Inclusion Modification in C104Cr Saw Wire Steel with Different Cerium Content
Abstract
1. Introduction
2. Experiments
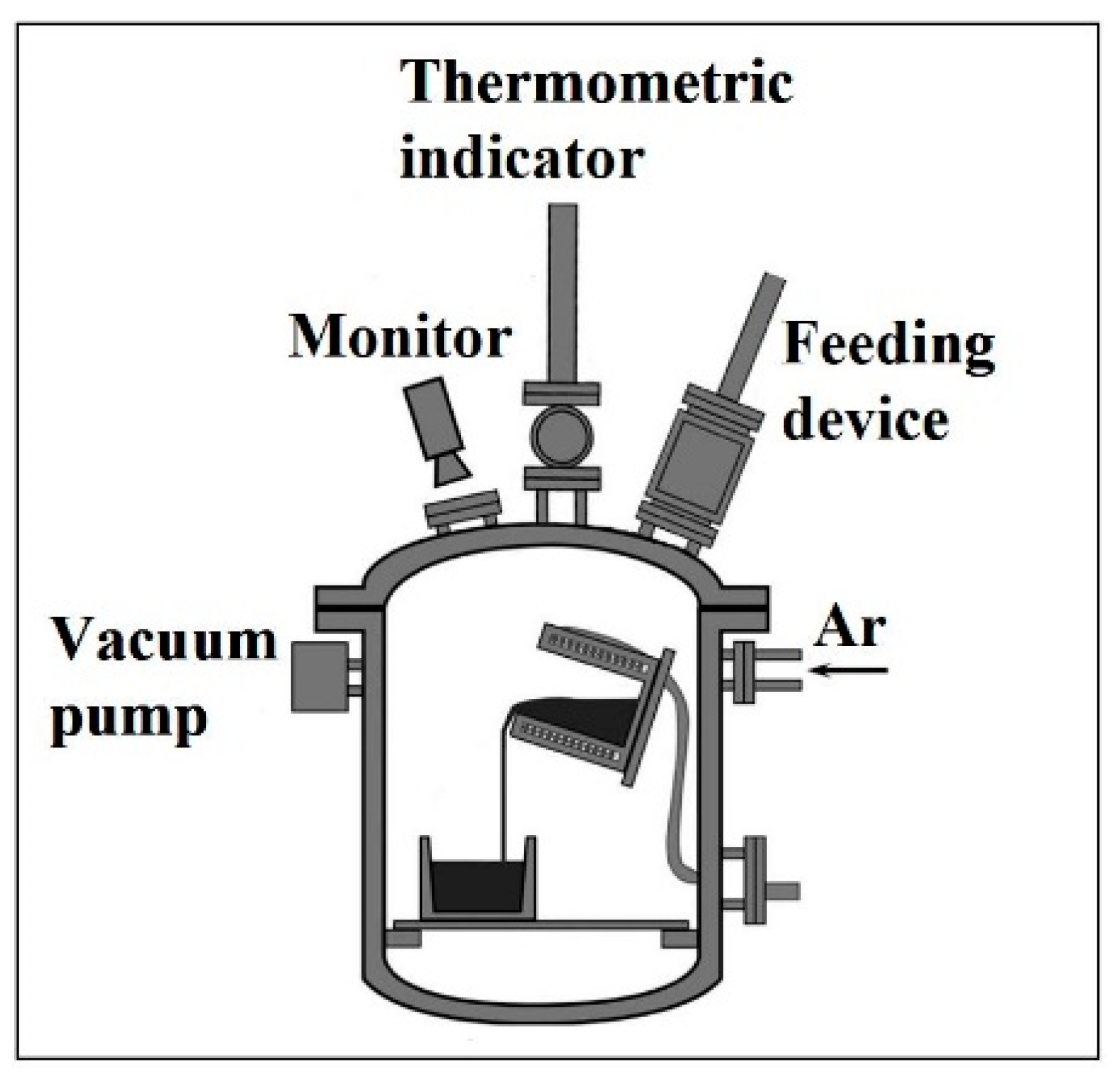
2.1. Experimental Procedure
2.2. Analysis Methods
3. Results and Discussion
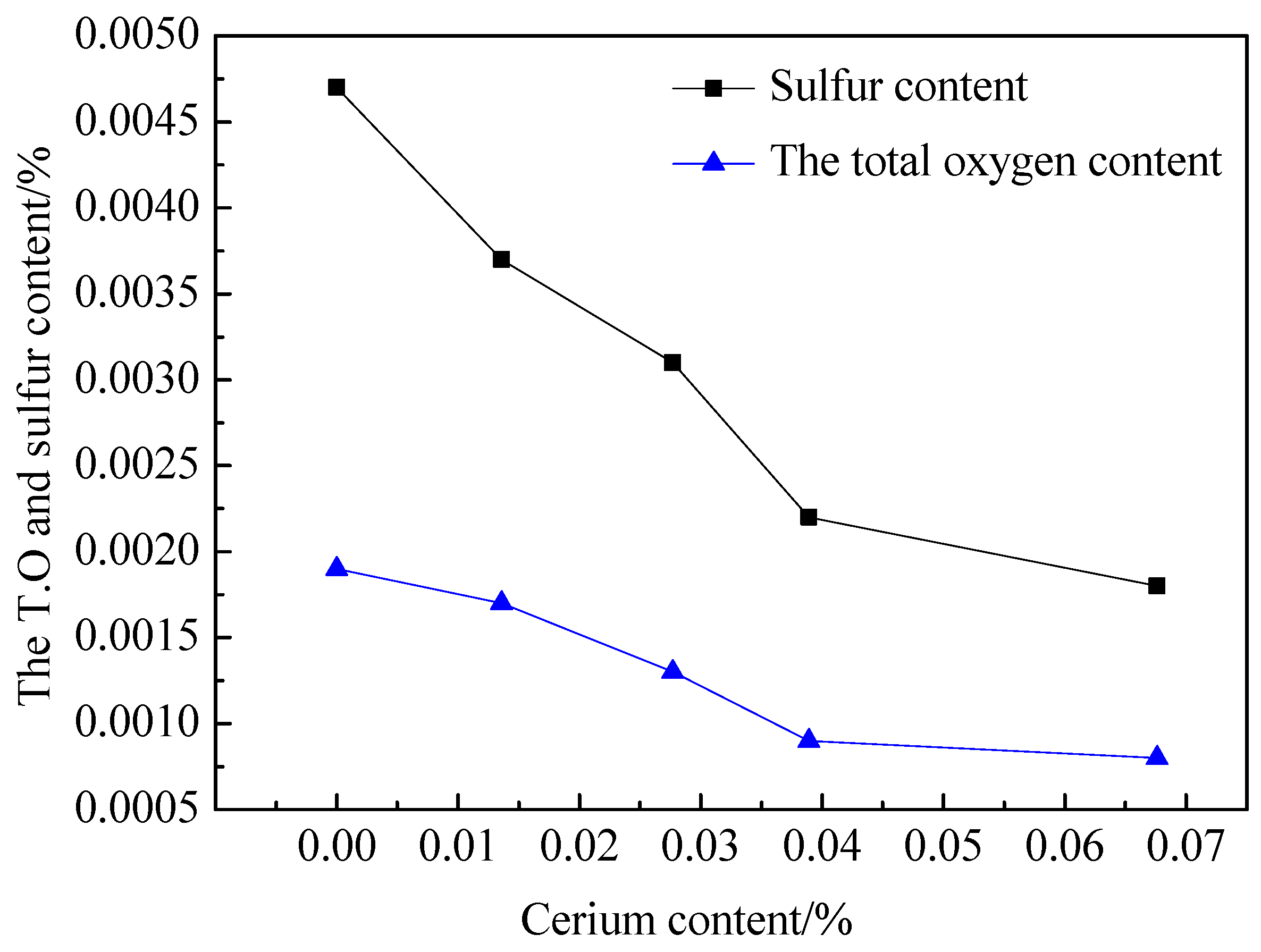
3.1. The Effect of the Cerium Addition on S and Total Oxygen (T.O) Content in C104Cr Steel
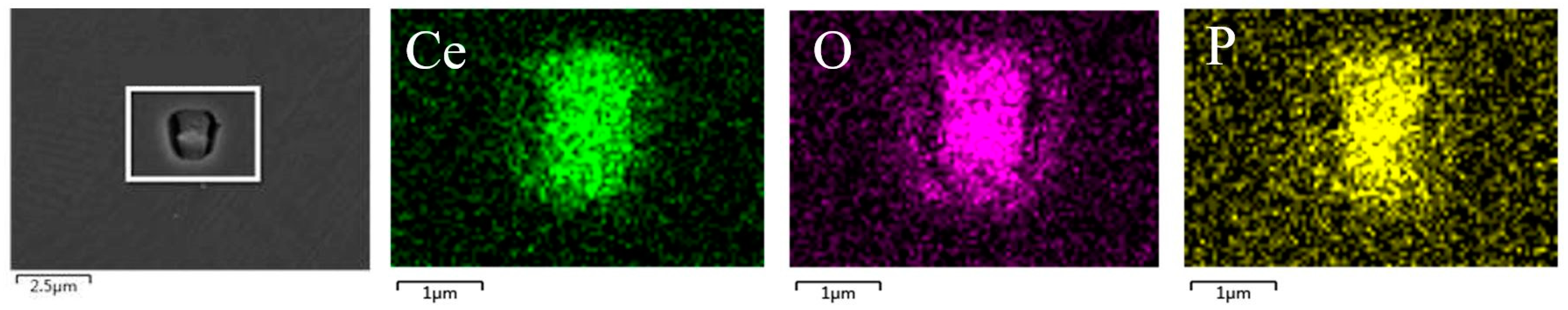
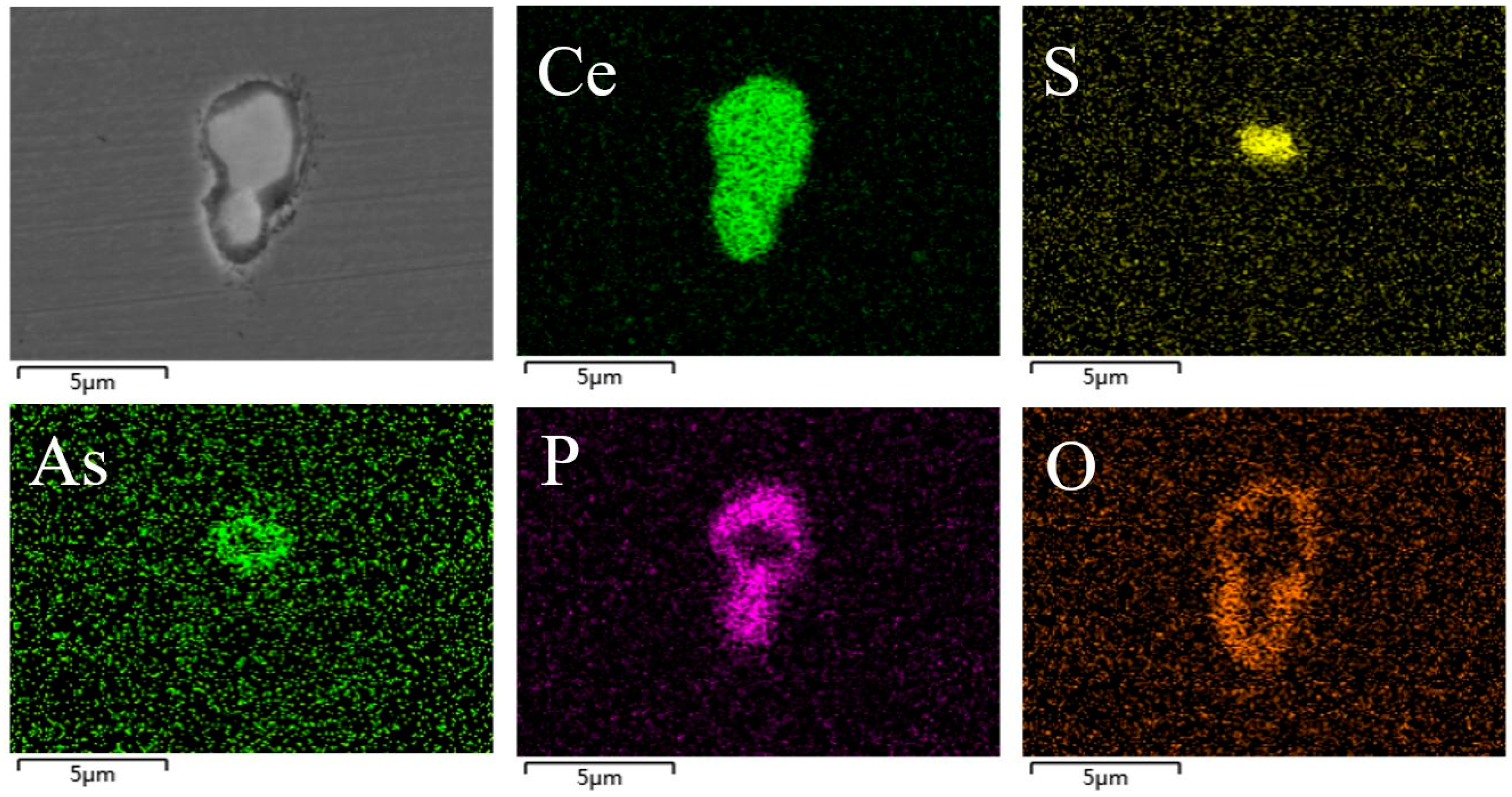
3.2. The Effect of the Cerium Addition on the Characteristics of Inclusions
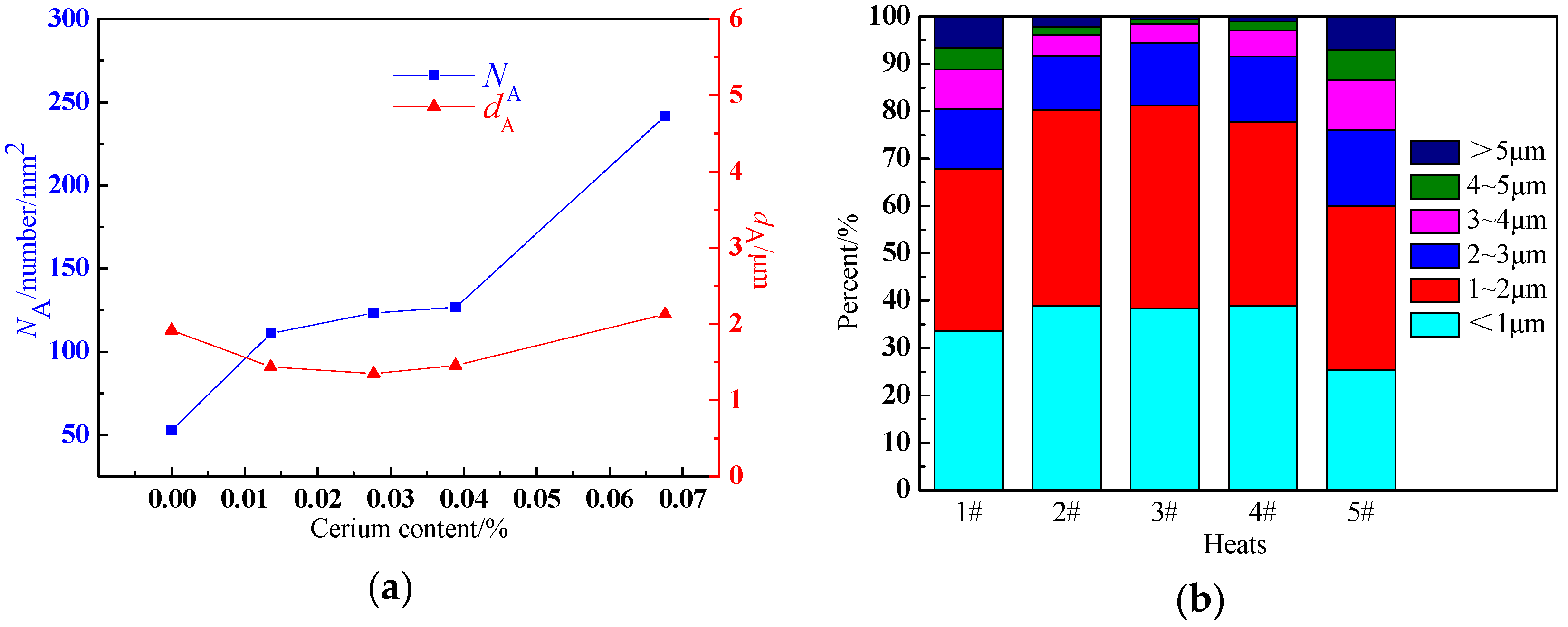
3.3. The Effect of the Cerium Addition on the Number, Size, and Distribution of Inclusions
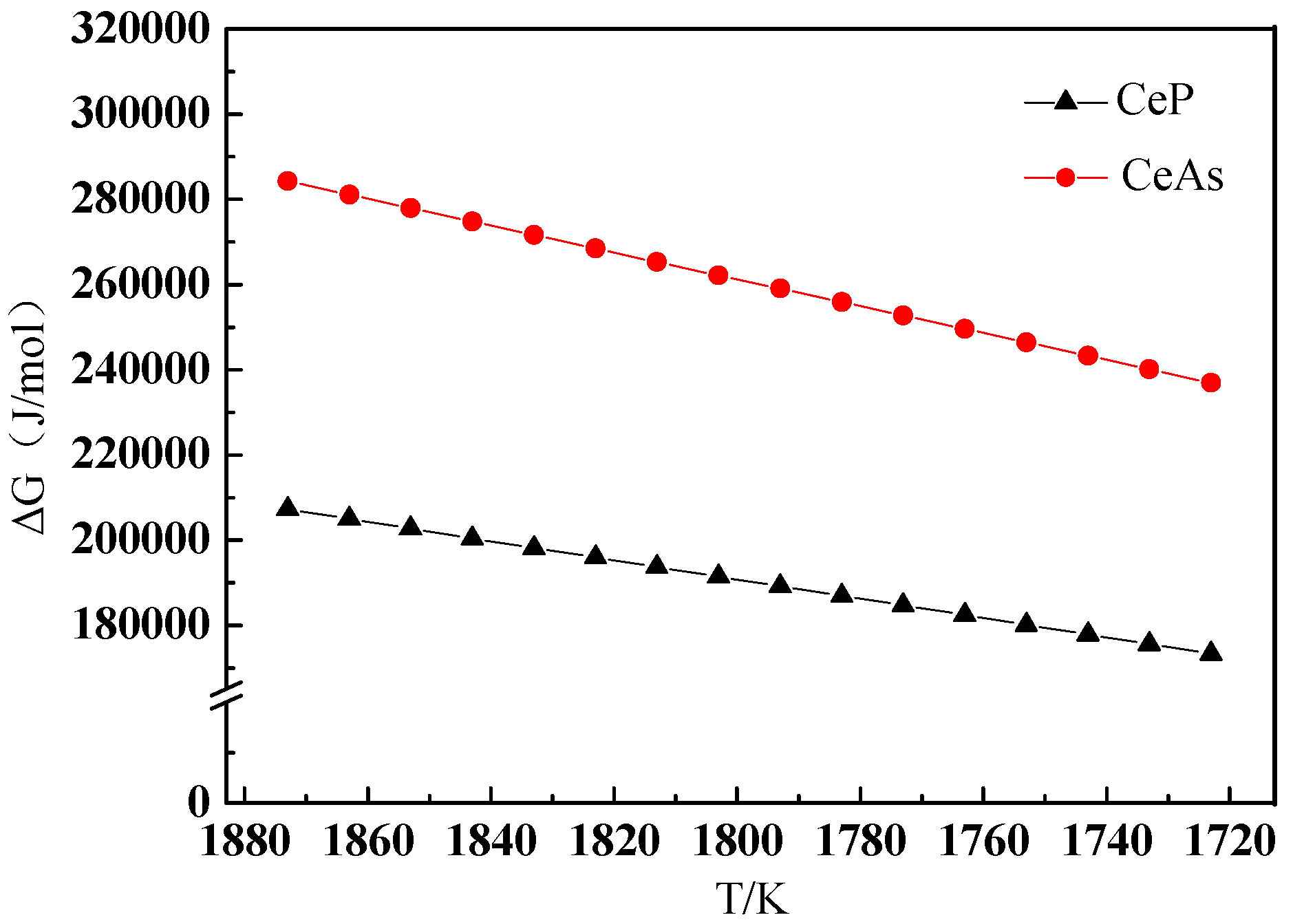
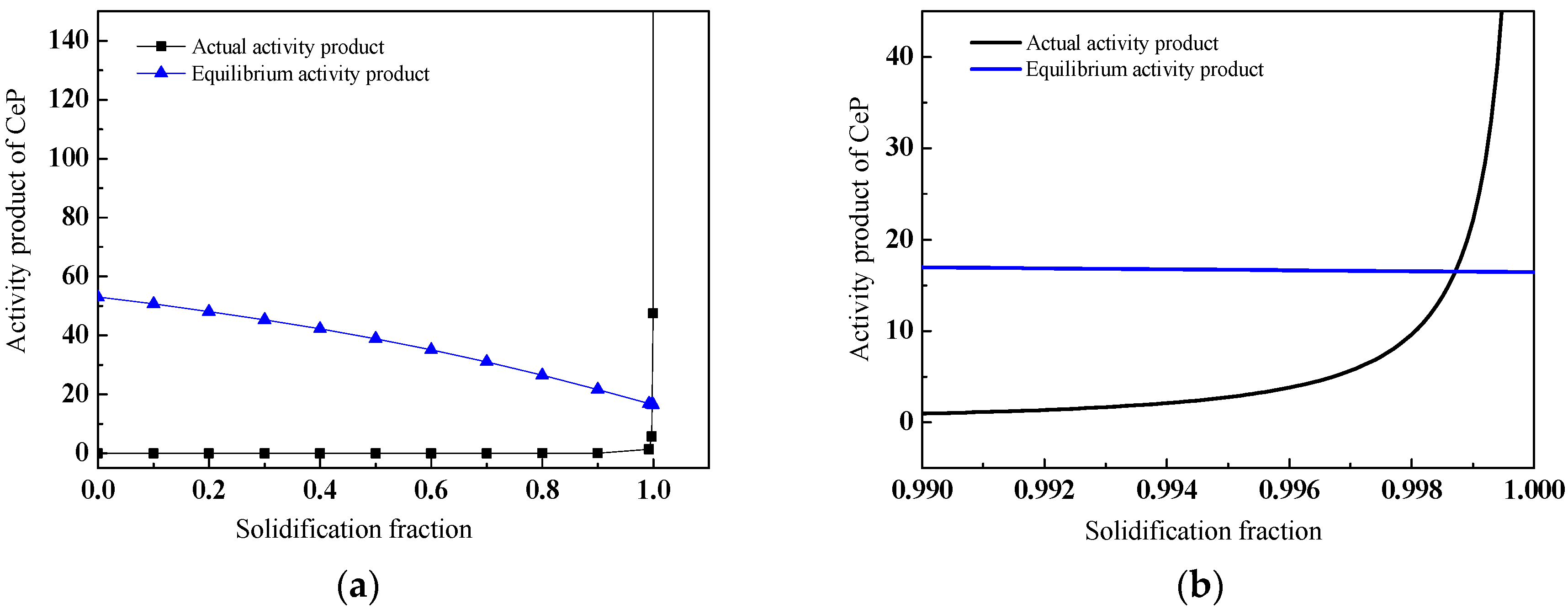
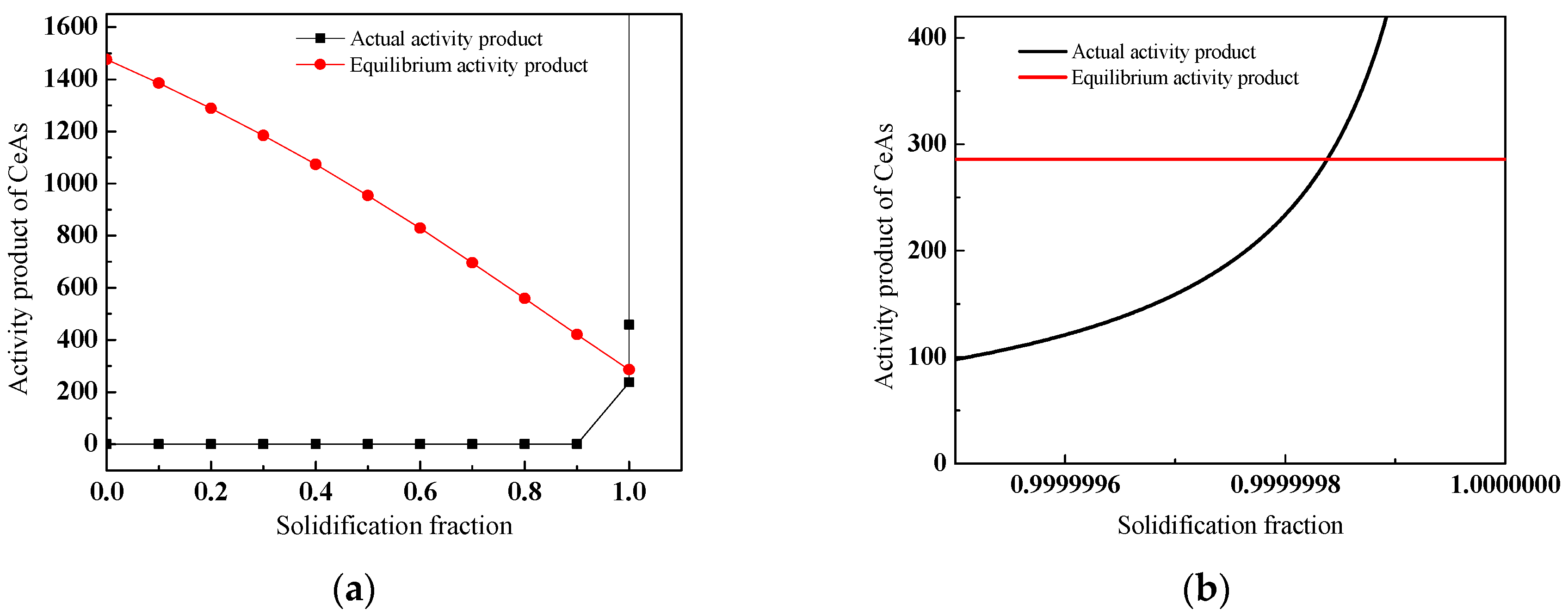
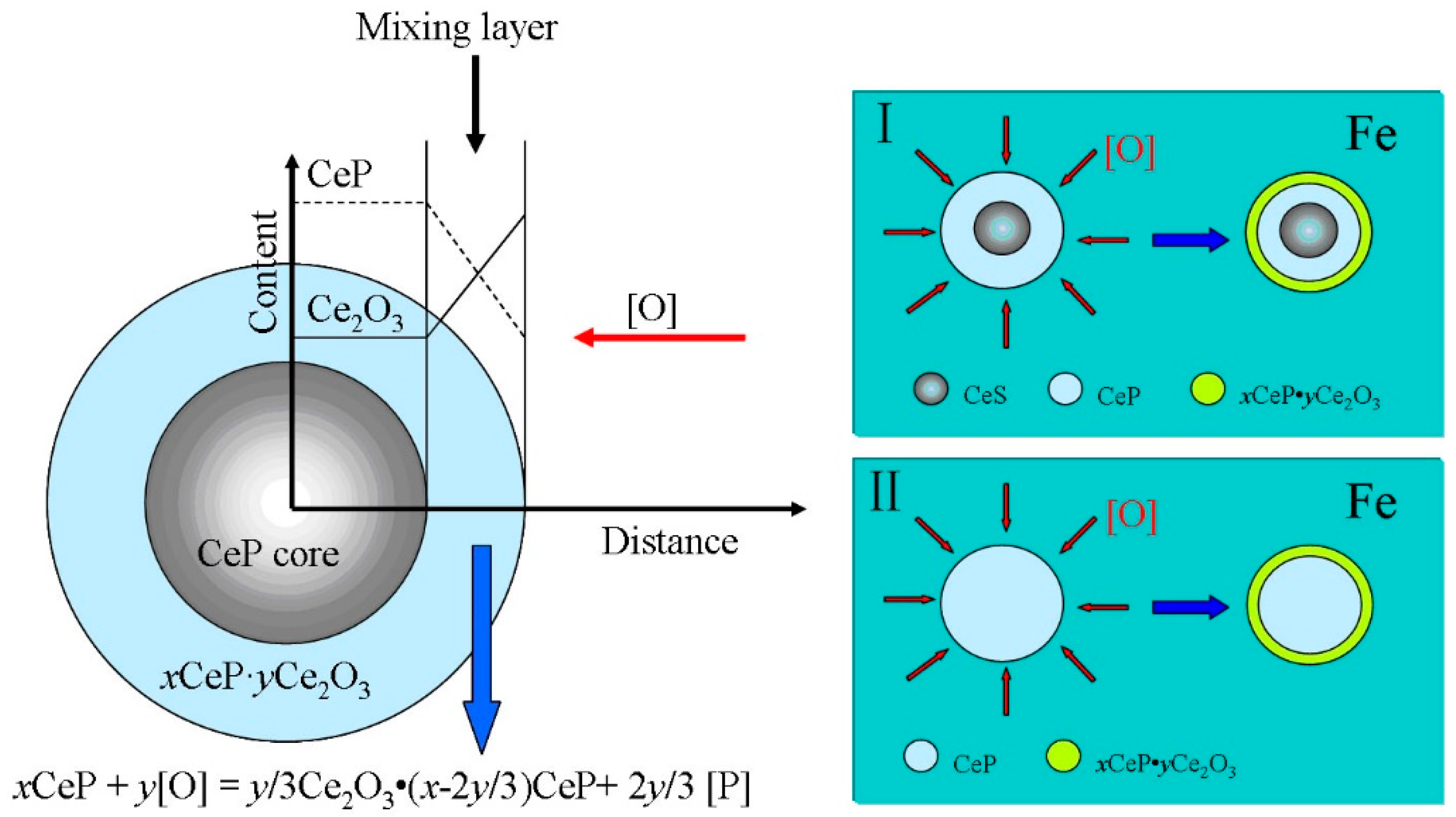
3.4. Discussion on Evolution Mechanism of CeP, CeAs, and Cerium Composite Inclusions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, X. New Products and New Technology of Special Steel; Metallurgical Industry Press: Beijing, China, 2016; p. 270. ISBN 978-7-5024-7268-9. [Google Scholar]
