Role of Chemical Composition in Corrosion of Aluminum Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Mechanical Properties
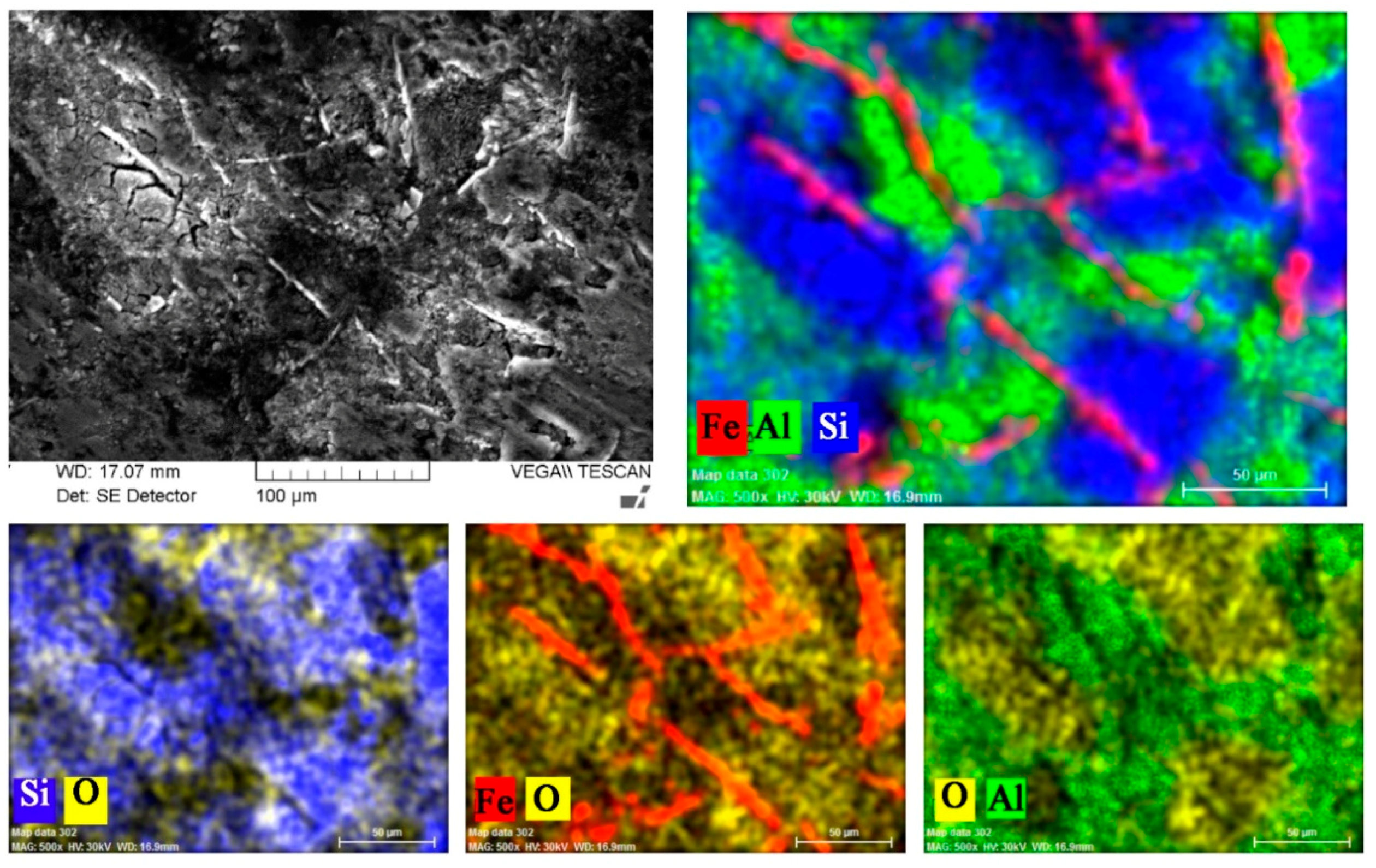
