Phase and Morphology Transformations in Sulfur-Fixing and Reduction Roasting of Antimony Sulfide
Abstract
:1. Introduction
2. Experimental
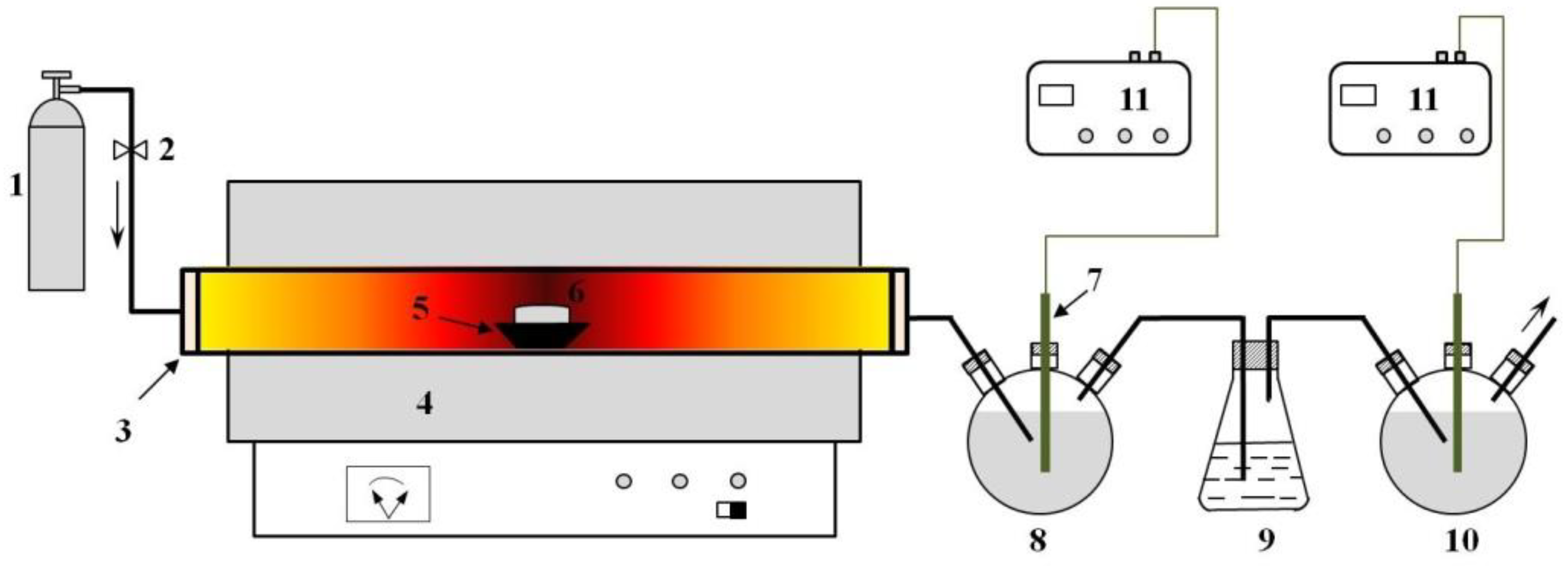
2.1. Materials and Instruments
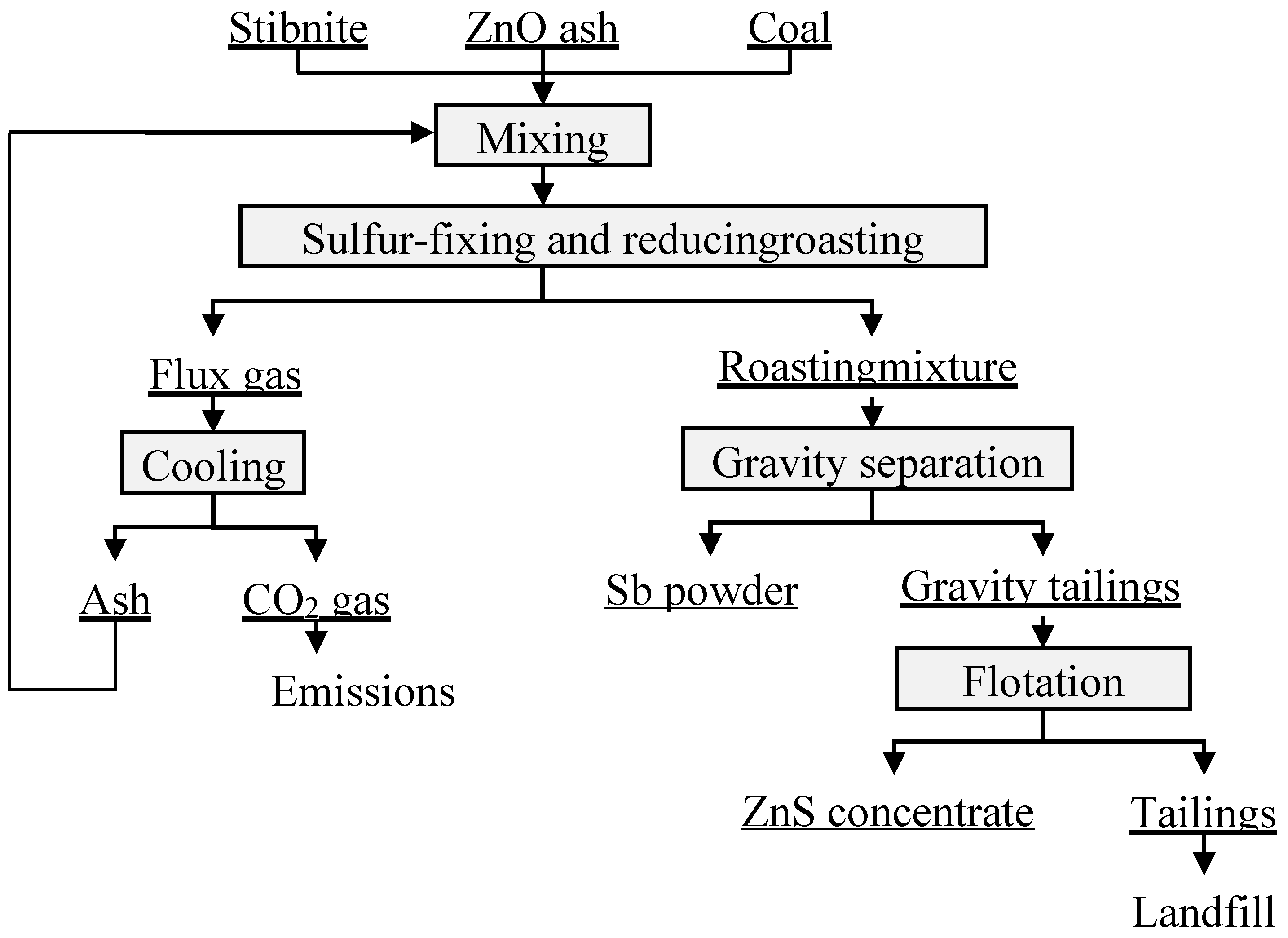
2.2. Methods
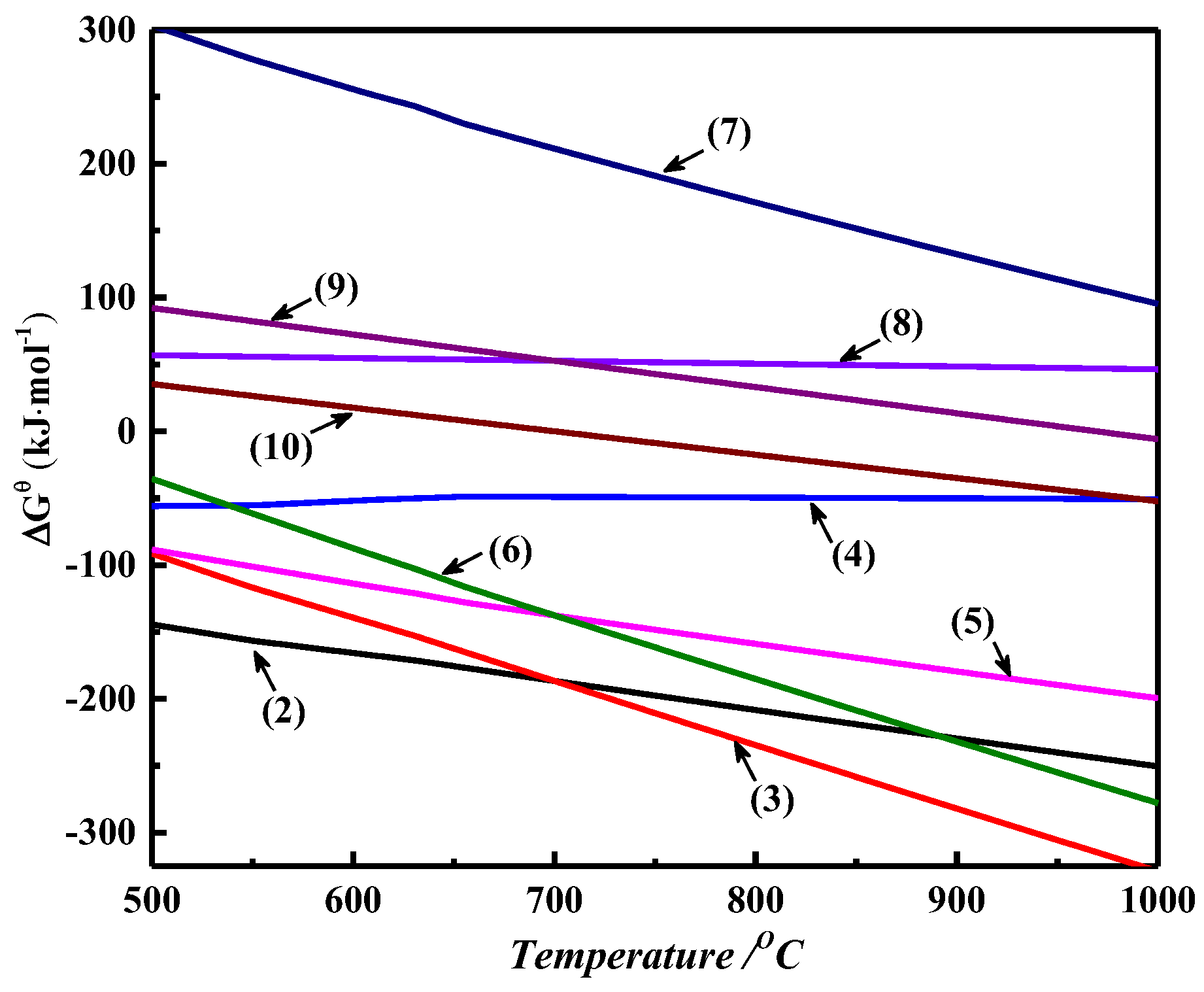
2.3. Thermodynamic Calculations
3. Results and Discussion
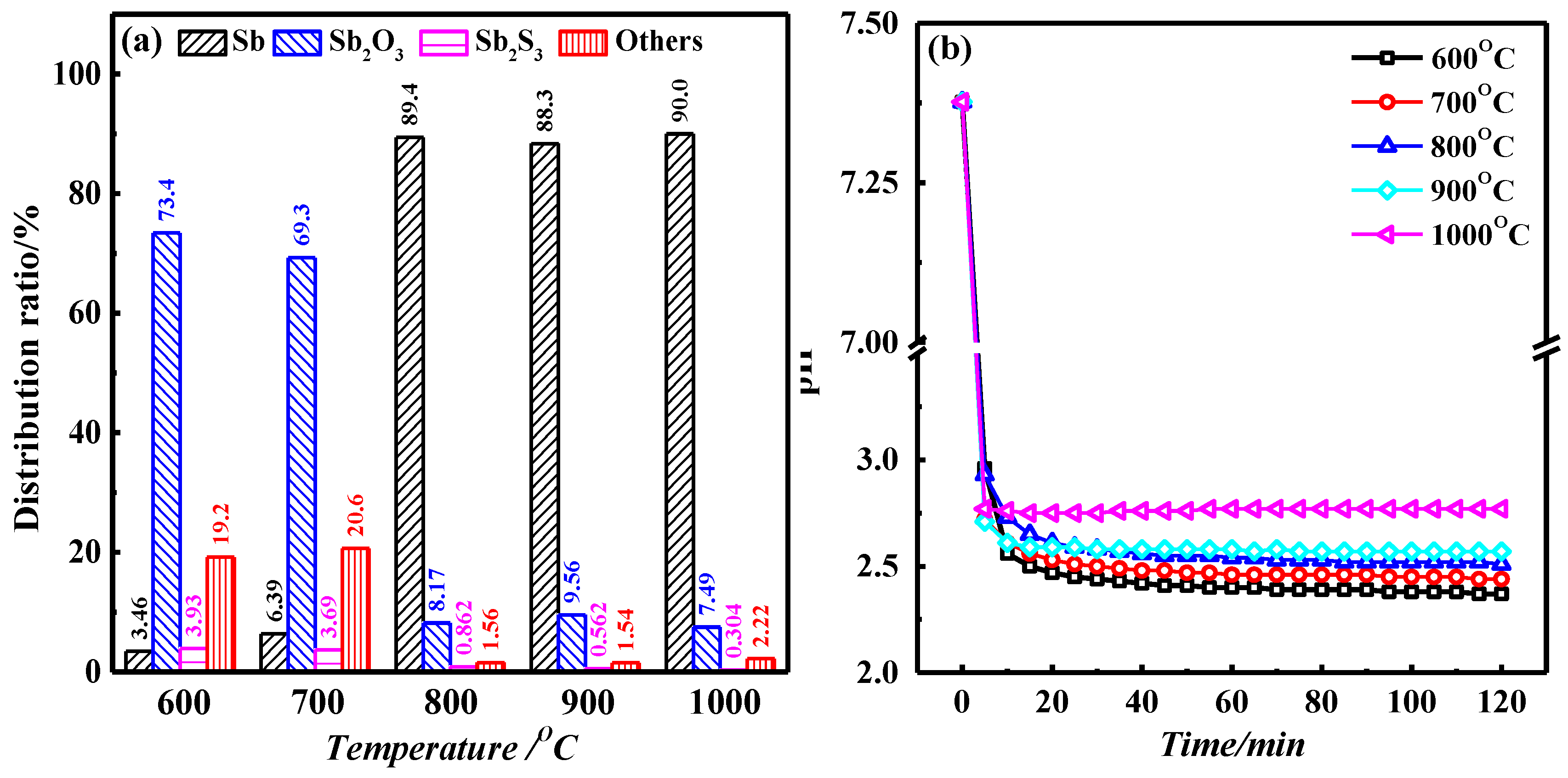
3.1. Effect of Temperature on Roasting Results
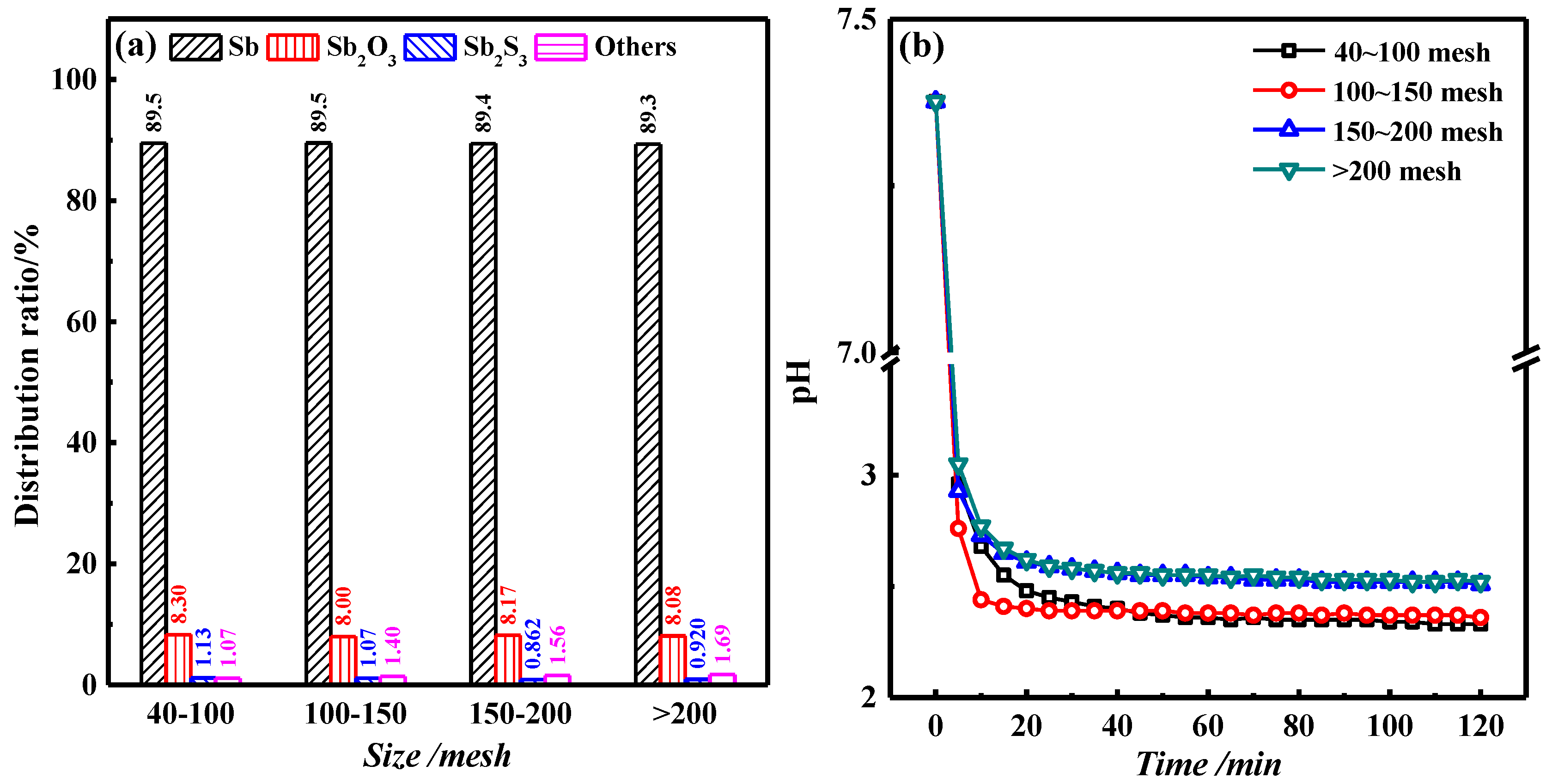
3.2. Effect of C Particle Size on Roasting Results
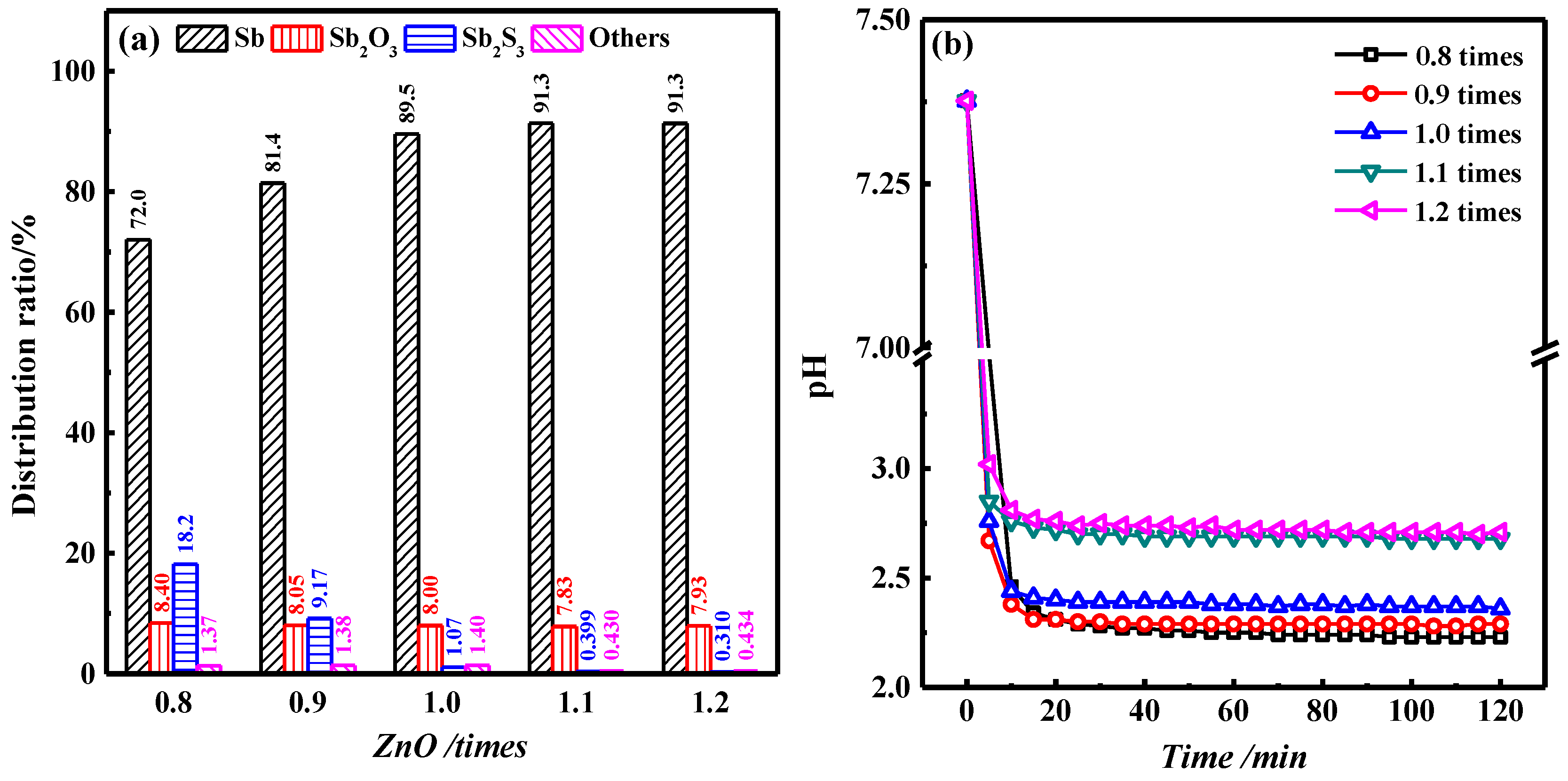
3.3. Effect of ZnO Content on Roasting Results
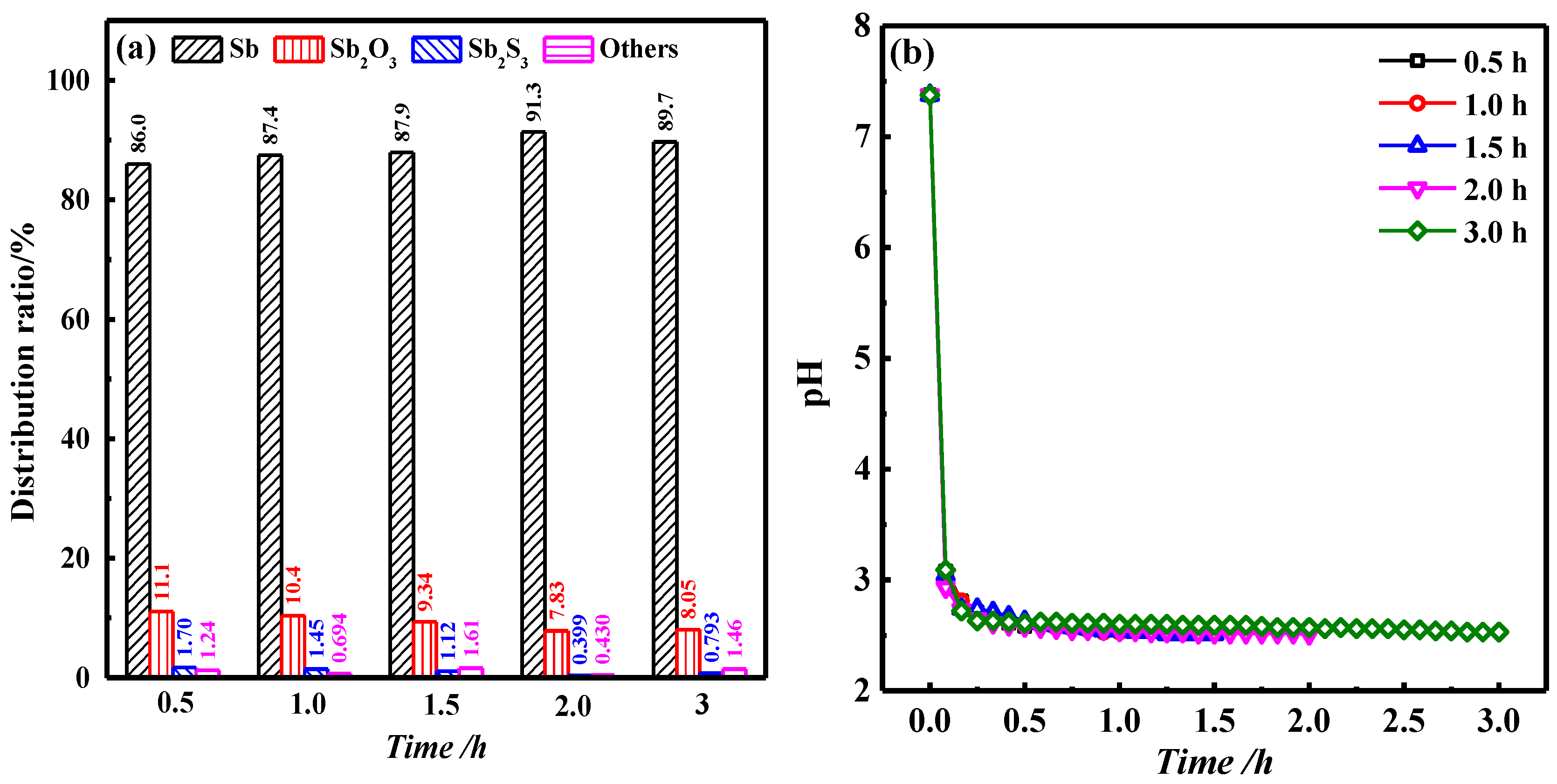
3.4. Effect of Time on Roasting Results
3.5. Effect of Cooling Mode on Roasting Results
3.6. Energy Consumption Comparision
