Abstract
In this work, the effect of pH (3, 7 and 10) on the stress corrosion cracking behavior of 6082 aluminum alloy, in a 0.3 M sodium chloride (NaCl) aqueous based solution was investigated. The stress corrosion cracking behavior was studied with slow strain rate testing, whereas failure analysis of the fractured surfaces was used to identify the dominant degradation mechanisms. The experimental results clearly indicated that stress corrosion cracking behavior of this aluminum alloy strongly depends on the pH of the solution. In particular, the highest drop in ultimate tensile strength and ductility was observed for the alkaline pH, followed by the acidic, whereas the lowest susceptibility was observed in the neutral pH environment. This observation is attributed to a change in the dominant stress corrosion cracking mechanisms.
1. Introduction
Wrought aluminum-magnesium-silicon alloys (6xxx) have been extensively used in the automotive, shipbuilding and construction industry [,,]. One of the most popular aluminum-magnesium-silicon alloys is 6082, since it has good corrosion resistance, light weight, excellent fabricability and low cost [,,]. Despite the excellent properties of this alloy, one the most common risks of failure is due to the synergism between mechanical loading and corrosion, as in the case of stress corrosion cracking (SCC). Indeed, such aggressive conditions are frequently encountered in various industrial and technological applications (e.g., structural and engine components in passenger and transport ships) where 6082 aluminum alloy components are used. It has been clearly shown that the majority of aluminum alloys are susceptible to SCC [,,,,,,,,]. For this reason, many researchers have tried to improve the SCC resistance of aluminum alloys by utilizing different approaches such as thermomechanical [] and post heat [] treatments, as well as surface modifications [] and deposition of coatings [].
Despite the many existing studies on the SCC behavior of aluminum alloys [,,,,,,,,,,,,], very little is actually known about the SCC susceptibility of the widely applied 6082 aluminum alloy. In addition, most of the previous work has been performed under neutral pH environments, neglecting the significant effect of pH on the occurring mechanisms. Indeed, it has been found [] that the pH of the environment can have a strong influence on the SCC behavior of Al alloys. This is considered extremely important for the aluminum alloys that are applied in e.g., the chemical and/or nuclear industry.
Recent reports have shown that the properties of 6082 alloy are strongly dependent on the surrounding environment. For example, the sliding wear behavior of this alloy can significantly change under an aqueous environment, whereas the addition of inhibitors also has a strong effect []. In addition, the microstructure is closely related to the SCC behavior of wrought aluminum alloys. It has been proved that heat treatments [] and forming processes [] of aluminum-magnesium or similar alloys can result in changes in the size and distribution of precipitates, which have a strong effect on the SCC behavior. Indeed, intermetallic phases are known to improve the mechanical performance of aluminum alloys [,], but can also lead to localized galvanic corrosion [,,]. In addition, the size and distribution of grains should be taken into consideration as grain boundaries are easy paths for the diffusion of halide ions [], but also present obstacles to dislocation movement and multiplication during mechanical deformation []. Thus, it is of key importance to correlate the microstructure with the functionality of the alloy.
In this research, a detailed investigation of the effect of a Cl− based environment with alkaline, neutral and acidic pH on the stress corrosion cracking behavior of 6082 alloy is investigated. The aim is to establish a structure-property relationship by evaluating the synergism between mechanical loading and corrosion. This is achieved by performing slow strain rate tests (SSRT) [] and linking them to the SCC mechanisms. Finally, it should be mentioned that, to date there is no published data on the effect of pH on the stress corrosion cracking resistance of the extensively applied 6082 aluminum alloy.
2. Materials and Methods
The material used in this study was a commercially supplied 6082 wrought aluminum alloy sheet. Its wt.% chemical composition was 1.1% Si, 1% Mg, 0.7% Mn, 0.1% Cu, 0.5% Fe and Al made up the balance. Specimens of 40 mm × 20 mm × 2 mm were cut from this sheet for the corrosion experiments. Standardized ASTM E-8M tensile specimens were also cut from the same sheet. A schematic of the tensile sample is given in Figure 1a. All specimens were then mechanical grinded with SiC paper with increasing finishes, until the resulting roughness was approximately 0.4 μm (measured with a Marr Perthen Profilometer—Mahr GmbH, Göttingen, Germany). The roughness tests were considered essential so as to ensure that the surface topography of all samples was similar.
