scholar] [CrossRef]
- Abd El Haleem, S.M.; Abd El Aal, E.E.; Abd El Wanees, S.; Diab, A. Environmental factors affecting the corrosion behaviour of reinforcing steel: I. The early stage of passive film formation in Ca(OH)2 solutions. Corros. Sci. 2010, 52, 3875–3882. [Google Scholar]
- Duarte, R.G.; Castela, A.S.; Neves, R.; Freire, L.; Montemor, M.F. Corrosion behavior of stainless steel rebars embedded in Concrete: an electrochemical impedance spectroscopy study. Electrochim. Acta 2014, 124, 218–224. [Google Scholar] [CrossRef]
- Asunción, B.; Velasco, F.; Manuel, T.C. Influence of the Alkaline Reserve of Chloride-containing Mortars on the 6-Year Corrosion Behavior of Corrugated UNS S32304 and S32001 Stainless Steels. Metals 2019, 9, 686. [Google Scholar]
- Shi, T.; Zheng, L.W.; Xu, X.C. Evaluation of alkali reactivity of concrete aggregates via AC impedance spectroscopy. Constr. Build. Mater. 2017, 145, 548–554. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Palaniswamy, N. Analysis of the electrochemical phenomenon at the rebar-concrete interface using the electrochemical impedance spectroscopic technique. Mag. Concr. Res. 2010, 62, 177–189. [Google Scholar] [CrossRef]
- Dong, B.Q.; Shi, G.Y.; Dong, P.; Ding, W.J.; Teng, X.J.; Qin, S.F.; Liu, Y.Q.; Xing, F.; Hong, S.X. Visualized tracing of rebar corrosion evolution in concrete with x-ray micro-computed tomography method. Cem. Concr. Compos. 2018, 92, 102–109. [Google Scholar] [CrossRef]