3.2. Microstructure of Experimental Materials
3.3. Corrosion Behavior
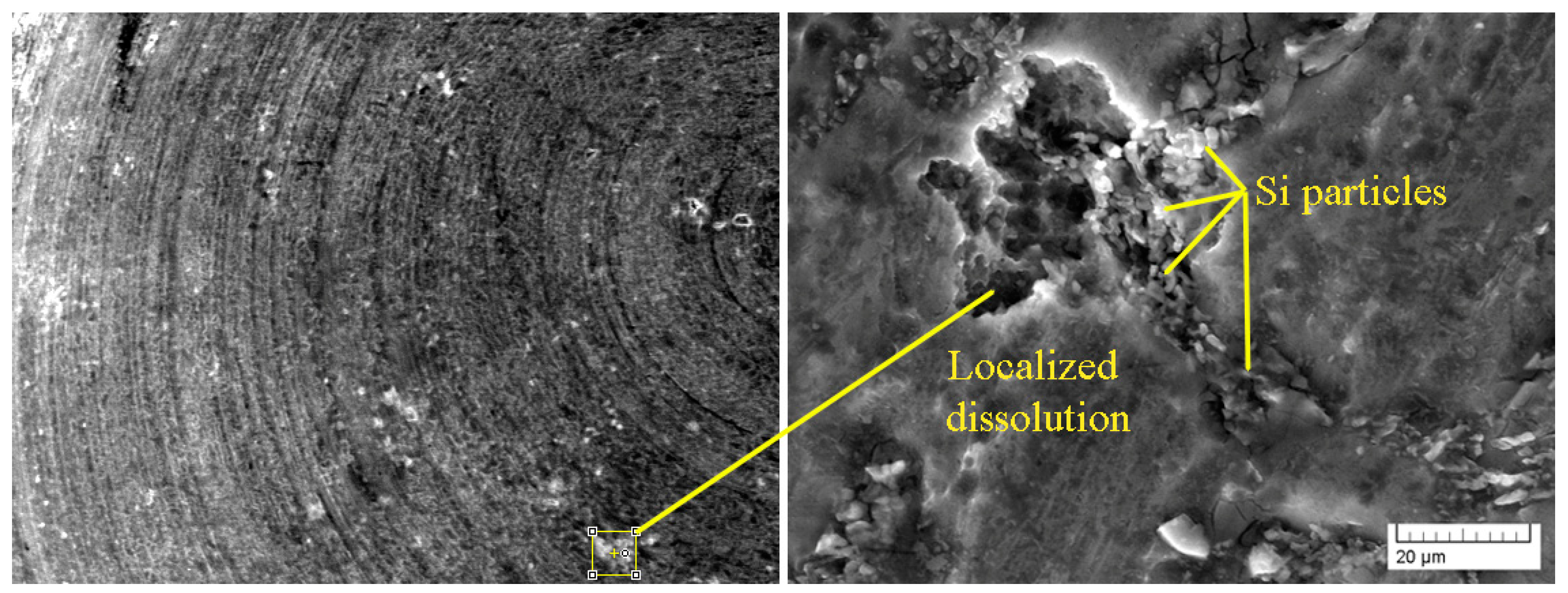
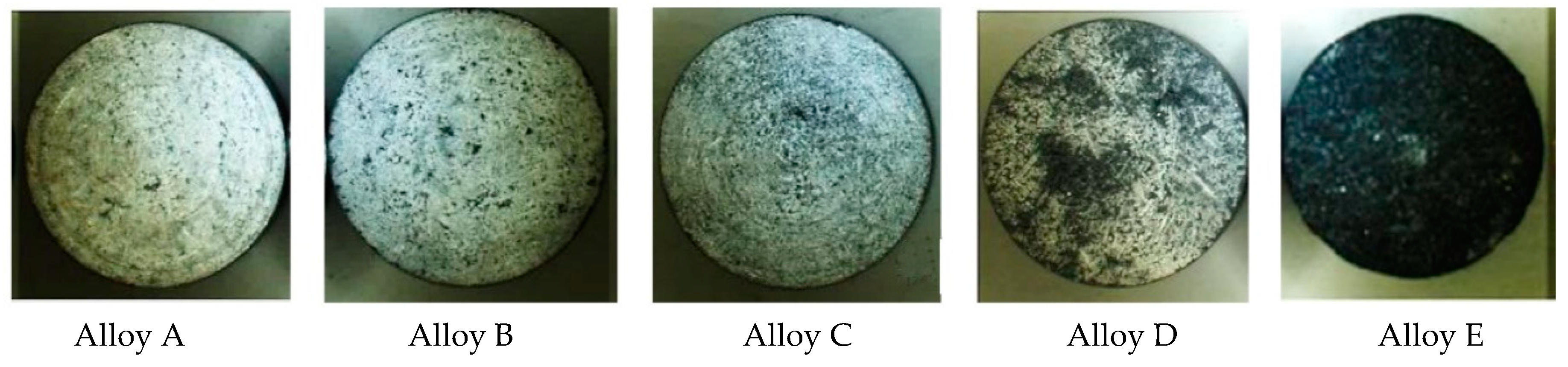
3.3.1. Exposure Test
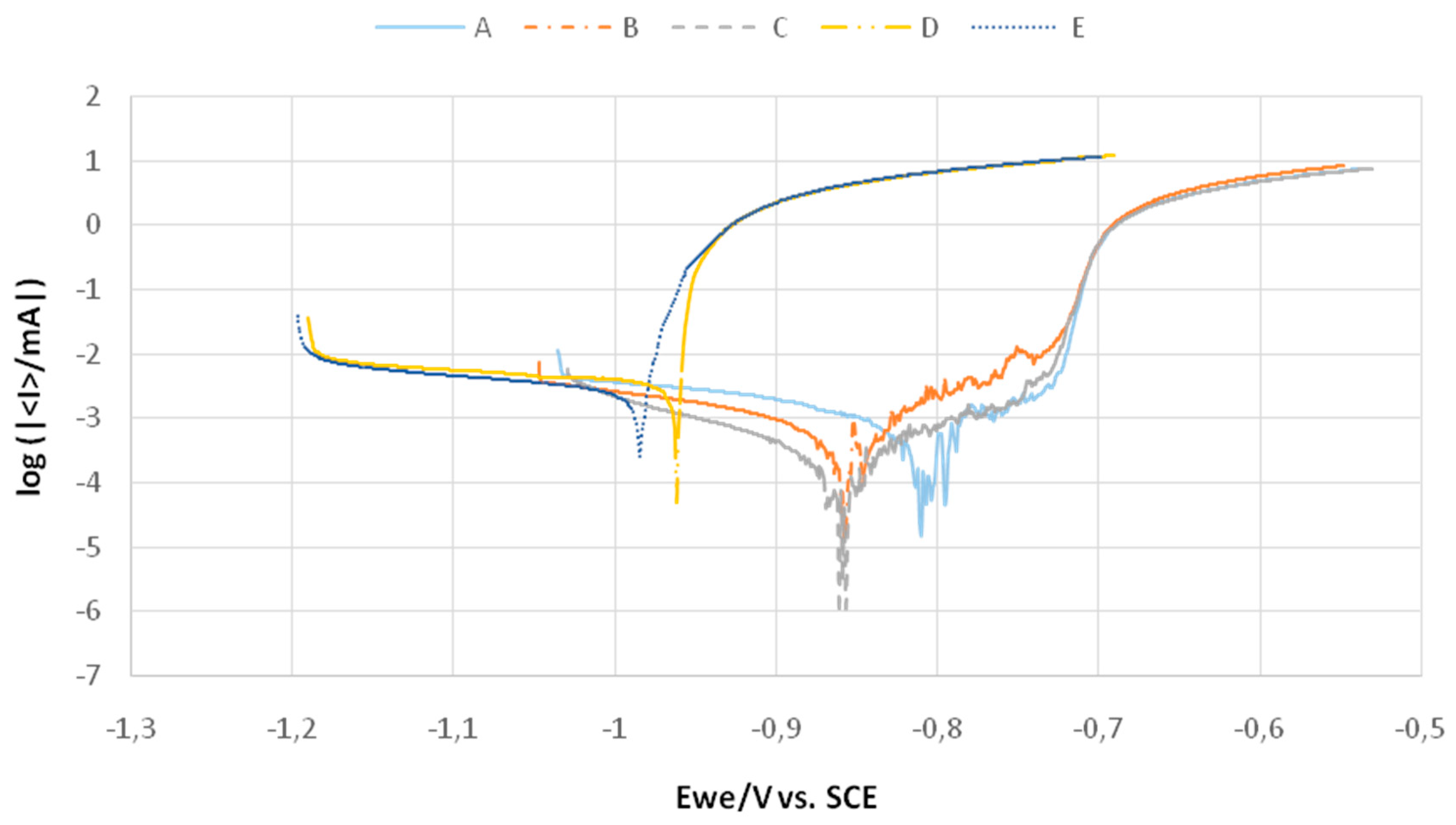
3.3.2. Potentiodynamic Test
3.3.3. Audi Test
4. Conclusions
- Various chemical compositions greatly influence the studied properties of the tested aluminum alloys.