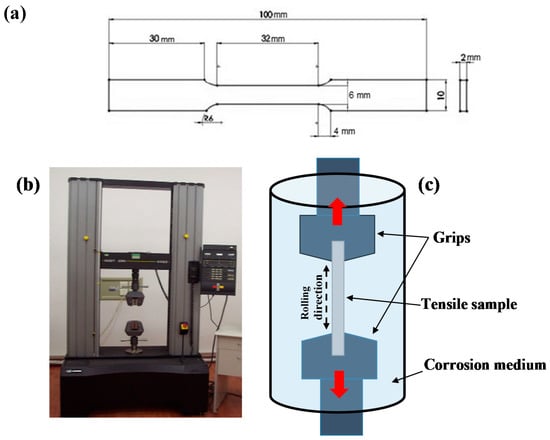
Figure 1.
(a) ASTM E-8M tensile specimen; (b) Instron 4482 apparatus; and (c) schematic of stress corrosion setup.
After grinding, the specimens were annealed at 415 °C for 2 h in an Ar inert furnace and then slowly cooled to room temperature to remove residual stresses and bring them to O conditions (reference condition). Following the annealing, a T6 treatment was performed in the same furnace. This T6 process consisted of solution treatment at 550 °C for 1 h, followed by water quenching at room temperature and then aging 160 °C for 18 h. To reveal the microstructure of this alloy, chemical etching was performed by dipping in Graff’s reagent (84 mL H2O, 10 mL HNO3, 0.5 mL HF, 3 g Cr2O3) for 30 min and then rinsing with distilled water and ethanol.
The stress corrosion cracking (SCC) behavior of T6 treated 6082 aluminum alloy was determined by slow strain rate tests (SSRT) performed in an Instron 4482 (Instron, Norwood, MA, USA) apparatus (Figure 1b). Prior to testing, the grips were isolated to avoid the formation of any galvanic coupling (between the specimen and the grip) and/or contamination of the solution due to corrosion of the grips. The SSRT experiments were conducted in 0.3 M sodium chloride (NaCl) solution with three different pH values (pH = 3, 7 and 10) at 25 °C. A schematic of the SCC cell is given in Figure 1c. For each test 2 L of corrosive solution was used, and the pH of the solution was measured before and after the end of each test. In all experiments a strain rate of 10−6 s−1 was used. Reference tests were also performed in air at the same strain rate. For each pH and for the reference test in air, three independent tests were performed, and the values presented are the average of those results. Potentiostatic tests were also performed to investigate the evolution of the open circuit potential (OCP) of T6 treated aluminum alloy in the different pH by using a Potentiostat-Galvanostat and standardized electrochemical cells by EG&G (Edgerton, Germeshausen and Grier Inc., Gaithersburg, MD, USA). A standard calomel electrode (SCE) was used as the reference electrode and platinum as the counter electrode.
To identify the SCC mechanisms a Jeol 6100 Scanning Electron Microscope (JEOL, Akishima, Tokyo, Japan), connected to an energy dispersive X-ray analyzer (EDS) was used. In addition, the SCC products were investigated with a Siemens D5000 X-ray diffractometer (Bruker, Billerica, MA, USA) with Cu Ka radiation, Cu filter and a graphite monochromator.
3. Results and Discussion
Figure 2a shows the typical microstructure of a T6 treated 6082 aluminum alloy. Apart from the aluminum solid solution, two different intermetallic phases could be observed, namely Al96Fel0Mnl4Sil8 and All4Fe3Si3 []. These phases appear to be well distributed within the matrix of this alloy. Further electron backscattering analysis at the grain boundaries of the alloy revealed the presence of fine β″ (Mg5Si6) precipitates [], which are mainly dispersed along the grain boundaries (see Figure 2b).
