Kinetic Studies on Gas-Based Reduction of Vanadium Titano-Magnetite Pellet
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Oxidized VTM Pellets
2.2. Experimental Measurements
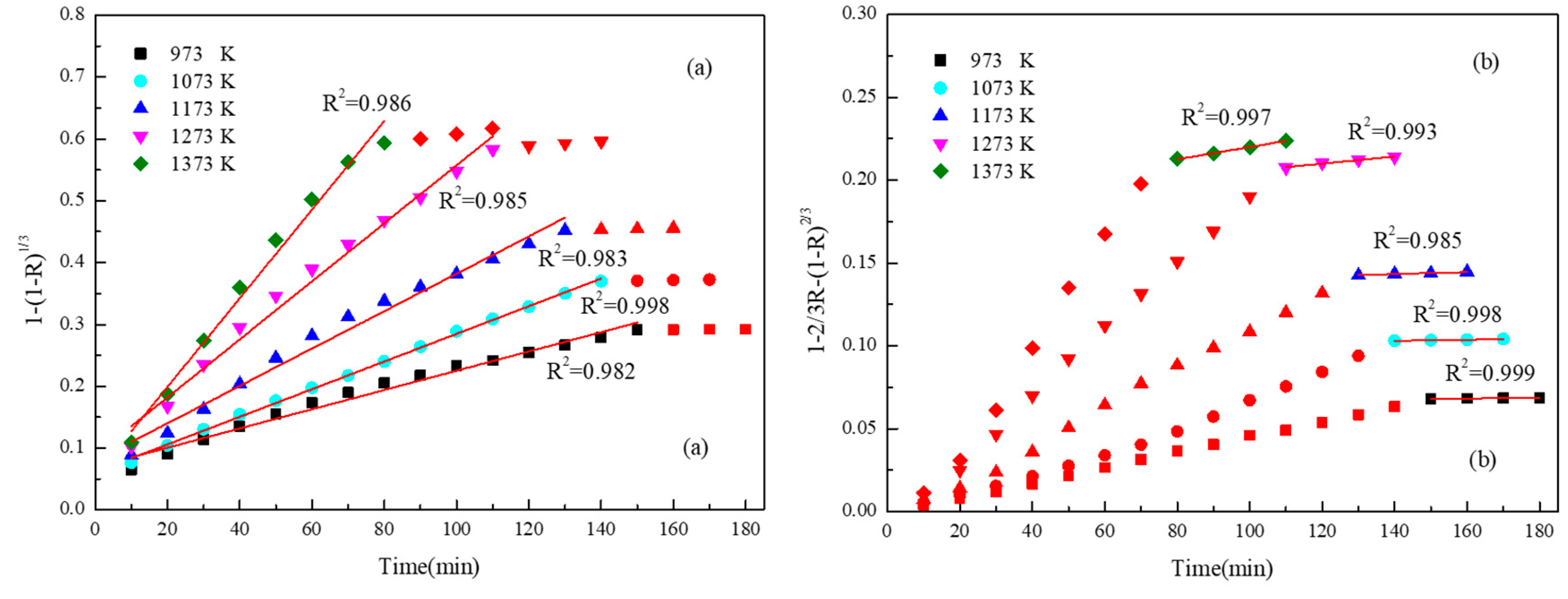
2.3. Reduction Kinetics Analysis
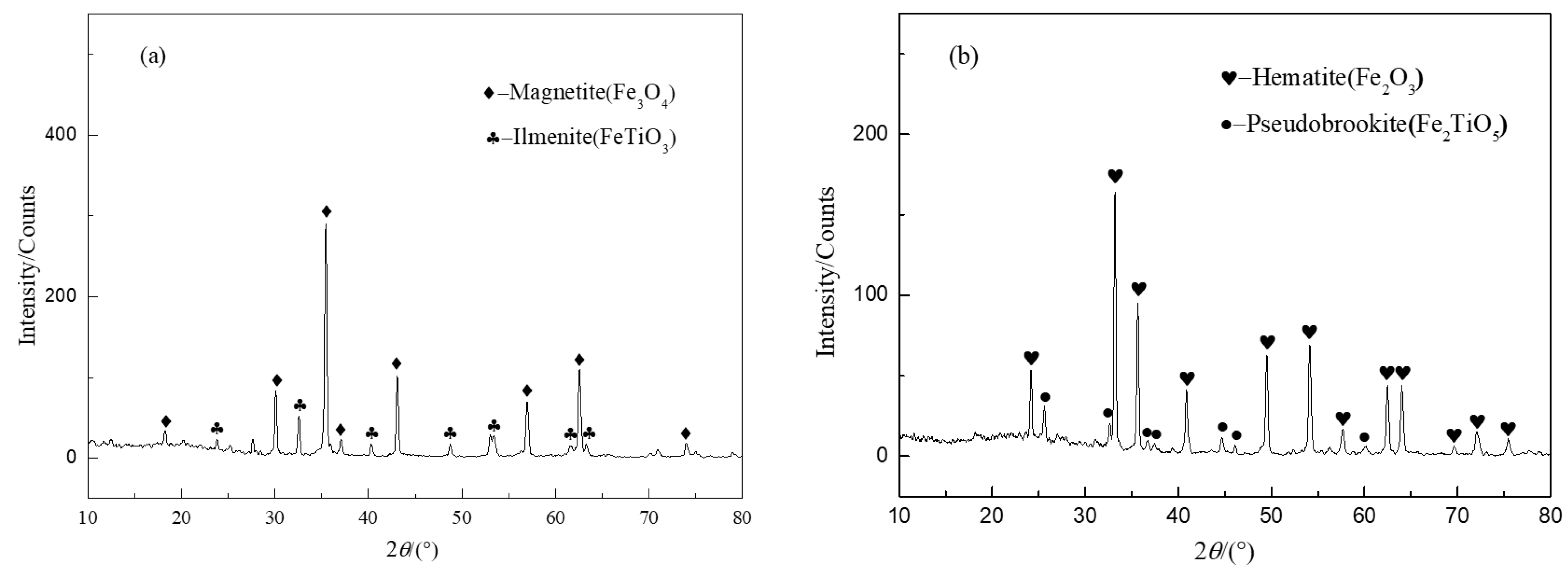
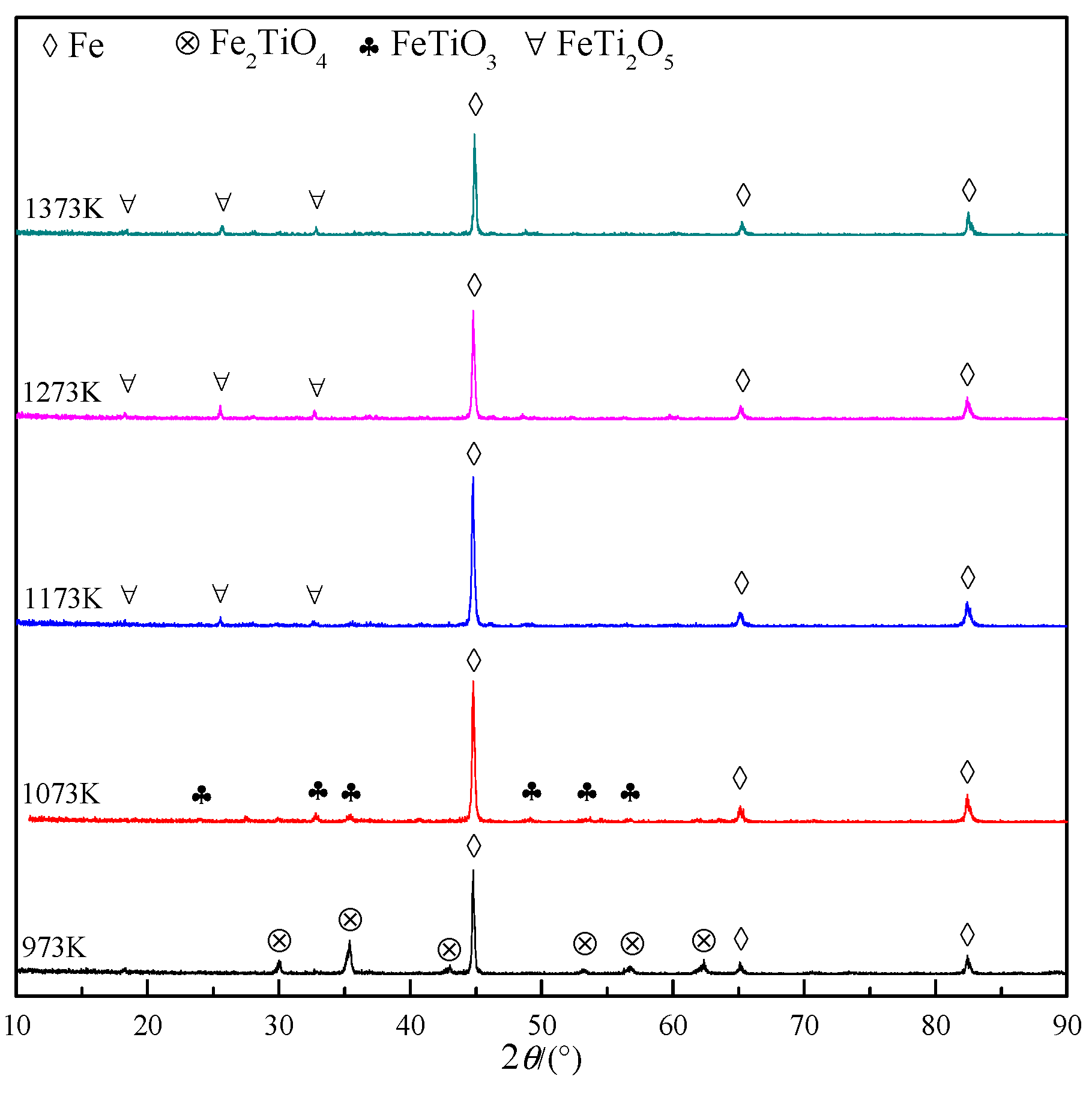
2.4. Characterization
3. Results and Discussion
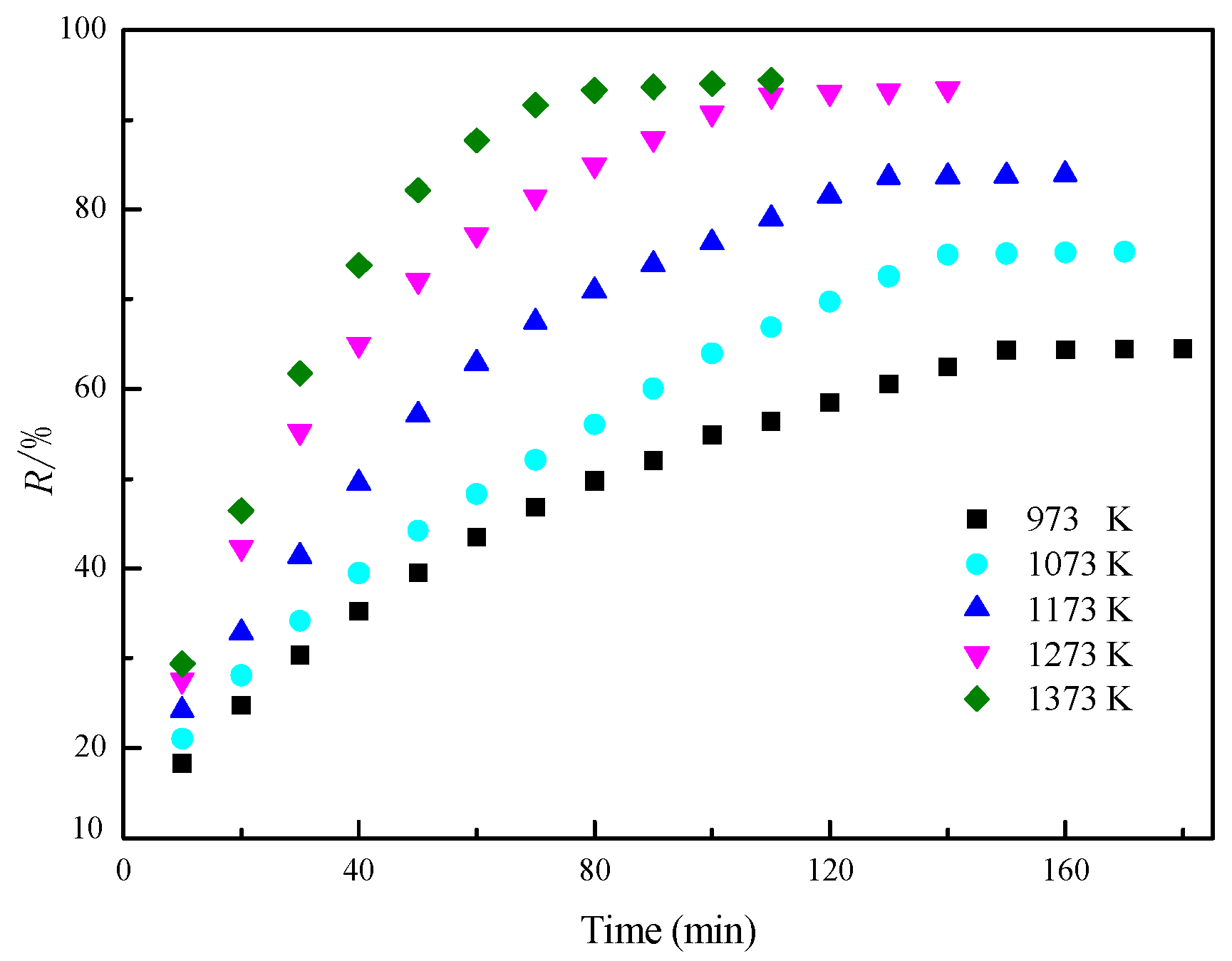
3.1. Reduction Temperature
3.2. Gas Composition
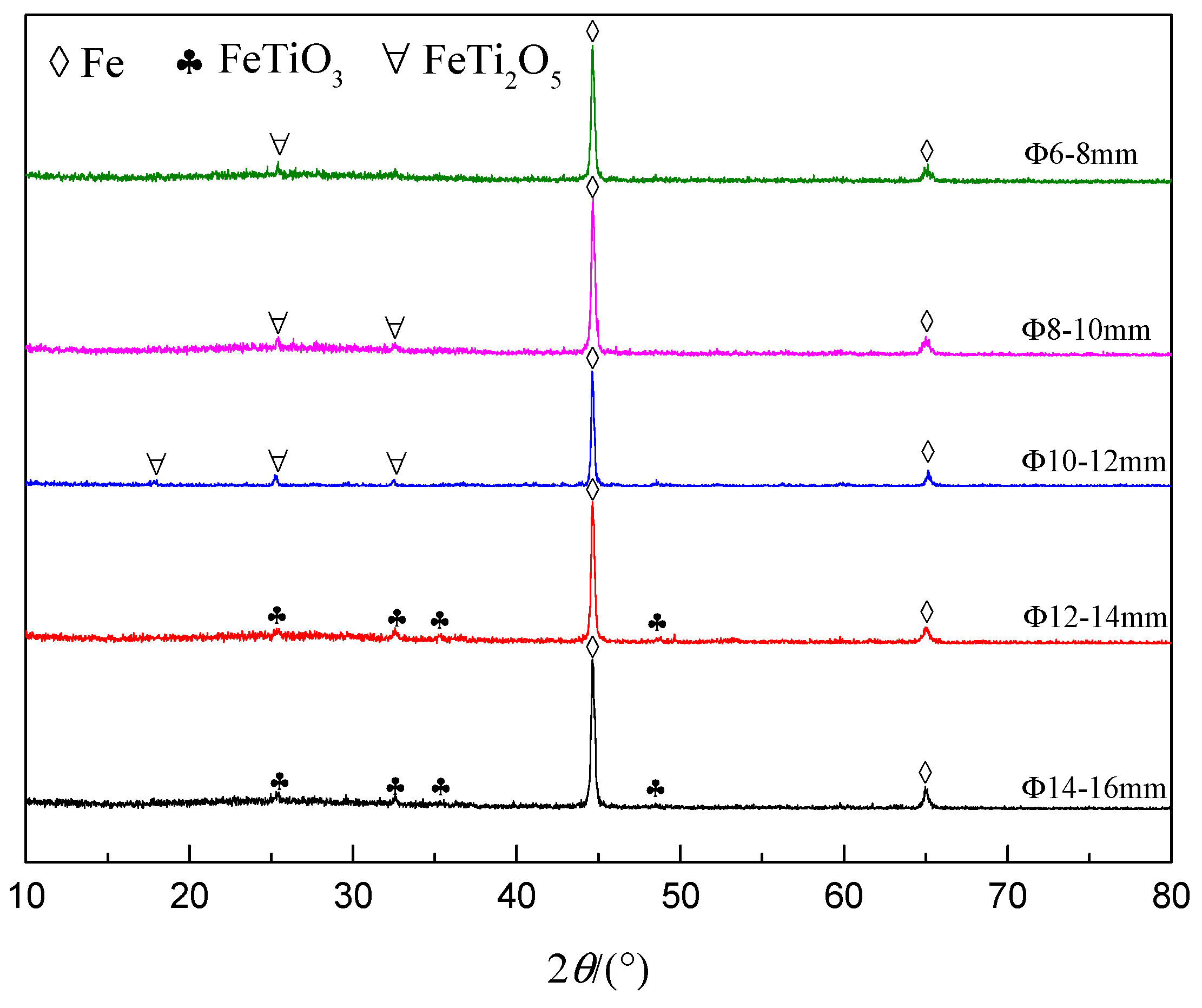
3.3. Pellet Size
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alfantazi, A.M.; Moskalyk, R.R. Processing of indium: A review. Miner. Eng. 2003, 16, 687–694. [Google Scholar] [CrossRef]
- Zhou, L.H.; Zeng, F.H. Reduction mechanisms of vanadium–titanomagnetite–non-coking coal mixed pellet. Ironmak. Steelmak. 2014, 38, 59–64. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xing, X.D.; Cao, M.M.; Jiao, K.X.; Wang, C.L.; Ren, S. Reduction kinetics of vanadium titano-magnetite carbon composite pellets adding catalysts under high temperature. J. Iron. Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Guo, L.; Yu, J.T.; Tang, J.K.; Lin, Y.H.; Guo, Z.C.; Tang, H.Q. Influence of coating MgO on sticking and functional mechanism during fluidized bed reduction of vanadium titano-magnetite. J. Iron. Steel Res. Int. 2015, 22, 464–472. [Google Scholar] [CrossRef]
- Bai, Y.Q.; Cheng, S.S.; Bai, Y.M. Analysis of Vanadium-bearing titanomagnetite sintering process by dissection of sintering bed. J. Iron Steel Res. Int. 2011, 18, 8–15. [Google Scholar] [CrossRef]
- Lv, X.; Lun, Z.; Yin, J.; Bai, C. Carbothermic reduction of vanadium titanomagnetite by microwave irradiation and smelting behavior. ISIJ Int. 2013, 53, 1115–1119. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, F.; Guo, Y.; Jiang, T.; Travyanov, A.Y.; Qiu, G. Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag. JOM 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Sticking behaviour of vanadium titano-magnetite oxidised pellets during gas-based reduction and its prevention. Ironmak. Steelmak. 2016, 44, 185–192. [Google Scholar] [CrossRef]
- Chen, D.S.; Song, B.; Wang, L.N.; Qi, T.; Wang, Y.; Wang, W.J. Solid state reduction of panzhihua titanomagnetite concentrates with pulverized coal. Miner. Eng. 2011, 24, 864–869. [Google Scholar] [CrossRef]
- Guo, Y.F.; Gao, Y.; Tao, J.; Qiu, G.Z. Solid-state reduction behavior of panzhihua ilmenite. J. Cent. South Univ. 2010, 41, 1639–1644. [Google Scholar]
- Cao, M.M.; Zhang, J.L.; Xing, X.D.; Wang, C.L.; Bai, Y.N.; Wen, Y.C. Reduction mechanism of vanadium titano-magnetite carbon composite pellets. Iron Steel 2012, 47, 5–12. [Google Scholar] [CrossRef]
- Chen, D.S.; Zhao, H.; Hu, G.P.; Qi, T.; Yu, H.D.; Zhang, F.Z.; Wang, L.N.; Wang, W.J. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite. J. Hazard. Mater. 2015, 294, 35–40. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Ying, Z.W.; Li, F.; Feng, C.; Liu, Z.G. Non-isothermal gas-based direct reduction behavior of high chromium vanadium-titanium magnetite pellets and the melting separation of metallized pellets. Metals 2017, 7, 153. [Google Scholar] [CrossRef]
- Tripathi, D.; Mani, V.; Pal, R.P. Vanadium in biosphere and its role in biological processes. Biol. Trace Elem. Res. 2018, 186, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloy. Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Xie, X.L.; Wang, S.; Zheng, F.Q. Gas-based reduction of vanadium titano-magnetite concentrate: Behavior and mechanisms. Int. J. Miner. Metall. Mater. 2017, 24, 10–17. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Cheng, C.Y. A synergistic solvent extraction system for separating copper from iron in high chloride concentration solutions. Hydrometallurgy 2012, 113–114, 155–159. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Preparation of Ti-rich material from titanium slag by activation roasting followed by acid leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 1174–1178. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Feng, C.; Tang, Y.T.; Liu, Z.G. Melting separation behavior and mechanism of high-chromium vanadium-bearing titanomagnetite metallized pellet got from gas-based direct reduction. ISIJ Int. 2016, 56, 210–219. [Google Scholar] [CrossRef]
- Mehdizadeh, A.M.; Klausner, J.F.; Barde, A.; Mei, R. Enhancement of thermochemical hydrogen production using an iron–silica magnetically stabilized porous structure. Int. J. Hydrogen Energy 2012, 37, 8954–8963. [Google Scholar] [CrossRef]
- Piotrowski, K.; Mondal, K.; Lorethova, H.; Stonawski, L.; Szymański, T.; Wiltowski, T. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process. Int. J. Hydrogen Energy 2005, 30, 1543–1554. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, M.; Wang, H.; Liu, Z.; Tang, J.; Ying, Z. Volumetric shrinkage characteristics and kinetics analysis of vanadium titanomagnetite carbon composite hot briquette during isothermal reduction. ISIJ Int. 2018, 58, 823–832. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S. Kinetics and mechanisms of direct reduction of iron ore-biomass composite pellets with hydrogen gas. Int. J. Hydrogen Energy 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- Huitu, K.; Helle, M.; Helle, H.; Kekkonen, M.; Saxén, H. Optimization of midrex direct reduced iron use in ore-based steelmaking. Steel Res. Int. 2015, 86, 456–465. [Google Scholar] [CrossRef]
- Kromhout, J.A.; Ludlow, V.; Mckay, S.; Normanton, A.S.; Thalhammer, M.; Ors, F.; Cimarelli, T. Physical properties of mould powders for slab casting. Ironmak. Steelmak. 2002, 29, 191–193. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Oxidation induration process and kinetics of hongge vanadium titanium-bearing magnetite pellets. Ironmak. Steelmak. 2016, 44, 294–303. [Google Scholar] [CrossRef]
- Sun, H.Y.; Dong, X.J.; She, X.F.; Xue, Q.G.; Wang, J.S. Reduction mechanism of titanomagnetite concentrate by carbon monoxide. J. Min. Metall. 2013, 49, 263–270. [Google Scholar] [CrossRef][Green Version]
- Huang, Z.C.; Ling-Yun, Y.I.; Hu, P.; Jiang, T. Effects of roast temperature on properties of oxide pellets and its gas-based direct reduction. J. Cent. South Univ. 2012, 43, 2889–2895. [Google Scholar]

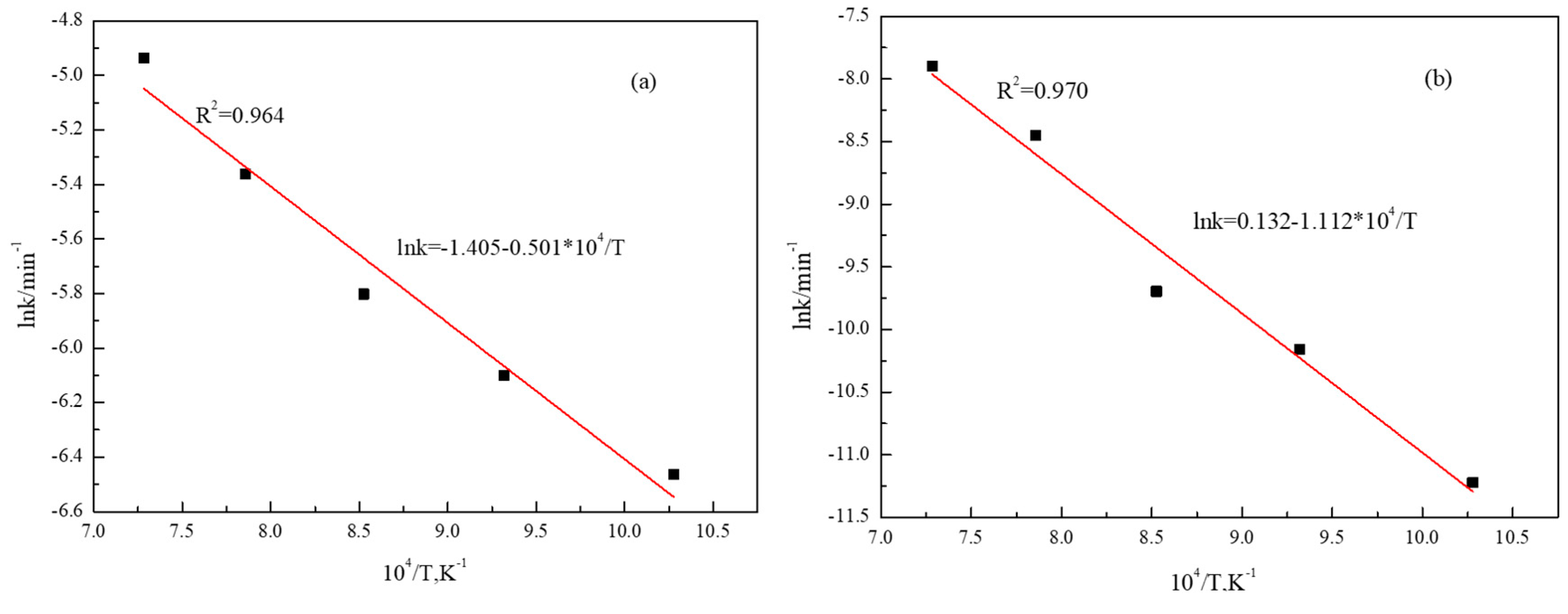

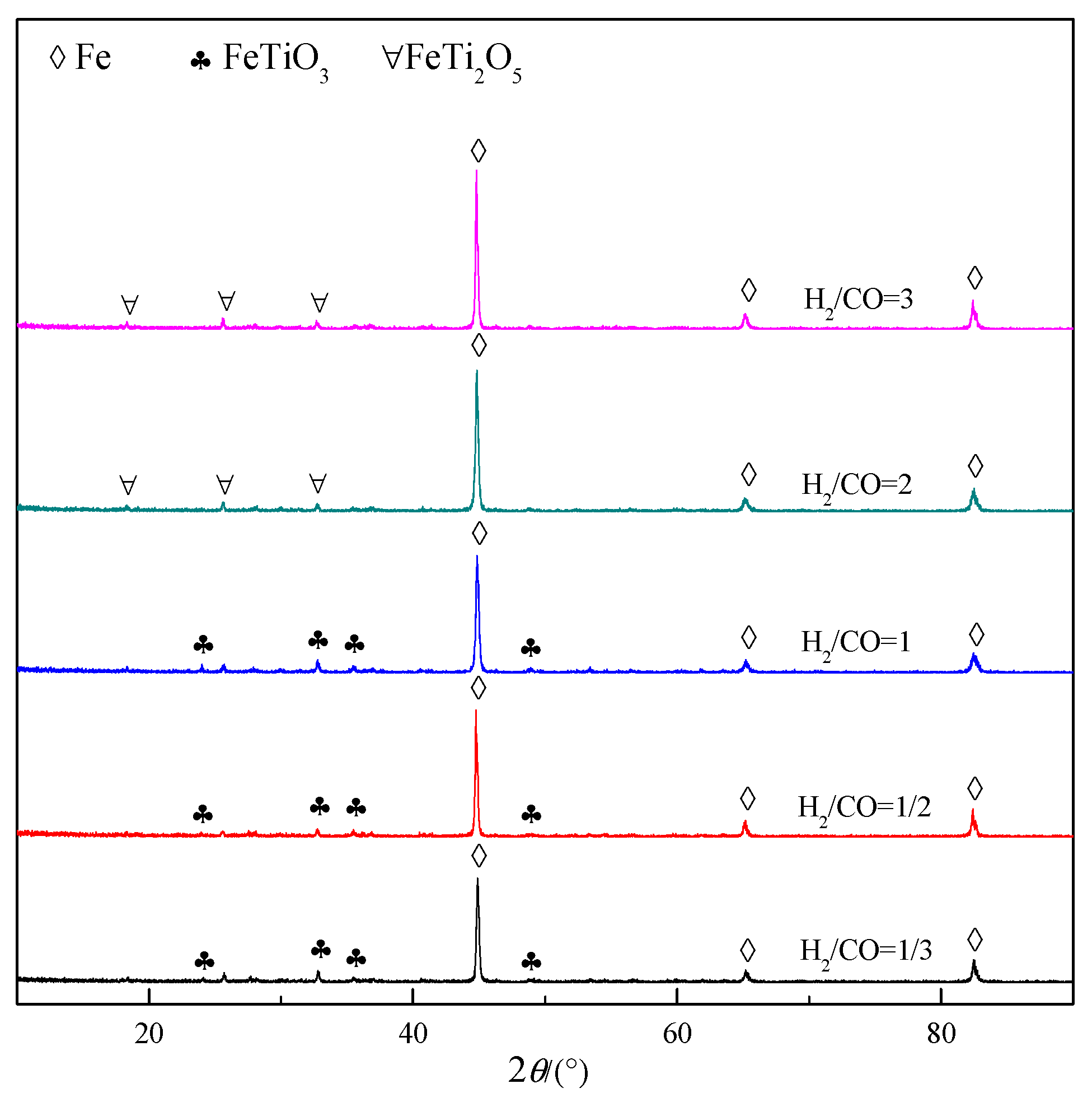
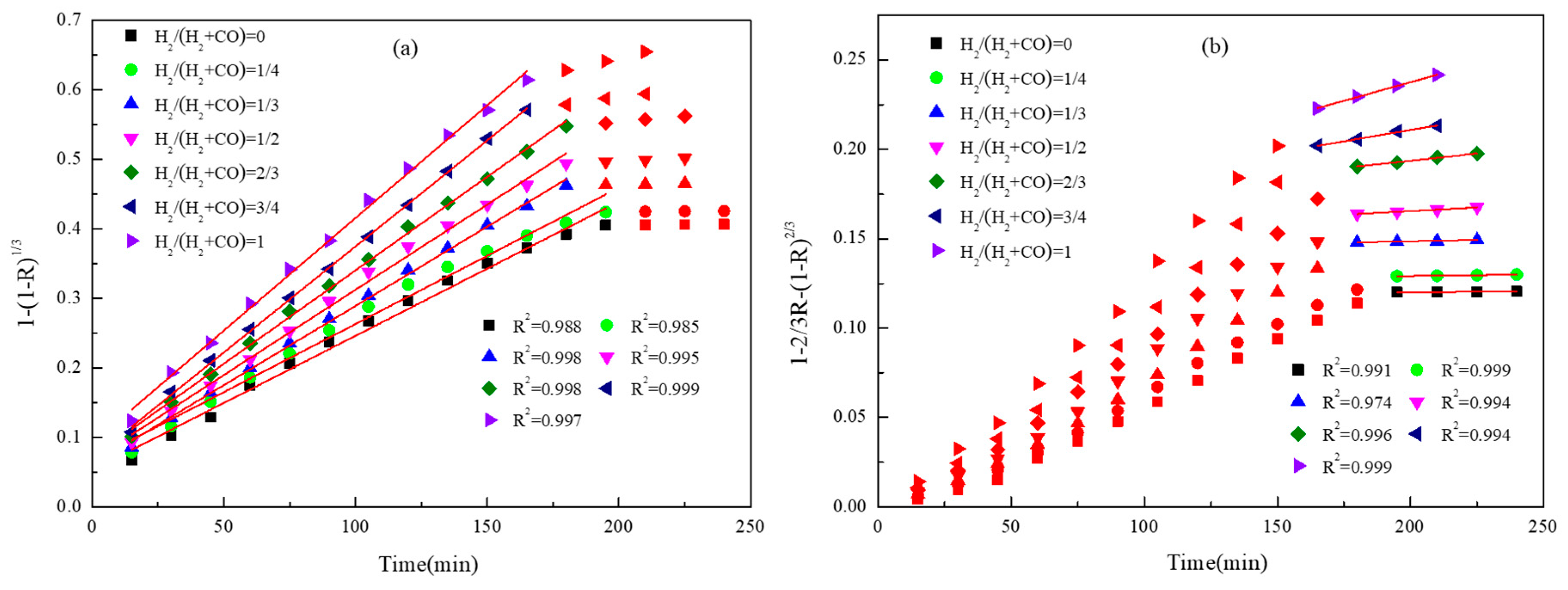
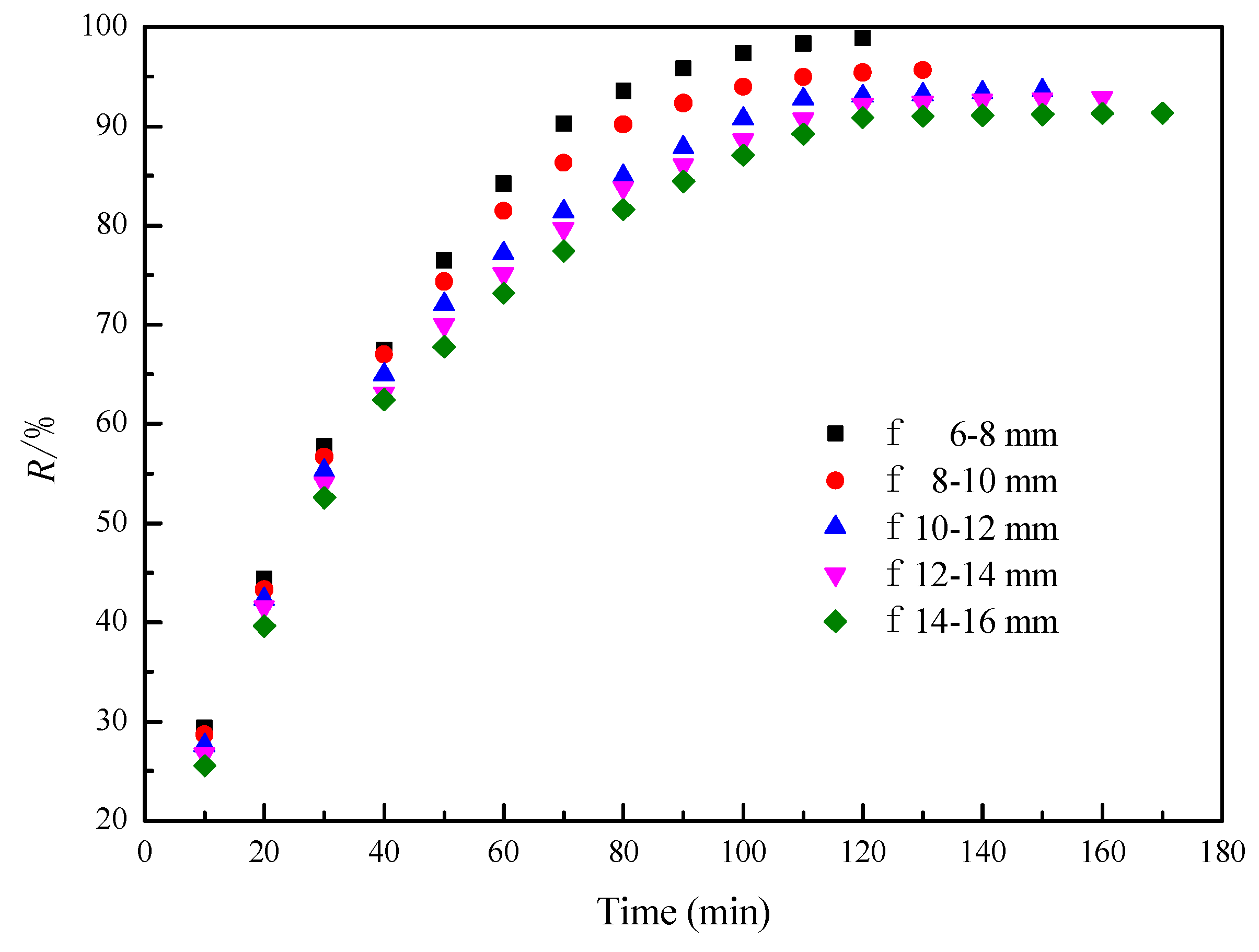

| TFe | FeO | TiO2 | SiO2 | Al2O3 | MgO | CaO | V2O5 | MnO | S |
|---|---|---|---|---|---|---|---|---|---|
| 45.50 | 0.59 | 13.40 | 8.42 | 6.54 | 3.28 | 1.40 | 0.54 | 0.32 | 0.037 |
| Temperature | 973 K | 1073 K | 1173 K | 1273 K | 1373 K |
|---|---|---|---|---|---|
| Intrinsic Chemical Reaction Control | 0.00156 | 0.00224 | 0.00302 | 0.00469 | 0.00717 |
| Diffusion Control | 1.34 × 10−5 | 3.88 × 10−5 | 6.15 × 10−5 | 2.13 × 10−4 | 3.70 × 10−4 |
| Pellets Diameter | 6–8 mm | 8–10 mm | 10–12 mm | 12–14 mm | 14–16 mm |
|---|---|---|---|---|---|
