Influence of Titanium Diboride Particle Size on Structure and Mechanical Properties of an Al-Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Master Alloys Production
2.2. Obtaining the Alloys
2.3. Rolling Technique
2.4. Methods of Analysis of Initial Materials and Alloys
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawazoe, M.; Shibata, T.; Mukai, T.; Higashi, K. Elevated temperature mechanical properties of A 5056 Al-Mg alloy processed by equal-channel-angular-extrusion. Scr. Mater. 1997, 36, 699–705. [Google Scholar] [CrossRef]
- Jones, R.H. The influence of hydrogen on the stress-corrosion cracking of low-strength Al-Mg alloys. JOM 2003, 55, 42–46. [Google Scholar] [CrossRef]
- Lee, S.; Utsunomiya, A.; Akamatsu, H.; Neishi, K.; Furukawa, M.; Horita, Z.; Langdon, T.G. Influence of scandium and zirconium on grain stability and superplastic ductilities in ultrafine-grained Al–Mg alloys. Acta Mater. 2002, 50, 553–564. [Google Scholar] [CrossRef]
- Filatov, Y.A.; Yelagin, V.I.; Zakharov, V.V. New Al–Mg–Sc alloys. Mater. Sci. Eng. A 2000, 280, 97–101. [Google Scholar] [CrossRef]
- Ahmad, Z. The properties and application of scandium-reinforced aluminum. JOM 2003, 55, 35–39. [Google Scholar] [CrossRef]