- Dong, B.Q.; Fang, G.H.; Liu, Y.Q.; Dong, P.; Zhang, J.C.; Xing, F.; Hong, S.X. Monitoring reinforcement corrosion and corrosion-induced cracking by X-ray microcomputed tomography method. Cem. Concr. Res. 2017, 100, 311–321. [Google Scholar] [CrossRef]
- Lee, H.S.; Yang, H.M.; Singh, J.K.; Prasad, S.K.; Yoo, B. Corrosion mitigation of steel rebars in chloride contaminated concrete pore solution using inhibitor: An electrochemical investigation. Constr. Build. Mater. 2018, 173, 443–451. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.H.; Ke, W. The influence of cooling processes on the corrosion performance of the rebar scale. Constr. Build. Mater. 2010, 24, 275–282. [Google Scholar] [CrossRef]
- Sun, W.; Tieu, A.K.; Jiang, Z.; Lu, C.; Zhu, H. Surface characteristics of oxide scale in hot strip rolling. J. Mater. Process Technol. 2003, 140, 76–83. [Google Scholar] [CrossRef]
- Burke, D.P.; Higginson, R.L. Characterization of multi-component scale by electron back scattered diffraction (EBSD). Scripta Mater. 2000, 42, 277–281. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Wu, Y.Y.; Jin, W.L. Distribution of millscale on corroded steel bars and penetration of steel corrosion products in concrete. Corros. Sci. 2013, 66, 160–168. [Google Scholar] [CrossRef]