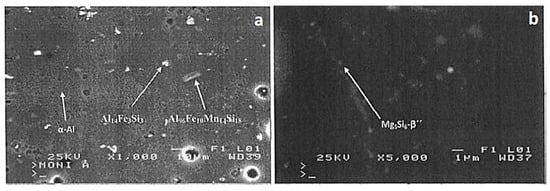
Figure 2.
SEM micrographs showing (a) the microstructure of the T6 treated 6082 aluminum alloy and (b) the distribution of β″ (Mg5Si6) precipitates along the grain boundaries of this alloy.
Figure 3 shows indicative stress strain curves obtained from the slow strain rate test (SSRT) experiments in the 0.3 M sodium chloride (NaCl) solution with different pH values (3,7 and 10). From this figure it can be seen that in all SCC cases a decrease of ductility (ε%) was observed, Table 1. This drop was more pronounced when the test was performed in an alkaline environment (pH = 10). In particular, the ductility of T6 treated 6082 aluminum alloy decrease from 12.1% (reference test in air) to 8.4%. This indicates a significant loss of ductility in the range of 30.6%. When the test was performed in an acidic environment (pH = 3), the ductility decreased from 12.1% to 9.2% (24% ductility loss) and in a neutral environment (pH = 7) it decreased to 11.3% (6.6% ductility loss). These results clearly illustrate the risk of embrittlement, even for ductile alloys such as aluminum alloys.
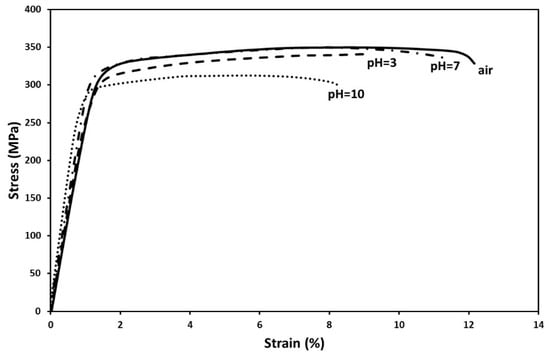
Figure 3.
Effect of the pH of 0.3 M NaCl solution on the stress-strain curves of T6 treated 6082 aluminum alloy, obtained from the slow strain rate test (SSRT).

Table 1.
Effect of the pH of 0.3 M NaCl solution on the ultimate tensile strength and ductility of T6 treated 6082 aluminum alloy.
In addition, when comparing the ultimate tensile strength (UTS) of 6082 alloy obtained from the SCC tests with that of the reference test in air, a significant decrease was observed only in the case of the alkaline environment, Table 1. A small drop was observed in the case of the acidic environment, whereas no difference was found in the UTS when performing tests in a neutral pH. To understand the different SCC behavior of T6 treated 6082 aluminum alloy in respect to the pH of the solution, fractography and surface analysis were performed to identify the main failure mechanisms and study the influence of microstructure on the SCC behavior of this alloy.
Figure 4 presents SEM fractographies of 6082 aluminum alloy specimens after SSRT experiments in air (reference), acidic, neutral and alkaline environments respectively. In air, the main failure mechanisms were ductile (formation of dimples) and intergranular fracture (Figure 4a). A similar fracture pattern was also observed for the alloy tested under a neutral pH (Figure 4c). This confirms the similar mechanical behavior presented in Figure 3. However, localized brittle fracture zones were found in the outer layer of 6082 aluminum alloy after SSRT testing in the acidic and alkaline environment (Figure 4b,d, respectively). In both cases, the ductile fracture mechanism is maintained in the deeper layers of the alloy.
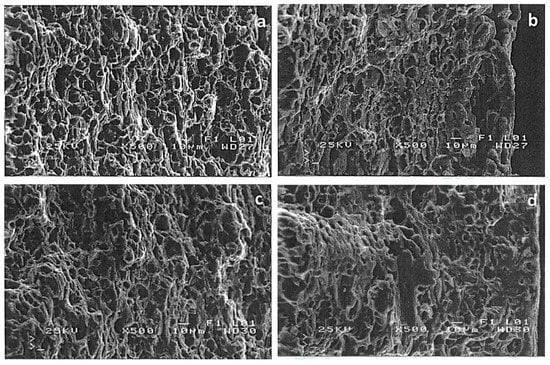
Figure 4.