- Yan, S.J.; Dai, S.L.; Zhang, X.Y.; Yang, C.; Hong, Q.H.; Chen, J.Z.; Lin, Z.M. Investigating aluminum alloy reinforced by graphene nanoflakes. Mater. Sci. Eng. A 2014, 612, 440–444. [Google Scholar] [CrossRef]
- Vorozhtsov, S.; Minkov, L.; Dammer, V.; Khrustalyov, A.; Zhukov, I.; Promakhov, V.; Vorozhtsov, A.; Khmeleva, M. Ex situ introduction and distribution of nonmetallic particles in aluminum melt: Modeling and experiment. JOM 2017, 69, 2653–2657. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Shen, Y.; Hu, W.; Luo, L. Dissimilar friction stir welding of Ti–6Al–4V alloy and aluminum alloy employing a modified butt joint configuration: Influences of process variables on the weld interfaces and tensile properties. Mater. Des. 2014, 53, 838–848. [Google Scholar] [CrossRef]
- Zhukov, I.; Promakhov, V.; Vorozhtsov, S.; Kozulin, A.; Khrustalyov, A.; Vorozhtsov, A. Influence of Dispersion Hardening and Severe Plastic Deformation on Structure, Strength and Ductility Behavior of an AA6082 Aluminum Alloy. JOM 2018, 70, 2731–2738. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Qian, M.; Eskin, D.G.; Das, A. On the microstructural refinement in commercial purity Al and Al-10 wt % Cu alloy under ultrasonication during solidification. Mater. Des. 2017, 132, 266–274. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.L.; Liu, J.C.; Li, H.X.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. The influences of grain size and morphology on the hot tearing susceptibility, contraction, and load behaviors of AA7050 alloy inoculated with Al-5Ti-1B master alloy. Metal. Mater. Trans. A 2016, 47, 4024–4037. [Google Scholar] [CrossRef]
- Mahamani, A.; Jayasree, A.; Mounika, K.; Reddi, K.; Sakthivelan, N. Evaluation of mechanical properties of AA6061-TiB2/ZrB2 in-situ metal matrix composites fabricated by K2TiF6-KBF4-K2ZrF6 reaction system. Int. J. Microstruct. Mater. Prop. 2015, 10, 185–200. [Google Scholar]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Ezatpour, H.R.; Torabi Parizi, M.; Sajjadi, S.A.; Ebrahimi, G.R.; Chaichi, A. Microstructure, mechanical analysis and optimal selection of 7075 aluminum alloy based composite reinforced with alumina nanoparticles. Mater. Chem. Phys. 2016, 178, 119–127. [Google Scholar] [CrossRef]