- In terms of the effect of higher Fe content, the mechanical properties of AlZn10Si8Mg and AlSi7Mg0.3 cast alloys are not significantly influenced by it. The presence of hard and brittle Fe-needle phases leads to slightly improved mechanical properties. Therefore, the use of such materials does not significantly influence the mechanical properties of resulting alloys.
- The microstructure of both types of experimental materials is typically hypoeutectic, involving an α-matrix, eutectic and intermetallic phases. The α-phase’s finesses and content were similar in the same experimental material. The silicon present is in the form of small grains of poorly rounded, thickened grains that were observed on the periphery of dendrites α-phase. Out of the intermetallic phases were in microstructure of AlSi7Mg0.3 cast alloys observed: Al5FeSi, Al15(FeMg)2Si2 and Mg2Si. The Mg2Si, Al2CuMg, Al5FeSi, AlFeMnSiNi and Al-MgZn2-Cu in AlZn10Si8Mg cast alloy. In both experimental materials were observed increase lengths and amounts of Fe-needle phases as a reaction to increased Fe content in the microstructure.
- Worse corrosion properties were documented in case of AlZn10Si8Mg cast alloys, compared to experimental AlSi7Mg0.3 by all carried out experiments. During their exposure and Audi tests, it showed that higher Fe contents decrease corrosion resistance of experimental materials, but with regard to Si content. Based on the results of potentiodynamic a test was found that higher Fe amounts in AlSi7Mg0.3 casts alloys decelerate corrosion kinetic. These results correlate with Osório’s work [33]. All applied tests showed better corrosion resistance in AlSi7Mg0.3 cast alloys. But it was also found that the above depends not only on the Fe content, but also on the proportion of Fe and Si in the Al alloys as reported by Osório [6] in his work.
Author Contributions
Funding
Conflicts of Interest
References
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Rosso, M.; Peter, I.; Castella, C.; Molina, R. Properties of AlZn10Si8Mg alloys for high performances application. In Light Metals; Springer: Cham, Switzerland, 2014; pp. 213–218. [Google Scholar]
- Oztürk, I.; Agaoglu, G.H.; Erzi, E.; Dispinar, D.; Orhan, G. Effects of strontium addition on the microstructure and corrosion behavior of A356 aluminum alloy. J. Alloys Compd. 2018, 763, 384–391. [Google Scholar] [CrossRef]
- Arrabal, R.; Mingo, B.; Pardo, A.; Mohedano, M.; Matykina, E.; Rodriguez, I. Pitting corrosion of rheocast A356 aluminum alloy in 3.5 wt. % NaCl solution. Corros. Sci. 2013, 73, 342–355. [Google Scholar] [CrossRef]
- Osório, W.R.; Garcia, L.R.; Goulart, P.R.; Garcia, A. Effects of eutectic modification and T4 heat treatment on mechanical properties and corrosion resistance of an Al–9 wt. % Si casting alloy. Mater. Chem. Phys. 2007, 106, 343–349. [Google Scholar] [CrossRef]
- Osório, W.R.; Goulart, P.R.; Garcia, A. Effect of silicon content on microstructure and electrochemical behavior of hypoeutectic Al–Si alloys. Mater. Lett. 2008, 62, 365–369. [Google Scholar] [CrossRef]
- Cecchel, S.; Cornacchia, G.; Gelfi, M. Corrosion behavior of primary and secondary AlSi high pressure die casting alloys. Mater. Corros. 2017, 68, 961–969. [Google Scholar] [CrossRef]
- Tahamtan, S.; Boostani, A.F. Evaluation of pitting corrosion of thixoformed A356 alloy using a simulation model. Trans. Nonferrous Met. Soc. China 2010, 20, 1702–1706. [Google Scholar] [CrossRef]