SEM analysis of the fractured surface of T6 treated 6082 aluminum alloy, after SSRT testing in (a) air, (b) pH = 3, (c) pH = 7 and (d) pH = 10.
Further analysis of the brittle zones shown in Figure 4b,d revealed cleavage-like morphologies [], Figure 5a,b, respectively. In particular, at the surface layers of T6 treated 6082 Al alloy after SSRT testing in acidic pH, a brittle cleavage (flat surface patterns indicated by red arrows in Figure 5a) and ductile fracture identified by microvoids/dimples (indicated by yellow arrows in Figure 5a) were observed. This pattern is attributed to the presence of precipitates along the grain boundaries (Figure 2b) which provide an easy path for crack propagation via anodic dissolution [,]. It is believed that, the large difference in potential between the precipitates and aluminum matrix can create strong galvanic couples []. These galvanic couples can cause sharp crack advancement, resulting in an intergranular SCC mechanism []. In addition, brittle fracture by cleavage (indicated by blue arrows in Figure 5b) can be seen at the surface layers of the alloy after SSRT testing in alkaline pH. This brittle pattern is a result of both pinning of dislocations by the solute hydrogen atoms and to the formation and fracture of aluminum hydrides [,].
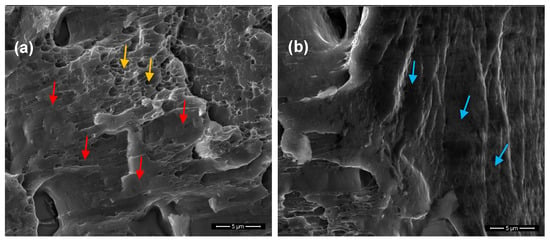
Figure 5.
SEM analysis of brittle zones on the outer surface of the fractured surface of T6 treated 6082 aluminum alloy, after SSRT testing in (a) pH = 3 and (b) pH = 10.
Analysis of the surface near the fractured area for all tested samples are presented in Figure 6. Some grinding lines due to the initial grinding can be discerned. In this figure, some distinctive patterns can be observed in each environment. For the 6082 aluminum alloy, which was SSRT tested in an acidic environment, an extended layer of corrosion products was observed. This layer was cracked due to tensile stresses (as indicated in Figure 6b). The corrosion products on the surface of the alloy were identified by XRD analysis to be mainly aluminum oxides (Al2O3), see Figure 7. However, when the SSRT tests were performed in a neutral pH, very limited corrosion products were observed (Figure 6c) and the surface morphology appeared to be very similar to that of the reference sample (experiment in air), see Figure 6a,c. In the case of SSRT testing in an alkaline pH, intergranular corrosion phenomena were seen (Figure 6d). This observation is attributed to the existence of precipitates along the grain boundaries, which have a different corrosion potential than that of the aluminum matrix. In particular, the β″ (Mg5Si6) precipitates might have a similar corrosion potential to the aluminum matrix, but intermetallic phases such a-(AlFeMnSi) and Al-Si-Mg have a higher corrosion potential when compared to the same matrix. Thus, galvanic corrosion can take place at the grain boundaries. Furthermore, some aluminum oxides (Al2O3) were detected on the surface of the alloy after SSRT test in pH = 10, but to a lesser extent in comparison to those formed in the acidic environment (Figure 6).
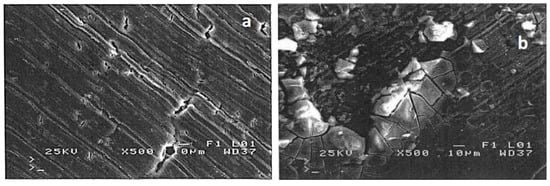

Figure 6.
SEM analysis of the surface near the fractured area of T6 treated 6082 aluminum alloy, after SSRT testing in (a) air, (b) pH = 3, (c) pH = 7 and (d) pH = 10.