- Vorozhtsov, S.A.; Eskin, D.G.; Tamayo, J.; Vorozhtsov, A.B.; Promakhov, V.V.; Averin, A.A.; Khrustalyov, A.P. The application of external fields to the manufacturing of novel dense composite master alloys and aluminum-based nanocomposites. Metal. Mater. Trans. A 2015, 46, 2870–2875. [Google Scholar] [CrossRef]
- Mousavian, R.T.; Khosroshahi, R.A.; Yazdani, S.; Brabazon, D.; Boostani, A.F. Fabrication of aluminum matrix composites reinforced with nano-to micrometer-sized SiC particles. Mater. Des. 2016, 89, 58–70. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Qi, L.; Wang, Y.; Nie, J.F. Phase-field simulation of Orowan strengthening by coherent precipitate plates in an aluminum alloy. Metal. Mater. Trans. A 2015, 46, 3287–3301. [Google Scholar] [CrossRef]
- Eskin, D.G.; Al-Helal, K.; Tzanakis, I. Application of a plate sonotrode to ultrasonic degassing of aluminum melt: Acoustic measurements and feasibility study. J. Mater. Proc. Technol. 2015, 222, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Xuan, Y.; Nastac, L. The role of ultrasonic cavitation in refining the microstructure of aluminum based nanocomposites during the solidification process. Ultrasonics 2018, 83, 94–102. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, S.; Lü, S.; Xiong, X.; Du, R.; An, P. Improvement of particles distribution of in-situ 5 vol % TiB2 particulates reinforced Al-4.5 Cu alloy matrix composites with ultrasonic vibration treatment. J. Alloys Compd. 2017, 692, 1–9. [Google Scholar] [CrossRef]
- Promakhov, V.V.; Khmeleva, M.G.; Zhukov, I.A.; Platov, V.V.; Khrustalyov, A.P.; Vorozhtsov, A.B. The Impact of Particle Reinforcement with Al2O3, TiB2, and TiC and Severe Plastic Deformation Treatment on the Combination of Strength and Electrical Conductivity of Pure Aluminum. Metals 2019, 65, 9. [Google Scholar]
- Zhukov, I.A.; Ziatdinov, M.K.; Vorozhtsov, A.B.; Zhukov, A.S.; Vorozhtsov, S.A.; Promakhov, V.V. Self-propagating high-temperature synthesis of Al and Ti borides. Rus. Phys. J. 2016, 59, 1324–1326. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Promakhov, V.V.; Matveev, A.E.; Platov, V.V.; Khrustalev, A.P.; Dubkova, Y.A.; Vorozhtsov, S.A.; Potekaev, A.I. Principles of Structure and Phase Composition Formation in Composite Master Alloys of the Al–Ti–B4C Systems Used for Aluminum Alloy Modification. Rus. Phys. J. 2018, 60, 2025–2031. [Google Scholar] [CrossRef]