- Song, D.; Sun, W.; Jiang, J.Y.; Ma, H.; Zhang, J.C.; Chen, Z.J. Corrosion Behavior of Cr Micro-alloyed Corrosion-resistant Rebar in Neutral Cl−-containing Environment. J. Iron Steel Res. Int. 2016, 23, 608–617. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Ren, H.Y.; Dai, H.; Jin, W.L. Composition and expansion coefficient of rust based on X-ray diffraction and thermal analysis. Corros. Sci. 2011, 53, 1646–1658. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yu, J.; Wu, Y.Y.; Jin, W.L. Critical thickness of rust layer at inner and out surface cracking of concrete cover in reinforced concrete structures. Corros. Sci. 2012, 59, 316–323. [Google Scholar] [CrossRef]
- Zhua, K.Y.; Vasselb, A.; Brissetc, F.; Lud, K.; Lua, J. Nanostructure formation mechanism of a-titanium using SMAT. Acta Mater. 2004, 52, 4101–4110. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scripta Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, H.Y.; Tang, J.; Guo, J.J.; Pi, J.L.; Ye, K.L. Corrosion properties of phase reversion induced nano/ultrafine grained AISI 304 metastable austenite stainless steel. Mater. Res. Bullet. 2018, 107, 421–429. [Google Scholar] [CrossRef]
- Song, D.; Jiang, J.H.; Guan, X.N.; Qiao, Y.X.; Li, X.B.; Chen, J.Q.; Sun, J.P.; Ma, A.B. Effect of Surface Nanocrystallization on Corrosion Resistance of the Conformed Cu–0.4%Mg Alloy in NaCl Solution. Metals 2018, 8, 765. [Google Scholar] [CrossRef]
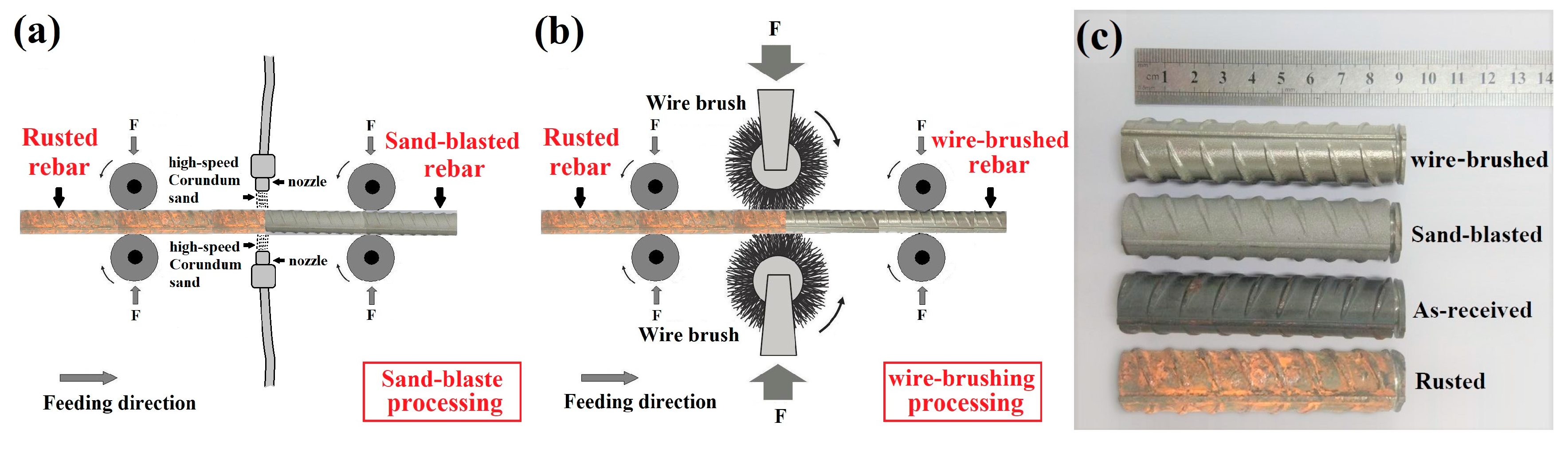
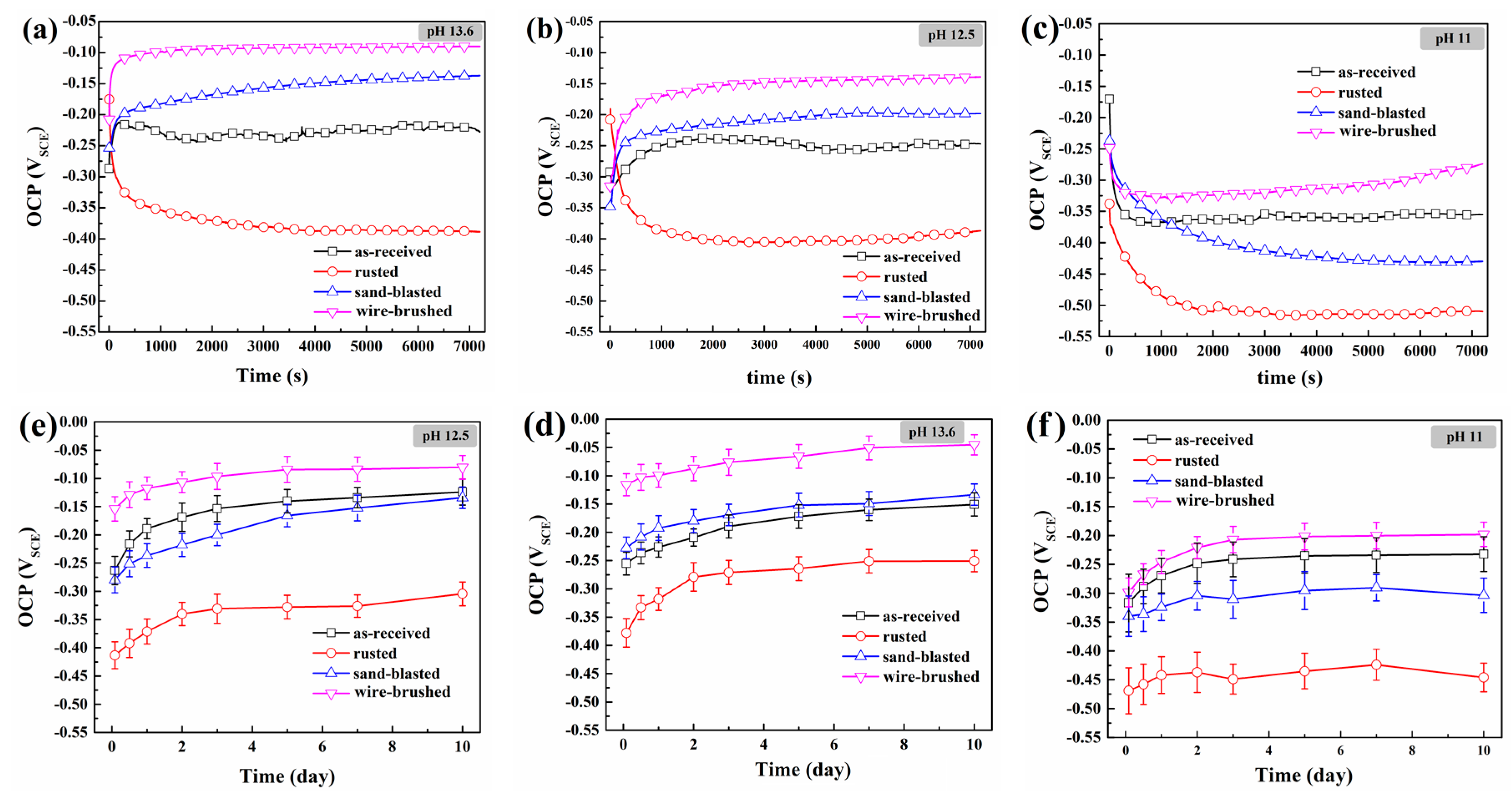