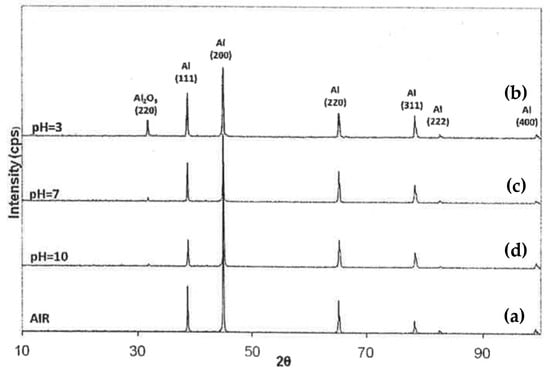
Figure 7.
X-ray diffraction (XRD) analysis of the surface of T6 treated 6082 aluminum alloy, after SSRT testing in (a) air, (b) pH = 3, (c) pH = 7 and (d) pH = 10.
From the fractographic analysis of the samples after SSRT testing it is evident that the change in pH resulted in different surface morphologies. To get a better insight into the effect of corrosion on the surface of 6082 aluminum alloy and to better understand the synergism between corrosion and wear, potentiostatic corrosion experiments were performed. Figure 8 shows the evolution of open circuit potential (OCP) of T6 treated 6082 aluminum alloy as a function of immersion time, for the three examined pH values of 0.3 M sodium chloride (NaCl) aqueous solution. From this figure, it can be observed that the OCP of the specimens at pH = 7 and 10 was more noble than that of pH = 3. Indeed, it is known that the pH of the corrosion environment can affect the stability of the oxide film (Al2O3), according to the Pourbaix diagram []. In particular, in neutral pH values a stable Al2O3 oxide film is formed, passivating 6082 aluminum alloy and hindering the diffusion of the Cl− halide ions. Therefore, the corrosion phenomena are more intense in the acidic pH, when compared to neutral. In addition, hydrogen uptake will occur in an environment with alkaline pH []. However, it should be also taken into consideration that stress corrosion cracking involves the synergism between electrochemical and mechanical aspects. For example, mechanical stresses can accelerate the diffusion of halide and/or hydrogen ions enhancing corrosion and/or embrittlement phenomena []. On the other hand, the formation of corrosion products and surface defects (e.g., pits, blisters) are known to degrade the mechanical performance of materials [].
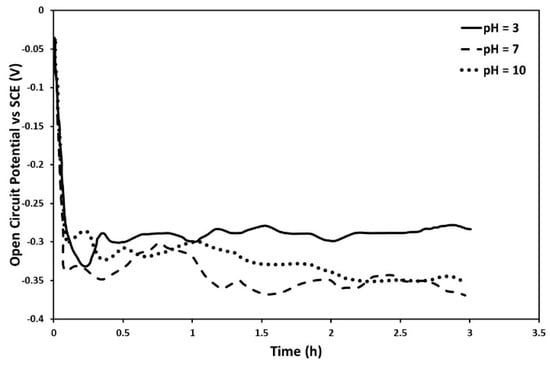
Figure 8.
Effect of pH of 0.3 M NaCl solution on the open circuit potential (ocp) of T6 treated 6082 aluminum alloy as a function of immersion time.
To better compare these different SCC conditions, the ratio of time-to-fracture (rtf) index was evaluated. This index is calculated according to the equation rtf = tfe/tfc, where tfe and tfc are the time to failure for the testing and control (air) environments, respectively. Figure 9a shows the rtf value of T6 treated 6082 aluminum alloy as a function of the pH of solution. From this figure it can be seen that this alloy is more susceptible to stress corrosion cracking in the alkaline environment, whereas the influence was very small at the neutral pH. In addition, the effect of pH on the stress corrosion resistance of T6 treated 6082 Al alloy was quantitatively assessed by using the susceptibility index ISCC []. This index is calculated by the following equation ISCC = [(εair – εscc)/εair] × 100, where εair is the elongation after testing in air (reference test) and εscc is the elongation after the SCC test. This index can be also described in terms of UTS []. The SCC susceptibility index is given in Figure 9b. Similar to the rtf index, the ISCC index also confirms that the alkaline, followed by the acidic pH has the strongest influence on both the ductility and UTS of the alloy.