- Belov, N.A. Effect of eutectic phases on the fracture behavior of high-strength castable aluminum alloys. Met. Sci. Heat Treat. 1995, 37, 237–242. [Google Scholar] [CrossRef]
- Sun, N.; Apelian, D. Friction stir processing of aluminum cast alloys for high performance applications. JOM 2011, 63, 44–50. [Google Scholar] [CrossRef]
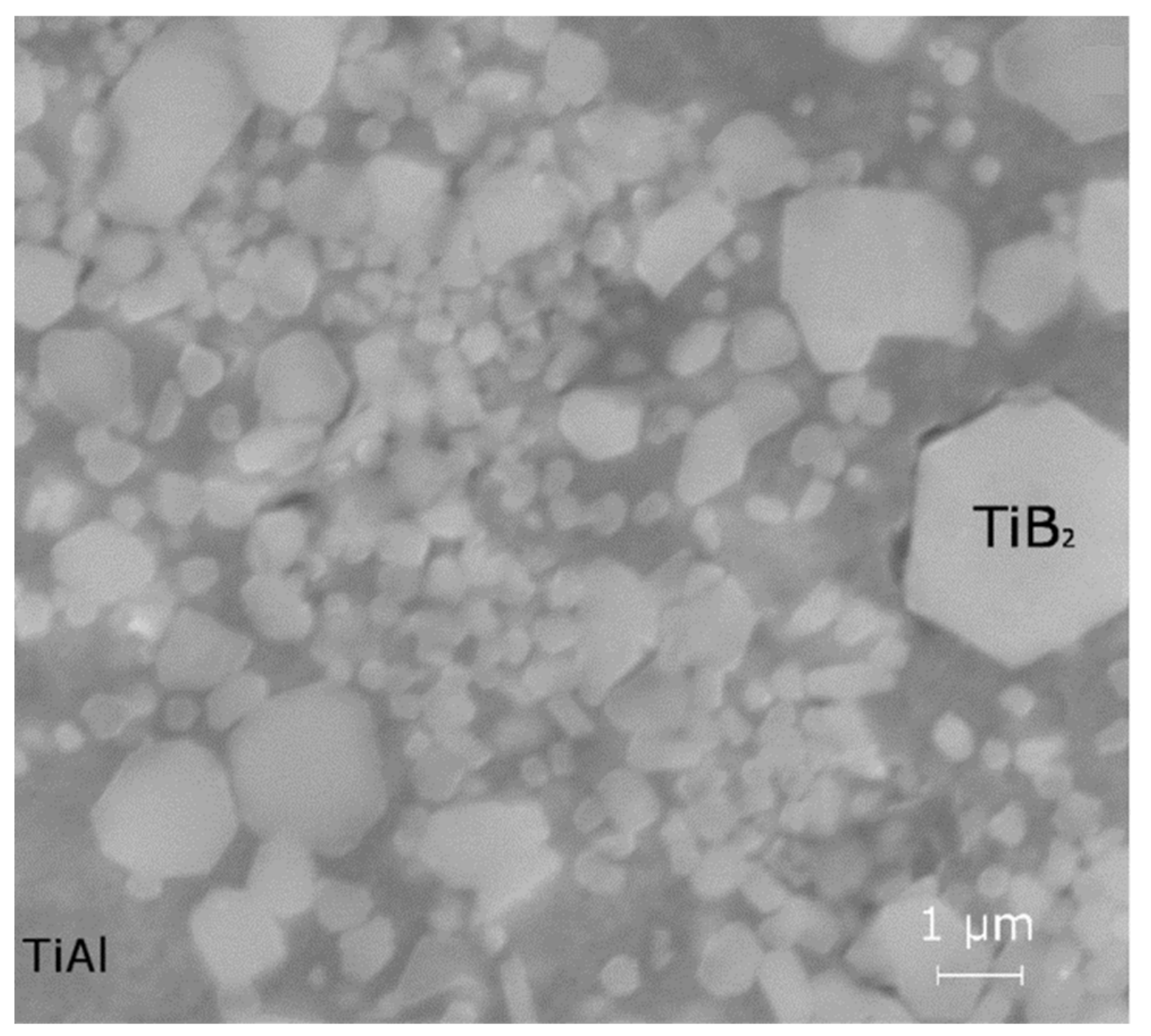
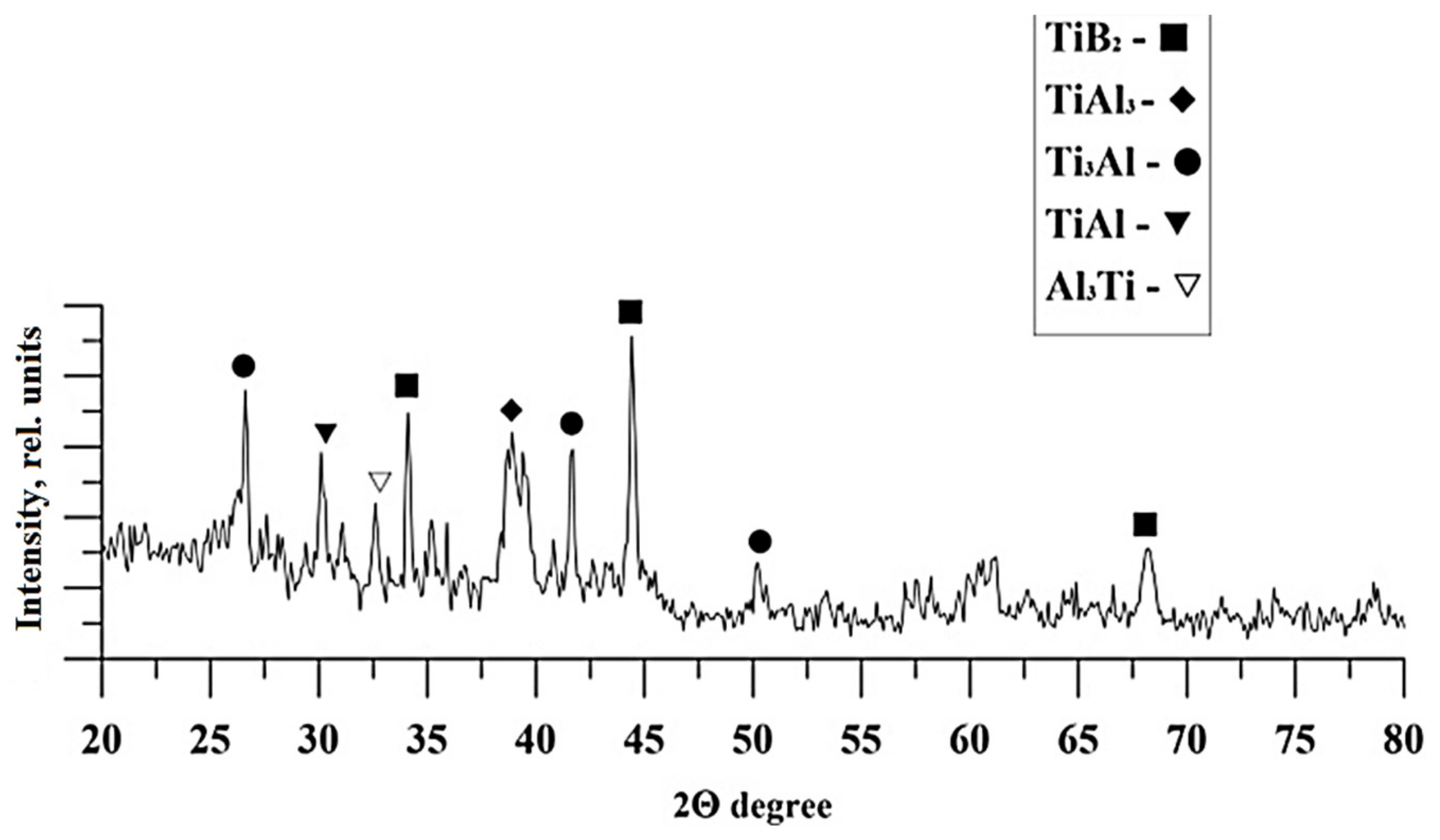


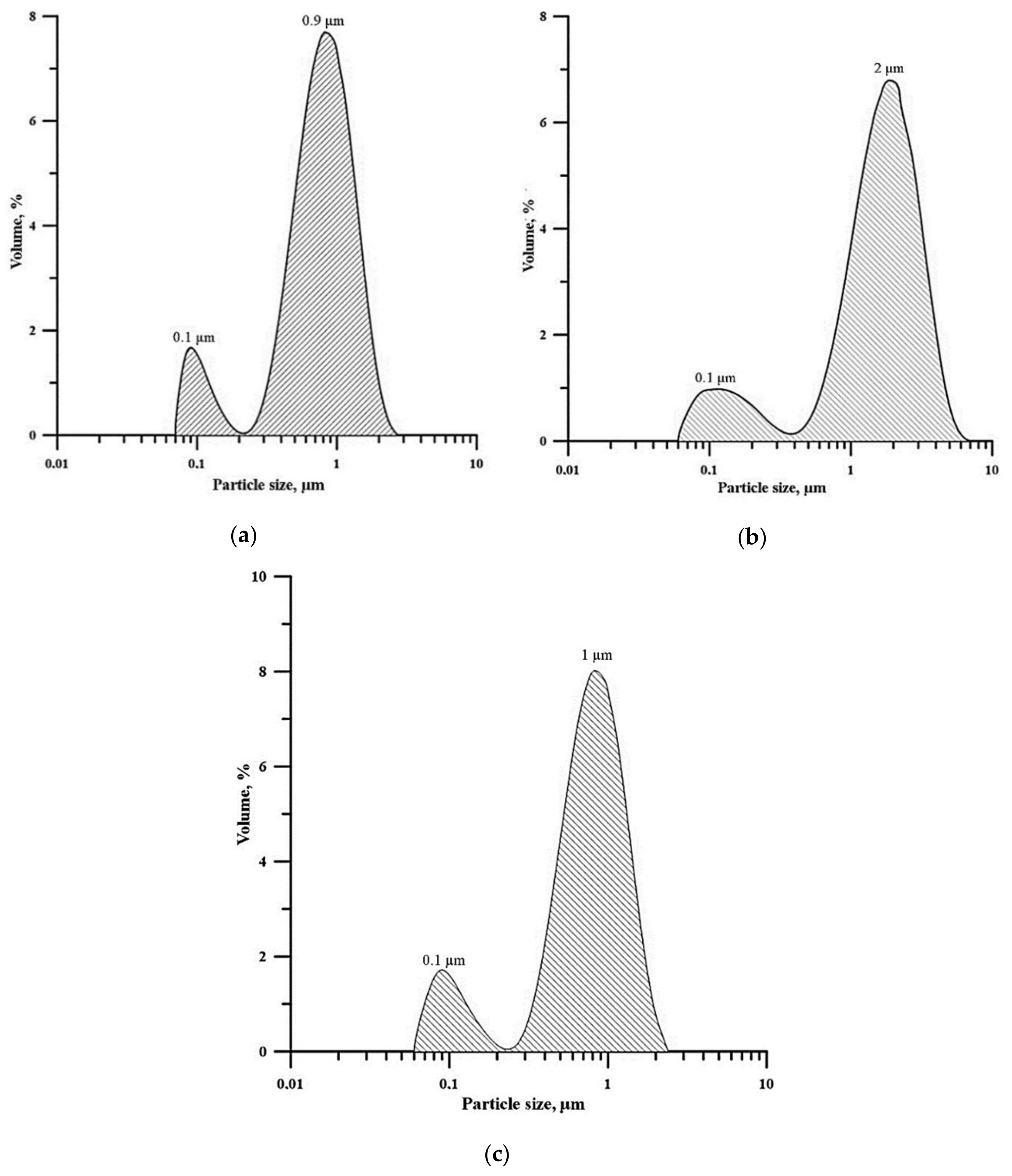
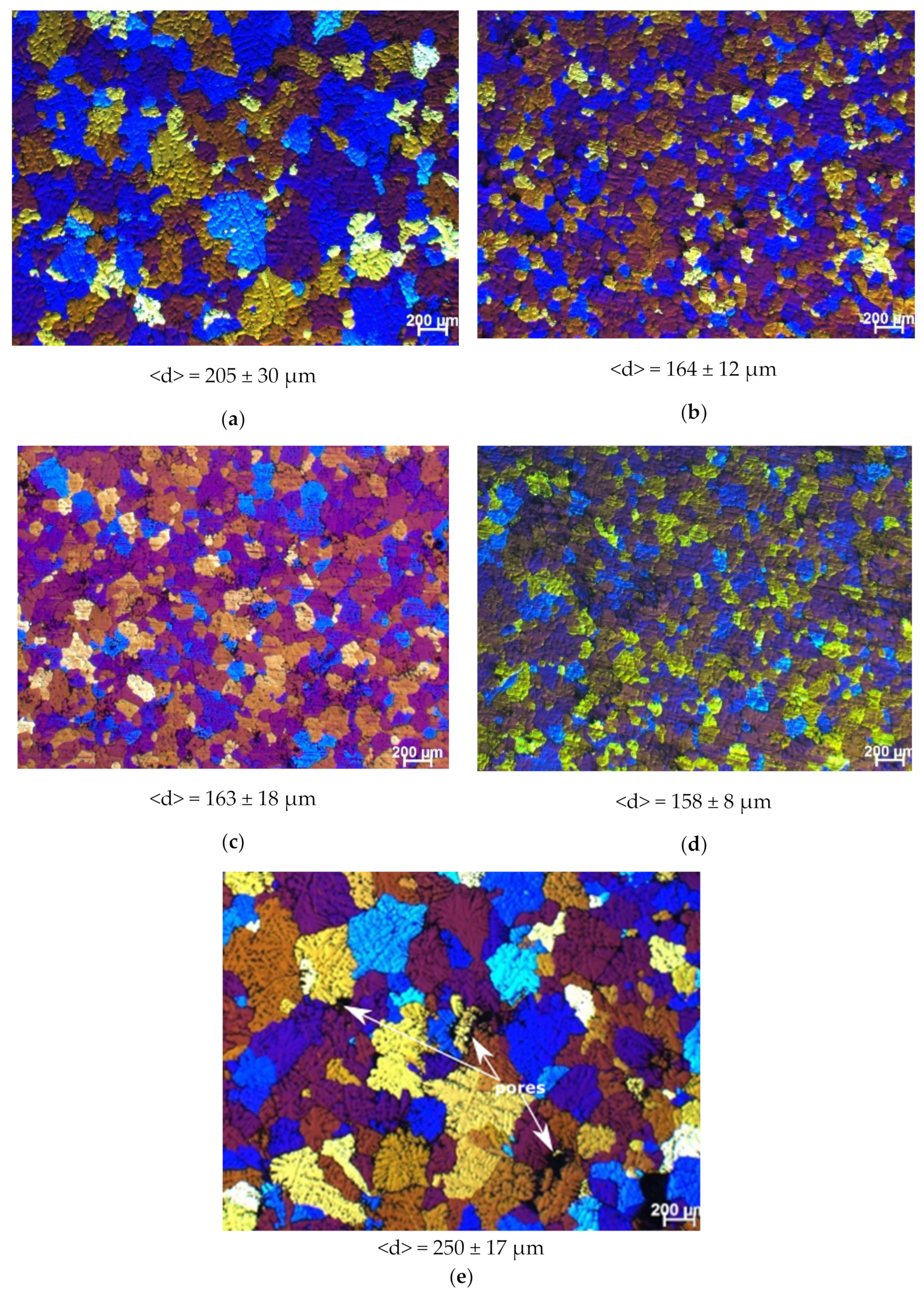

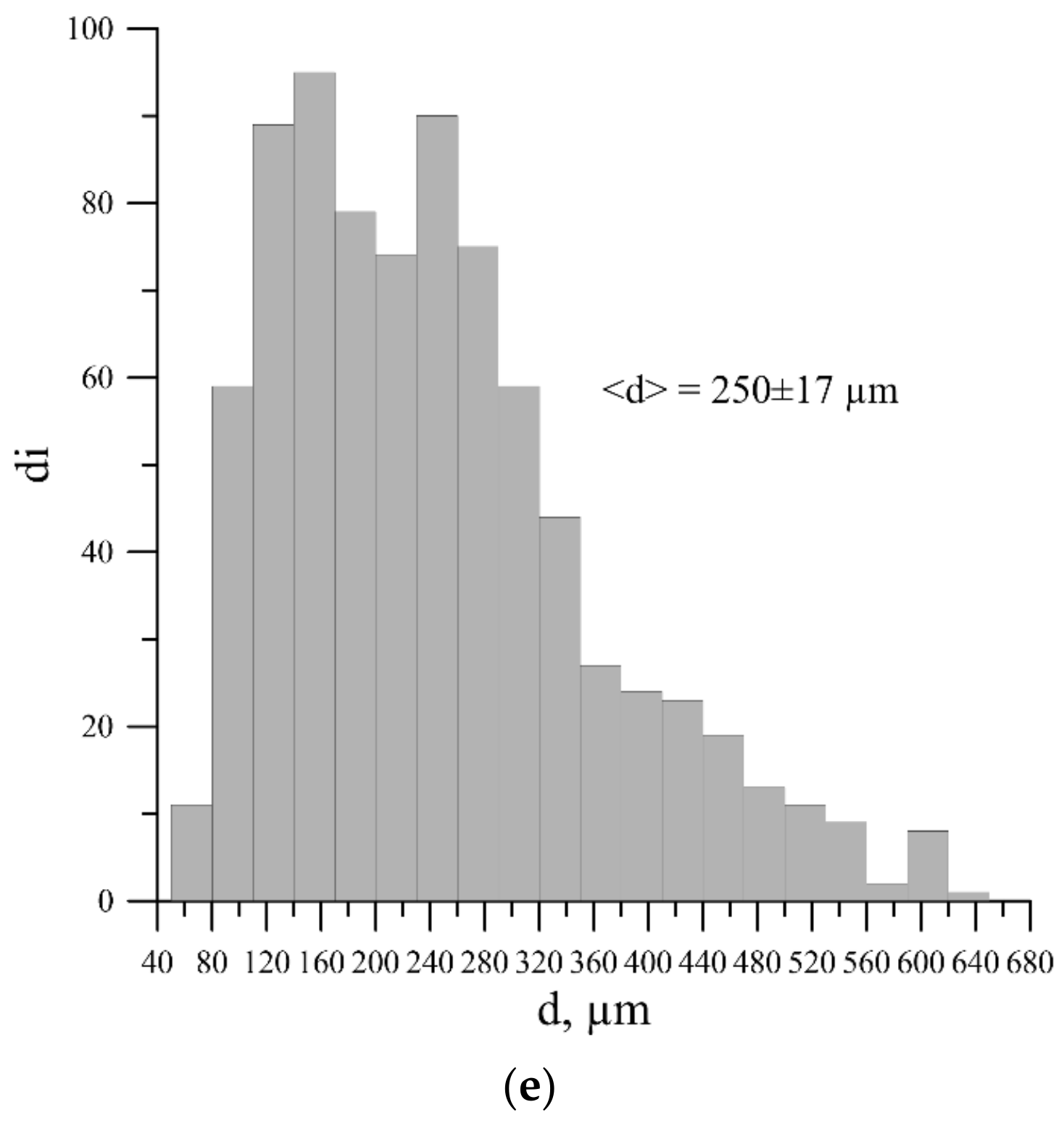
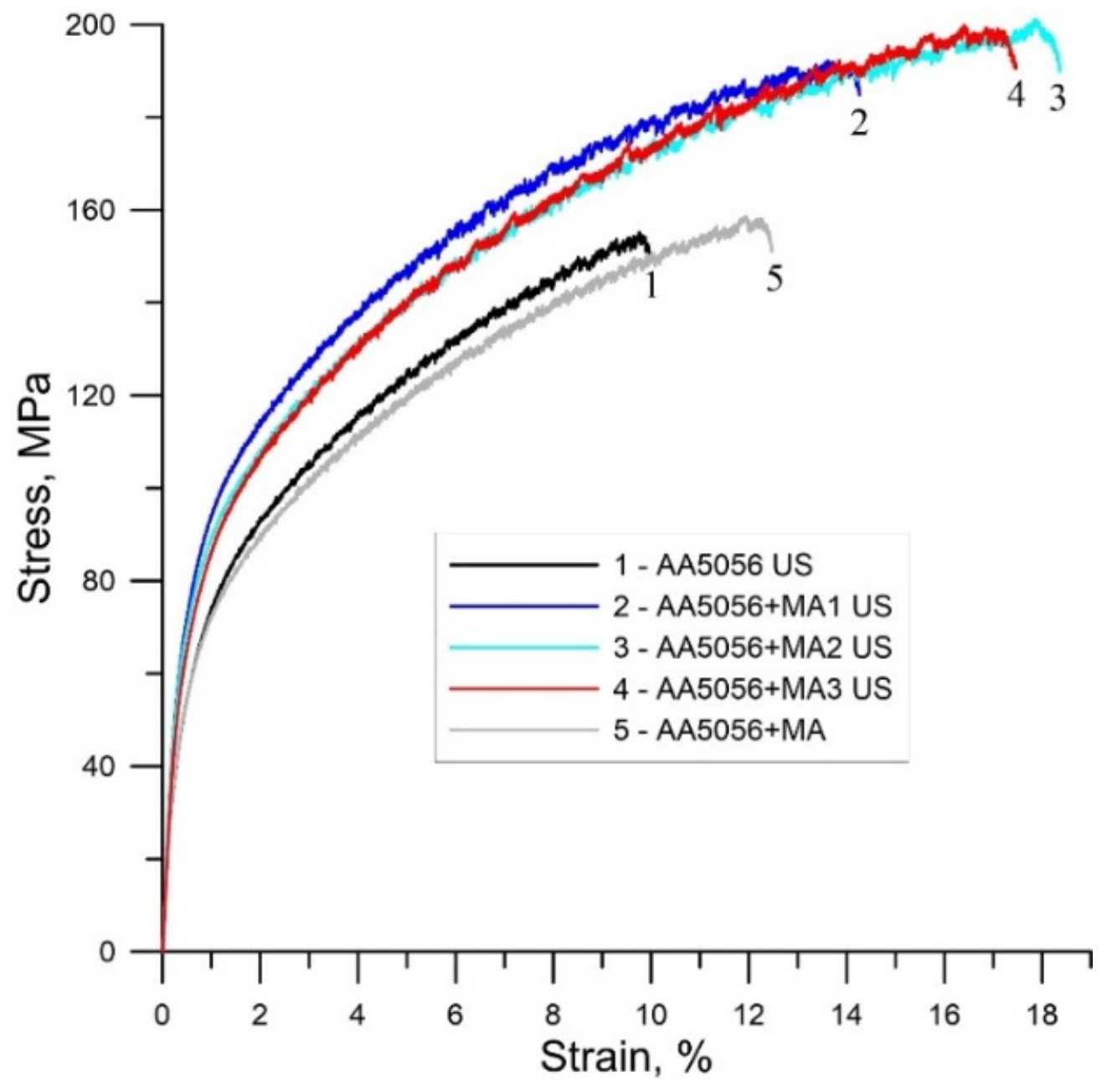
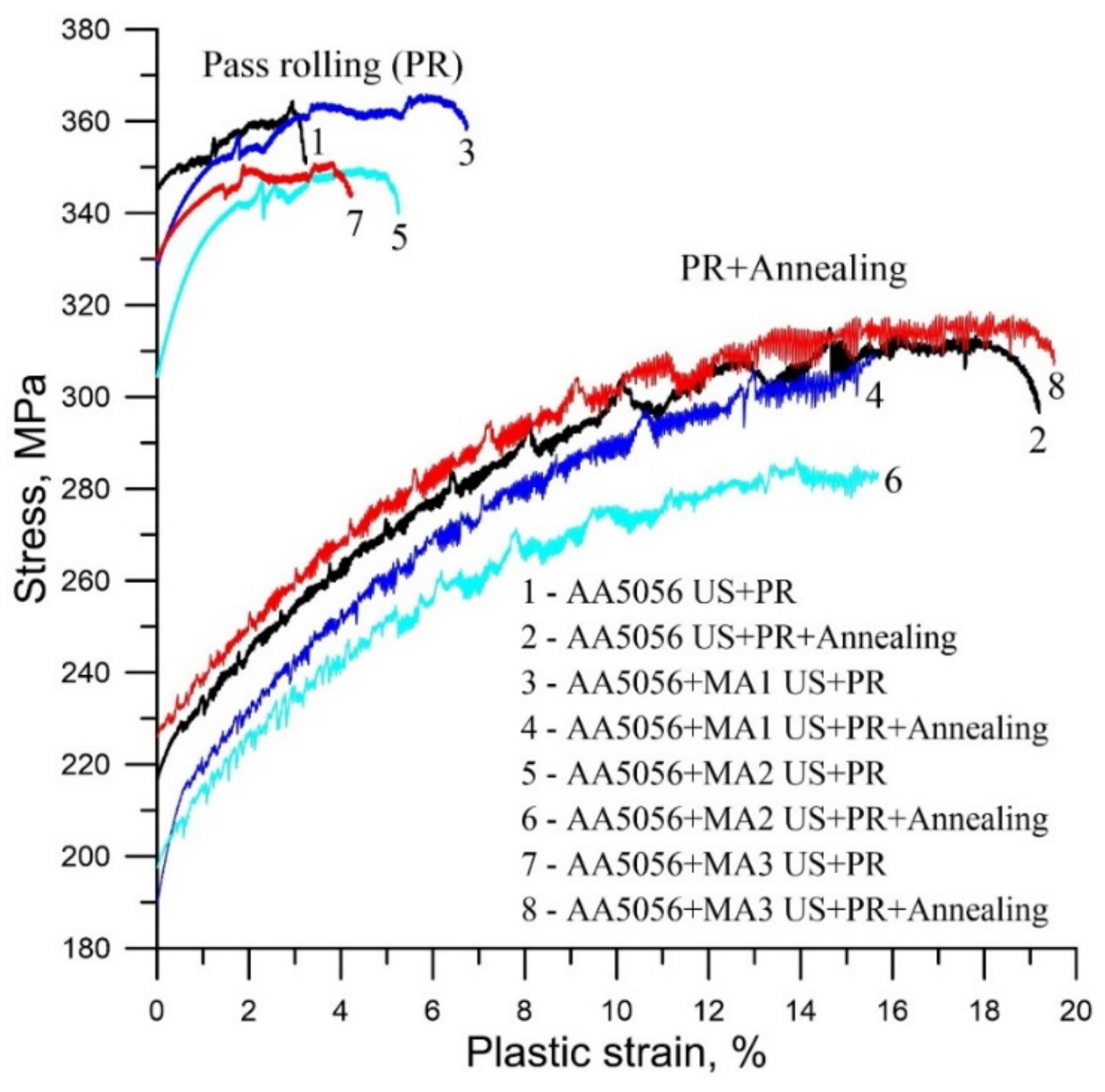
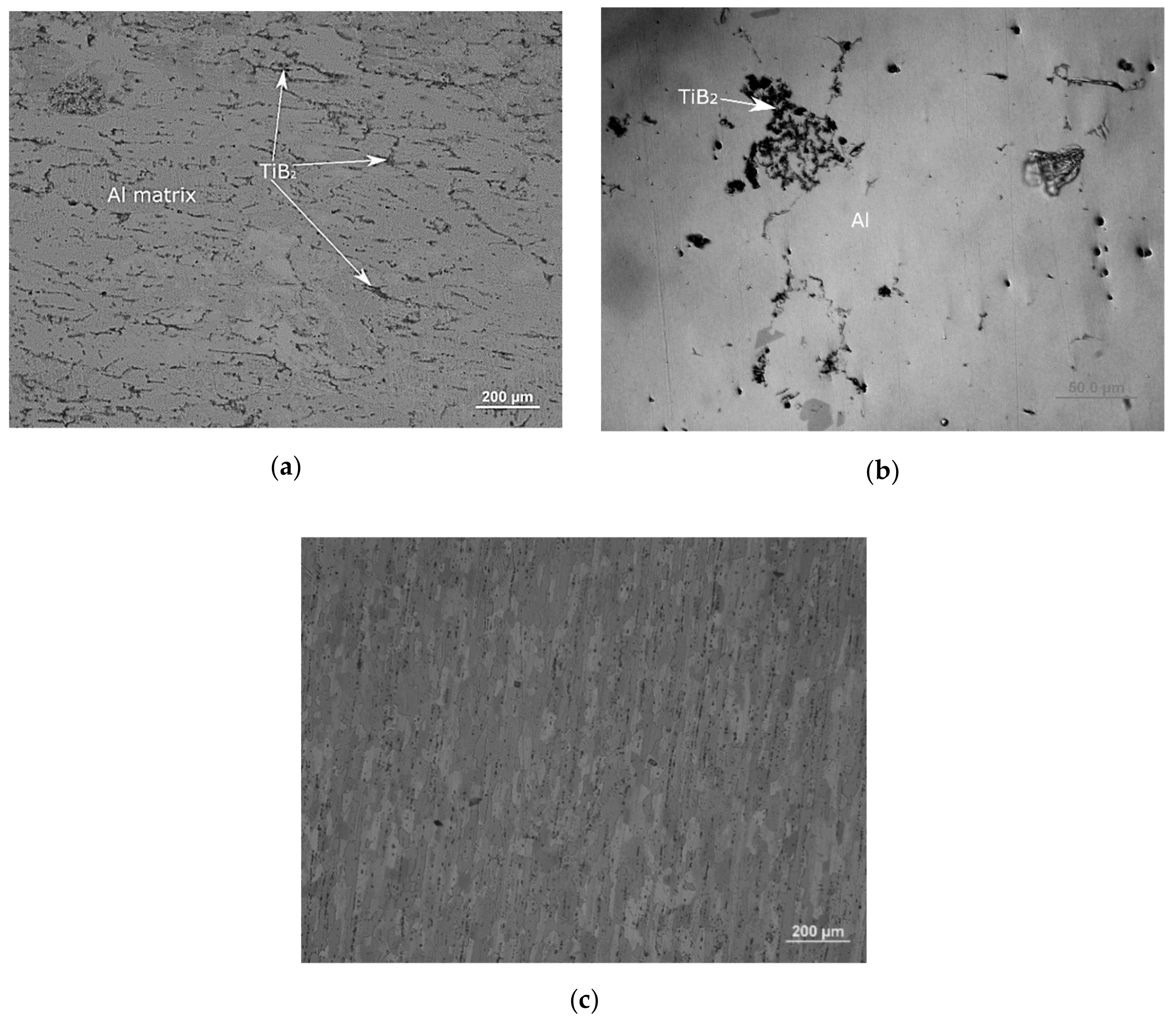
| Master-Alloy | Phase | Phase Content, wt% | Lattice Parameter, Å |
|---|---|---|---|
| 1 | TiB2 | 30 | a = 3.0296, c = 3.2260 |
| Al3Ti | 26 | a = 4.0123 | |
| Ti3Al | 9 | a = 5.6683, c = 4.5854 | |
| TiAl | 35 | a = 4.0115 | |
| 2 | TiB2 | 30 | a = 3.0293, c = 3.2257 |
| Al3Ti | 40 | a = 3.9484, c = 8.4989 | |
| Ti3Al | 14 | a = 5.6640, c = 4.6344 | |
| TiAl | 16 | a = 4.0278 | |
| 3 | TiAl | 57 | a = 4.0319 |
| TiB2 | 43 | a = 3.0140, c = 3.2000 |
| Alloy Matrix | Ultrasonic Treatment | Master Alloy | TiB2 Particle Quantity in a Master Alloy, % | TiB2 Particle Quantity in 1 kg of an Alloy |