- Kirhara, K. Production technology of wire rod for high tensile strength steel cord. Kobelco Technol. Rev. 2011, 30, 62–65. [Google Scholar]
- Li, Y.; Chen, C.; Jiang, Z.; Sun, M.; Hu, H.; Li, H. Application of alkali oxides in LF refining slag for enhancing inclusion removal in C96V saw wire steel. ISIJ Int. 2018, 58, 1232–1241. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.; Su, Y. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Yang, L.; Gao, X. Effect of Ce on inclusions and impact property of 2Cr13 stainless steel. J. Iron Steel Res. Int. 2010, 17, 59–64. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Wang, Q.; Chou, K. Evolution mechanisms of MgO·Al2O3 inclusions by Cerium in spring steel used in fasteners of high-speed railway. ISIJ Int. 2015, 55, 970–975. [Google Scholar] [CrossRef]
- Wen, B.; Song, B.; Pan, N.; Shu, Q.; Hu, X.; Mao, J. Influence of Ce on characteristics of inclusions and microstructure of pure iron. J. Iron Steel Res. Int. 2011, 18, 38–44. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, H.; Xie, J. Recent progress and new ideas for accelerating research in rare earth steel. J. Iron Steel Res. 2017, 29, 513–529. [Google Scholar]
- Wang, L. Application Prospects and Behavior of RE in new generation high strength steels with superior toughness. J. Chin. Rare Earth Soc. 2004, 22, 48–54. [Google Scholar]
- Zhi, J.; Liu, L.; Chen, J.; Li, Z. Effect of RE on Inclusion of BNbRE Rail Steel. J. Chin. Rare Earth Soc. 2004, 22, 181–184. [Google Scholar]
- Zhao, X.; Jiang, J.; Li, G.; Li, F. Kinetics of formation of large rare earth inclusion in steels. J. Chin. Rare Earth Soc. 2004, 22, 142–144. [Google Scholar]
- Xin, W.; Song, B.; Song, M.; Song, G. Effect of cerium on characteristic of inclusions and grain boundary segregation of arsenic in iron melts. Steel Res. Int. 2015, 86, 1430–1438. [Google Scholar] [CrossRef]
- Wang, C. Effect of Rare Earth Lanthanum and Magnesium on Cleanliness and Properties of Typical Special Steels. Ph.D. Thesis, Northeastern University, Shenyang, China, 2017. [Google Scholar]
- Liang, Y.; Che, Y. Handbook of Thermodynamic Data for Inorganic Matter; NEU Press: Shenyang, China, 1993; p. 107. ISBN 7-81006-532-7. [Google Scholar]
- Li, N.; Lu, Q.; Wang, Y.; Zhu, Z.; Xiang, L.; Qiu, S. Effect of Ce on inclusions modification in 2. 9%Si-0. 8%A1 non-oriented electrical steel. J. Iron Steel Res. 2017, 29, 570–576. [Google Scholar]