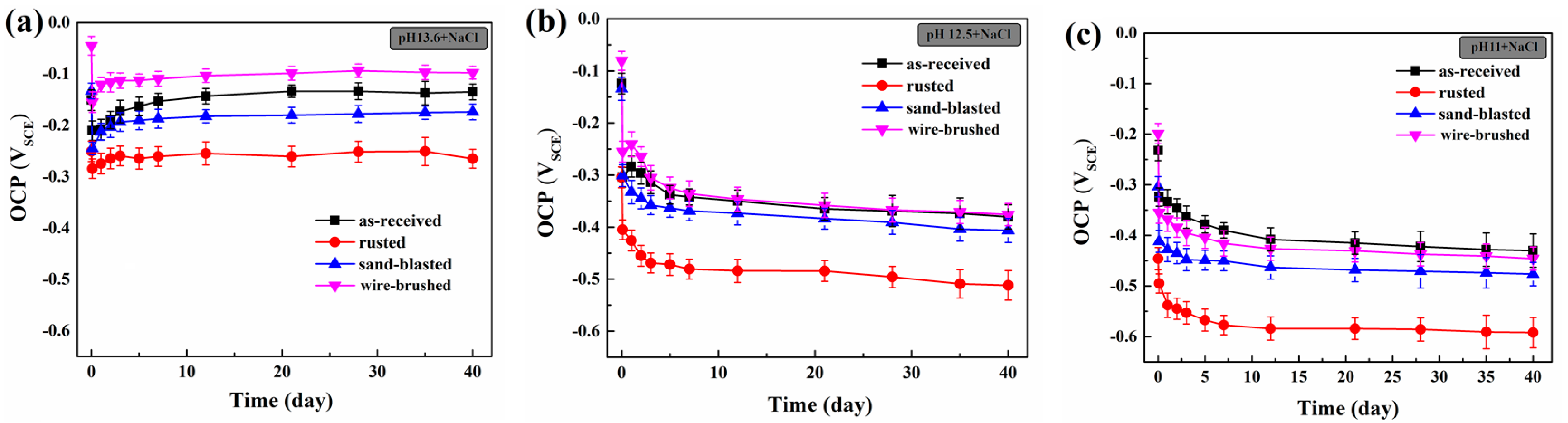
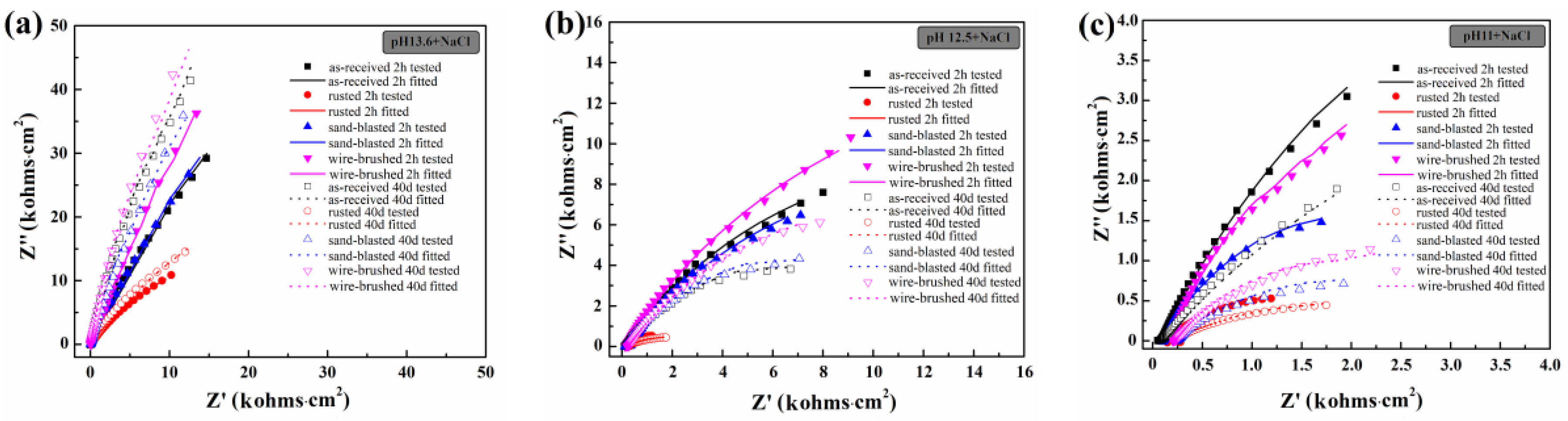

| Fe | C | Si | Mn | Cr | S | P | Ni | Cu | V |
|---|---|---|---|---|---|---|---|---|---|
| Balance | 0.22 | 1.44 | 0.02 | 0.022 | 0.025 | 0.025 | 0.01 | 0.01 | 0.038 |
| Simulated Concrete Pore Solutions | Concentration (mol∙dm−3) | pH Value | |||
|---|---|---|---|---|---|
| Ca(OH)2 | NaOH | KOH | NaHCO3 | ||
| pH–13.6 SCPS | 0.001 | 0.2 | 0.6 | 0 | 13.6 |
| pH–12.5 SCPS | saturated | 0 | 0 | 0 | 12.5 |
| pH–11 SCPS | saturated | 0 | 0 | appropriate | 11 |
| Rebar Sample and Cl−-Containing SCPS | Ecorr (mVSCE) | icorr (μA∙cm−2) | Corrosion Rate (μm∙y−1) | Epit (mVSCE) | |
|---|---|---|---|---|---|
| as-received | pH–13.6 | –129 ± 6 | 0.106 ± 0.005 | 1.229 ± 0.058 | 458 ± 15 |
| pH–12.5 | –395 ± 10 | 1.250 ± 0.008 | 14.495 ± 0.093 | −8 ± 20 | |
| pH–11 | −420 ± 10 | 2.220 ± 0.010 | 25.743 ± 0.116 | −180 ± 10 | |
| rusted | pH–13.6 | −214 ± 8 | 0.940 ± 0.008 | 10.900 ± 0.096 | 225 ± 13 |
| pH–12.5 | −508 ± 13 | 4.060 ± 0.023 | 47.078 ± 0.657 | −258 ± 15 | |
| pH–11 | −577 ± 13 | 8.510 ± 0.055 | 98.681 ± 0.638 | - | |
| sand-blasted | pH–13.6 | −169 ± 5 | 0.122 ± 0.005 | 1.415 ± 0.058 | 415 ± 10 |
| pH–12.5 | −406 ± 10 | 1.580 ± 0.008 | 18.325 ± 0.093 | −35 ± 10 | |
| pH–11 | −467 ± 12 | 4.740 ± 0.021 | 54.965 ± 0.244 | −270 ± 12 | |
| wire-brushed | pH–13.6 | −111 ± 5 | 0.054 ± 0.002 | 0.626 ± 0.023 | 507 ± 17 |