|---|---|---|---|---|
| AA5056 | + | - | - | - |
| AA5056 | - | МA1 | 30 | 4 × 1020 ± 6 × 1010 |
| AA5056 | + | МA1 | 30 | 4 × 1020 ± 6 × 1010 |
| AA5056 | + | МA2 | 32 | 4 × 1020 ± 6 × 1010 |
| AA5056 | + | МA3 | 43 | 4.5 × 1020 ± 7.3 × 1010 |
| Alloy | σ0.2, MPa | σB, MPa | δ, % |
|---|---|---|---|
| AA5056 US | 57 ± 4 | 155 ± 11 | 11.5 ± 0.8 |
| AA5056 US + MA1 | 74 ± 7 | 192 ± 14 | 14.5 ± 0.4 |
| AA5056 US + MA2 | 71 ± 6 | 201 ± 12 | 18.8 ± 0.6 |
| AA5056 US + MA3 | 69 ± 8 | 200 ± 10 | 17.8 ± 0.5 |
| Alloy | σ0.2, MPa | σB, MPa | δ, % |
|---|---|---|---|
| Pass rolling | |||
| AA5056 US | 345 ± 11 | 369 ± 16 | 9.6 ± 0.3 |
| AA5056 US + MA1 | 328 ± 13 | 365 ± 17 | 12.3 ± 0.4 |
| AA5056 US + MA2 | 304 ± 16 | 349 ± 19 | 8.4 ± 0.2 |
| AA5056 US + MA3 | 330 ± 12 | 350 ± 17 | 10 ± 0.3 |
| Pass rolling + annealing | |||
| AA5056 US | 217 ± 9 | 311 ± 11 | 22.2 ± 0.3 |
| AA5056 US + MA1 | 189 ± 10 | 309 ± 13 | 17.9 ± 0.1 |
| AA5056 US + MA2 | 197 ± 7 | 286 ± 10 | 18.9 ± 0.2 |
| AA5056 US + MA3 | 226 ± 8 | 316 ± 12 | 22.4 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrustalyov, A.P.; Kozulin, A.A.; Zhukov, I.A.; Khmeleva, M.G.; Vorozhtsov, A.B.; Eskin, D.; Chankitmunkong, S.; Platov, V.V.; Vasilyev, S.V. Influence