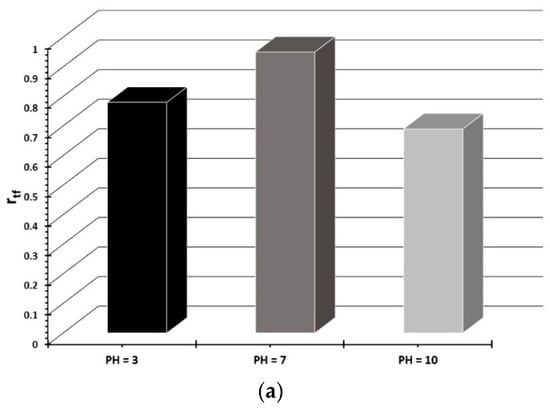
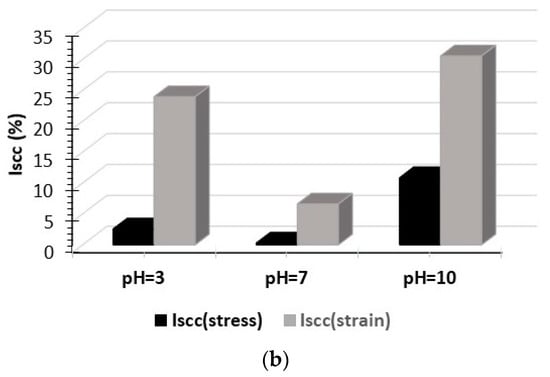
Figure 9.
Effect of pH of 0.3 M NaCl solution on (a) the ratio of time-to-fracture (rtf) index and (b) the ISCC susceptibility index of T6 treated 6082 aluminum alloy.
To date, it is still unknown if the main stress corrosion cracking mechanism in Al-Mg-Si alloys is the easy path corrosion or the hydrogen embrittlement []. The authors believe that, in the case of acidic pH value (pH = 3) an intergranular corrosion cracking mechanism takes place. On the other hand, when the SCC tests were performed in an alkaline environment (pH = 10), a hydrogen embrittlement mechanism is dominant. This can also be explained by McCaferty’s theory [,]. According to this theory in alkaline environments, when the pH is higher than the pzc, the surface of the aluminum oxide (Al2O3) has a net negative charge and cations (H+) can be adsorbed. After this, the cations can penetrate or dissolve through the oxide film and dissolve or form hydrides with the underlying metal. It has been proved that solute hydrogen atoms often act as dislocation pinning sites, which increase the surface hardness []. This theory explains the high arrogation loss of the material when tested in alkaline environments and the low rtf value.
4. Conclusions
This research deals with the effect of pH on the stress corrosion cracking behavior of 6082 alloy in a NaCl solution. The main outcomes of this work are summarized in the following points:
- The pH of the NaCl solution had a strong influence on the ultimate tensile strength and ductility of T6 treated 6082 Al alloy. In particular, a drop in the ultimate tensile strength and ductility was recorded in the alkaline pH, followed by the acidic. In the neutral pH, the influence was minimal when compared to the reference sample (experiments in air).
- Different stress corrosion cracking mechanisms occurred in the different pHs. In particular, a brittle and intergranular mode was observed on the outer layers of the alloy in the acidic pH, galvanic corrosion together with hydrogen embrittlement in the alkaline pH and mild intergranular corrosion along with ductile fracture in the neutral pH.
- The thickness of the brittle layer was observed to be dependent on the pH conditions, as it was thicker in the alkaline environment, followed by the acidic, whereas it was very limited in the neutral pH.
- In all examined SCC cases, an aluminum oxide (Al2O3) film was detected on the surface of T6 treated 6082 Al aluminum.