- Liu, Y.; Wang, L.; Guo, J.; Hu, X.; Chou, K. Thermodynamic analysis of cerium inclusion formed in spring steel used in fastener of high-speed railway. Chin. J. Nonferrous Met. 2013, 23, 720–726. [Google Scholar]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-O-S-As inclusions in high carbon steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Xin, W. Effect of Arsenic on the Properties of Steel and Improvement by Adding Rare Earth. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Clyne, T.; Kurz, W. Solute redistribution during solidification with rapid solid state diffusion. Metall. Trans. A 1981, 12, 965–971. [Google Scholar] [CrossRef]
- Won, Y.; Thomas, B. Simple model of microsegregation during solidification of steels. Metall. Mater. Trans. A 2001, 32, 1755–1767. [Google Scholar] [CrossRef]
- Han, Z.; Cai, K.; Han, Z.; Cai, K. Study on a mathematical model of microsegregation in continuously cast slab. Acta Metall. Sin. 2000, 36, 869–873. [Google Scholar]
- Kim, K.; Han, H.; Yeo, T.; Lee, Y.; Oh, K.; Lee, D. Analysis of surface and internal cracks in continuously cast beam blank. Ironmak. Steelmak. 1997, 24, 249–256. [Google Scholar]
- Zhong, X.; Hu, H.; Liu, C. The measurement of cerium equilibrium partition coefficient K0 and influence of cerium on value of manganese and silicon K0 in high manganese steel. Chin. J. Eng. 1984, 3, 21–22. [Google Scholar]
- Li, W. Thermodynamics of the formation of rare-earth inclusions in steel. Iron Steel 1986, 21, 7–12. [Google Scholar]
- Božić, B.; Lučić, R. Diffusion in iron-arsenic alloys. J. Mater. Sci. 1976, 11, 887–891. [Google Scholar] [CrossRef]
- Ma, Z.; Janke, D. Characteristics of oxide precipitation and growth during solidification of deoxidized steel. ISIJ Int. 1998, 38, 46–52. [Google Scholar] [CrossRef]




| No | C | Si | Mn | Cr | Ce | N | T.O | S | P | As |
|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 1.043 | 0.16 | 0.42 | 0.19 | 0.0000 | 0.0011 | 0.0019 | 0.0047 | 0.0079 | 0.0046 |
| 2# | 1.049 | 0.16 | 0.41 | 0.18 | 0.0136 | 0.0012 | 0.0017 | 0.0037 | 0.0071 | 0.0022 |
| 3# | 1.045 | 0.16 | 0.42 | 0.19 | 0.0277 | 0.0011 | 0.0013 | 0.0031 | 0.0074 | 0.0021 |
| 4# | 1.042 | 0.16 | 0.42 | 0.19 | 0.0389 | 0.0009 | 0.0009 | 0.0022 | 0.0072 | 0.0021 |