| pH–12.5 | −394 ± 10 | 1.095 ± 0.006 | 12.697 ± 0.069 | 5 ± 15 | |
| pH–11 | −440 ± 12 | 3.485 ± 0.020 | 40.412 ± 0.232 | −240 ± 18 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Yang, F.; Guo, M.; Zhao, S.; Hao, J.; Chen, Z.; Sun, J.; Xu, Y.; Jiang, J. Surface Modification of Rusted Rebar and Enhanced Passivation/Anticorrosion Performance in Simulated Concrete Pore Solutions with Different Alkalinity. Metals 2019, 9, 1050. https://doi.org/10.3390/met9101050
Song D, Yang F, Guo M, Zhao S, Hao J, Chen Z, Sun J, Xu Y, Jiang J. Surface Modification of Rusted Rebar and Enhanced Passivation/Anticorrosion Performance in Simulated Concrete Pore Solutions with Different Alkalinity. Metals. 2019; 9(10):1050. https://doi.org/10.3390/met9101050
Chicago/Turabian StyleSong, Dan, Falin Yang, Mingzhi Guo, Sujing Zhao, Jun Hao, Zhaojun Chen, Jiapeng Sun, Yifeng Xu, and Jinyang Jiang. 2019. "Surface Modification of Rusted Rebar and Enhanced Passivation/Anticorrosion Performance in Simulated Concrete Pore Solutions with Different Alkalinity" Metals 9, no. 10: 1050. https://doi.org/10.3390/met9101050
APA StyleSong, D., Yang, F., Guo, M., Zhao, S., Hao, J., Chen, Z., Sun, J., Xu, Y., & Jiang, J. (2019). Surface Modification of Rusted Rebar and Enhanced Passivation/Anticorrosion Performance in Simulated Concrete Pore Solutions with Different Alkalinity. Metals, 9(10), 1050. https://doi.org/10.3390/met9101050





