Effect of Primary Crystals on Pore Morphology during Semi-Solid Foaming of A2024 Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Master Alloys
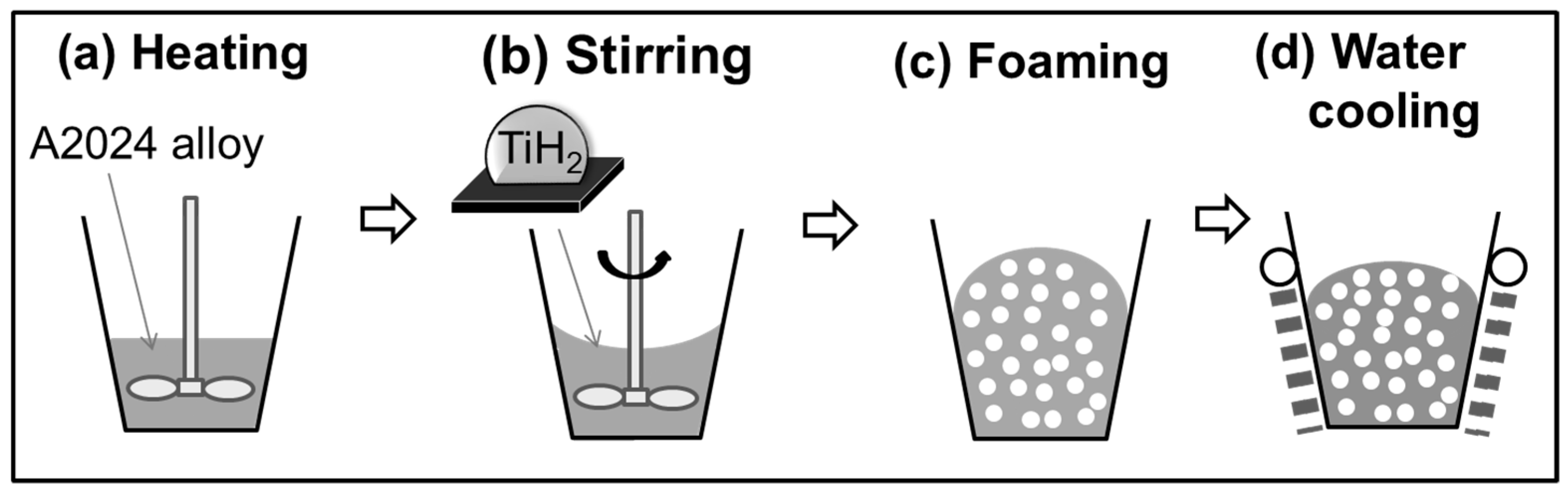
2.2. Fabrication of A2024 Alloy Foam in a Semi-Solid State