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ravinder, S.M.; Thomas, F.; George, P.D. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony recovery from end-of-life products and industrial process residues: A critical review. J. Sustain. Metall. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Yang, T.Z.; Xie, B.Y.; Liu, W.F.; Zhang, D.C.; Chen, L. Enriched of gold in antimony matte by direct smelting of refractory gold concentrate. JOM 2018, 70, 1018–1023. [Google Scholar] [CrossRef]
- Anderson, C.G. The metallurgy of antimony. Chem. Erde-Geochem. 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Yang, J.G.; Tang, C.B.; Chen, Y.M.; Tang, M.T. Separation of antimony from a stibnite concentrate through a low-temperature smelting process to eliminate SO2 emission. Metall. Mater. Trans. B 2011, 42B, 30–36. [Google Scholar] [CrossRef]
- Zhao, T.C. Aantimony, 1st ed.; Metallurgical Industry Press: Beijing, China, 1987. [Google Scholar]
- Habashi, F. Handbook of Extractive Metallurgy; WILEY-VCH: Weinheim, Germany, 1997; Volume II. [Google Scholar]
- Liu, W.; Luo, H.L.; Qing, W.Q.; Zheng, Y.X.; Yang, K.; Han, J.W. Investigation into oxygen-enriched bottom-blown stibnite and direct reduction. Metall. Mater. Trans. B 2014, 45, 1281–1290. [Google Scholar] [CrossRef]
- Chen, M.; Dai, X. Microscopic study of the phase transformation during the oxygen-enriched direct smelting of jamesonite concentrate. JOM 2018, 70, 41–46. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Yang, J.G.; Zhang, W.H. One-step extraction of antimony from low-grade stibnite in sodium carbonate-sodium chloride binary molten salt. J. Clean. Prod. 2015, 93, 134–139. [Google Scholar] [CrossRef]
- Ye, L.G.; Hu, Y.J.; Xia, Z.M.; Tang, C.B.; Chen, Y.M.; Tang, M.T. Solution behavior of ZnS and ZnO in eutectic Na2CO3-NaCl molten salt used for Sb smelting. J. Cent. South. Univ. 2017, 24, 1269–1274. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.M.; Xue, H.T.; Tang, C.B.; Yang, S.H.; Tang, M.T. One-step extraction of antimony in low temperature from stibnite concentrate using iron oxide as sulfur-fixing agent. Metals 2016, 6, 153. [Google Scholar] [CrossRef]
- Padilla, R.; Chambi, L.C.; Ruiz, M.C. Antimony production by carbothermic reduction of stibnite in presence of lime. J. Min. Metall. Sect. B-Metall. 2014, 50, 5–13. [Google Scholar] [CrossRef]
- Padilla, R.; Aracena, A.; Ruiz, M.C. Kinetics of stibnite (Sb2S3) oxidation at roasting temperatures. J. Min. Metall. Sect. B-Metall. 2014, 50, 127–132. [Google Scholar] [CrossRef]
- Mahlangu, T.; Gudyanga, F.P.; Simbi, D.J. Reductive leaching of stibnite (Sb2S3) flotation concentrates using metallic iron in a hydrochloric acid medium II: Kinetics. Hydrometallurgy 2007, 88, 132–142. [Google Scholar] [CrossRef]
- Yang, J.G.; Wu, Y.T. A hydrometallurgical process for the separation and recovery of antimony. Hydrometallurgy 2014, 143, 68–74. [Google Scholar] [CrossRef]
- Awe, S.A.; Sundkvist, J.E.; Bolin, N.J.; Sandstrom, A. Process flow sheet development for recovering antimony from Sb-bearing copper concentrates. Miner. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandstrom, A. Selective leaching of arsenic and antimony from a tetrahedriterich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sari, A.; Tuzen, M. Effective adsorption of antimony (III) from aqueous solution by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Wang, Q.M.; Guo, X.Y.; Tian, Q.H.; Jiang, T.; Chen, M.; Zhao, B.J. Effects of Matte grade on the distribution of minor elements (Pb, Zn, As, Sb, and Bi) in the bottom blown copper smelting process. Metals 2017, 7, 502. [Google Scholar] [CrossRef]