| Intrinsic Chemical Reaction Control | 0.00694 | 0.0057 | 0.00469 | 0.00423 | 0.00405 |
| Diffusion Control | 1.66 × 10−3 | 6.35 × 10−4 | 2.12 × 10−4 | 1.58 × 10−4 | 7.64 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chen, W.; Mi, L.; Jiao, Y.; Wang, X. Kinetic Studies on Gas-Based Reduction of Vanadium Titano-Magnetite Pellet. Metals 2019, 9, 95. https://doi.org/10.3390/met9010095
Chen J, Chen W, Mi L, Jiao Y, Wang X. Kinetic Studies on Gas-Based Reduction of Vanadium Titano-Magnetite Pellet. Metals. 2019; 9(1):95. https://doi.org/10.3390/met9010095
Chicago/Turabian StyleChen, Junwei, Weibin Chen, Liang Mi, Yang Jiao, and Xidong Wang. 2019. "Kinetic Studies on Gas-Based Reduction of Vanadium Titano-Magnetite Pellet" Metals 9, no. 1: 95. https://doi.org/10.3390/met9010095
APA StyleChen, J., Chen, W., Mi, L., Jiao, Y., & Wang, X. (2019). Kinetic Studies on Gas-Based Reduction of Vanadium Titano-Magnetite Pellet. Metals, 9(1), 95. https://doi.org/10.3390/met9010095