- Ye, H. An Overview of the development of Al-Si-Alloy based material for engine applications. J. Mater. Eng. Perform. 2003, 12, 288–297. [Google Scholar] [CrossRef]
- Chena, C.L.; West, G.; Thomsonb, R.C. Characterisation of intermetallic phases in multicomponent Al-Si casting alloys for engineering applications. Mater. Sci. Forum 2006, 519–521, 359–364. [Google Scholar] [CrossRef]
- Donatus, U.; Thompson, G.E.; Omotoyinbo, J.A.; Alaneme, K.K.; Aribo, S.; Agbabiaka, O.G. Corrosion pathways in aluminum alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 55–62. [Google Scholar] [CrossRef]
- Tupaj, M.; Orłowicz, A.W.; Mróz, M.; Trytek, A.; Markowska, O. The effect of cooling rate on properties of intermetallic phase in a complex Al-Si alloy. Arch. Foundry Eng. 2016, 16, 125–128. [Google Scholar] [CrossRef]
- Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Beta Al5FeSi phase platelets-porosity formation relationship in A319.2 type alloys. Inter. J. Met. Cast. 2018, 12, 55–70. [Google Scholar] [CrossRef]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E. Iron intermetallic phases in the Al corner of the Al–Si–Fe system. Metall. Mater. Trans. A 2003, A34, 807–825. [Google Scholar] [CrossRef]
- Kral, M. A crystallographic identification of intermetallic phases in Al-Si alloys. Mater. Lett. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- Shabestari, S.G. The effect of iron and manganese on the formation of intermetallic compounds in aluminum–silicon alloys. Mater. Sci. Eng. 2004, A383, 289–298. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Taylor, J.A.; Schaffer, G.B.; Stjohn, D.H. The role of iron in the formation of porosity in Al-Si-Cu–based casting alloys: Part II. a phase-diagram approach. Metall. Mater. Trans. A 1999, 30A, 1651–1655. [Google Scholar] [CrossRef]
- Závodská, D.; Tillová, E.; Guagliano, M.; Chalupová, M.; Kucharikova, L. Effect of porosity on the fatigue behavior of AlZn10Si8Mg casting alloys in a high cycle region. Procedia Eng. 2017, 192, 988–993. [Google Scholar] [CrossRef]
- Kučera, V.; Vojtěch, D. Influence of heat treatment on corrosion behavior and mechanical properties of the AA 7075 alloy. Manuf. Technol. 2017, 17, 747–752. [Google Scholar]
- Linder, J. The influence of surrounding environment on the fatigue properties for a high pressure die cast AlSi9Cu3 alloy. Fatigue Fract. Eng. Mater. Struct. 2007, 30, 759–765. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Macciò, S.; Luciano, G.; Canepa, E.; Traverso, P. Thermal and corrosion behavior of as cast Al-Si alloys with rare earth elements. J. Alloys Compd. 2017, 695, 2180–2189. [Google Scholar] [CrossRef]
- Camicia, G.; Timelli, G. Grain refinement of gravity die cast secondary AlSi7Cu3Mg alloys for automotive cylinder heads. Trans. Nonferrous Met. Soc. China 2016, 26, 1211–1221. [Google Scholar] [CrossRef]
- Vicen, M.; Fabian, P.; Tillová, E. Self-hardening AlZn10Si8Mg aluminum alloy as an alternative replacement for AlSi7Mg0.3 aluminum alloy. Arch. Foundry Eng. 2017, 17, 139–142. [Google Scholar] [CrossRef]
- Ďuriníková, E.; Tillová, E.; Chalupová, M. Phase and structure characteristics of recycled AlZn10Si8Mg cast alloy. Manuf. Technol. 2011, 11, 11–17. [Google Scholar]
- Tillová, E.; Chalupová, M.; Kuchariková, L.; Závodská, D.; Belan, J.; Vaško, A. Use of microscopy in the study of self-hardening al-alloy for automotive application. Manuf. Technol. 2016, 16/5, 1174–1179. [Google Scholar]
- Moustafa, M.A. Effect of iron content on the formation of β-Al5FeSi and porosity in Al–Si eutectic alloys. J. Mater. Process. Technol. 2009, 209, 605–610. [Google Scholar] [CrossRef]