| 5# | 1.043 | 0.17 | 0.42 | 0.19 | 0.0676 | 0.0008 | 0.0008 | 0.0018 | 0.0068 | 0.0020 |
| C | Si | Mn | O | Cr | Al | Ce | P | S | Mg | N | As | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 0.14 | 0.08 | −0.012 | −0.34 | −0.024 | 0.043 | −0.0026 | 0.051 | 0.046 | −0.07 | 0.11 | 0.043 |
| O | −0.45 | −0.131 | −0.021 | −0.2 | −0.04 | −3.9 | −0.57 | −0.07 | −0.133 | −300 | 0.057 | - |
| Al | 0.091 | 0.0056 | 0.012 | −6.6 | 0.025 | 0.045 | −0.043 | 0.05 | 0.03 | −0.13 | −0.053 | - |
| Ce | −0.077 | - | 0.13 | −5.03 | - | −2.25 | −0.003 | 1.746 | −39.8 | - | - | - |
| P | 0.13 | 0.12 | 0 | 0.13 | - | 0.037 | - | 0.062 | 0.028 | - | 0.094 | - |
| S | 0.11 | 0.063 | −0.026 | −0.27 | - | −0.035 | −0.231 | 0.029 | −0.028 | −1.82 | 0.01 | 0.0041 |
| As | 0.25 | - | - | - | - | - | - | - | - | - | - | - |
| i | Al | C | O | Ce | S | P | As |
|---|---|---|---|---|---|---|---|
| fi | 1.23 | 1.41 | 0.30 | 0.71 | 1.29 | 1.41 | 1.82 |
| Reaction | ΔGθ, J·mol−1 |
|---|---|
| [Ce] + [N] = CeN(s) | −172,890 + 81.09T |
| [Ce] + 2[C] = CeC2(s) | −131,000 + 121.40T |
| [Ce] + [P] = CeP(s) | −215,534 + 157.74T |