Author Contributions
C.N.P. conceived and designed the experiments, C.N.P. and E.G. wrote the paper, C.N.P. and K.I.G. analyzed the data, P.G.O. performed the experiments.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Society for Metals; Metals Handbook Committee. Metals Handbook, Properties and Selection: Nonferrous Alloys and Pure Metals, 9th ed.; ASM: Metals Park, OH, USA, 1979. [Google Scholar]
- Polmear, I.J. Light Alloys: Metallurgy of the Light Metals, 3rd ed.; MMS: London, UK, 1995. [Google Scholar]
- Panagopoulos, C.N.; Georgiou, E.P.; Gavras, A.G. Corrosion and wear of 6082 Al alloy. Tribol. Int. 2009, 42, 886–889. [Google Scholar] [CrossRef]
- Kramer, L.S.; Blair, T.P.; Blough, S.D.; Fisher, J.J.; Pickens, J.R. Stress-Corrosion Cracking Susceptibility of Various Product Forms of Aluminum Alloy 2519. J. Mater. Eng. Perform. 2002, 11, 645–650. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.K.; Park, J.C. The corrosion and mechanical properties of Al alloy 5083-H116 in metal inert gas welding based on slow strain rate test. Surf. Coat. Technol. 2010, 205, S73–S78. [Google Scholar] [CrossRef]
- Balogun, S.A.; Esezobor, D.E.; Adeosun, S.O. Stress Corrosion Cracking of Cast 6063 and Deep Drawn 1017 Aluminum Utensils In Lycopersicum esculentum. J. Mater. Eng. Perform. 2007, 16, 720–725. [Google Scholar] [CrossRef]
- Ghosh, R.; Venugopal, A.; Ramesh Narayanan, P.; Sharma, S.C.; Venkitakrishnan, P.V. Environmentally assisted cracking resistance of Al-Cu-Li alloy AA2195 using slow strain rate test in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2017, 27, 241–249. [Google Scholar] [CrossRef]
- Obert, B.; Ngo, K.; Hashemi, J.; Ekwaro-Osire, S.; Sivam, T.P. An Investigation of the Reduction in Tensile Strength and Fatigue Life of Pre-Corroded 7075-T6 Aluminum Alloy. J. Mater. Eng. Perform. 2000, 9, 441–448. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Khaled, M.; Karatas, C. Corrosion Properties and Morphology of Laser Melted Aluminum Alloy 8022 Surface. J. Mater. Eng. Perform. 2009, 18, 1–7. [Google Scholar] [CrossRef]
- Lin, J.-C.; Liao, H.-L.; Jehng, W.-D.; Chang, C.-H.; Lee, S.-L. Effect of heat treatments on the tensile strength and SCC-resistance of AA7050 in an alkaline saline solution. Corros. Sci. 2006, 48, 3139–3156. [Google Scholar] [CrossRef]
- Popovic, M.; Romhanji, E. Stress corrosion cracking susceptibility of Al-Mg alloy sheet with high Mg content. J. Mat. Proc. Technol. 2002, 125–126, 275–280. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Z.Y. Effect of pre-strain on microstructure and stress corrosion cracking of over-aged 7050 aluminum alloy. J. Alloys Compd. 2009, 469, 445–450. [Google Scholar] [CrossRef]
- Ghosh, R.; Venugopal, A.; Sankaravelayudham, P.; Panda, R.; Sharma, S.C.; George, K.M.; Raja, V.S. Effect of Thermomechanical Treatment on the Environmentally Induced Cracking Behavior of AA7075 Alloy. J. Mater. Eng. Perform. 2015, 24, 545–555. [Google Scholar] [CrossRef]
- Ghosh, R.; Venugopal, A.; Sudarshan Rao, G.; Ramesh Narayanan, P.; Pant, B.; Cherian, R.M. Effect of Temper Condition on the Corrosion and Fatigue Performance of AA2219 Aluminum Alloy. J. Mater. Eng. Perform. 2018, 27, 423–433. [Google Scholar] [CrossRef]
- Yu, J.; Gou, G.; Zhang, L.; Zhang, W.; Chen, H.; Yang, Y.P. Ultrasonic Impact Treatment to Improve Stress Corrosion Cracking Resistance of Welded Joints of Aluminum Alloy. J. Mater. Eng. Perform. 2016, 25, 3046–3056. [Google Scholar] [CrossRef]