2.3. Evaluation of Pores and Primary Crystal Formation
2.4. Analysis of the Pure Ti Distribution
3. Results and Discussion
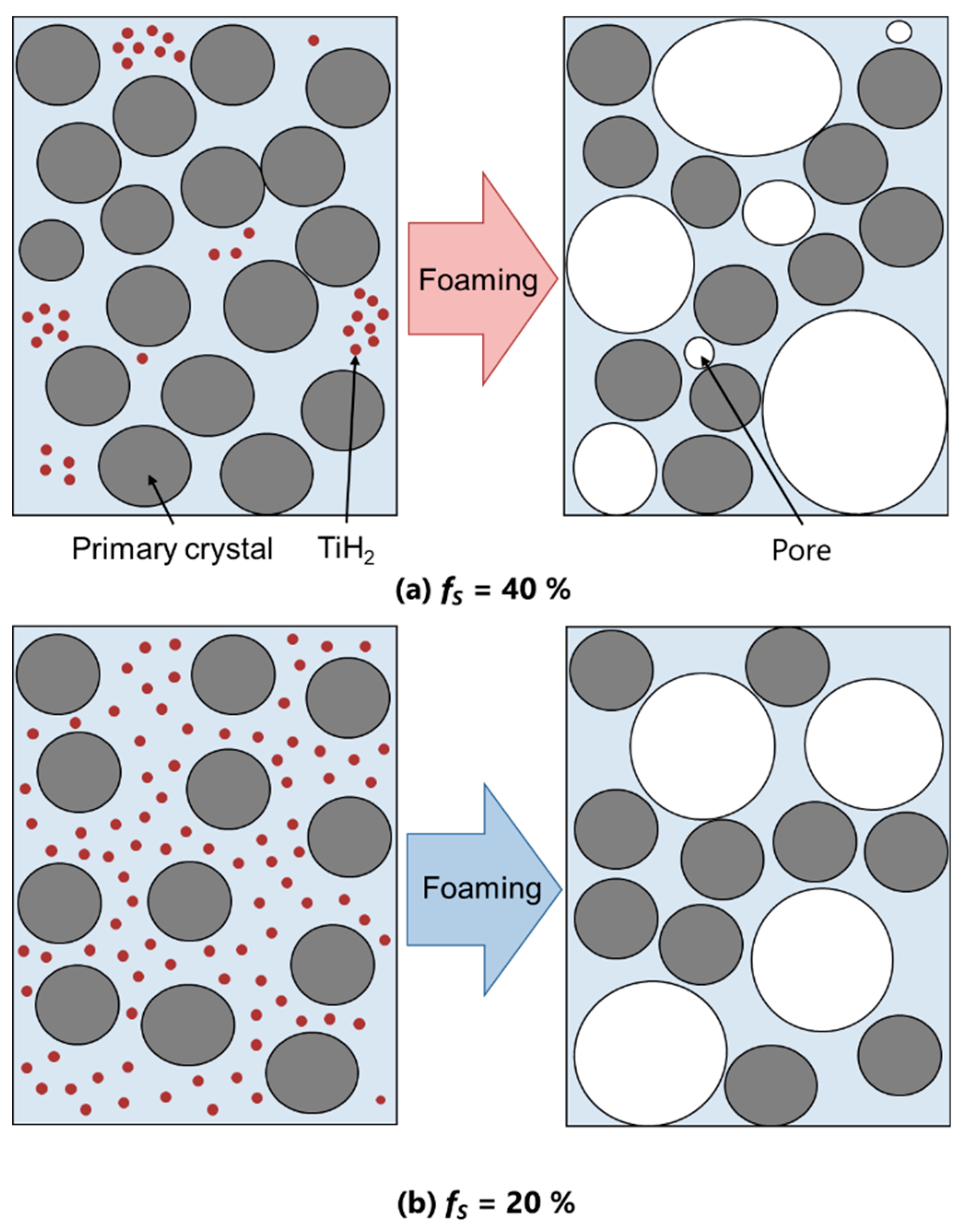
3.1. Pore Formation on the A2024 Alloy Foams