| [Ce] + [As] = CeAs(s) | −302,040 + 237.2T |
| [Ce] + 2[O] = CeO2(s) | −852,720 + 249.96T |
| [Ce] + 3/2[O] = 1/2Ce2O3(s) | −714,380 + 179.74T |
| [Ce] + [O] + 1/2[S] = 1/2Ce2O2S(s) | −675,700 + 165.50T |
| [Ce] + [S] = CeS(s) | −422,100 + 120.38T |
| [Ce] + 3/2[S] = 1/2Ce2S3(s) | −536,420 + 163.86T |
| [Ce] + 4/3[S] = 1/3Ce3S4(s) | −497,670 + 146.30T |
| [Ce] + 3/2[C] = 1/2Ce2C3(s) | −112,000 + 102.90T |
| [Ce] + 3[O] + [Al] = CeAlO3(s) | −1,366,460 + 364.3T |
| [Ce] + Al2O3(s) = CeAlO3(s) + [Al] | −423,900 − 247.30T |
| Cerium Content/% | Typical Inclusions | |
|---|---|---|
| 0% (1#) |  | 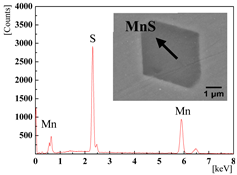 |
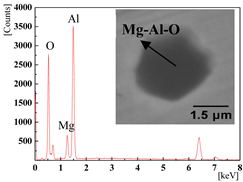 | 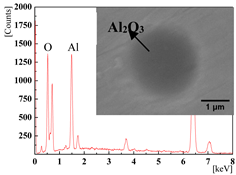 | |
| 0.0136% (2#) and 0.0277% (3#) | 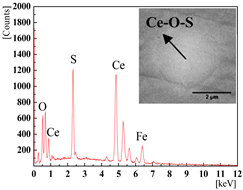 | 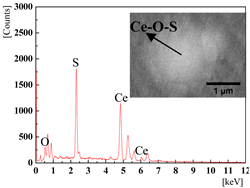 |
| 0.0389% (4#) and 0.0277% (5#) |  | 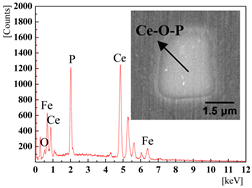 |
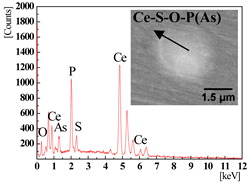 | 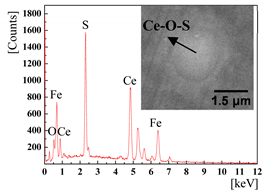 | |
| Element | k | DS/(m2·s−1) |
|---|---|---|
| C | 0.34 | 7.61 × 10−6exp(−134,557/RT) |
| Si | 0.52 | 3 × 10−5exp(−251,458/RT) |
| Mn | 0.78 | 5.5 × 10−6exp(−249,366/RT) |