of Titanium Diboride Particle Size on Structure and Mechanical Properties of an Al-Mg Alloy. Metals 2019, 9, 1030. https://doi.org/10.3390/met9101030
Khrustalyov AP, Kozulin AA, Zhukov IA, Khmeleva MG, Vorozhtsov AB, Eskin D, Chankitmunkong S, Platov VV, Vasilyev SV. Influence of Titanium Diboride Particle Size on Structure and Mechanical Properties of an Al-Mg Alloy. Metals. 2019; 9(10):1030. https://doi.org/10.3390/met9101030
Chicago/Turabian StyleKhrustalyov, Anton P., Alexander A. Kozulin, Ilya A. Zhukov, Marina G. Khmeleva, Alexander B. Vorozhtsov, Dmitry Eskin, Suwaree Chankitmunkong, Vladimir V. Platov, and Sergey V. Vasilyev. 2019. "Influence of Titanium Diboride Particle Size on Structure and Mechanical Properties of an Al-Mg Alloy" Metals 9, no. 10: 1030. https://doi.org/10.3390/met9101030
APA StyleKhrustalyov, A. P., Kozulin, A. A., Zhukov, I. A., Khmeleva, M. G., Vorozhtsov, A. B., Eskin, D., Chankitmunkong, S., Platov, V. V., & Vasilyev, S. V. (2019). Influence of Titanium Diboride Particle Size on Structure and Mechanical Properties of an Al-Mg Alloy. Metals, 9(10), 1030. https://doi.org/10.3390/met9101030