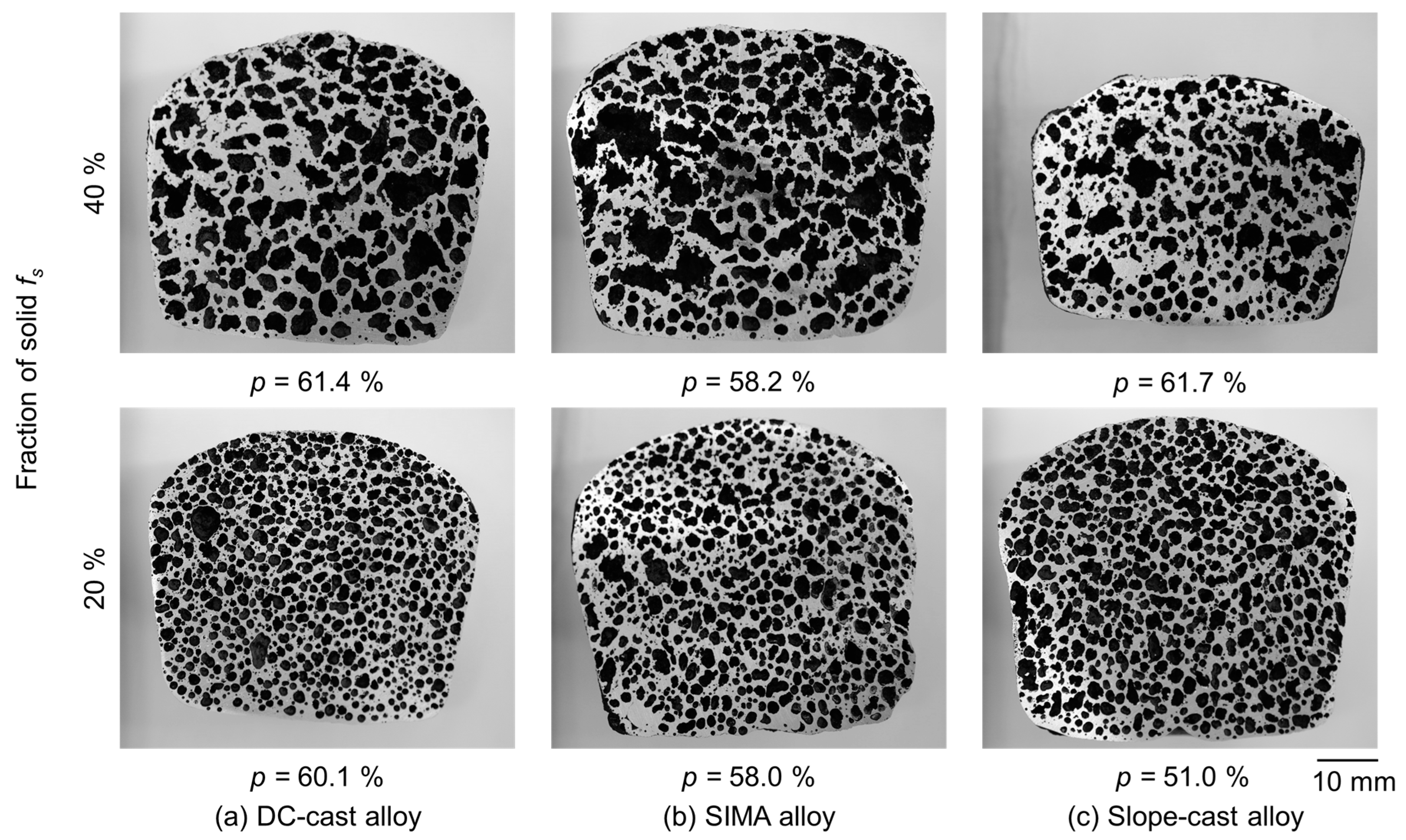
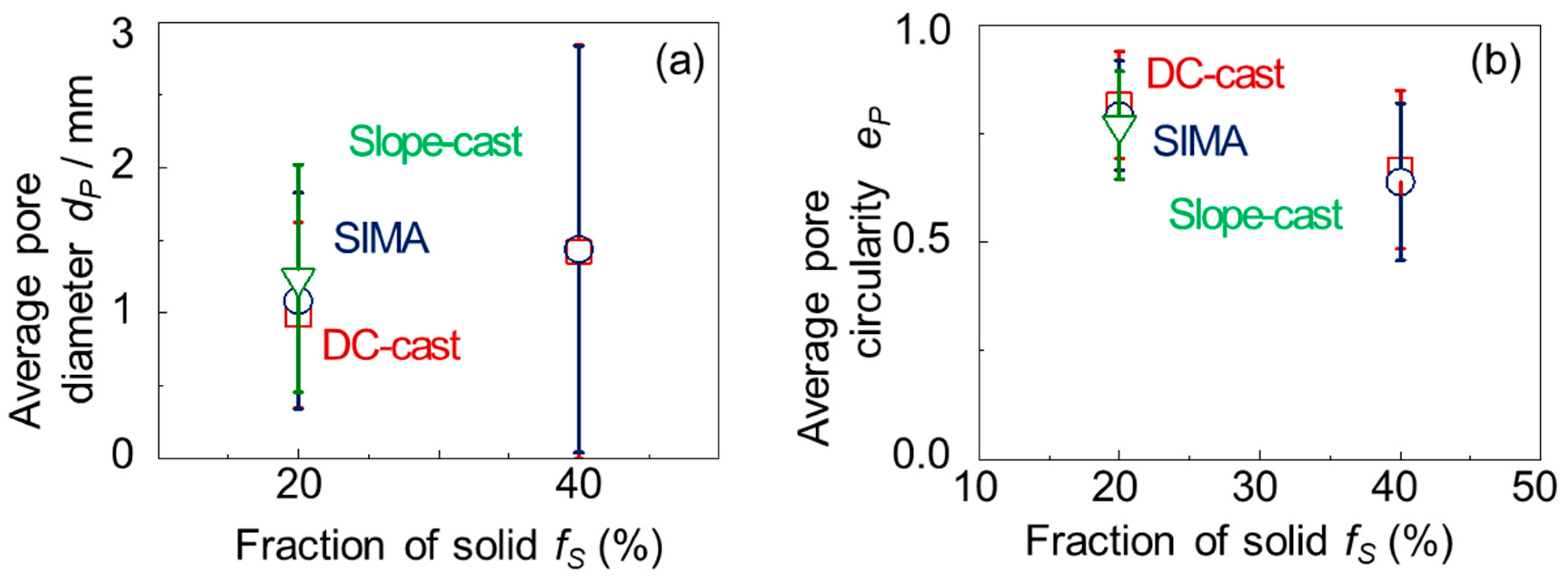
3.2. Macro-Pores of the A2024 Alloys Fabricated in a Semi-Solid State
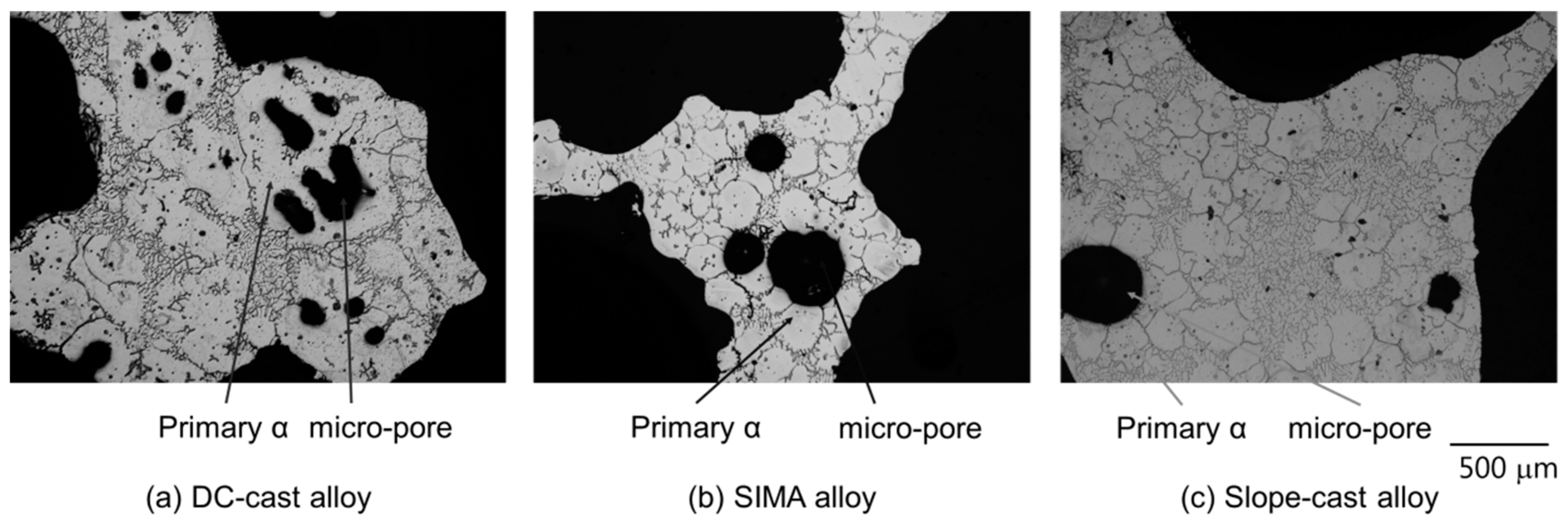
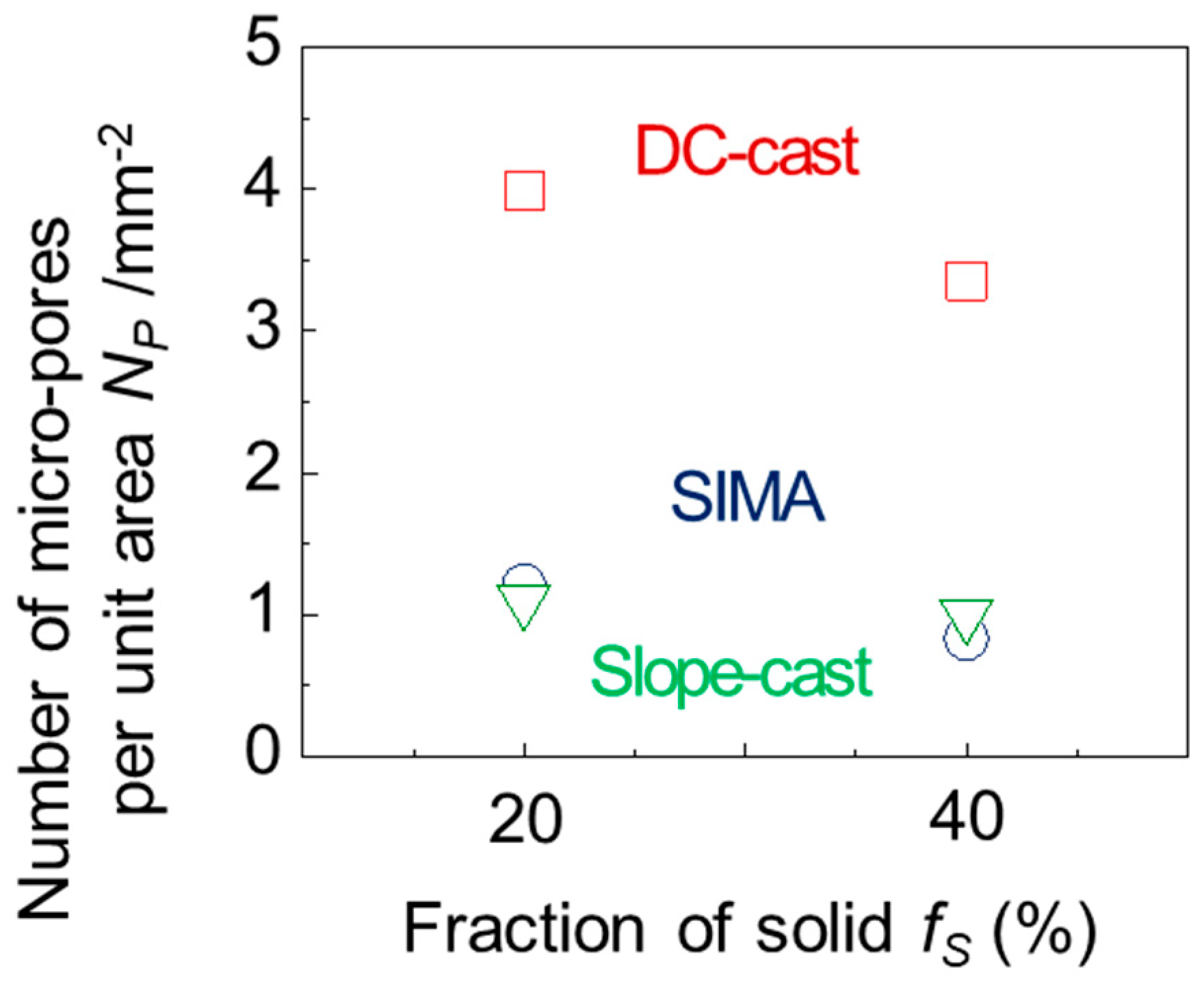