| P | 0.13 | 1.0 × 10−6exp(−182,841/RT) |
| S | 0.035 | 2.4 × 10−4exp(−214,639/RT) |
| O | 0.022 | 3.71 × 10−6exp(−23,050/RT) |
| Ce | 0.05 | 1 × 10−20 |
| As | 0.33 | 58 × 10−6exp(−58,900/RT) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, M.; Jiang, Z.; Chen, C.; Chen, K.; Huang, X.; Sun, S.; Li, H. Inclusion Modification in C104Cr Saw Wire Steel with Different Cerium Content. Metals 2019, 9, 54. https://doi.org/10.3390/met9010054
Li Y, Sun M, Jiang Z, Chen C, Chen K, Huang X, Sun S, Li H. Inclusion Modification in C104Cr Saw Wire Steel with Different Cerium Content. Metals. 2019; 9(1):54. https://doi.org/10.3390/met9010054
Chicago/Turabian StyleLi, Yang, Meng Sun, Zhouhua Jiang, Changyong Chen, Kui Chen, Xinfeng Huang, Shen Sun, and Huabing Li. 2019. "Inclusion Modification in C104Cr Saw Wire Steel with Different Cerium Content" Metals 9, no. 1: 54. https://doi.org/10.3390/met9010054
APA StyleLi, Y., Sun, M., Jiang, Z., Chen, C., Chen, K., Huang, X., Sun, S., & Li, H. (2019). Inclusion Modification in C104Cr Saw Wire Steel with Different Cerium Content. Metals, 9(1), 54. https://doi.org/10.3390/met9010054