- Zdzislaw, A.; Katarzyna, N. Environmental mobility of trace elements present in dusts emitted from Zn-Pb metallurgical processes. Environ. Earth Sci. 2016, 75, 956–961. [Google Scholar] [CrossRef]
- Yu, L.; Fu, B. The Analysis Handbook of Nonferrous Metallurgy; Metallurgical Industry Press: Beijing, China, 2004. [Google Scholar]
- Ouyang, Z.; Liu, S.F.; Tang, C.B.; Chen, Y.F.; Ye, L.G. Kinetics studies for sulfur-fixing and roasting reduction of antimony sulfide for direct antimony extraction. Vacuum 2019, 159, 358–366. [Google Scholar] [CrossRef]
- Lu, G.W. Handbook of Mineral Processing Designing; Metallurgical Industry Press: Beijing, China, 2000. [Google Scholar]



| Reductions | No. |
|---|---|
| Sb2S3 + 3ZnO + 1.5C = 2Sb + 3ZnS + 1.5CO2(g) | (2) |
| Sb2S3 + 3ZnO + 3C = 2Sb + 3ZnS + 3CO(g) | (3) |
| Sb2S3 + 3ZnO = Sb2O3 + 3ZnS | (4) |
| Sb2O3 + 1.5C = 2Sb + 1.5CO2(g) | (5) |
| Sb2O3 + 3C = 2Sb + 3CO(g) | (6) |