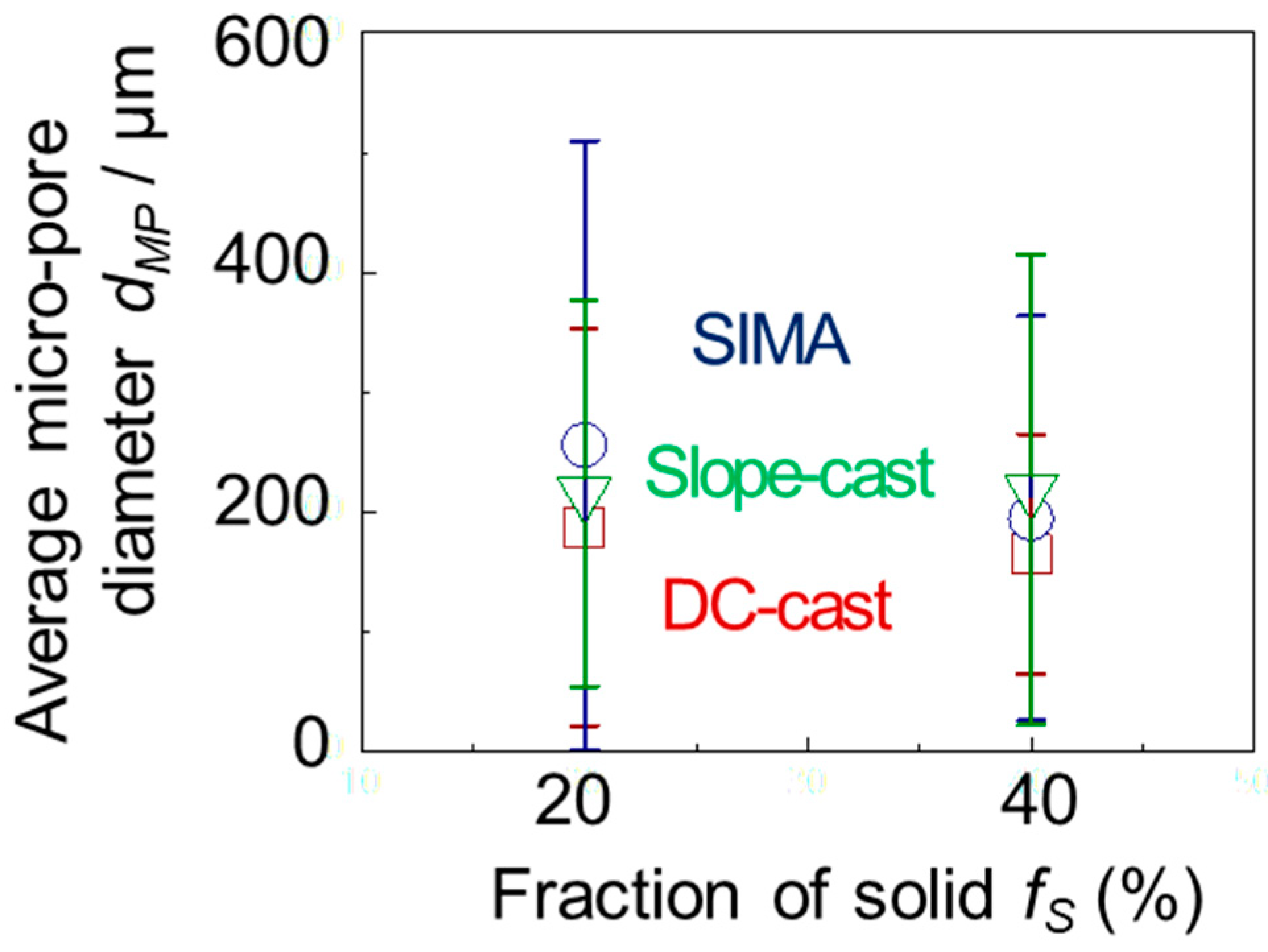
3.3. Micro-Pores of the A2024 Alloys Fabricated in a Semi-Solid State
3.4. Effect of Primary Crystals on Micro-Pore Formation
4. Conclusions
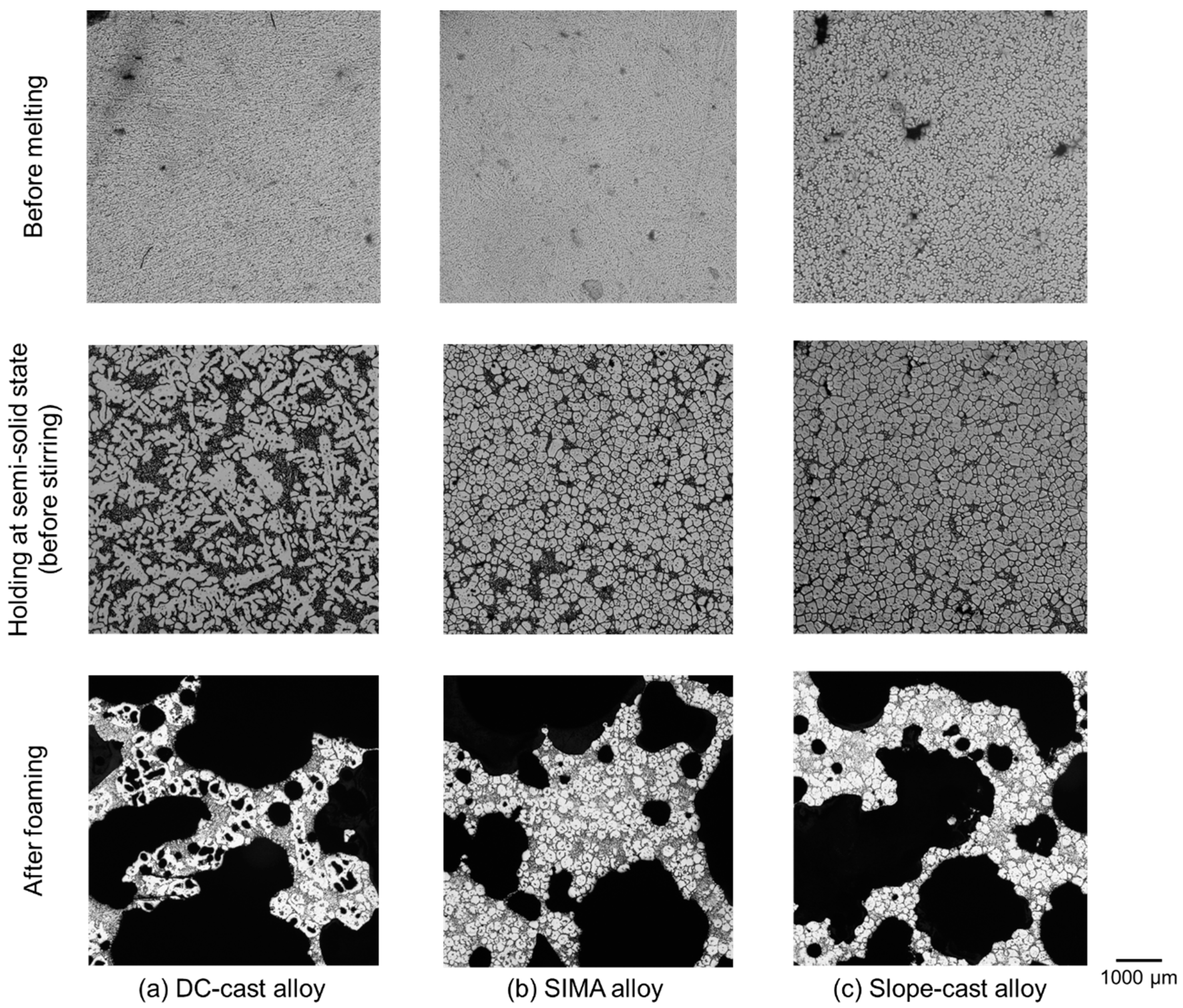