- Ferraro, S.; Timelli, G. Influence of sludge particles on the tensile properties of die-cast secondary aluminum alloys. Metall. Mater. Trans. B 2015, 46B, 1022–1034. [Google Scholar] [CrossRef]
- Aziz, P.M.; Godard, H.P. Pitting corrosion characteristics of aluminum—The influence of iron and silicon. Corrosion 1954, 10, 269–272. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Pardo, A.; Matykina, E.; Skeldon, P. 3D study of intermetallics and their effect on the corrosion morphology of rheocast aluminum alloy. Mater. Charact. 2016, 112, 122–128. [Google Scholar] [CrossRef]
- Khireche, S.; Boughrara, D.; Kadri, A.; Hamadou, L.; Benbrahim, N. Corrosion mechanism of Al, Al–Zn and Al–Zn–Sn alloys in 3 wt. % NaCl solution. Corros. Sci. 2014, 87, 504–516. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International: Metals Park, OH, USA, 1993; pp. 579–622. [Google Scholar]
- Osório, W.R.; Goulart, P.R.; Santos, G.A.; Neto, C.M.; Garcia, A. Effect of dendritic arm spacing on mechanical properties and corrosion resistance of Al 9 Wt Pct Si and Zn 27 Wt Pct Al Alloys. Metall. Mater. Trans. A 2006, 37A, 2525–2538. [Google Scholar] [CrossRef]
- Gharavi, F.; Matori, K.A.; Yunus, R.; Othman, N.K. Corrosion behavior of friction stir welded lap joints of AA6061-T6 aluminum alloy. Mater. Res. 2014, 17, 672–681. [Google Scholar] [CrossRef] [Green Version]
- Knight, S.P.; Salagaras, M.; Trueman, A.R. The study of intergranular corrosion in aircraft aluminum alloys using X-ray tomography. Corros. Sci. 2011, 53, 727–734. [Google Scholar] [CrossRef]






| Alloy | Si | Fe | Cu | Mn | Mg | Zn | Ti | Na | Al | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 7.028 | 0.123 | 0.013 | 0.009 | 0.354 | 0.036 | 0.123 | 0.002 | 92.253 | balance |
| B | 7.34 | 0.454 | 0.021 | 0.009 | 0.302 | 0.02 | 0.118 | 0.004 | 91.673 | balance |
| C | 7.315 | 0.655 | 0.03 | 0.01 | 0.292 | 0.028 | 0.12 | 0.005 | 91.486 | balance |
| D | 8.703 | 0.150 | 0.008 | 0.013 | 0.381 | 10.001 | 0.05 | 0.002 | 80.64 | balance |
| E | 8.831 | 0.559 | 0.008 | 0.019 | 0.319 | 9.335 | 0.049 | 0.002 | 80.828 | balance |
| Alloy | Ecorr (mV) | icorr (µA/cm2) | rcorr (mm/year) |
|---|---|---|---|
| A | −824.333 ±34 | 0.85367 ±0.0259 | 0.022 ±0.001 |
| B | −833.333 ±32 | 0.821 ±0.0137 | 0.01333 ±0.001 |
| C | −850.666 ±11 | 0.49133 ±00.161 | 0.008067 ±0.001 |
| D | −984 ±23 | 2.457 ±0.0567 | 0.04 ±0.003 |
| E | −1022.33 ±38 | 2.49133 ±0.0192 | 0.04 ±0.002 |
| Alloy | Corrosion rate (g/m2) |
|---|---|
| A | 0.00768 |
| B | 0.175957 |
| C | 0.132133 |
| D | 0.23014 |
| E | 0.840229 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchariková, L.; Liptáková, T.; Tillová, E.; Kajánek, D.; Schmidová, E. Role of Chemical Composition in Corrosion of Aluminum Alloys. Metals 2018, 8, 581. https://doi.org/10.3390/met8080581
Kuchariková L, Liptáková T, Tillová E, Kajánek D, Schmidová E. Role of Chemical Composition in Corrosion of Aluminum Alloys. Metals. 2018; 8(8):581. https://doi.org/10.3390/met8080581
Chicago/Turabian StyleKuchariková, Lenka, Tatiana Liptáková, Eva Tillová, Daniel Kajánek, and Eva Schmidová. 2018. "Role of Chemical Composition in Corrosion of Aluminum Alloys" Metals 8, no. 8: 581. https://doi.org/10.3390/met8080581
APA StyleKuchariková, L., Liptáková, T., Tillová, E., Kajánek, D., & Schmidová, E. (2018). Role of Chemical Composition in Corrosion of Aluminum Alloys. Metals, 8(8), 581. https://doi.org/10.3390/met8080581