| Sb2S3 + 2Sb2O3 = 6Sb + 3SO2(g) | (7) |
| ZnO + CO(g) = Zn + CO2(g) | (8) |
| ZnO + C = Zn + CO(g) | (9) |
| C + CO2(g) = 2CO(g) | (10) |
| Points | Sb | Zn | S | O |
|---|---|---|---|---|
| 1 | 96.00 | - | - | 4.00 |
| 2 | 20.22 | 49.16 | 28.03 | 2.60 |
| 3 | 90.13 | 4.08 | 1.64 | 4.15 |
| 4 | 41.18 | 36.67 | 19.55 | 2.6 |
| 5 | 100 | - | - | - |
| 6 | 1.06 | 69.44 | 30.50 | - |
| The Conventional Pyrometallurgy Process | Metallurgy and Beneficiation Combined Process | ||
|---|---|---|---|
| Unit operation | Energy cost GJ·t−1 | Unit operation | Energy cost GJ·t−1 |
| Oxidising-roasting | 3.05 × 1010 | Roasting | 3.28 × 1010 |
| Reduction-smelting | 1.45 × 1010 | Gravity separation | 4.69 × 108 |
| Flotation separation | 5.50 × 108 | ||
| Total costing = | 4.5 × 1010 | Total costing = | 3.38 × 1010 |
| CO2 emission | 4.62 t | CO2 emission | 3.29 t |
| SO2 generation | 0.81 t | SO2 generation | 0.04 t |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Z.; Ye, L.; Tang, C.; Chen, Y. Phase and Morphology Transformations in Sulfur-Fixing and Reduction Roasting of Antimony Sulfide. Metals 2019, 9, 79. https://doi.org/10.3390/met9010079
Ouyang Z, Ye L, Tang C, Chen Y. Phase and Morphology Transformations in Sulfur-Fixing and Reduction Roasting of Antimony Sulfide. Metals. 2019; 9(1):79. https://doi.org/10.3390/met9010079
Chicago/Turabian StyleOuyang, Zhen, Longgang Ye, Chaobo Tang, and Yifeng Chen. 2019. "Phase and Morphology Transformations in Sulfur-Fixing and Reduction Roasting of Antimony Sulfide" Metals 9, no. 1: 79. https://doi.org/10.3390/met9010079
APA StyleOuyang, Z., Ye, L., Tang, C., & Chen, Y. (2019). Phase and Morphology Transformations in Sulfur-Fixing and Reduction Roasting of Antimony Sulfide. Metals, 9(1), 79. https://doi.org/10.3390/met9010079