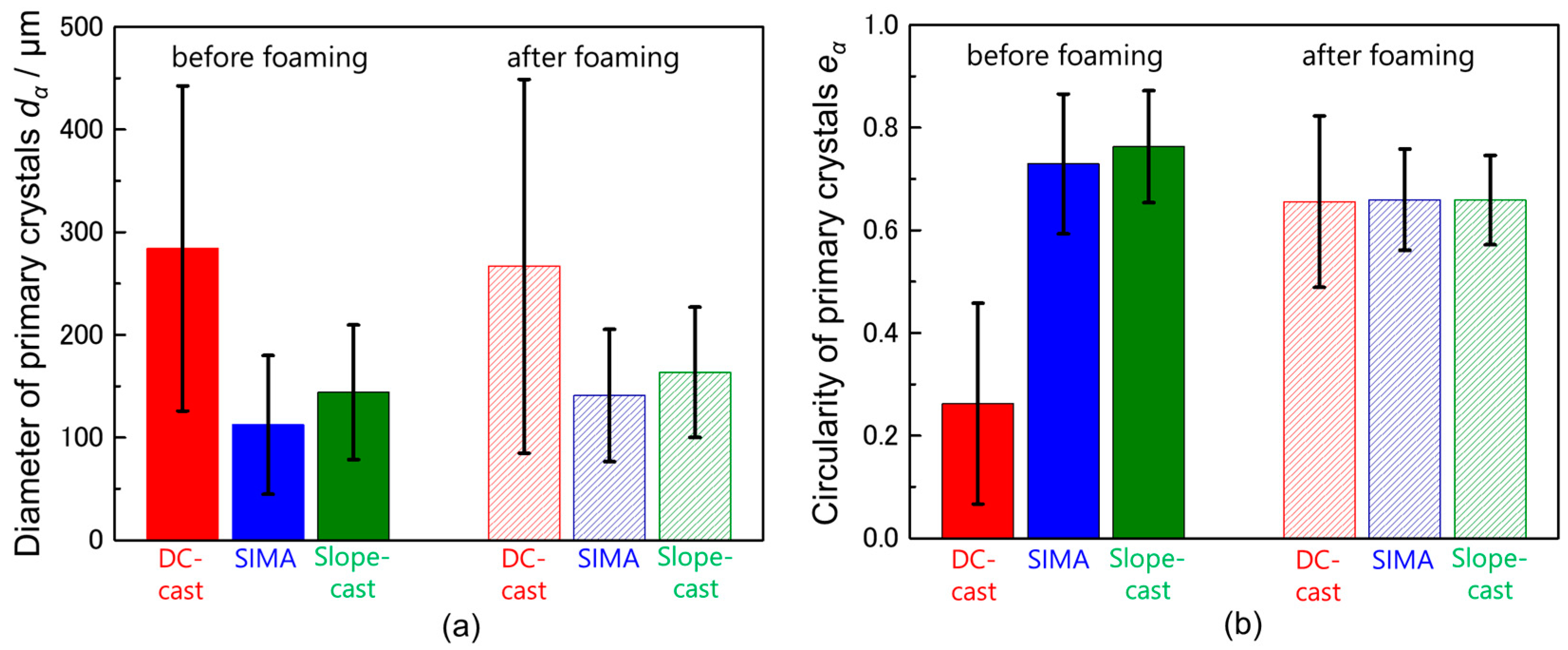
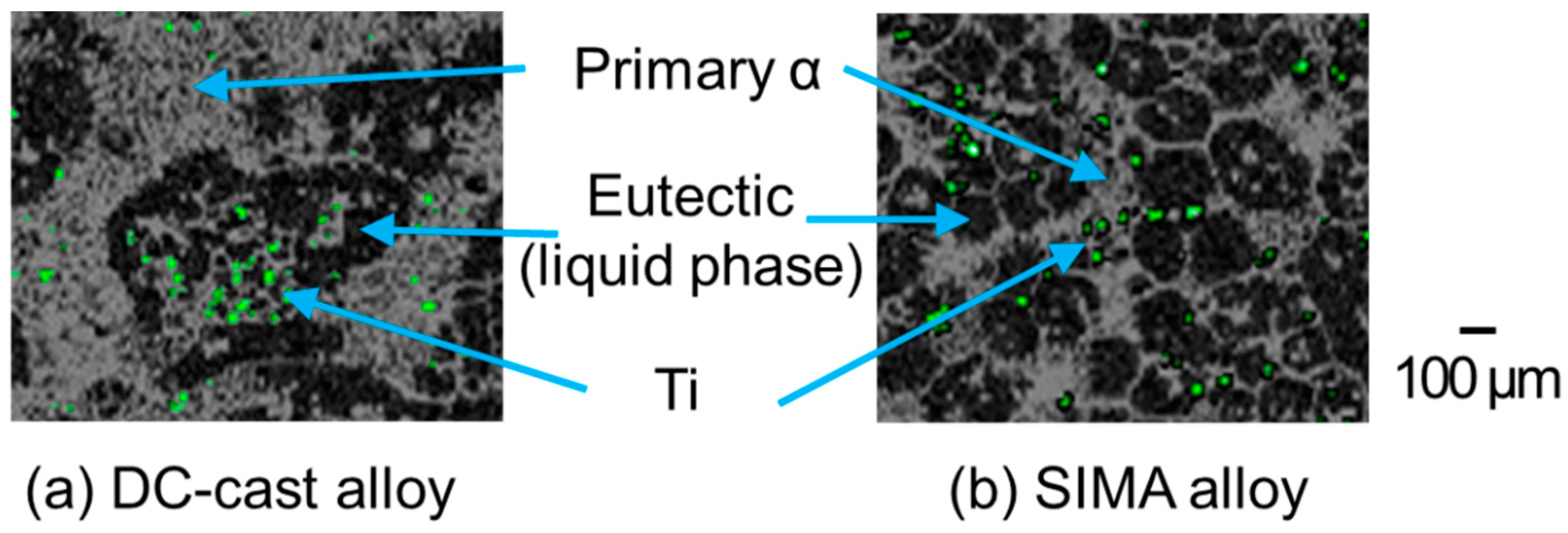
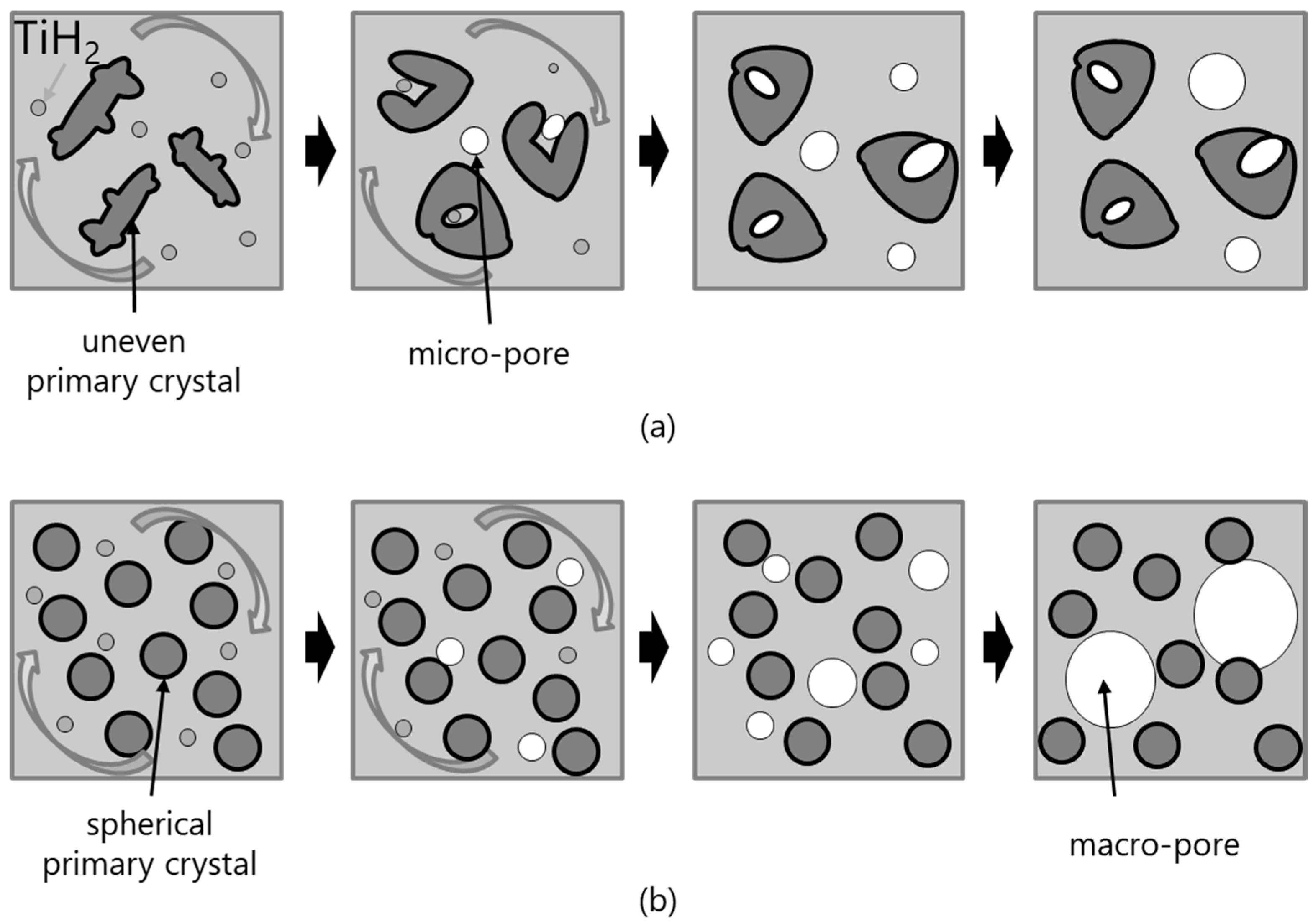
- In the DC-cast alloy, coarse, dendritic, and uneven primary crystals were present before stirring. They were subsequently bent and spheroidized via stirring. On the other hand, the primary crystals in the SIMA and slope-cast alloys maintained their fine and spherical shape before and after stirring.
- The differences between the uneven primary crystals and the fine/spherical primary crystals before stirring had little effect on macro-pore morphology. The size of the primary crystals was significantly smaller than that of the macro-pores.