- Schubbe, J.J.; Westmoreland, S.N. Effects of Chromate and Non-Chromate Coating Systems on Environmentally Assisted Fatigue of an Aluminum Alloy. J. Mater. Eng. Perform. 2014, 23, 3534–3540. [Google Scholar] [CrossRef]
- Rout, P.K.; Ghosh, M.M.; Ghosh, K.S. Effect of solution pH on electrochemical and stress corrosion cracking behavior of a 7150 Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2014, 604, 156–165. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P.; Giannakopoulos, K. The effect of heat treatment on the corrosion behaviour of 319 cast aluminium alloy. Mater. Corros. 2009, 60, 415–418. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P. Cold rolling and lubricated wear of 5083 aluminium alloy. Mater. Des. 2010, 31, 1050–1055. [Google Scholar] [CrossRef]
- Georgiou, E.P.; Celis, J.P.; Panagopoulos, C.N. The Effect of Cold Rolling on the Hydrogen Susceptibility of 5083 Aluminum Alloy. Metals 2017, 7, 45. [Google Scholar] [CrossRef]
- Kim, S.J.; Jang, S.K. A Slow Strain Rate Test Experiment to Evaluate the Characteristics of High-Strength Al-Mg Alloy for Application in Ships. Mater. Sci. Forum 2006, 510–511, 162–165. [Google Scholar] [CrossRef]
- Aginagalde, A.; Gomez, X.; Galdos, L.; Garcia, C. Heat Treatment Selection and Forming Strategies for 6082 Aluminum Alloy. J. Eng. Mat. Technol. 2009, 131, 044501. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. Atomic model for GP-zones in a 6082 Al-Mg-Si system. Acta Mater. 2001, 49, 321–328. [Google Scholar] [CrossRef]
- American Society for Metals; Metals Handbook Committee. Metals Handbook, Metallography and Microstructures, 9th ed.; ASM: Metals Park, OH, USA, 1985. [Google Scholar]
- Lotto, C.A. Stress corrosion cracking: Characteristics, mechanisms and experimental study. Int. J. Adv. Manuf. Technol. 2017, 93, 3567–3582. [Google Scholar] [CrossRef]
- Argade, G.R.; Kumar, N.; Mishra, R.S. Stress corrosion cracking susceptibility of ultrafine grained Al-Mg-Sc alloy. Mater. Sci. Technol. A 2013, 565, 80–89. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P. The effect of hydrogen charging on the mechanical behaviour of 5083 wrought aluminum alloy. Corros. Sci. 2007, 49, 4443–4451. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H. Effect of artificial aging on intergranular corrosion of extruded AlMgSi alloy with small Cu content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering, 3rd ed.; McGraw-Hill Book Company: New York, NY, USA, 1987. [Google Scholar]
- Ilin, D.N.; Saintier, N.; Olive, J.M.; Abgrall, R.; Aubert, I. Simulation of hydrogen diffusion affected by stress-strain heterogeneity in polycrystalline stainless steel. Int. J. Hydrogen Energy 2014, 39, 2418–2422. [Google Scholar] [CrossRef]
- Padekar, B.S.; Singh Raman, P.K.; Raja, V.S.; Paul, L. Stress corrosion cracking of recent rare-earth containing magnesium alloy, EV31A, and a common Al-containing alloy, AZ91E. Corros. Sci. 2013, 71, 1–9. [Google Scholar] [CrossRef]
- Haruna, T.; Kouno, T.; Fujimoto, S. Electrochemical conditions for environment-assisted cracking of 6061 Al alloy. Corros. Sci. 2005, 47, 2441–2449. [Google Scholar] [CrossRef]
- McCafferty, E. The electrode kinetics of pit initiation on aluminum. Corros. Sci. 1995, 37, 481–492. [Google Scholar] [CrossRef]
- McCafferty, E. Sequence of steps in the pitting of aluminum by chloride ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]
- Rozenak, P.; Ladna, B.; Bimbaum, H.K. SIMS study of deuterium distribution in chemically charged aluminum containing oxide layer defects and trapping sites. J. Alloys Compd. 2006, 415, 134–142. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).