- In the DC-cast alloy, micro-pores existed inside the primary crystals after foaming. On the other hand, micro-pores were present between the primary crystals in the SIMA and slope-cast alloys. The number of micro-pores in the SIMA and slope-cast alloys was 1/3 of that in the DC-cast alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.J.; Ashby, M.F. Cellular Solids Structure and Properties, 2nd ed.; Pergamon Press: Oxford, UK, 1997; pp. 163–197. [Google Scholar]
- Fukui, T.; Saito, M.; Nonaka, Y.; Suzuki, S. Fabrication of foams of A2024 and A7075 and influence of porosity and heat treatment on their mechanical properties. Trans. Jpn. Soc. Mech. Eng. 2014, 80, SMM0248. (In Japanese) [Google Scholar] [CrossRef]
- Fukui, T.; Nonaka, Y.; Suzuki, S. Fabrication of Al-Cu-Mg Alloy Foams Using Mg as Thickener through Melt Route and Reinforcement of Cell Walls. Proc. Mat. Sci. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS Aluminum Foam: Production Process, Properties, and Applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Ueno, H.; Akiyama, S. Effects of calcium addition on the foamability of molten aluminum. J. Jpn. Inst. Light Met. 1987, 37, 42–47. [Google Scholar] [CrossRef]
- Hanafusa, T.; Ohishi, K. Making of Porous Metallic Material by the Semi-Solid Aluminum Alloy. Rpt. Hiroshima Prefect. Technol. Res. Inst. West Reg. Ind. Res. Center 2010, 23, 28–29. (In Japanese) [Google Scholar]
- Sekido, K.; Kitazono, K. Effect of Foaming Condition on Cell Morphology of Superplastic Zn-22Al Alloy Foams Manufactured through Melt Foaming Process. J. Jpn. Inst. Met. Mater. 2013, 77, 497–502. [Google Scholar] [CrossRef]
- Heim, K.; García-Moreno, F.; Banhart, J. Particle size and fraction required to stabilise aluminum alloy foams created by gas injection. Scr. Mater. 2018, 153, 54–58. [Google Scholar] [CrossRef]
- Young, K.P.; Kyonka, C.P.; Courtois, F. Fine Grained Metal Composition. U.S. Patent US4415374A, 15 November 1983. [Google Scholar]
- Haga, T.; Kapranos, P. Billetless simple thixoforming process. J. Mater. Process. Technol. 2002, 130–131, 581–586. [Google Scholar] [CrossRef]
- Sirong, Y.; Dongcheng, L.; Kim, N. Microstructure evolution of SIMA processed Al2024. Mater. Sci. Eng. A 2006, 420, 165–170. [Google Scholar] [CrossRef]
- Kattamis, T.Z.; Piccone, T.J. Rheology of Semisolid Al-4.5%Cu-1.5%Mg Alloy. Mater. Sci. Eng. A 1991, 131, 265–272. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kang, C.G.; Lee, S.M. Effect of viscosity on microstructure characteristic in rheological behavior of wrought aluminum alloys by compression and stirring process. Mater. Sci. Technol. 2010, 26, 20–30. [Google Scholar] [CrossRef]
- Mukherjee, M.; García-Moreno, F.; Jiménez, C.; Rack, A.; Banhart, J. Microporosity in aluminum foams. Acta Mater. 2018, 131, 156–168. [Google Scholar] [CrossRef]
- Ohgaki, T.; Toda, H.; Kobayashi, M.; Uesugi, K.; Niinomi, M.; Akahori, T.; Kobayashi, T.; Makii, K.; Aruga, Y. In situ observations of compressive behavior of aluminum foams by local tomography using high-resolution X-rays. Philos. Mag. 2006, 86, 4417–4438. [Google Scholar] [CrossRef]
- Toda, H.; Ohgaki, T.; Uesugi, K.; Kobayashi, M.; Kuroda, N.; Kobayashi, T.; Niinomi, M.; Akahori, T.; Makii, K.; Aruga, Y. Quantitative Assessment of Microstructure and its Effects on Compression Behavior of Aluminum Foams via High-Resolution Synchrotron X-Ray Tomography. Metall. Mater. Trans. A 2006, 37, 1211–1219. [Google Scholar] [CrossRef]
- Chen, H.I.; Chen, J.C.; Liao, J.J. The influence of shearing conditions on the rheology of semi-solid magnesium alloy. Mater. Sci. Eng. A 2008, 487, 114–119. [Google Scholar] [CrossRef]


| Element | Cu | Mg | Mn | Fe | Si | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| mass% | 4.44 | 1.60 | 0.67 | 0.15 | 0.08 | <0.01 | <0.01 | <0.01 | bal. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwahara, T.; Saito, M.; Osaka, T.; Suzuki, S. Effect of Primary Crystals on Pore Morphology during Semi-Solid Foaming of A2024 Alloys. Metals 2019, 9, 88. https://doi.org/10.3390/met9010088
Kuwahara T, Saito M, Osaka T, Suzuki S. Effect of Primary Crystals on Pore Morphology during Semi-Solid Foaming of A2024 Alloys. Metals. 2019; 9(1):88. https://doi.org/10.3390/met9010088
Chicago/Turabian StyleKuwahara, Takashi, Mizuki Saito, Taro Osaka, and Shinsuke Suzuki. 2019. "Effect of Primary Crystals on Pore Morphology during Semi-Solid Foaming of A2024 Alloys" Metals 9, no. 1: 88. https://doi.org/10.3390/met9010088
APA StyleKuwahara, T., Saito, M., Osaka, T., & Suzuki, S. (2019). Effect of Primary Crystals on Pore Morphology during Semi-Solid Foaming of A2024 Alloys. Metals, 9(1), 88. https://doi.org/10.3390/met9010088




